Abstract
For arc welding of high-strength and cold-resistant steels, the author developed an advanced design of steel wire with a micro-composite coating of a nickel matrix and nanoparticles of LaF3 and LaB6, which improves the metallurgical influence of rare-earth elements (REE) and forms refractory sulphides and oxides of REE, as well as boron nitride. The addition of 0.1–0.3 wt% La in the weld pool leads to an increase in the content of the refractory compounds La2O3, LaO2, and LaS, and to the reduction in the content of the low-melting and brittle oxides and sulphides SiO2, SiO, MnO, MnS, and SiS. The use of steel wire with the composite coating of LaF3 and LaB6 allows for microstructural refinement when welding S960QL bainitic-martensitic steel and X70 API bainitic steel, and increases the impact toughness of the welds by 1.17–1.6 times.
1. Introduction
Development in the Arctic region requires the application of advanced high-strength and cold-resistant steels, the weldability of which is more complicated due to low-temperature embrittlement and hydrogen-assisted cold cracks (HAC). The focus of research on the weldability of advanced steels is welding metallurgy [1,2], including the development of special welding consumables with rare-earth metals (REEs) [3].
REEs reduce sensitivity to HAC and increase the impact toughness of the weld metal due to microstructural refinement, the formation of acicular ferrite, and a high affinity for sulphur and oxygen [4,5,6]. Wang et al. [6] performed arc welding of HSLA steel 10CrNi3MoV, with a 14 mm thickness, using flux-cored wire with a 0.3–1 wt% rare-earth concentrate of the total flux weight in the wire. When the content of the REE-alloy was 0.3 wt%, the tensile strength of the welds increased from 680 to 750 MPa, and the impact energy increased from 25 to 36 J at −40 °C. Similar positive results were achieved by researchers in welding and casting [7].
Yu et al. [8] improved the ductility and refined the microstructure of the high-temperature alloy Fe-43Ni by adding La2O3 with a residual content in the casting of 0.01–0.04 wt% La. The microstructural refinement was promoted by the formation of dispersed inclusions of La2O2S with an increase in the content of lanthanum up to 0.025 wt%, which provided effective nucleation centers for the crystallization. Similar results have been achieved by other researchers [9,10], who have reported the formation of spherical inclusions of La2O3, La2O2S and LaS instead of elongated inclusions of MnS.
Since REEs have a high affinity not only for oxygen, but also for sulphur, the role of refractory oxides and sulphides should be taken into account when considering the microstructural refinement mechanism of high-strength steels. For example, the formation of the complex oxysulphides Ce2O2S and La2O2S, which have a high melting temperature, density, and thermodynamic stability, and a low Gibbs free energy [11,12,13,14]. Non-metallic inclusions in high-strength steel welding promote the intragranular nucleation of acicular ferrite, increasing the fatigue crack propagation energy and decreasing the critical temperature of the ductile–brittle transition [15].
It is known that the addition of boron, leading to the formation of borides and carboborides, also has a positive effect on the properties and microstructure of cold-resistant austenitic steel 316L with a low residual nitrogen content of up to 0.04% and boron content of up to 0.004%. This effect is associated with boron solubility in γ-Fe up to 0.02% and in α-Fe up to 0.0081%, and the formation of the borides Me2B, Me5B3, Me3(C, B), Me23(C, B)6, and FeMo2B4 [16,17]. The addition of REEs in the welding of the flux-cored wire and solid wires also makes it possible to improve the technological properties of the welding arc and the arc stability, and to reduce spattering of the CO2 shielding gas [18,19,20,21].
The transition of REEs from the welding consumable into the weld pool is accompanied by loss of REEs through evaporation and oxidation; therefore, to achieve an effective residual content of REE in the molten pool, the content of REE in the electrode wire should be at least 0.3–1.6 wt% [3]. The content of REE in the molten weld pool undergoes a further reduction as a result of the REE metallurgical reactions with sulphur, oxygen, and nitrogen, which are characterized by the transition coefficient of an alloying element from the weld pool in the weld metal [22]. In the work of Goldstein and Mizin [23], it is noted that the residual content of REE in the weld metal is 0.02% and 0.05% when adding 0.1% and 0.25% of the REE content to the weld pool, respectively; that is, the transition coefficient from weld pool in the weld metal is 0.2. The effect of grain refinement is observed when the residual content of REEs is 0.02–0.16 wt% in the weld [24,25,26,27]. For example, Zhu and Yan [28] improved the microstructure and reduced sensitivity to cracks in 441 steel welding by adding 0.024% cerium. Therefore, a positive effect was observed with an initial content of 0.1–0.8% REEs in the weld pool, before metallurgical reactions had commenced [3].
2. Materials and Methods
The arc welding described here was focused on using samples of X70 API pipe steel (CHTPZ, Chelyabinsk, Russia) of 300 mm × 150 mm × 21.3 mm with G3Si1 wire (ESAB, Gothenburg, Sweden) of 1.2 mm diameter, and S960QL steel (SSAB, Stockholm, Sweden) of 350 mm × 100 mm × 8 mm with Union X96 wire (Boehler Welding, Hamm, Germany) of 1.0 mm diameter, with multilayer vertical weld depositions that were 60–80 mm high and 180 mm long, with 316L wire (ESAB, Sweden) of 1.0 mm and G3Si1 of 1.2 mm diameter. In the arc welding and deposition of samples in a shielding mixture of 82% Ar and 18% CO2, the authors used a Comau Smart NC-16-1.65 robot (Comau S.p.A., Grugliasco, Italy) with a Fronius TransPuls Synergic 4000 CMT source (Fronius, Wels, Austria), according to the welding parameters shown in Table 1.

Table 1.
Welding parameters for S960QL, X70 API welding and the multilayer weld deposition.
Thermodynamic modelling of the metallurgical reactions and phase composition were determined on the basis of the thermodynamic data of individual substances, using the modelling program “Terra” (Bauman Moscow State Technical University, Moscow, Russia) and FactSage (CRCT, Montreal, Canada) [29,30,31]. Mechanical testing was conducted on a Tinius Olsen Model 602 machine (Walter+Bai AG, Löhningen, Switzerland) in accordance with the GOST 6996-66 standard, using the PH450 pendulum impact testing system (Walter+Bai AG, Löhningen, Switzerland) in accordance with the ISO 148-1:2016 standard Charpy V-notch tests and the EMCOTEST DuraScan-20 hardness tester (EMCO-TEST PrufmaSchinen GmbH, Kuchl, Austria) in accordance with the ISO 6507-1:2018 standard. The chemical composition was determined using a Bruker Q4 TASMAN optical emission spectrometer (Bruker, Karlsruhe, Germany). The metallographic analysis was conducted using the optical microscope Reichert-Jung Me F3A (Leica Microsystems, Wetzlar, Germany), Zeiss Axiovert 200 MAT (Carl Zeiss AG, Oberkochen, Germany), and scanning electron microscope SUPRA 55VP WDS (Carl Zeiss, Oberkochen, Germany), SEM TESCAN MIRA 3 (Tescan Orsay Holding, Brno, Czech Republic).
3. Results and Discussion
3.1. REE Compound Properties
As pure metallic powders of REEs have high chemical activity, it is preferable to use refractory REE compounds with negative Gibbs free energies for microalloying of the weld pool and grain refinement, through the heterogeneous nucleation mechanism of non-metallic inclusions [14,15]. Table 2 and Table 3 and Figure 1 and Figure 2 detail the physical properties and Gibbs free energies of the forming refractory and high–density REE compounds.

Table 2.
Physical properties of refractory oxides of REEs [32,33,34,35].

Table 3.
Physical properties of refractory sulphides of REEs [32,33,34].
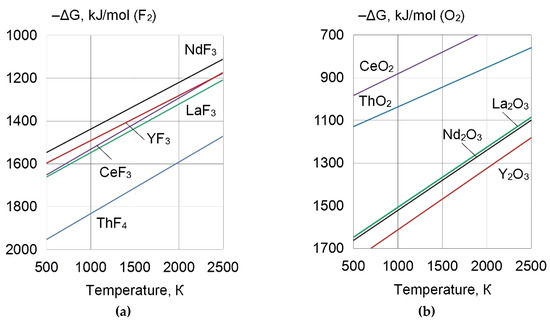
Figure 1.
Gibbs free energies of the formation of (a) fluorides and (b) oxides of REEs.
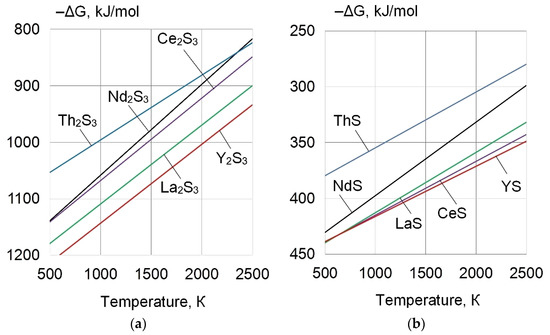
Figure 2.
Gibbs free energies of the formation of (a,b) sulphides of REEs.
3.2. Metallurgical Reactions in the Weld Pool
The presence of REE La, Ce, Y, and Th in the weld pool promotes strong metallurgical reactions—deoxidation of FeO and desulfurization of FeS by the formation of refractory REE oxides and sulphides according to reactions (1–8), which have high equilibrium constants, as shown in Figure 3.
3FeO + 2La = La2O3 + 3Fe
2FeO + 2Ce = CeO2 + 2Fe
3FeO + 2Y = Y2O3 + 3Fe
2FeO + 2Th = ThO2 + 2Fe
3FeS + 2La = La2S3 + 3Fe
3FeS + 2Ce = Ce2S3 + 3Fe
3FeS + 2Y = Y2S3 + 3Fe
3FeS + 2Th = Th2S3 + 3Fe
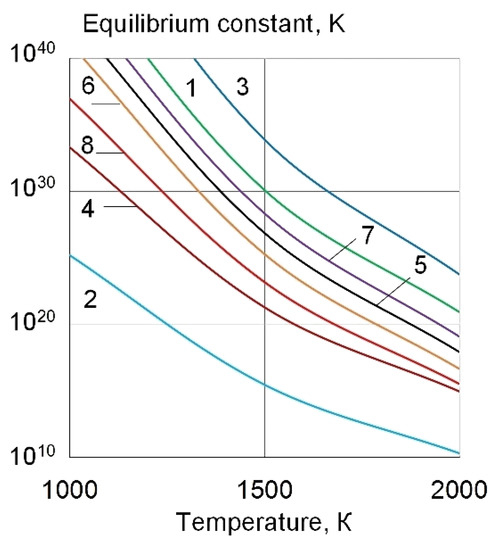
Figure 3.
The equilibrium constants of the (1–4) deoxidation and (5–8) desulfurization reactions in the interaction of the REEs of FeO and FeS.
Thermodynamic modelling of the equilibrium phase composition of the weld pool, in accordance with the maximal solubility of the impurity substances S, O, and N in the program "Terra", confirms that the addition of 0.1–0.3% La and 0.01–0.03 B leads to the formation of refractory REE sulphides and oxides, which are I type modifiers of the microstructure [3,23], as shown in Figure 4 and Figure 5. At the same time, the content of S, O, N, MnO, MnS, SiO, SiO2, SiS, and CrS decreases. The presence of these substances leads to the formation of low-melting eutectics at the grain boundaries, and reduces the impact toughness.
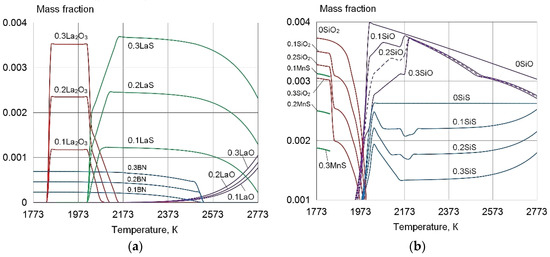
Figure 4.
The equilibrium phase composition of the weld pool of G3Si1 type, wt%: 0.09C, 0.8Si, and 1.3Mn with the addition of La and B: (a) La2O3, LaO, LaS, BN; (b) SiO2, SiO, MnS. Corresponding contents of La and B are indicated before compounds, wt%: (0) without REE; (0.1) (0.1La+0.01B); (0.2) (0.2La+0.02B); (0.3) (0.3La+0.03B).
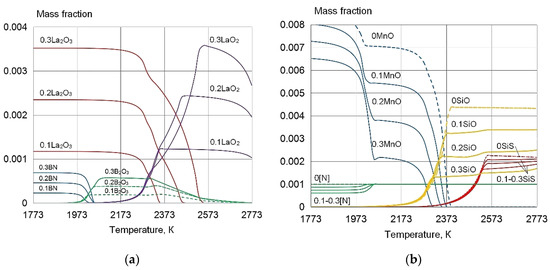
Figure 5.
The equilibrium phase composition of the weld pool of 316L type, wt%: 0.03C, 0.5Si, 2.0Mn, 19.0Cr, 12.0Ni, and 3.0Mo in the addition of La and B: (a) La2O3, LaO2, B2O3, BN; (b) MnO, SiO, SiS, (N). Corresponding contents of La and B are indicated before compounds, wt%: (0) without REE; (0.1) (0.1La+0.01B); (0.2) (0.2La+0.02B); (0.3) (0.3La+0.03B).
3.3. Wires with Composite Coating
Standard wires of G3Si1, Union X96, and 316L were treated using advanced electrochemical technology, in Ni-electrolytes with 60% Ni(BF4)2 and 10% LaF3 or LaB6. This led to the formation of composite coatings of about 6 μm in thickness from a nickel matrix and LaF3 and LaB6 nanodispersed particles less than 0.7 μm in size, giving an overall content of REE compounds of 0.3–0.4 wt% in the solid wire. Figure 6 presents the design of the composite electrode wire, with an electronic and optical view of the macrostructure of the wire surface showing the particles of LaB6. The microstructure of the wire’s composite coating and the distribution of chemical elements in the composite coating are shown in Figure 7.
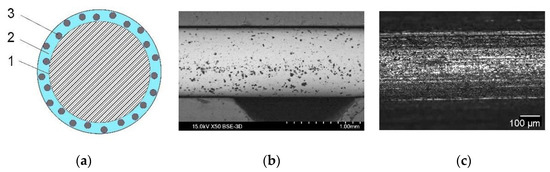
Figure 6.
Wire with composite coating: (a) The design of the composite wire: (1) solid wire, (2) Ni-matrix, (3) nanoparticles of REE compounds; (b) SEM and (c) optical macrostructure of the composite Ni-coating on the surface of the wire with particles of lanthanum boride, LaB6.
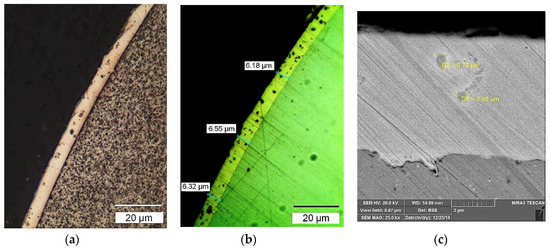
Figure 7.
The microstructure of composite coatings: optical (a,b) and SEM images (c) of the microstructure of composite coatings on the Union X96 wire: (a) Ni-LaF3 coating; (b) Ni-LaB6 coating; (c) LaF3 particles in an Ni-matrix.
According to the X-ray spectral analysis of the composite coatings, the content of La and F was 31.3% and 15.3%, respectively, as shown in Table 4.

Table 4.
X–ray spectral analysis of the composite coatings, wt%.
The investigation of the macrostructure of the deposited metal shows that the weld deposition of the vertical layers leads to microporosity; however, the presence of lanthanum and fluorine vapour in the arc improves solidity and reduces the levels of microporosity in the weld depositions, as shown in Figure 8.
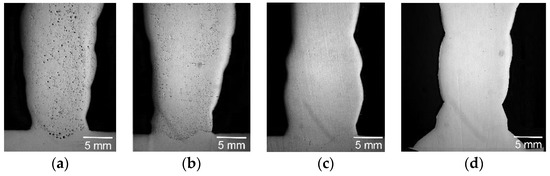
Figure 8.
The optical macrostructure of the multilayer weld deposition: (a) 316L wire; (b) 316L wire with Ni-LaB6 coating; (c) G3Si1 wire; (d) G3Si1 wire with Ni-LaF3 coating of 6 μm in thickness.
Lanthanum fluoride and boride were found to have a significant effect on the microstructure of the weld metal. The analysis of the microstructure of the deposited metal showed that the use of composite wires with nanodispersed particles of LaF3 and LaB6 leads to microstructural refinement, a decrease in the average grain size for the G3Si1 wire from 40–60 μm to 12–28 μm and from 40–80 μm to 20–35 μm for the 316L wire, and improvement in the shape and the distribution of the microstructural phases, as shown in Figure 9 and Figure 10.
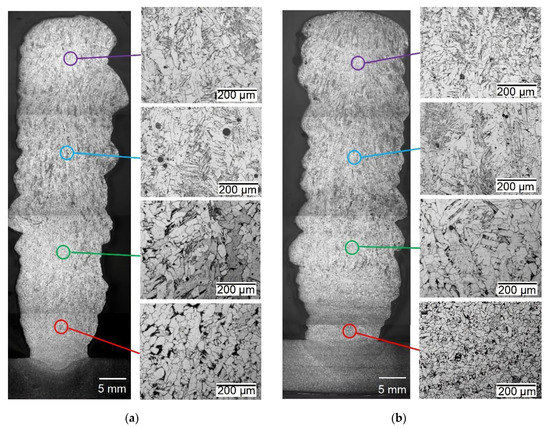
Figure 9.
The optical microstructure of the multilayer weld deposition: (a) G3Si1 standard wire; (b) G3Si1 wire with Ni-LaF3 coating of 6 μm in thickness.
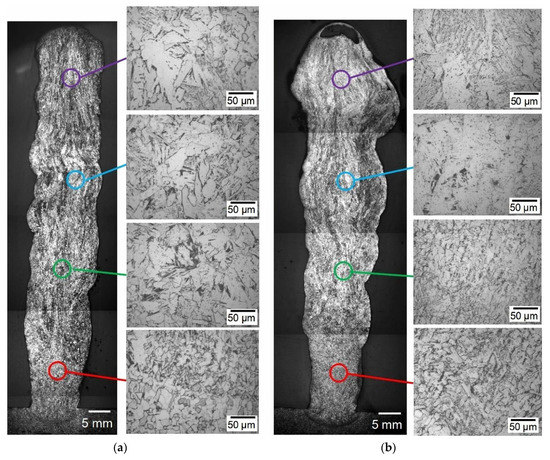
Figure 10.
The optical microstructure of the multilayer weld deposition: (a) 316L standard wire; (b) 316L wire with Ni-LaB6 coating of 6 μm in thickness.
Table 5 shows the chemical composition of S960QL welds with Union X96 wires coated in Ni-LaF3 and Ni-LaB6, indicating a transfer of La from the composite coating in the weld at a content of 0.01–0.04 wt%.

Table 5.
Chemical composition of S960QL welds, wt%.
When using composite coatings of LaF3 and LaB6 particles in the welding of S960QL steel, it is possible to observe the microstructural refinement in different zones of the weld—corresponding to a decrease in the average grain size from 8–28 μm to 6–12 μm—including the weld metal, the transition zone from the weld to the HAZ (heat-affected zone) and in the HAZ, as shown in Figure 11.
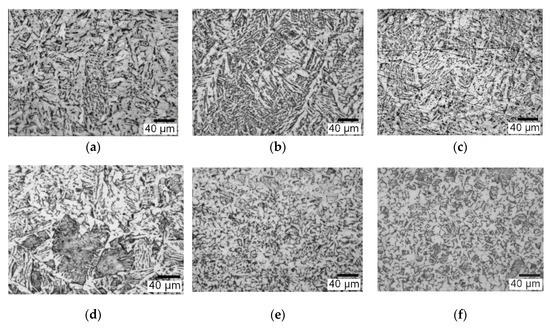
Figure 11.
The optical microstructure of the weld metal (a–c) and of the HAZ (d–f) in the welding of S960QL steel with Union X96 wire: (a,d) Union X96 standard wire; (b,e) Union X96 wire with Ni-LaF3 coating; (c,f) Union X96 wire with Ni-LaB6 coating of 6 μm in thickness.
Mechanical tests showed that the use of composite coatings with LaF3 and LaB6 particles in the welding of S960QL steel led to an increase in the yield strength, hardness, and average impact toughness of the weld from 45 to 54–66 J at −40 °C, as shown in Table 6.

Table 6.
The mechanical properties of the welds of S960QL steel with Union X96 wire.
Table 7 shows the chemical composition of X70 API welds with G3Si1 wire with coatings of Ni-LaF3 and Ni-LaB6, which indicates the transfer of La and Ni from the composite coating in the weld at a content of 0.01–0.03 wt% La and 0.16–0.21 Ni.

Table 7.
Chemical composition of X70 API welds, wt%.
A similar positive effect of microstructural refinement in different zones of the weld was achieved during X70 API pipe steel welding with G3Si1 wire with coatings of Ni-LaF3 and Ni-LaB6, as shown in Figure 12.
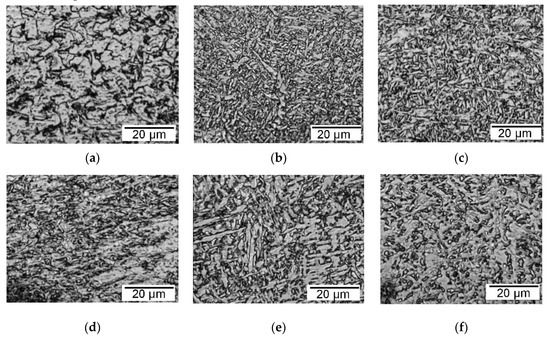
Figure 12.
The optical microstructure of the weld metal (a–c) and of the HAZ (d–f) in the welding of X70 API steel with G3Si1 wire: (a,d) G3Si1 standard wire; (b,e) G3Si1 wire with Ni-LaF3 coating; (c,f) G3Si1 wire with Ni-LaB6 coating of 6 μm in thickness.
The analysis of the microstructure in the field area of 0.014 mm2 showed an increase in the proportion of acicular and polygonal ferrite in the bainitic microstructure, and a decrease in the grain size in the weld metal. In particular, the average grain area in the weld decreased from 25 to 11–12 μm2. In the HAZ, the average grain area decreased from 29 to 14–16 μm2.
As a result of the positive effect of microstructural refinement, the mechanical tests showed that the use of composite coatings with LaF3 and LaB6 particles during X70 API steel welding led to an increase in the yield strength from 526 to 572 MPa, the average impact toughness in the weld from 87 to 143 J and in the HAZ from 143 to 174 J at −20 °C, as shown in Table 8.

Table 8.
The mechanical properties of the welds of X70 API steel with G3Si1 wire.
4. Conclusions
- (1)
- The advanced design of steel wire with a micro-composite coating of a nickel matrix and nanoparticles of REE compounds allows improvement of the microstructure and the properties of the welds during arc welding or wire-arc additive manufacturing of high-strength and cold-resistant steels. Adding LaF3 and LaB6 into the matrix of the composite coating of the wire at a content of 0.4 wt% increases the transition coefficient of lanthanum from the wire to the weld pool, and the metallurgical effect of REE on the nucleation mechanism of the nonmetallic inclusions.
- (2)
- The REE and boron transition from the wire composite coating to the weld pool leads to strong metallurgical reactions, the formation of refractory sulphides and oxides of REEs and boron nitride, and a decrease in the content of low-melting and brittle sulphides and oxides. The maximum solubility of O, N and S, and the addition of 0.3 wt% La in the weld pool of G3Si1 pearlitic steel or 316 L austenitic steel, leads to an increase in the content of the refractory compounds La2O3, LaO2, and LaS to 0.0035 wt% each, which allows reduction of the content of SiO2, SiO, MnO, and MnS to 0.001–0.002 wt%, or by 3.3–3.5 times, and the SiS content by 1.6–2.2 times.
- (3)
- As a result, the pearlitic, bainitic, and austenitic microstructural refinement enables a reduction of the average grain size by 1.3–2 times, decreasing the width of the grain boundaries. The application of wires with a composite coating of LaF3 and LaB6 particles in the welding of S960QL bainitic-martensitic steel offers an increase in the impact toughness of the weld by 1.2–1.4 times, and in the welding of X70 API bainitic steel by 1.17–1.6 times.
Funding
This research was supported by the project ’Energy-efficient systems based on renewable energy for Arctic conditions’ (EFREA), KS1054, South-East Finland-Russia CBC Programme 2014–2020.
Acknowledgments
The author acknowledges Peter Mayr from Technical University of Munich and André Hälsig from Chemnitz University of Technology for the technical support.
Conflicts of Interest
The author declares no conflict of interest.
References
- Lippold, J.C. Welding Metallurgy and Weldability, 1st ed.; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Kou, S. Welding Metallurgy, 2nd ed.; Wiley: New Jersey, NJ, USA, 2003. [Google Scholar]
- Efimenko, N.G. Rare-Earth Metals in Welding Materials, 1st ed.; Collegium: Kharkov, Ukraine, 2017. (In Russian) [Google Scholar]
- Turkdogan, E.T. Fundamentals of Steelmaking, 1st ed.; Institute of Materials: London, UK, 1996. [Google Scholar]
- Abson, D.J. Acicular ferrite and bainite in C-Mn and low-alloy steel arc weld metals. Sci. Technol. Weld. Join. 2018, 23, 635–648. [Google Scholar] [CrossRef]
- Wang, K.; Lu, Q.; Jiang, Z.; Yi, Y.; Yi, J.; Niu, B.; Ma, J.; Hu, H. Effect of rare-earth elements on the corrosion resistance of flux-cored arc-welded metal with 10CrNi3MoV steel. Int. J. Corros. 2018, 1–12. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, J.; Chen, H.L.; Su, Y.H.; Kuo, C.L.; Su, Y.H.; Chen, S.H.; Lin, K.J.; Hsieh, P.H.; Hwang, W.S. Effects of rare earth metals on steel microstructures. Materials 2016, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Zhang, S.H.; Li, H.; Wang, S.B. Effects of rare earth lanthanum on the solidification structure and hot ductility of Fe-43Ni expansion alloy. High Temp. Mater. Process. 2018, 37, 261–269. [Google Scholar] [CrossRef]
- Wang, A.-Q.; Li, M.; Ma, D.-Q.; Wu, Q.-J.; Xie, J.-P. Effect of lanthanum on microstructures and properties of ASTM A216 steel. Kem. U Ind. 2016, 65, 11–16. [Google Scholar] [CrossRef]
- Guerra, F.V.; Bedolla-Jacuide, A.; Zuno-Silva, J.; Ortiz-Dominguez, M.; Ruiz-López, I.; Cardoso-Legorreta, E. Fatigue resistance improvement of a forging medium carbon steel using mishmetal (rare earths) as inclusions (MnS) modifier element. Nova Sci. 2016, 8, 97–125. [Google Scholar]
- Kimanov, B.M. Removing oxide and sulfide inclusions from molten steel by filtration. Steel Transl. 2008, 38, 641–646. [Google Scholar] [CrossRef]
- Hao, F.; Liao, B.; Li, D.; Dan, T.; Ren, X.; Yang, Q. Effects of rare earth oxide on hardfacing metal microstructure of medium carbon steel and its refinement mechanism. J. Rare Earths 2011, 29, 609–613. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, J.; Chen, H.L.; Su, Y.H.; Su, Y.H.; Hwang, W.S. Thermodynamic calculation among cerium, oxygen and sulfur in liquid iron. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Torkamani, H.; Raydan, S.; Garcia-Mateo, C.; Rassizadehghani, J.; Vivas, J.; Palizdar, Y.; San-Martin, D. The influence of La and Ce addition on inclusion modification in cast niobium microalloyed steels. Metals 2017, 7, 377. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in steels. In Theory and Practice, 3rd ed.; CRC Press: Boca Raton, USA, 2015. [Google Scholar]
- Wang, M.X.; He, L.X. Effect of boron on structure and properties of low carbon bainitic steels. ISIJ Int. 2002, 42, 38–46. [Google Scholar] [CrossRef]
- Da Rosa, G.; Maugis, P.; Portavoce, A.; Drillet, J.; Valle, N.; Lentzen, E.; Hoummada, K. Grain-boundary segregation of boron in high-strength steel studied by nano-SIMS and atom probe tomography. Acta Mater. 2020, 182, 226–234. [Google Scholar] [CrossRef]
- Kataoka, T.; Ikeda, R.; Ono, M.; Yasuda, K.; Hirata, Y. Effect of REM addition of wire on CO2 gas shielded arc phenomenon. Weld. Int. 2009, 23, 517–522. [Google Scholar] [CrossRef]
- Methong, T.; Yamaguchi, T.; Shigeta, M.; Tanaka, M.; Ikeda, R.; Matsushita, M.; Poopat, B. Effect of rare earth metal on plasma properties in GMAW using CO2 shielding gas. Welding in the World 2017, 61, 1039–1047. [Google Scholar] [CrossRef]
- Li, E.; Wang, N.; Wan, S. Application of lanthanum oxide and cerium oxide in E4303 electrode. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 5th Annual International Conference on Material Engineering and Application, Wuhan, China, 14–16 December 2018; IOP Publishing: Bristol, UK, 2019; Volume 484, pp. 1–7. [Google Scholar]
- Kataoka, T.; Ikeda, R.; Yasuda, K.; Amano, K. Effect of microalloying elements of wire on spatter generation in CO2 arc welding. Prepr. Natl. Meet. Jpn. Weld. Soc. 2003, 72, 80–81. (In Japanese) [Google Scholar]
- Parshin, S.G. Welding Metallurgy, 1st ed.; Politech-Press: Saint Petersburg, Russia, 2020. (In Russian) [Google Scholar]
- Goldstein, Y.E.; Mizin, V.G. Modification and Microalloying of Iron and Steel, 1st ed.; Metallurgia: Moscow, Russia, 1986. (In Russian) [Google Scholar]
- Gao, J.; Fu, P.; Liu, H.; Li, D. Effects of rare earth on the microstructure and impact toughness of H13 steel. Metals 2015, 5, 383–394. [Google Scholar] [CrossRef]
- Bartlett, L.N.; Avila, B.R. Grain refinement in lightweight advanced high-strength steel castings. Int. J. Met. 2016, 10, 401–420. [Google Scholar] [CrossRef]
- Haakonsen, F.; Solberg, J.K.; Klevan, O.S.; Eijk, C.V.D. Grain refinement of austenitic manganese steels. In AISTech 2011 Proceedings. Iron and Steel Technology Conference and Exhibition; AIST: Indianapolis, IN, USA, 2011; Volume 2, pp. 763–771. [Google Scholar]
- Ji, Y.; Zhang, M.-X.; Ren, H. Roles of lanthanum and cerium in grain refinement of steels during solidification. Metals 2018, 8, 884. [Google Scholar] [CrossRef]
- Zhu, S.; Yan, B. Effects of cerium on weld solidification crack sensitivity of 441 ferritic stainless steel. Metals 2019, 9, 372. [Google Scholar] [CrossRef]
- Chase, M.W. (Ed.) NIST-JANAF Thermochemical Tables, 4th ed.; NIST: New York, NY, USA, 1998.
- Gurvich, L.V.; Veits, I.V.; Medvedev, V.A.; Bergman, G.A.; Yungman, V.S.; Khachkuruzov, G.A.; Iorish, V.S.; Dorofeeva, O.V.; Osina, E.A.; Tolmach, P.I.; et al. (Eds.) Thermodynamic properties of individual substances. In Handbook. Tables of Thermodynamic Properties, 1st ed.; Volume 4. Part 2; Nauka: Moscow, Russia, 1982. (In Russian) [Google Scholar]
- Belov, G.V.; Trusov, B.G. Thermodynamic Modeling of Chemically Reacting Systems, 1st ed.; Bauman Moscow State Technical University: Moscow, Russia, 2013. (In Russian) [Google Scholar]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Brown, D.; Wedemeyer, H. Th Thorium: Compounds with S, Se, Te and B, 1st ed.; Springer: Berlin, Germany, 2013. [Google Scholar]
- Samsonov, G.V.; Vinitsky, I.M. Refractory Compounds; Metallurgia: Moscow, Russia, 1976. (In Russian) [Google Scholar]
- Samsonov, G.V.; Borisova, A.L.; Zhidkova, T.G. Physical and chemical properties of oxides. In Handbook, 1st ed.; Metallurgia: Moscow, Russia, 1978. (In Russian) [Google Scholar]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).