High Temperature Anti-Oxidation Behavior and Mechanical Property of Radio Frequency Magnetron Sputtered Cr Coating
Abstract
1. Introduction
2. Materials and Experimental Procedure
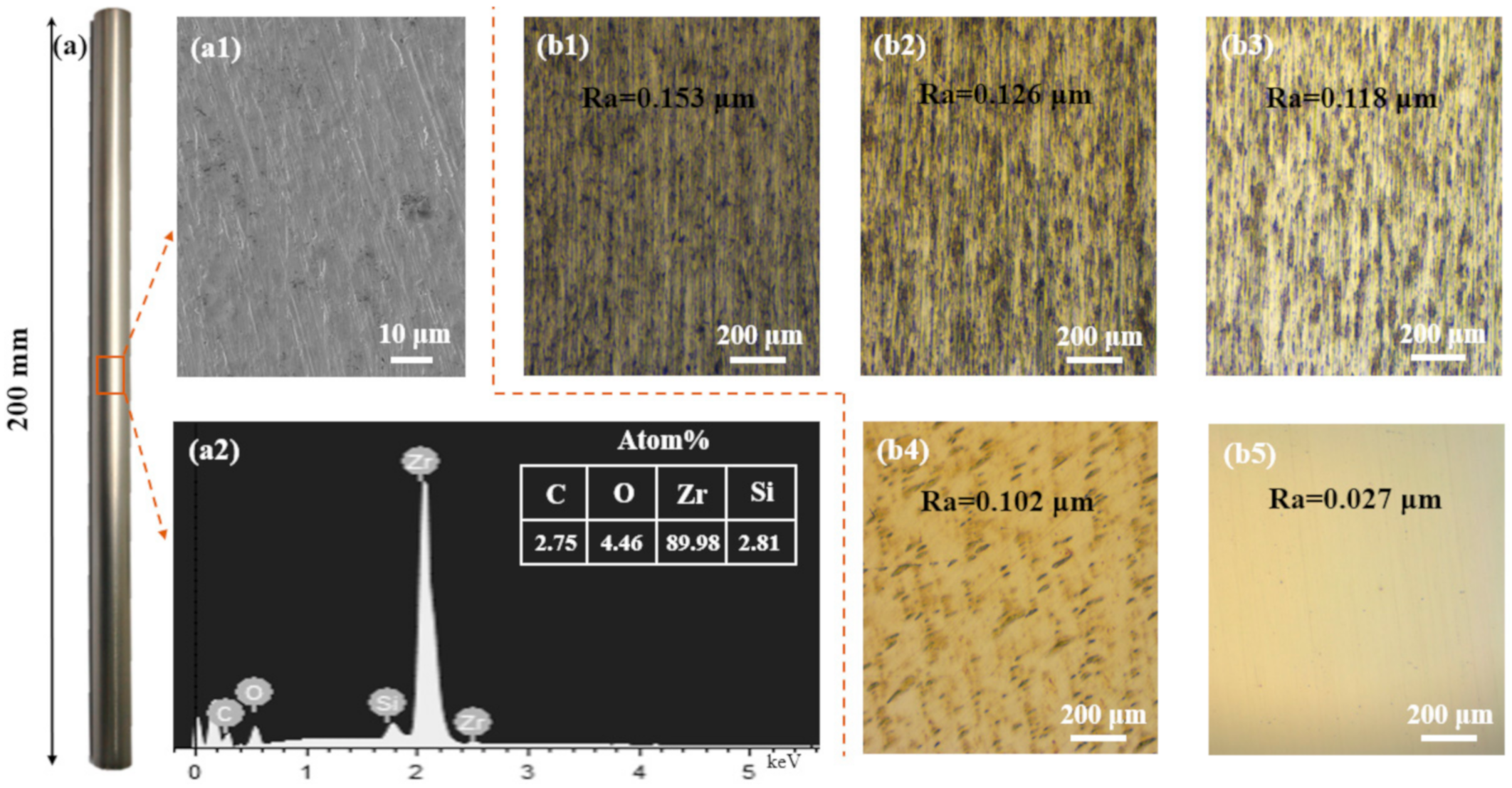
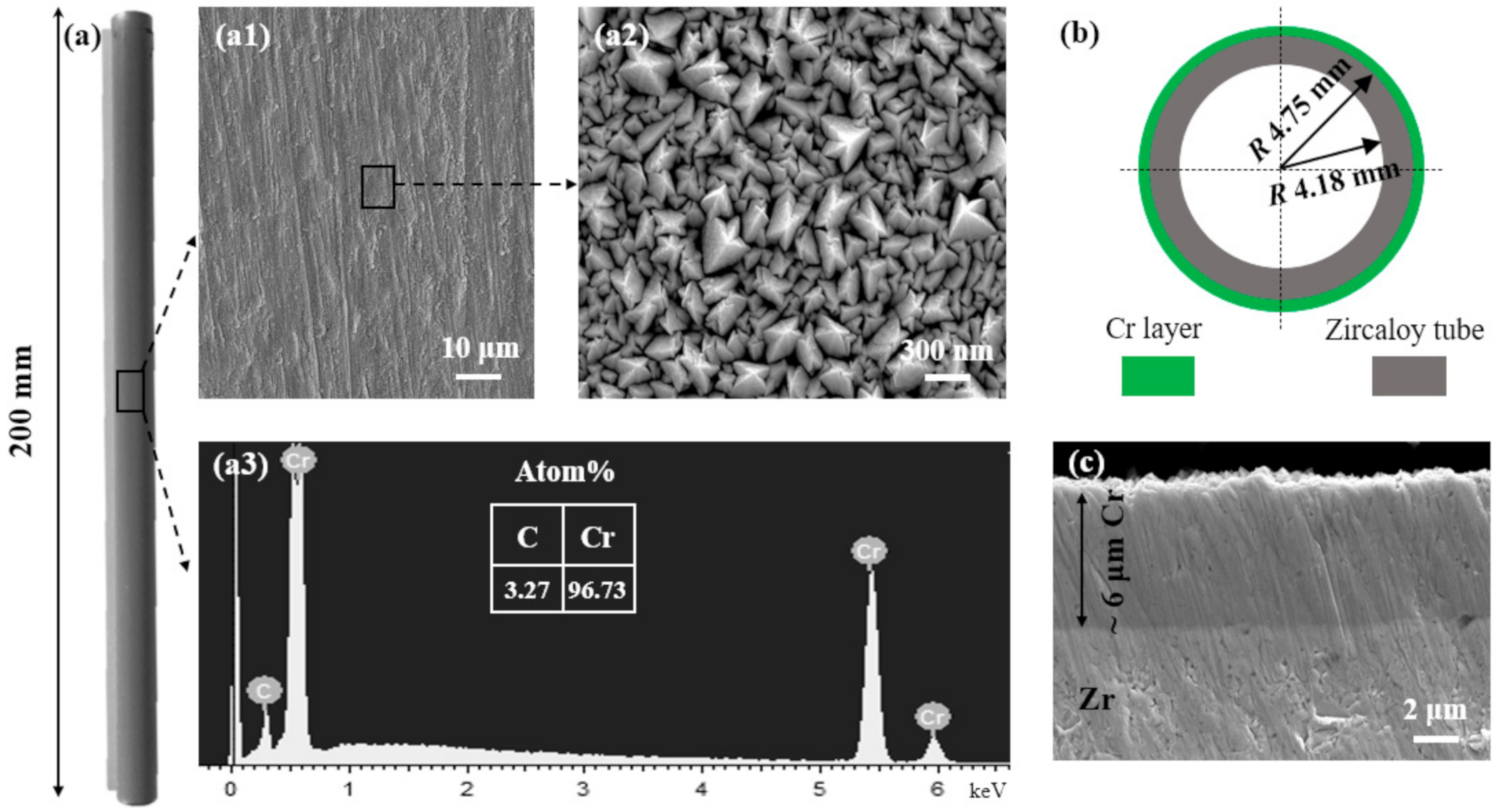
2.1. Materials and Coating Deposition
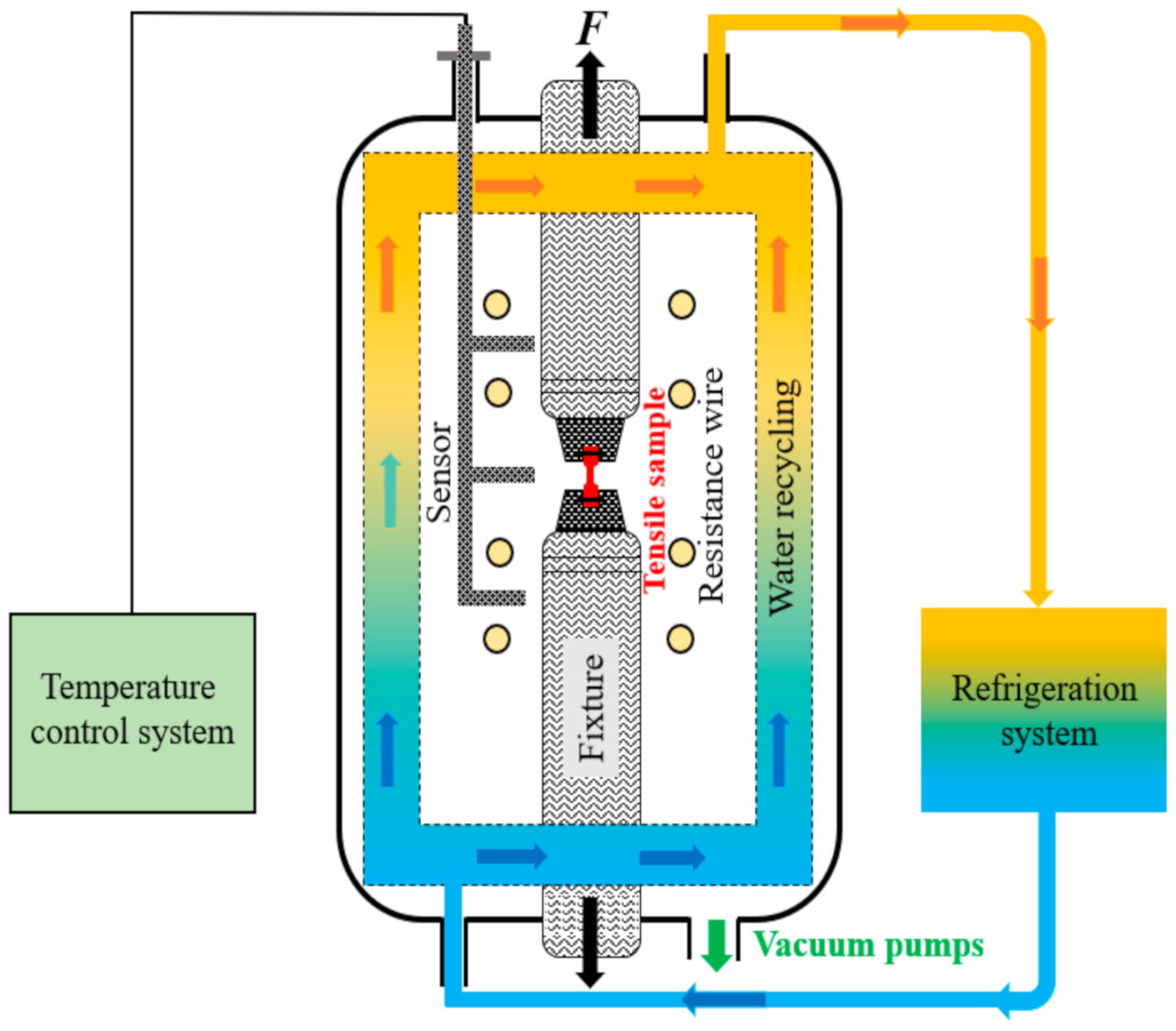
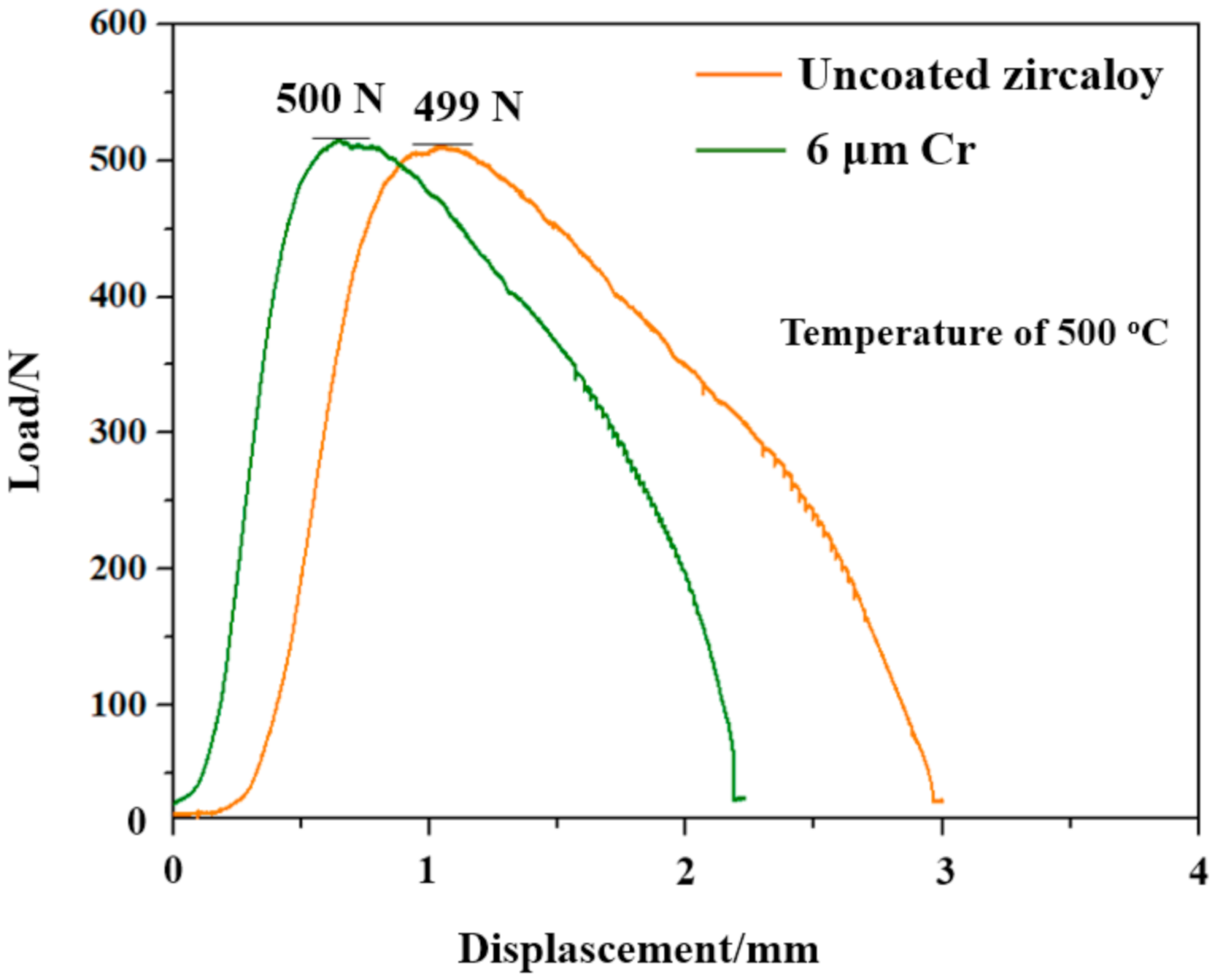
2.2. Tensile Tests
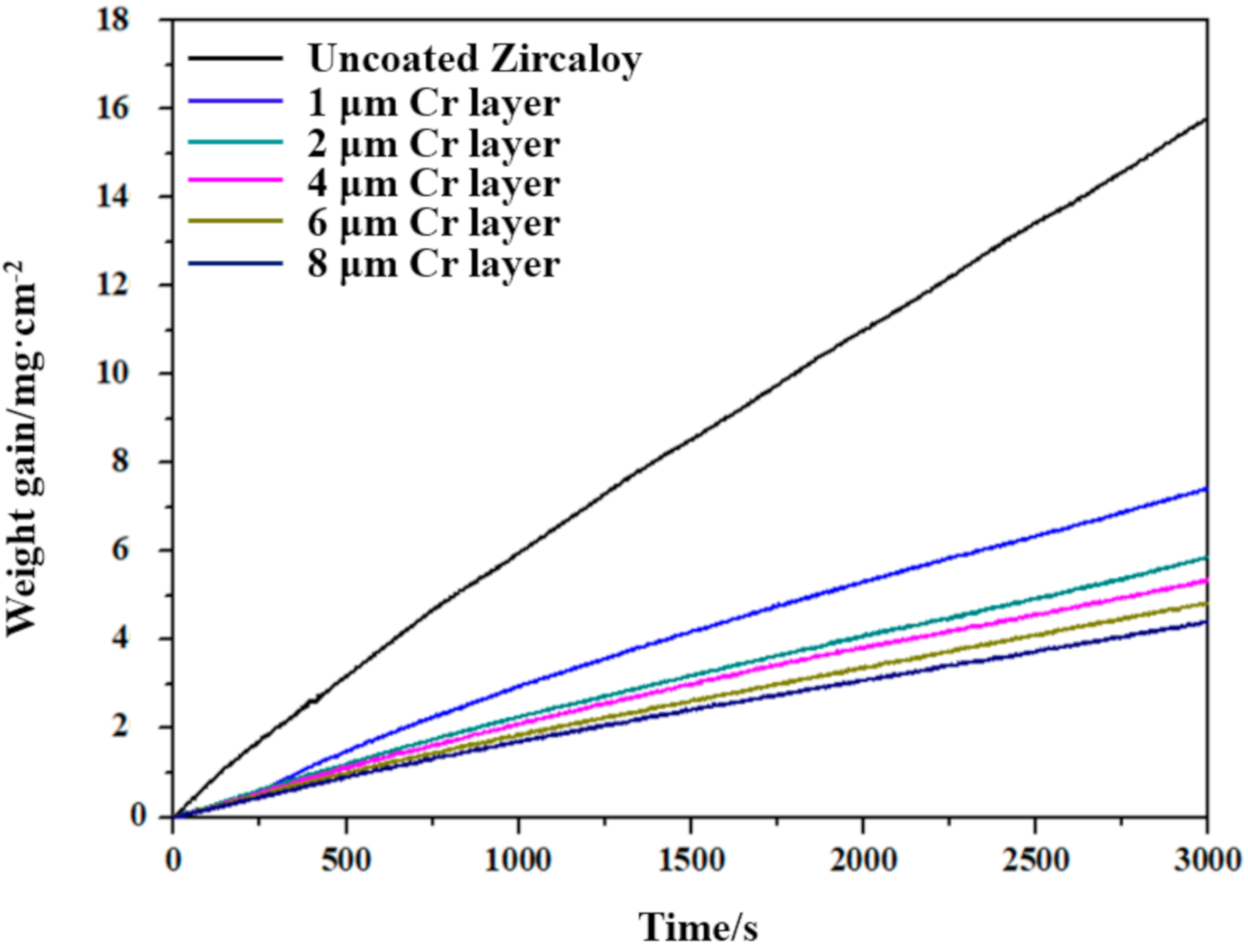
2.3. 1200 °C Steam Oxidation Tests
2.4. Characterization
3. Results and Discussion
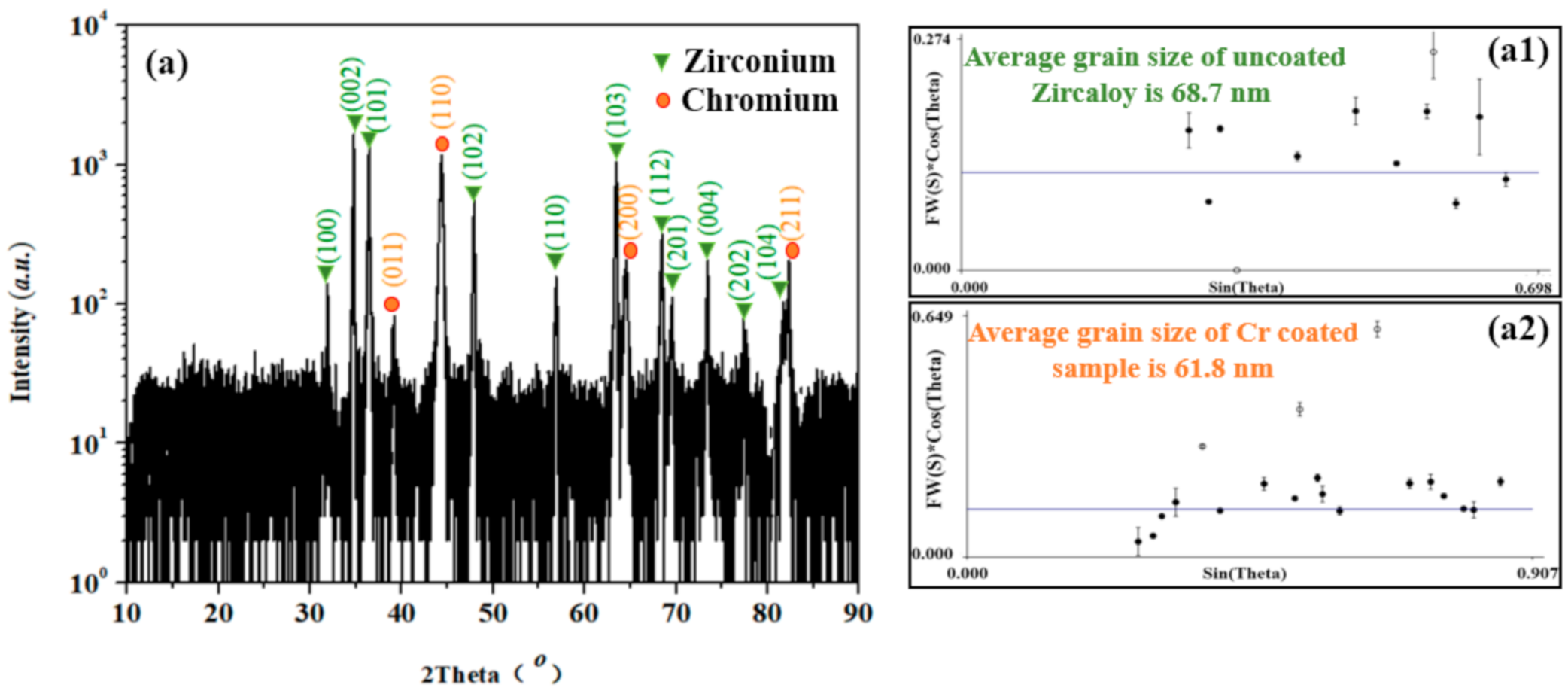
3.1. Morphology Structure and Phase Composition
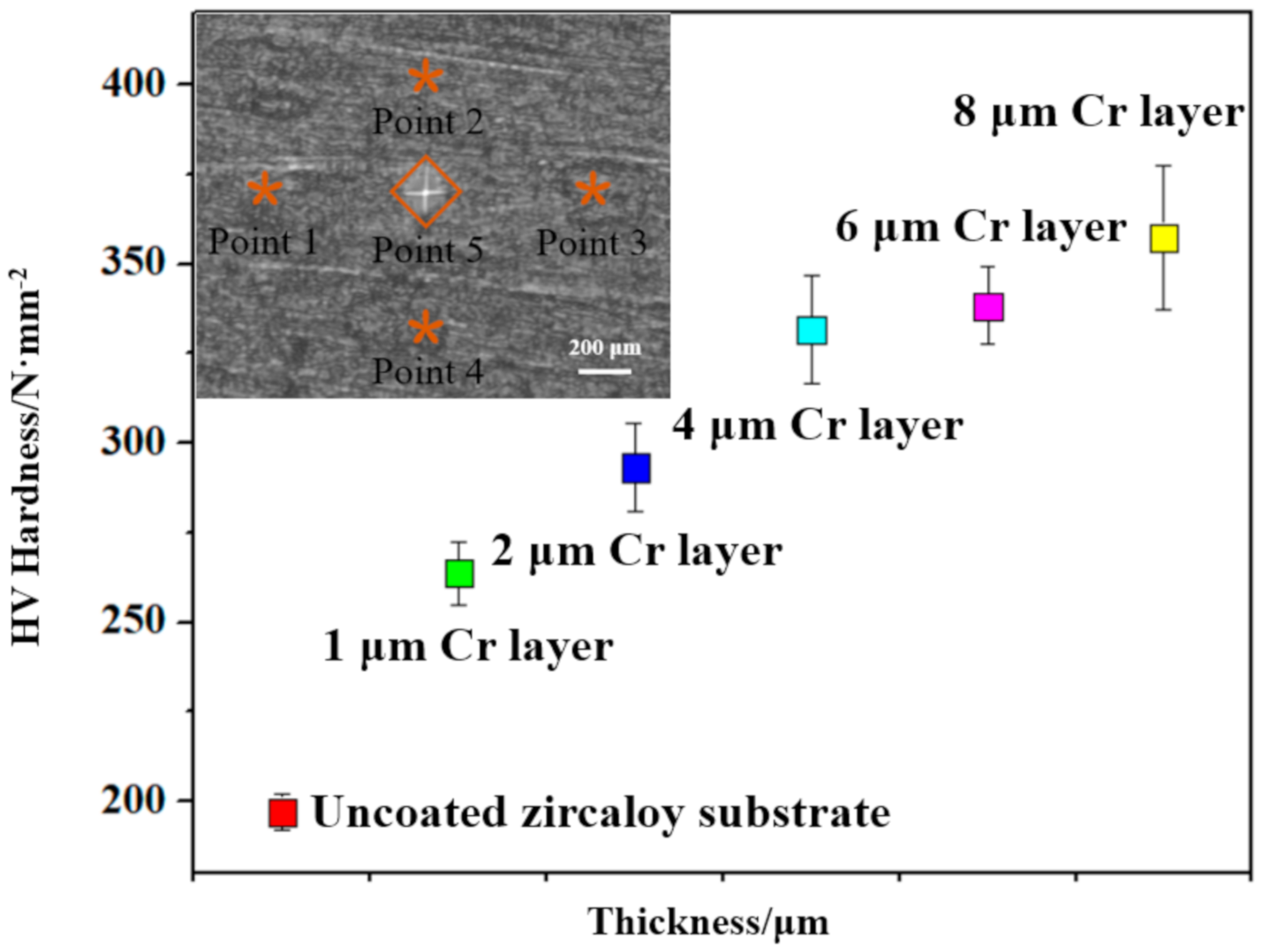
3.2. Mechanical Properties
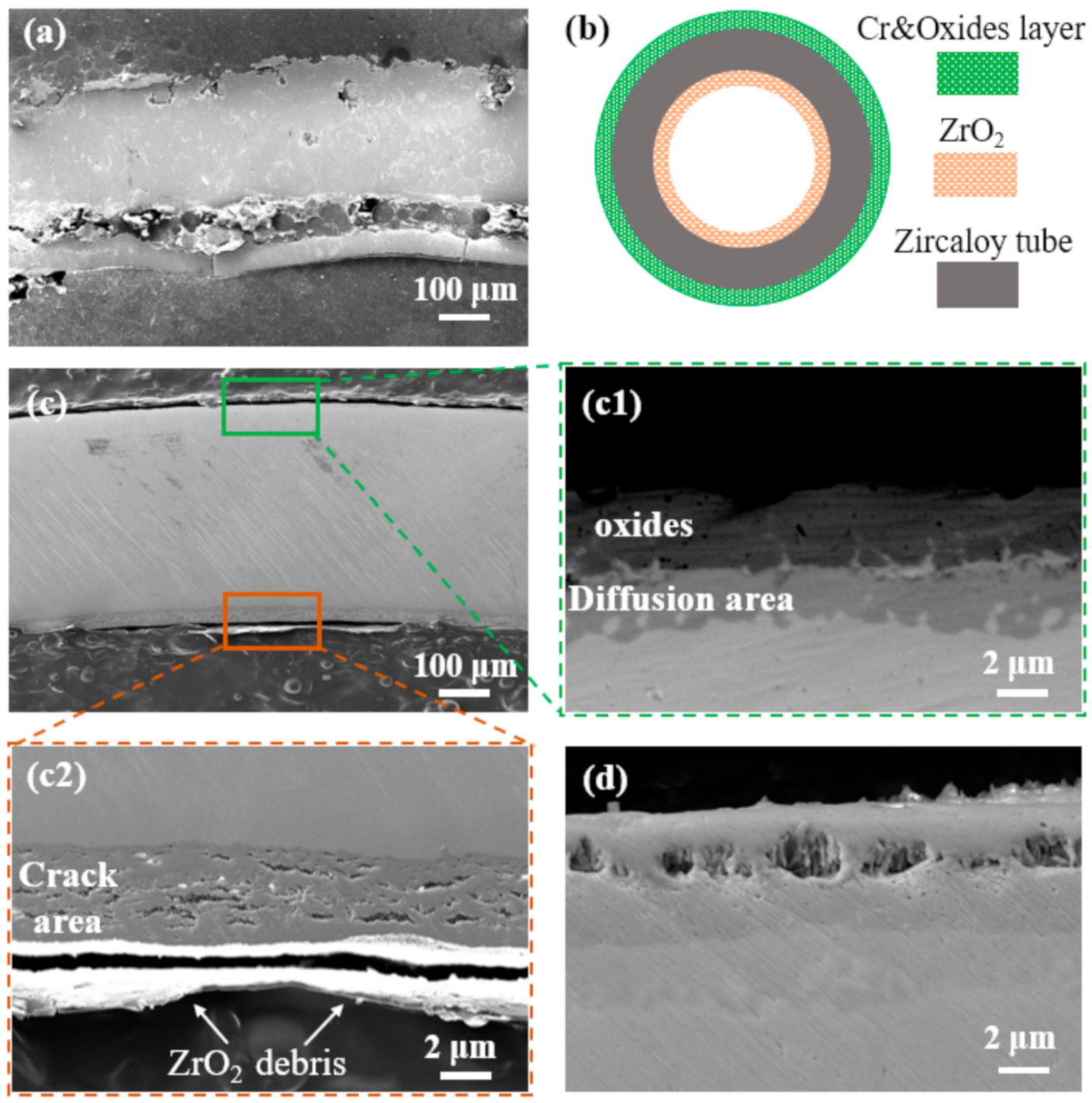
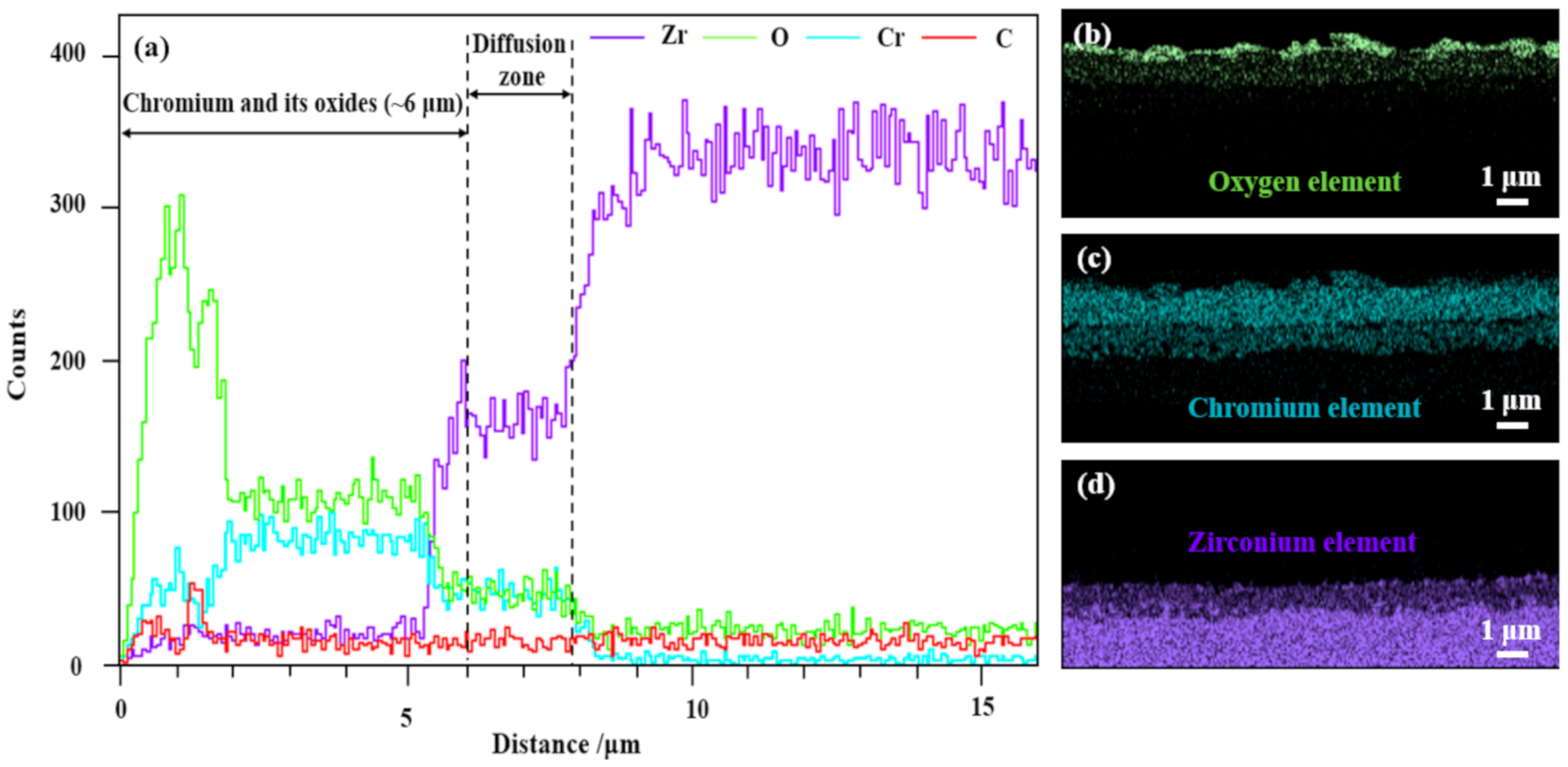
3.3. Oxidation Resistance Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Kimura, A.; Kasada, R.; Iwata, N.; Kishimoto, H.; Zhang, C.H.; Isselin, J.; Dou, P.; Lee, J.H.; Muthukumar, N.; Okuda, T.; et al. Development of Al added high-Cr ODS steels for fuel cladding of next generation nuclear systems. J. Nucl. Mater. 2011, 417, 176–179. [Google Scholar] [CrossRef]
- Sidelev, D.V.; Kashkarov, E.B.; Syrtanov, M.S.; Krivobokov, V.P. Nickel-chromium (Ni–Cr) coatings deposited by magnetron sputtering for accident tolerant nuclear fuel claddings. Surf. Coat. Technol. 2019, 369, 69–78. [Google Scholar] [CrossRef]
- Duan, Z.; Yang, H.; Satoh, Y.; Murakami, K.; Kano, S.; Zhao, Z.; Shen, J.; Abe, H. Current status of materials development of nuclear fuel cladding tubes for light water reactors. Nucl. Eng. Des. 2017, 316, 131–150. [Google Scholar] [CrossRef]
- Hallstadius, L.; Johnson, S.; Lahoda, E. Cladding for high performance fuel. Prog. Nucl. Energy 2012, 57, 71–76. [Google Scholar] [CrossRef]
- Cheng, B.; Kim, Y.J.; Chou, P. Improving accident tolerance of nuclear fuel with coated Mo-alloy cladding. Nucl. Eng. Technol. 2016, 48, 16–25. [Google Scholar] [CrossRef]
- Kim, I.; Khatkhatay, F.; Jiao, L.; Swadener, G.; Cole, J.I.; Gan, J.; Wang, H. TiN-based coatings on fuel cladding tubes for advanced nuclear reactors. J. Nucl. Mater. 2012, 429, 143–148. [Google Scholar] [CrossRef]
- Kuprin, A.S.; Belous, V.A.; Voyevodin, V.N.; Bryk, V.V.; Vasilenko, R.L.; Ovcharenko, V.D.; Reshetnyak, E.N.; Tolmachova, G.N.; V’yugov, P.N. Vacuum-arc chromium-based coatings for protection of zirconium alloys from the high-temperature oxidation in air. J. Nucl. Mater. 2015, 405, 400–406. [Google Scholar] [CrossRef]
- Daub, K.; van Nieuwenhove, R.; Nordin, H. Investigation of the impact of coatings on corrosion and hydrogen uptake of Zircaloy-4. J. Nucl. Mater. 2015, 467, 260–270. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef]
- Foroughi-Abari, A.; Xu, C.; Cadien, K.C. The effect of argon pressure, residual oxygen and exposure to air on the electrical and microstructural properties of sputtered chromium thin films. Thin Solid Film. 2012, 520, 1762–1767. [Google Scholar]
- Wang, Y.; Zhou, W.; Wen, Q.; Ruan, X.; Luo, F.; Bai, G.; Qing, Y.; Zhu, D.; Huang, Z.; Zhang, Y.; et al. Behavior of plasma sprayed Cr coatings and FeCrAl coatings on Zr fuel cladding under loss-of-coolant accident conditions. Surf. Coat. Technol. 2018, 344, 141–148. [Google Scholar]
- Su, R.; Zhang, H.; Meng, X.; Shi, L.; Liu, C. Synthesis of Cr2AlC thin films by reactive magnetron sputtering. Fusion Eng. Des. 2017, 125, 562–566. [Google Scholar]
- Pedersen, K.; Bøttiger, J.; Sridharan, M.; Sillassen, M.; Eklund, P. Texture and microstructure of Cr2O3 and (Cr,Al)2O3 thin films deposited by reactive inductively coupled plasma magnetron sputtering. Thin Solid Film. 2010, 518, 4294–4298. [Google Scholar]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.Y.; Koo, Y.H. Adhesion property and high-temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar]
- Kuprin, A.S.; Belous, V.A.; Voyevodin, V.N.; Vasilenko, R.L.; Ovcharenko, V.D.; Tolstolutskaya, G.D.; Kopanets, I.E.; Kolodiy, I.V. Irradiation resistance of vacuum arc chromium coatings for zirconium alloy fuel claddings. J. Nucl. Mater. 2018, 510, 163–167. [Google Scholar]
- Kane, K.A.; Stack PI, M.; Mouche, P.A.; Pillai, R.R.; Pint, B.A. Steam oxidation of chromium corrosion barrier coatings for sic-based accident tolerant fuel cladding. J. Nucl. Mater. 2020, 543, 152561. [Google Scholar]
- Ševeček, M.; Gurgen, A.; Seshadri, A.; Che, Y.; Wagih, M.; Phillips, B.; Champagne, V.; Shirvan, K. Development of Cr cold spray–coated fuel cladding with enhanced accident tolerance. Nucl. Eng. Technol. 2018, 50, 229–236. [Google Scholar]
- Hu, X.; Dong, C.; Wang, Q.; Chen, B.; Yang, H.; Wei, T.; Zhang, R.; Gu, W.; Chen, D. High-temperature oxidation of thick Cr coating prepared by arc deposition for accident tolerant fuel claddings. J. Nucl. Mater. 2019, 519, 145–156. [Google Scholar]
- Chen, Q.S.; Liu, C.H.; Zhang, R.Q.; Yang, H.Y.; Wei, T.G.; Wang, Y.; Li, Z.; He, L.X.; Wang, J.; Wang, L.; et al. Microstructure and high-temperature steam oxidation properties of thick Cr coatings prepared by magnetron sputtering for accident tolerant fuel claddings: The role of bias in the deposition process. Corros. Sci. 2020, 165, 108378. [Google Scholar]
- Li, G.; Liu, Y.; Zhang, Y.; Li, H.; Wang, X.; Zheng, M. Oxidation behavior of RF magnetron sputtered Cr-SiC-Cr composites coating on zircaloy fuel cladding. Mater. Res. Express 2019, 6, 096434. [Google Scholar]
- Yang, H.; Pan, S. Effect of Substrate Surface Roughness on Bond Strength of Coatings. Hot Work. Technol. 2008, 37, 118–121. [Google Scholar] [CrossRef]
- Hung, W.; Liu, M.; Li, Z.; Zeng, R. Thickness effects on corrosion and wear resistance properties of micro-arc discharge oxide coatings on AZ91D magnesium alloys. Trans. Nonferrous Met. Soc. China 2006, 16, 1827–1830. [Google Scholar]
- Yeom, H.; Maier, B.; Johnson, G.; Dabney, T.; Lenling, M.; Sridharan, K. High temperature oxidation and microstructural evolution of cold spray chromium coatings on Zircaloy-4 in steam environments. J. Nucl. Mater. 2019, 526, 151737. [Google Scholar]
- Wang, T.G.; Jeong, D.; Liu, Y.; Wang, Q.; Iyengar, S.; Melin, S.; Kim, K.H. Study on nanocrystalline Cr2O3 films deposited by arc ion plating: II. Mechanical and tribological properties. Surf. Coat. Technol. 2012, 206, 2638–2644. [Google Scholar]
- Barshilia, H.C.; Rajam, K.S. Growth and characterization of chromium oxide coatings prepared by pulsed-direct current reactive unbalanced magnetron sputtering. Appl. Surf. Sci. 2008, 255, 2925–2931. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Liu, Y.; Zhang, Y.; Li, H.; Wang, X.; Zheng, M.; Li, Y. High Temperature Anti-Oxidation Behavior and Mechanical Property of Radio Frequency Magnetron Sputtered Cr Coating. Metals 2020, 10, 1509. https://doi.org/10.3390/met10111509
Li G, Liu Y, Zhang Y, Li H, Wang X, Zheng M, Li Y. High Temperature Anti-Oxidation Behavior and Mechanical Property of Radio Frequency Magnetron Sputtered Cr Coating. Metals. 2020; 10(11):1509. https://doi.org/10.3390/met10111509
Chicago/Turabian StyleLi, Guangbin, Yanhong Liu, Yingchun Zhang, Huailin Li, Xiaojing Wang, Mingmin Zheng, and Yusha Li. 2020. "High Temperature Anti-Oxidation Behavior and Mechanical Property of Radio Frequency Magnetron Sputtered Cr Coating" Metals 10, no. 11: 1509. https://doi.org/10.3390/met10111509
APA StyleLi, G., Liu, Y., Zhang, Y., Li, H., Wang, X., Zheng, M., & Li, Y. (2020). High Temperature Anti-Oxidation Behavior and Mechanical Property of Radio Frequency Magnetron Sputtered Cr Coating. Metals, 10(11), 1509. https://doi.org/10.3390/met10111509




