Vacuum Brazing of 55 vol.% SiCp/ZL102 Composites Using Micro-Nano Brazing Filler Metal Fabricated by Melt-Spinning
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
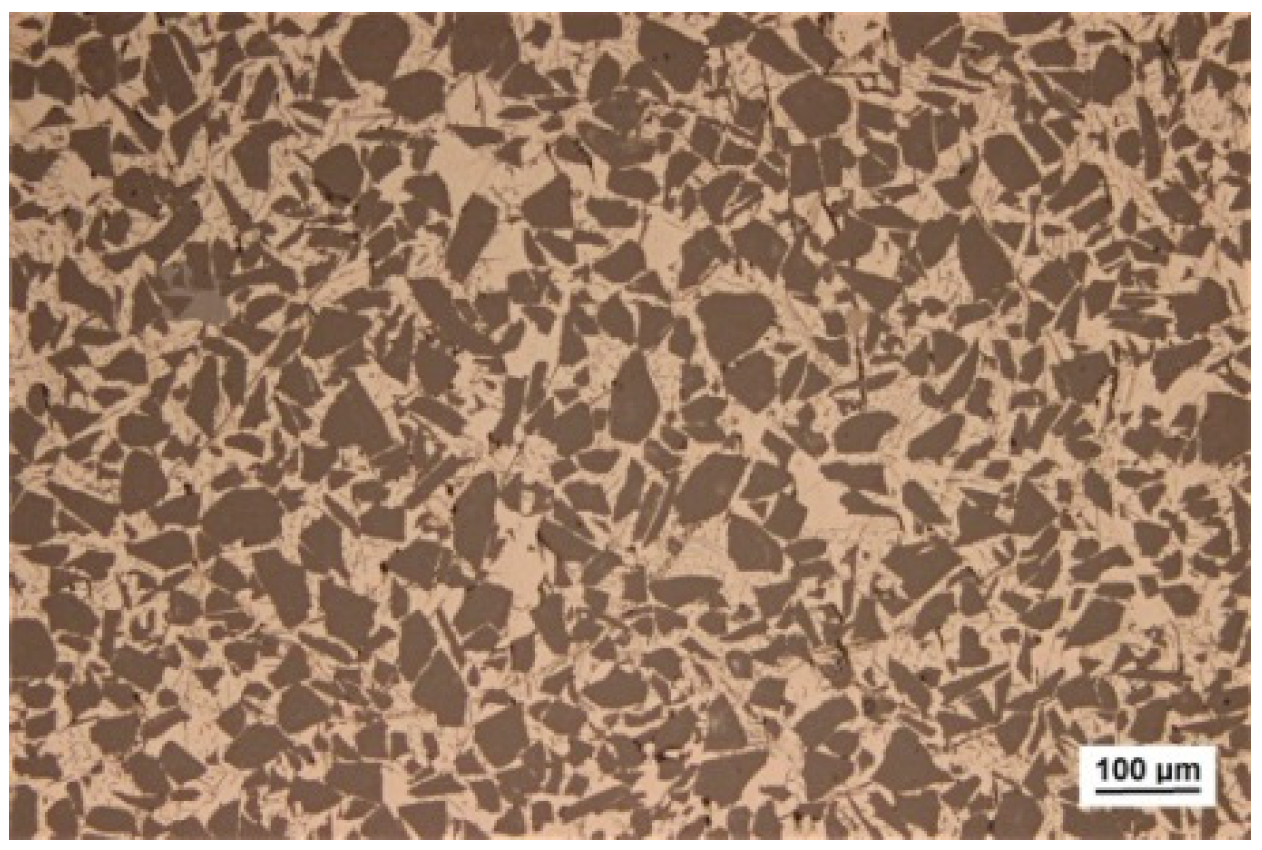
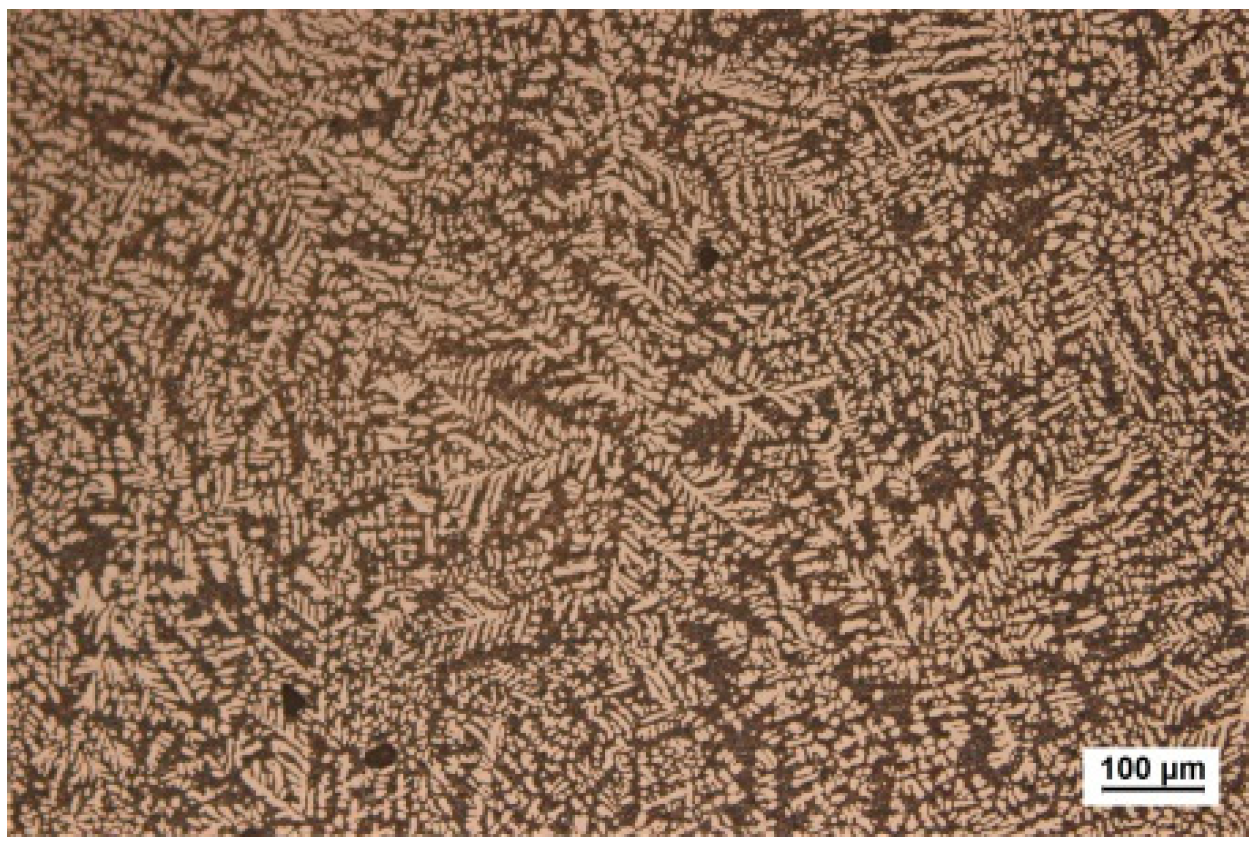
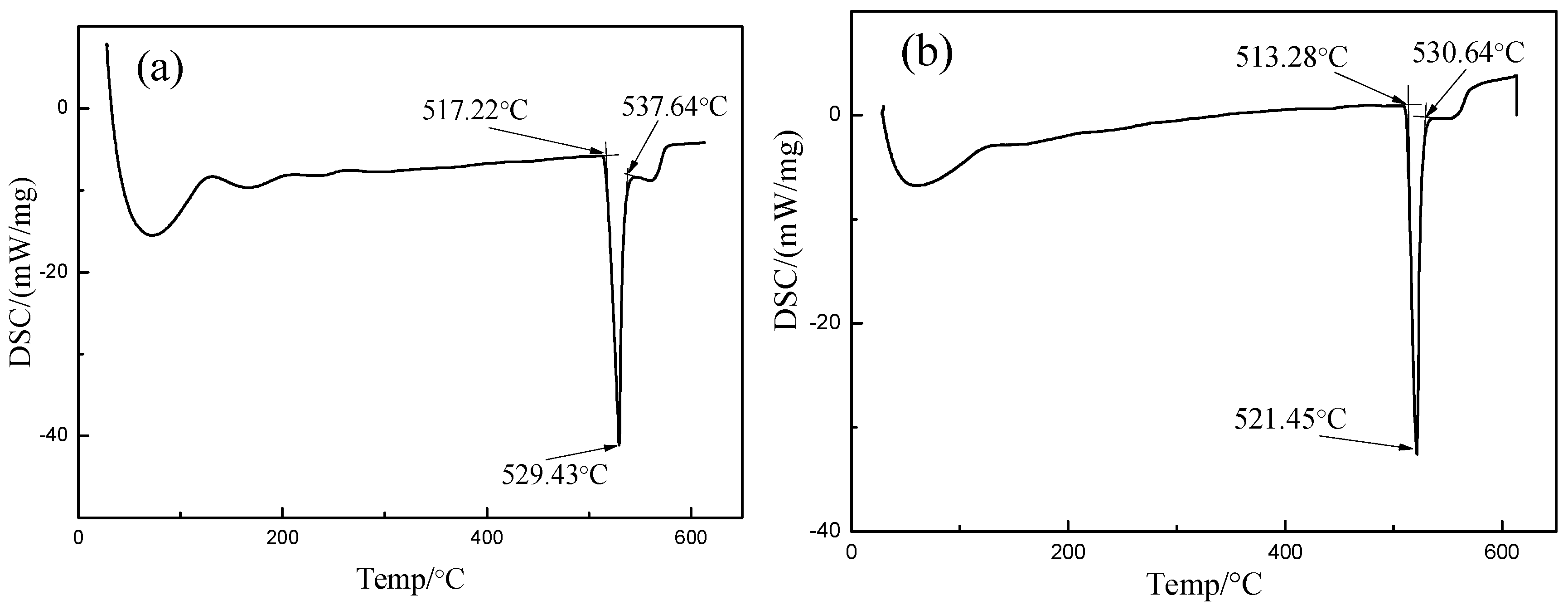
3.1. Thermodynamic Property and Microstructural Characteristics of the Brazing Filler Metal
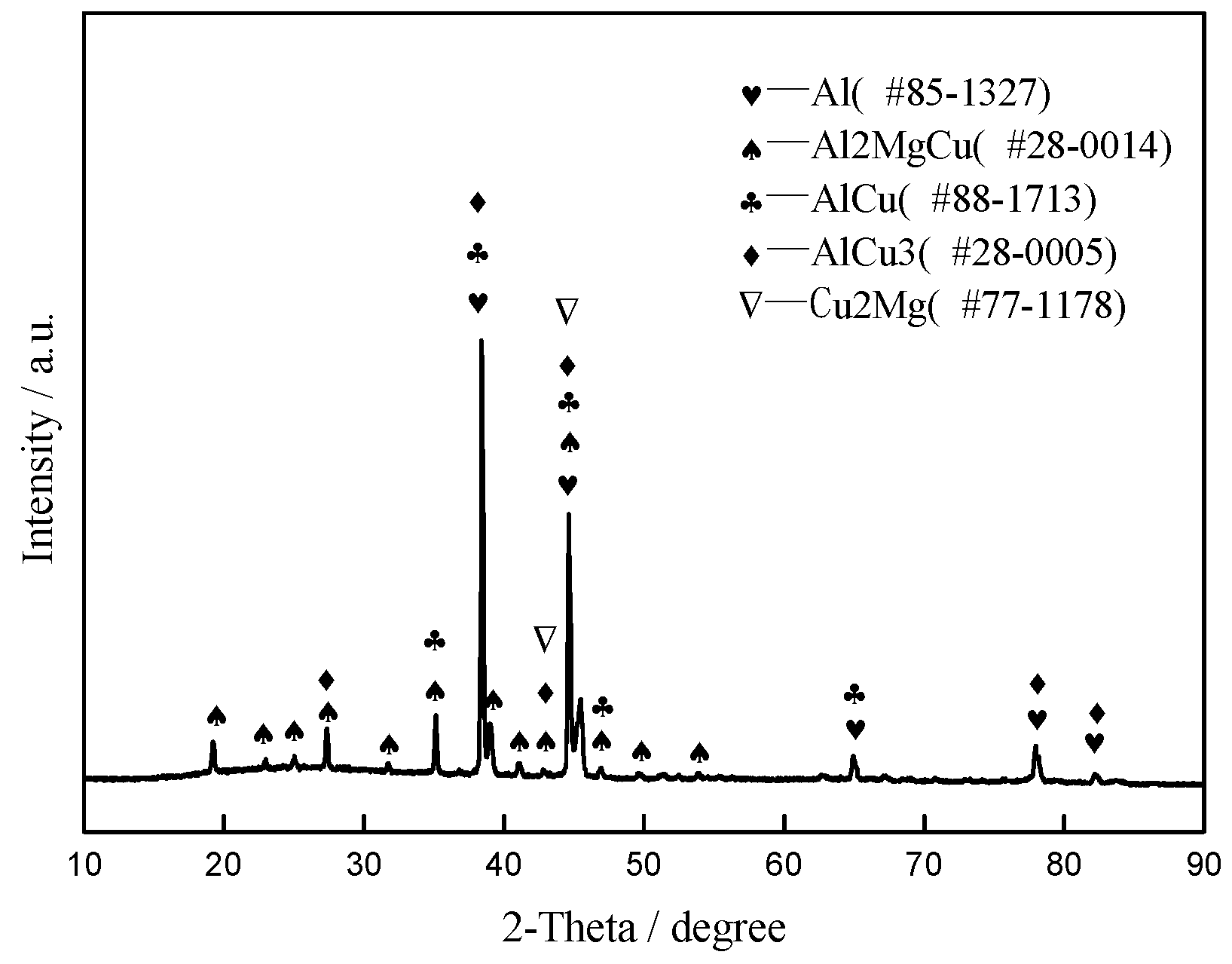
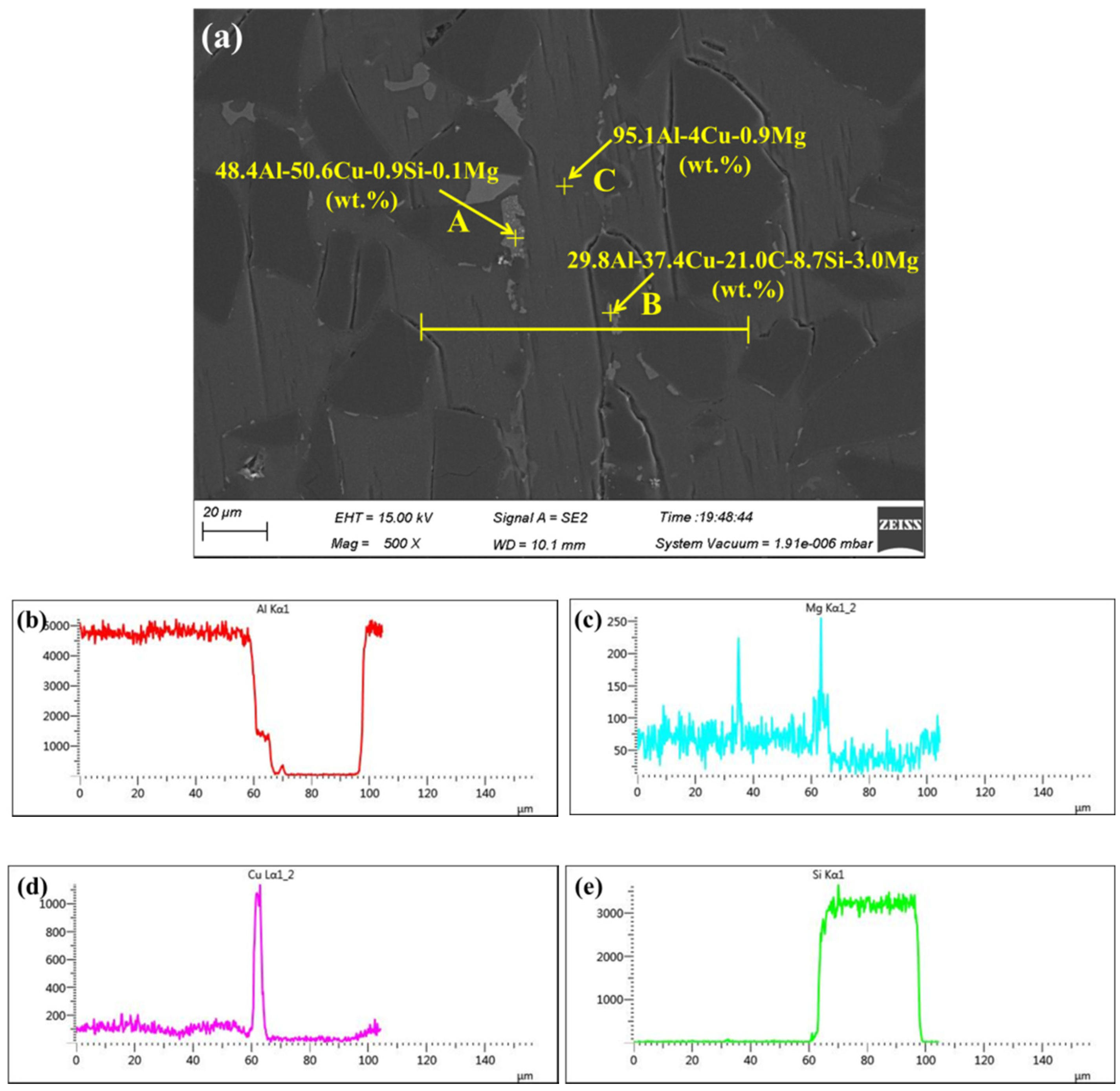
3.2. Microstructure and Element Distribution Analysis of Brazed Joints
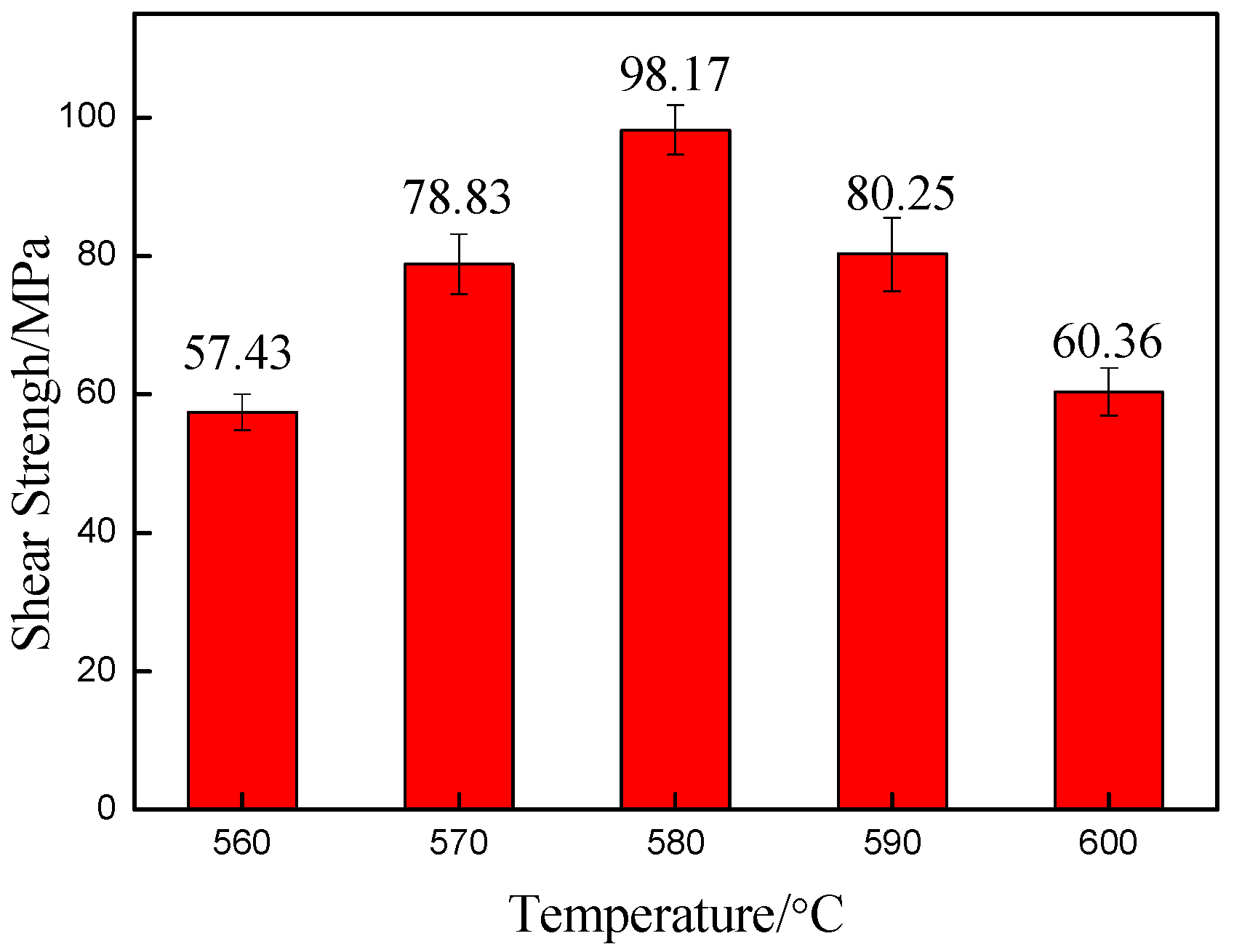
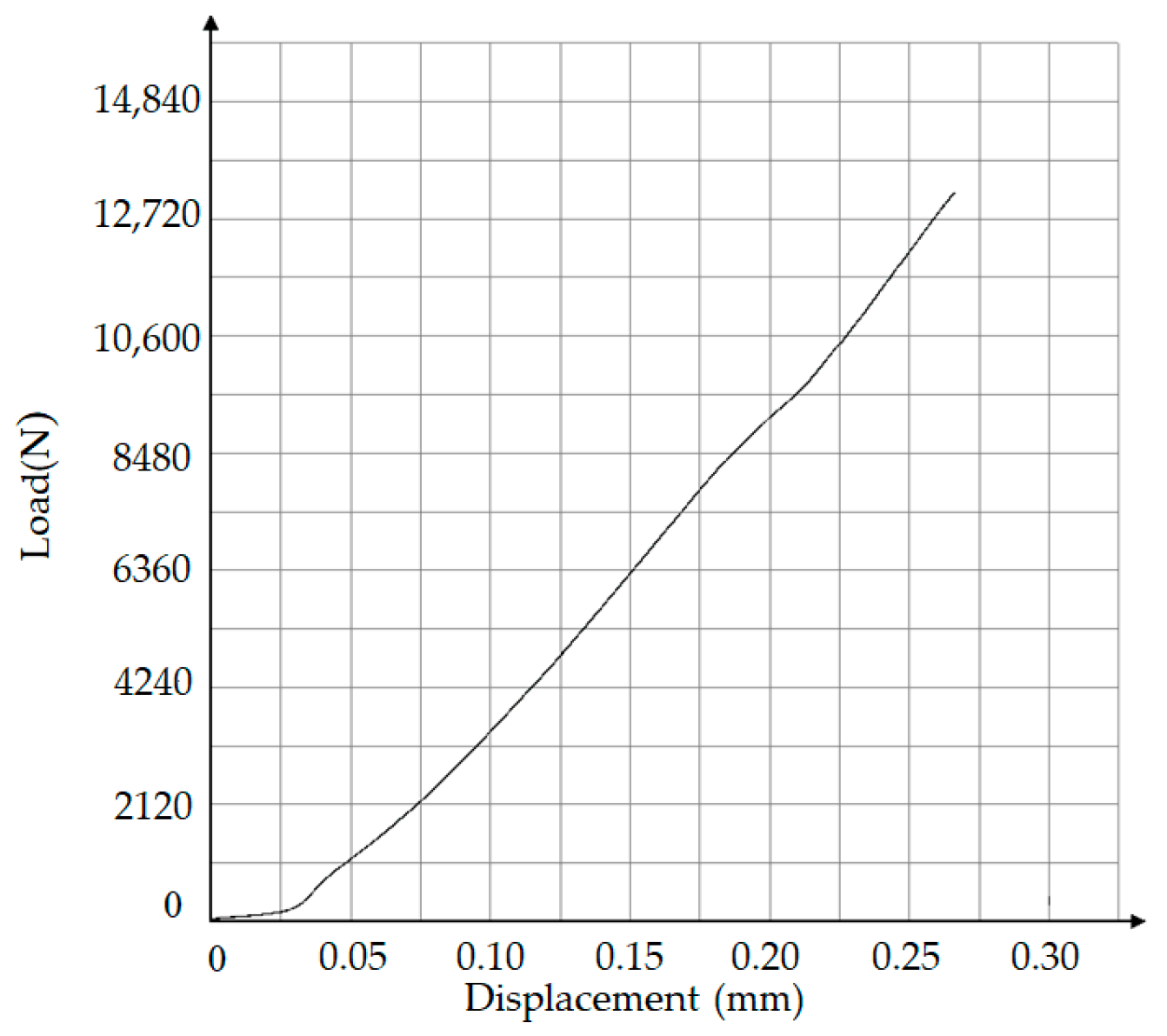
3.3. Mechanical Properties of Brazed Joints
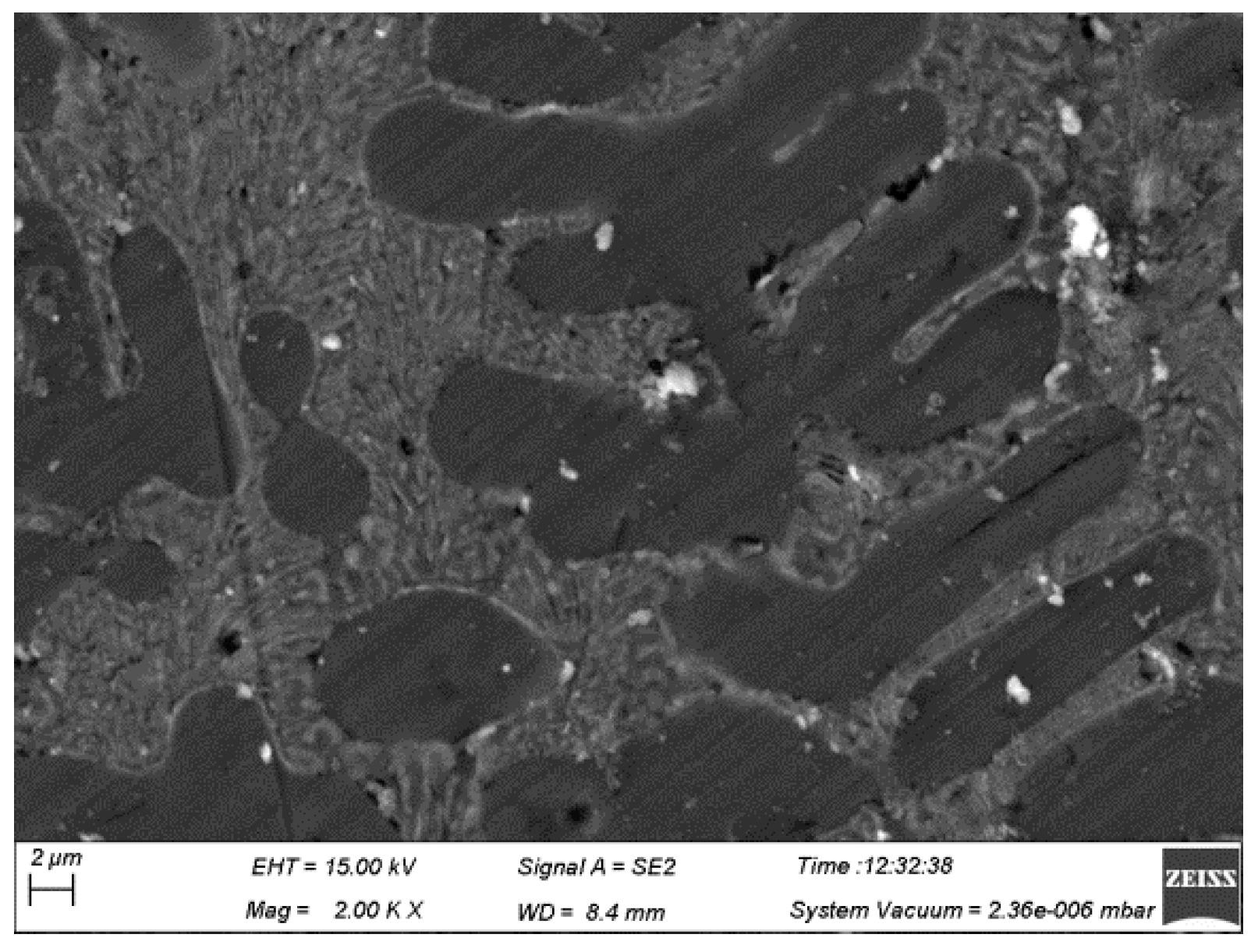
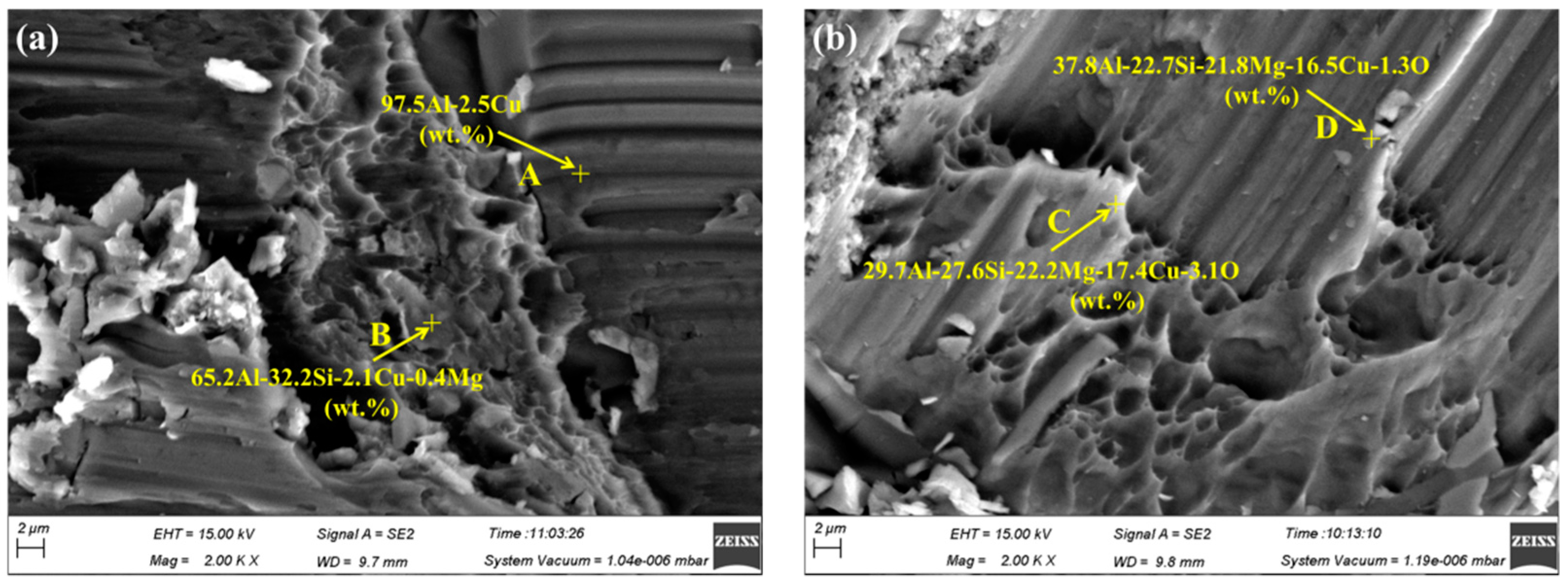
3.4. Fracture Analysis of Brazed Joints
4. Conclusions
- (1)
- Using melt-spinning technology, the foil-like brazing filler metal Al-17.0Cu-8.0Mg was obtained. The microstructure analysis indicated that the foil-like brazing filler metal mainly contained uniformed cellular nano grains, with a size less than 200 nm. The solidus and liquidus temperatures of the foil-like brazing filler metal decreased by 4 °C and 7 °C in comparison with the values of the as-cast brazing filler metal due to the nanometer size effect. Compared with the brazing filler metal prepared by conventional methods, the micro-nano brazing filler metal had higher toughness, lower melting point, and better diffusibility, which were more conducive to the wetting and spreading behavior between the brazing filler metal and base material.
- (2)
- The microstructure observation indicated that brazed joints were continuous and tightly bonded, without physical defects such as micro-voids and cracks when the brazing temperature exceeded 570 °C. Moreover, the width of the brazing seam became narrower and narrower with increasing brazing temperature owning to the strong interaction between the micro-nano brazing filler metal and 55 vol.% SiCp/ZL102 composites. Some white block phases mainly containing elements Al and Cu appeared near the brazing seam. The main diffusion path for Cu was the interface between the SiC particle and aluminum. After brazing, the chemical concentration gradient between the brazing filler metal and base material disappeared.
- (3)
- The maximum joint shear strength was achieved at 98.17 MPa at the brazing temperature of 580 °C. The joint shear strength increased in the temperature range of 560 °C to 580 °C. However, a further increase in the brazing temperature to 590 °C and 600 °C caused a decrease in shear strength to 80.25 MPa and 60.36 MPa, respectively. The excessive interaction in the joint and matrix caused the generation and growth of brittle intermetallics such as Al2Cu and AlCu having a remarkable influence on the shear strength.
- (4)
- The fracture morphology of the joint made at a brazing temperature of 580 °C was characterized by quasi-cleavage fracture. Fractures occurred in the brazing seam rather than the interface between the brazing filler metal and 55 vol.% SiCp/ZL102 composites since no SiC particles could be found in the fracture surface. There was no large blocked intermetallic compound in the joint, and this would be very beneficial in terms of increasing the joint mechanical properties.
Author Contributions
Funding
Conflicts of Interest
References
- Arami, H.; Simchi, A.; Reihani, S.M.S. Mechanical induced reaction in Al-CuO system for in-situ fabrication of Al based nanocomposites. J. Alloys Compd. 2008, 465, 151–156. [Google Scholar] [CrossRef]
- Lakra, S.; Bandyopadhyay, T.K.; Das, S.; Das, K. Synthesis and characterization of in-situ (Al-Al3Ti-Al2O3)/Al dual matrix composite. J. Alloys Compd. 2020, 842, 155–745. [Google Scholar] [CrossRef]
- Hekner, B.; Myalski, J.; Valle, N.; Botor-Probierz, A.; Sopicka-Lizer, M.; Wieczorek, J. Friction and wear behavior of Al-SiC(n) hybrid composites with carbon addition. Compos. Part B Eng. 2017, 108, 291–300. [Google Scholar] [CrossRef]
- Beffort, O.; Long, S.Y.; Cayron, C.; Kuebler, J.; Buffat, P.A. Alloying effects on microstructure and mechanical properties of high volume fraction SiC-particle reinforced Al-MMCs made by squeeze casting infiltration. Compos. Sci. Technol. 2007, 67, 737–745. [Google Scholar] [CrossRef]
- Wang, T.; Wu, X.; Zhang, G.; Dai, Y.; Xu, B.; Ruan, S. An experimental study on single-point diamond turning of a 55 vol% SiCp/Al composite below the ductile brittle transition depth of SiC. Int. J. Adv. Manuf. Technol. 2020, 108, 2255–2268. [Google Scholar] [CrossRef]
- Amirkhanlou, S.; Niroumand, B. Development of Al356/SiCp cast composites by injection of SiCp containing composite powders. Mater. Des. 2011, 32, 1895–1902. [Google Scholar] [CrossRef]
- Haghayeghi, R.; Kapranos, P. An investigation on physical and chemical refinement of aerospace aluminium alloys. Mater. Lett. 2013, 95, 121–124. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Lei, Y.; Nie, J.J.; Chen, X.; Zhang, Z. Effect of Ti-Al on microstructures and mechanical properties of plasma arc in-situ welded joint of SiCp/Al MMCs. Trans. Nonferrous Met. Soc. China 2008, 18, 809–813. [Google Scholar] [CrossRef]
- Niu, J.; Luo, X.; Tian, H.; Brnic, J. Vacuum brazing of aluminium metal matrix composite (55 vol.% SiCp/A356) using aluminium-based filler alloy. Mater. Sci. Eng. B Adv. 2012, 177, 1707–1711. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, D.; Tian, J.; Wang, D.; Wang, P.; Niu, J. Effects of Al-8.5Si-25Cu-xY filler metal foils on vacuum brazing of SiCp/Al composites. Mater. Sci. Technol. 2018, 34, 429–439. [Google Scholar] [CrossRef]
- Grilo, J.; Puga, H.; Carneiro, V.H.; Tohidi, S.D.; Barbosa, F.V.; Teixeira, J.C. Effect of Hybrid Ultrasonic and Mechanical Stirring on the Distribution of m-SiCp in A356 Alloy. Metals 2020, 10, 610. [Google Scholar] [CrossRef]
- Storjohann, D.; Barabash, O.M.; Babu, S.S.; David, S.A.; Sklad, P.S.; Bloom, E.E. Fusion and friction stir welding of aluminum-metal-matrix composites. Met. Mater. Trans. A 2005, 36, 3237–3247. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Gerlich, A.P. On the stability, microstructure, and mechanical property of powder metallurgy Al-SiC nanocomposites during similar and dissimilar laser welding. Mat. Sci. Eng. A 2019, 759, 688–702. [Google Scholar] [CrossRef]
- Hynes, N.R.J.; Prabhu, M.V.; Nagaraj, P. Joining of hybrid AA6063-6SiC(p)-3Gr(p) composite and AISI 1030 steel by friction welding. Def. Technol. 2017, 13, 338–345. [Google Scholar] [CrossRef]
- Maity, J.; Pal, T.K.; Maiti, R. Transient liquid phase diffusion bonding of 6061-15 wt% SiCp in argon environment. J. Mater. Process. Technol. 2009, 209, 3568–3580. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Du, Z.; Zhang, G. Application of AlMgGaLi foil for joining copper to SiCp/Al-MMCs for high-temperature and high-power electronics. Appl. Phys. A Mater. 2019, 125. [Google Scholar] [CrossRef]
- Xiao, J.; Li, S.; Bai, S.; Yan, J.; Xiong, D.; Tang, Y. Compression brazing of SiCp/Al composite using a semisolid Zn-Al-Cu filler metal based on the strain-induced melt activation process. JOM 2019, 71, 4931–4939. [Google Scholar] [CrossRef]
- Weis, S.; Elssner, M.; Wielage, B.; Wagner, G. Wetting behavior of AlAgCu brazing filler on aluminum matrix composites and stainless steel. Weld World 2017, 61, 383–389. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.C.; Chen, X.G.; Cui, Y. Ultrasonic dissolution of brazing of 55% SiCp/A356 composites. Trans. Nonferrous Met. Soc. China. 2010, 20, 746–750. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, H.; Feng, J.; Ji, F.; Niu, J.; Brnic, J. Flux-free diffusion joining of SiCp/6063 Al matrix composites using liquid gallium with nano-copper particles in atmosphere environment. Nanomaterials 2020, 10, 437. [Google Scholar] [CrossRef]
- Zuo, L.; Zhao, X.; Li, Z.; Zuo, D.; Wang, H. A review of friction stir joining of SiCp/Al composites. Chin. J. Aeronaut. 2020, 33, 792–804. [Google Scholar] [CrossRef]
- Wang, X.H.; Niu, J.T.; Guan, S.K.; Wang, L.J.; Cheng, D.F. Investigation on TIG welding of SiCp-reinforced aluminum-matrix composite using mixed shielding gas and Al-Si filler. Mat. Sci. Eng. A 2009, 499, 106–110. [Google Scholar]
- Pichumani, S.; Srinivasan, R.; Ramamoorthi, V. Mechanical properties, thermal profiles, and microstructural characteristics of Al-8%SiC composite welded using pulsed current TIG welding. J. Mech. Sci. Technol. 2018, 32, 1713–1723. [Google Scholar] [CrossRef]
- Muratoglu, M.; Yilmaz, O.; Aksoy, A. Investigation on diffusion bonding characteristics of aluminum metal matrix composites (Al/SiCp) with pure aluminum for different heat treatments. J. Mater. Process. Technol. 2006, 178, 211–217. [Google Scholar] [CrossRef]
- Sittaramane, A.; Mahendran, G. Optimal bonding conditions for diffusion bonding of aluminum metal matrix composites using taguchi based response surface methodlogy. J. Balk. Tribol. Assoc. 2016, 22, 2437–2446. [Google Scholar]
- Wu, M.; Chang, L.L.; Peng, Z.R.; Feng, Z.L.; Yong, W.N.; Wang, W.; Qu, X.H. Investigation of Al-based alloys for brazing SiCp/Al composites. Rare Met. 2011, 40, 132–135. [Google Scholar]
- Wang, P.; Xu, D.X.; Cheng, D.F.; Li, Q.; Niu, J.T. Active brazing filler metal on SiC particle reinforced aluminium matrix composites. Sci. Technol. Weld. Join. 2015, 20, 361–370. [Google Scholar] [CrossRef]
- Bangash, M.K.; Ubertalli, G.; Di Saverio, D.; Ferraris, M.; Jitai, N. Joining of Aluminium Alloy Sheets to Aluminium Alloy Foam Using Metal Glasses. Metals 2018, 8, 614. [Google Scholar] [CrossRef]
- Gao, Z.; Feng, J.G.; Wang, P.; Xu, C.Y.; Niu, J.T. Effect of brazing temperature on microstructure and performance of brazed joint of SiCp/Al composite. Ordnance Mater. Sci. Eng. 2018, 41, 57–61. [Google Scholar]
- Wang, P.; Gao, Z.; Li, J.; Cheng, D.; Niu, J. Research on reaction brazing of Ti layer-coated SiCp/Al composites using Al-based filler metal foil. Compos. Interfaces 2019, 26, 1057–1068. [Google Scholar] [CrossRef]
- Moller, C.; Grann, J. Understanding key process parameters of vacuum aluminum brazing. Adv. Mater. Process. 2014, 172, 15–19. [Google Scholar]




| Element | Si | Fe | Cu | Mn | Mg | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| wt.% | 10.0–13.0 | 0.0–0.7 | ≤0.30 | ≤0.50 | ≤0.10 | ≤0.10 | ≤0.20 | Balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, D.; Gao, Z.; Ba, X.; Wang, Z.; Niu, J. Vacuum Brazing of 55 vol.% SiCp/ZL102 Composites Using Micro-Nano Brazing Filler Metal Fabricated by Melt-Spinning. Metals 2020, 10, 1470. https://doi.org/10.3390/met10111470
Qiu D, Gao Z, Ba X, Wang Z, Niu J. Vacuum Brazing of 55 vol.% SiCp/ZL102 Composites Using Micro-Nano Brazing Filler Metal Fabricated by Melt-Spinning. Metals. 2020; 10(11):1470. https://doi.org/10.3390/met10111470
Chicago/Turabian StyleQiu, Dechao, Zeng Gao, Xianli Ba, Zhenjiang Wang, and Jitai Niu. 2020. "Vacuum Brazing of 55 vol.% SiCp/ZL102 Composites Using Micro-Nano Brazing Filler Metal Fabricated by Melt-Spinning" Metals 10, no. 11: 1470. https://doi.org/10.3390/met10111470
APA StyleQiu, D., Gao, Z., Ba, X., Wang, Z., & Niu, J. (2020). Vacuum Brazing of 55 vol.% SiCp/ZL102 Composites Using Micro-Nano Brazing Filler Metal Fabricated by Melt-Spinning. Metals, 10(11), 1470. https://doi.org/10.3390/met10111470




