The Effect of Molybdenum on Precipitation Behaviour in Austenite of Strip-Cast Steels Containing Niobium
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Hardness
3.2. Microstructure
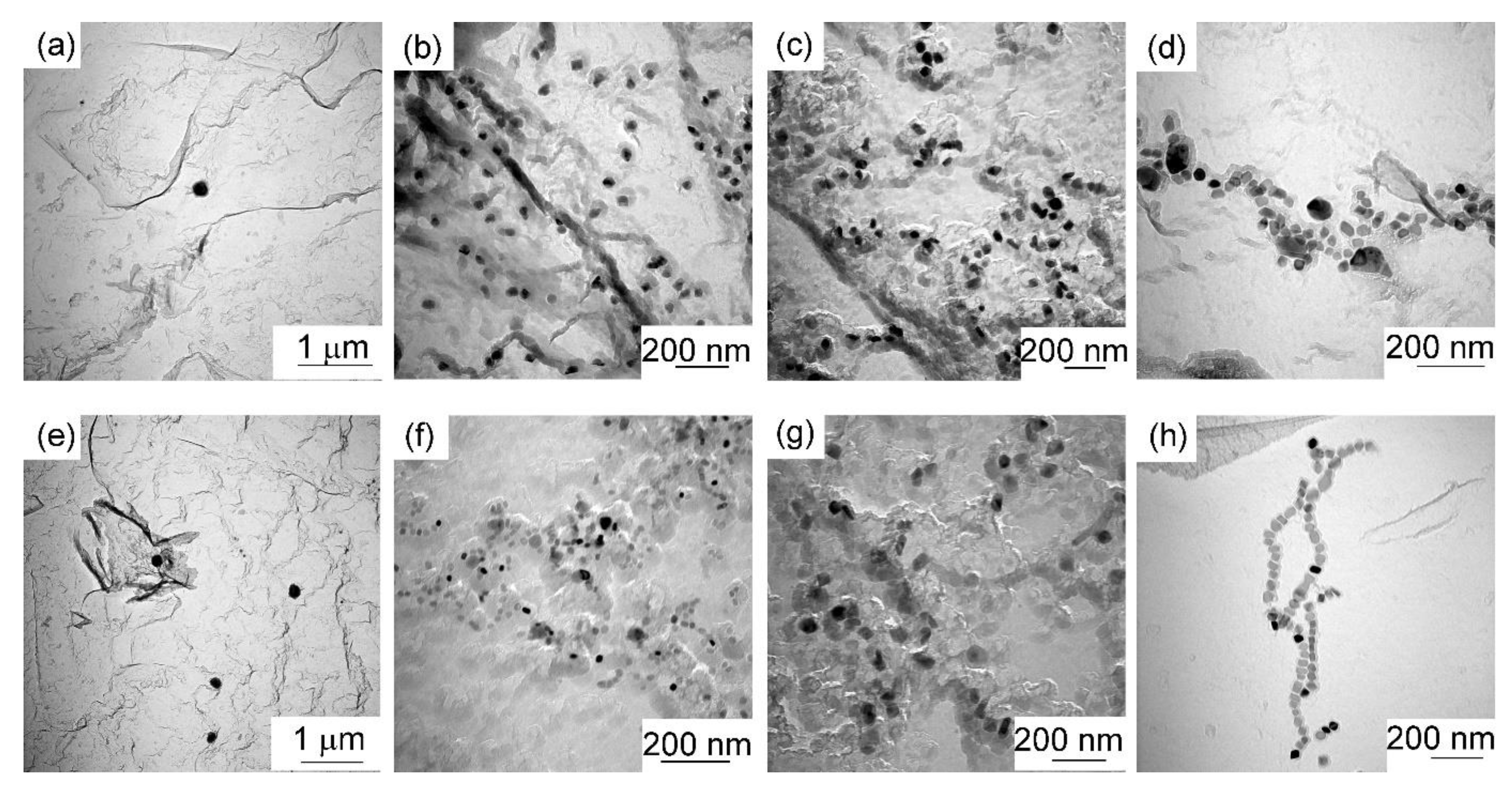
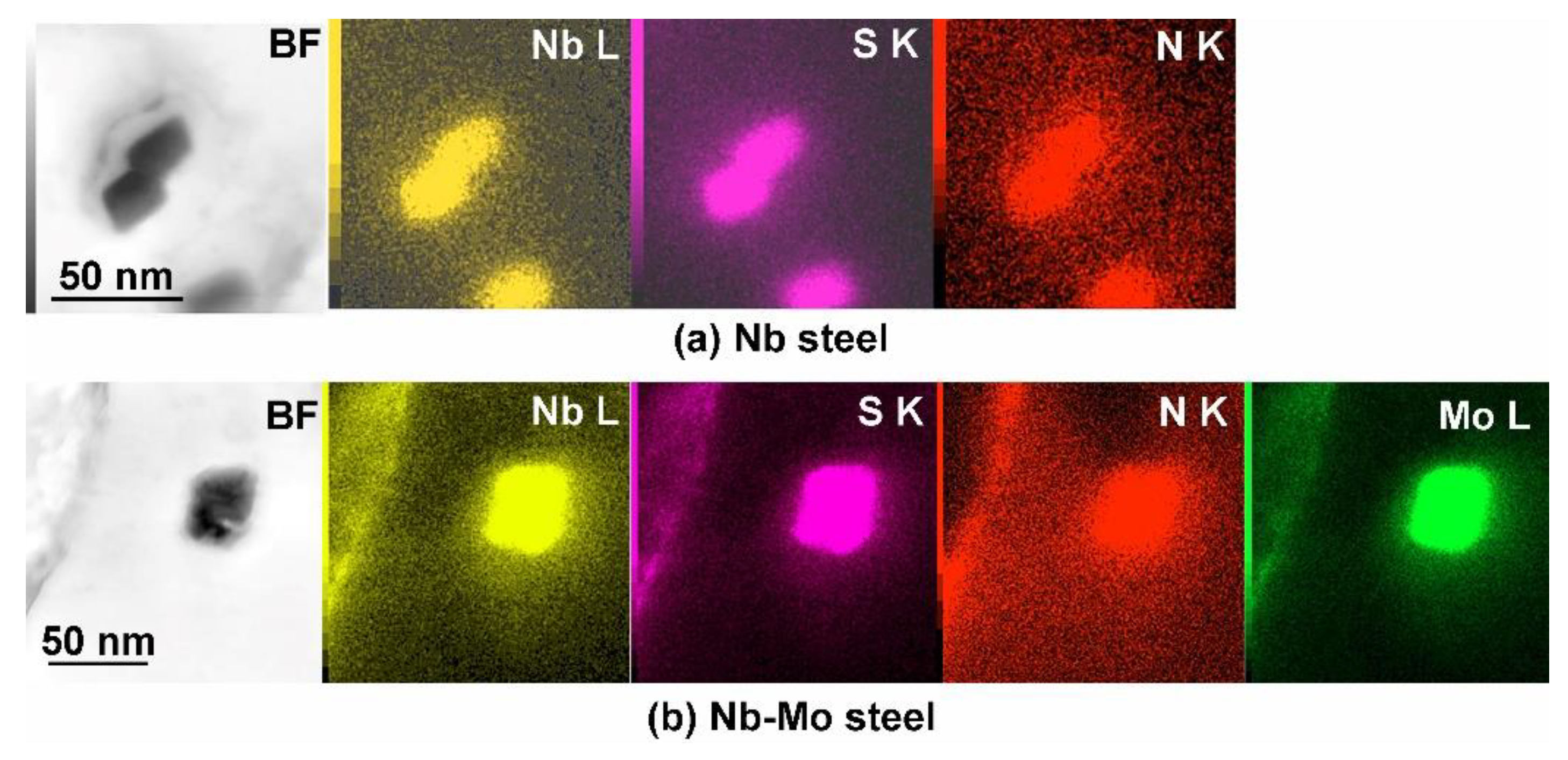
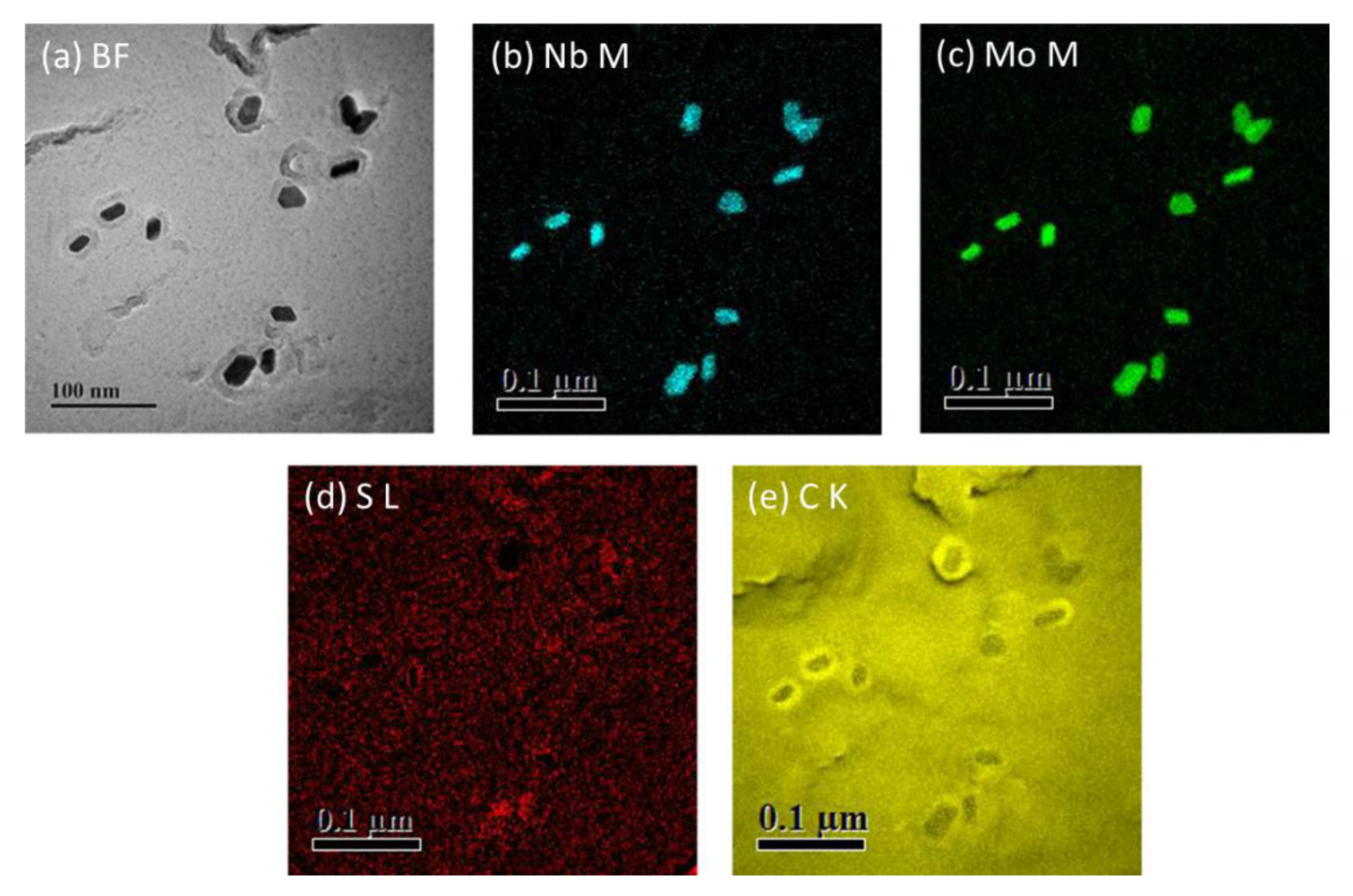
3.3. TEM
4. Discussion
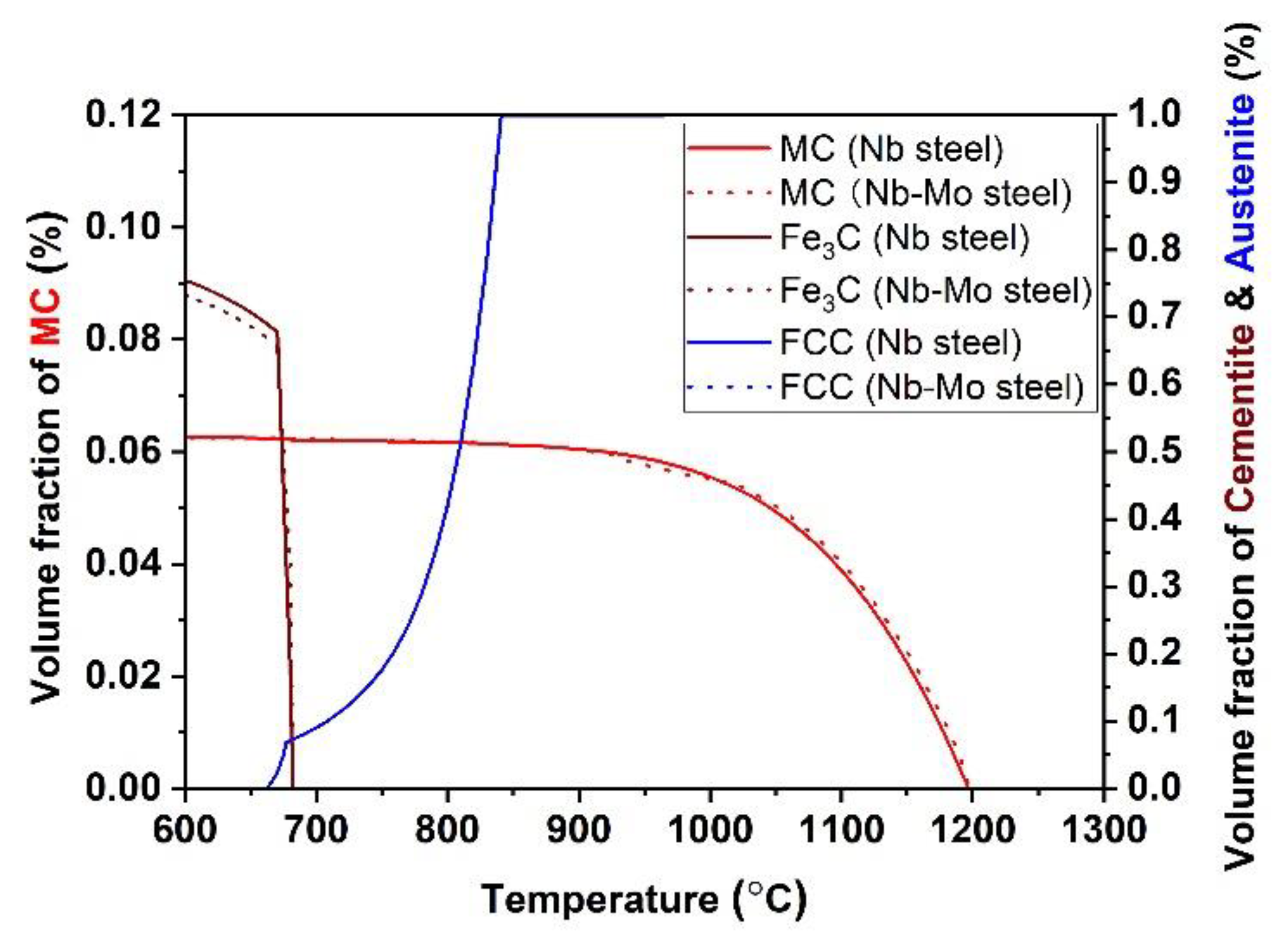
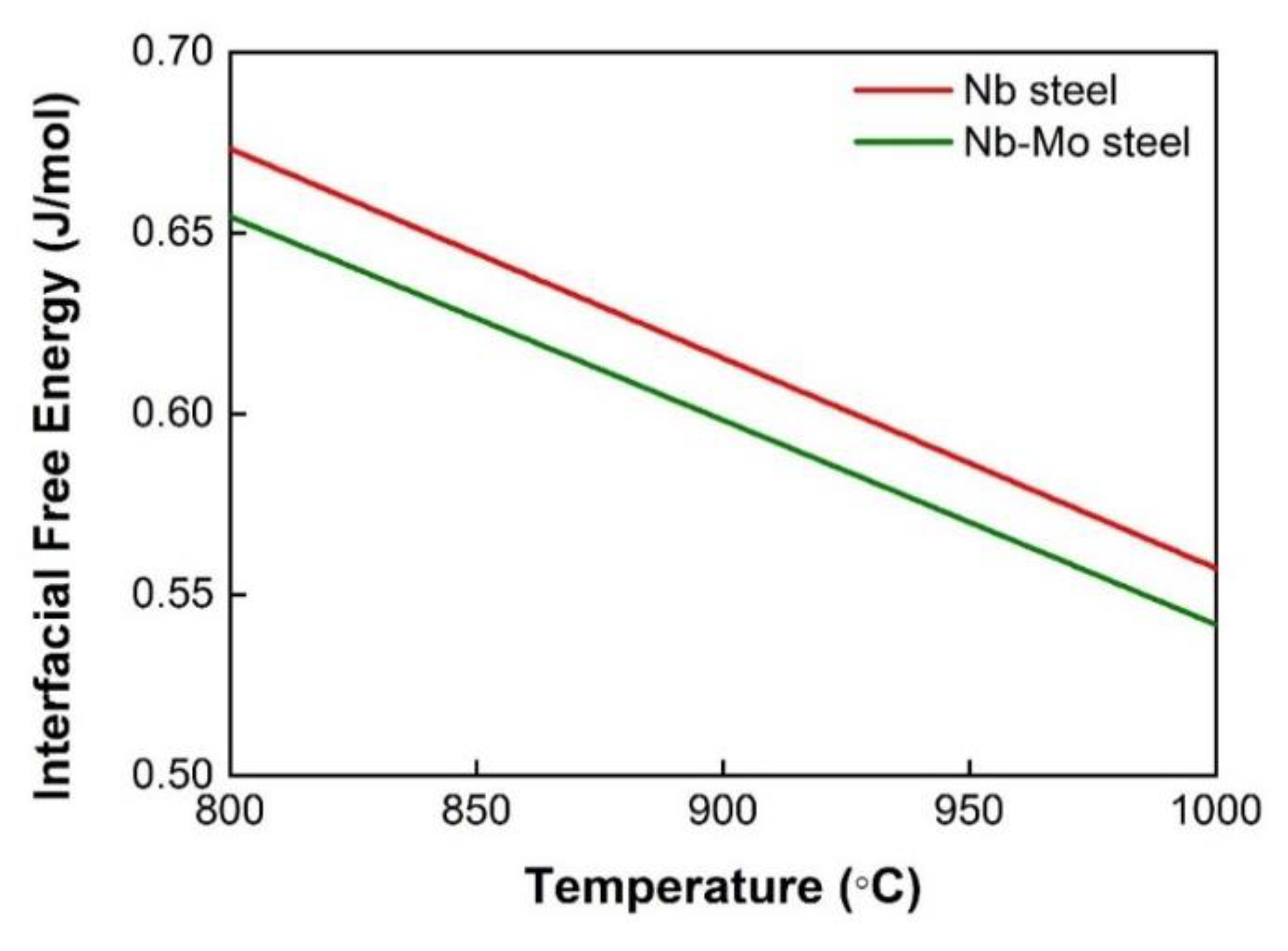
4.1. The Effect of Mo on the Thermodynamics of Precipitation
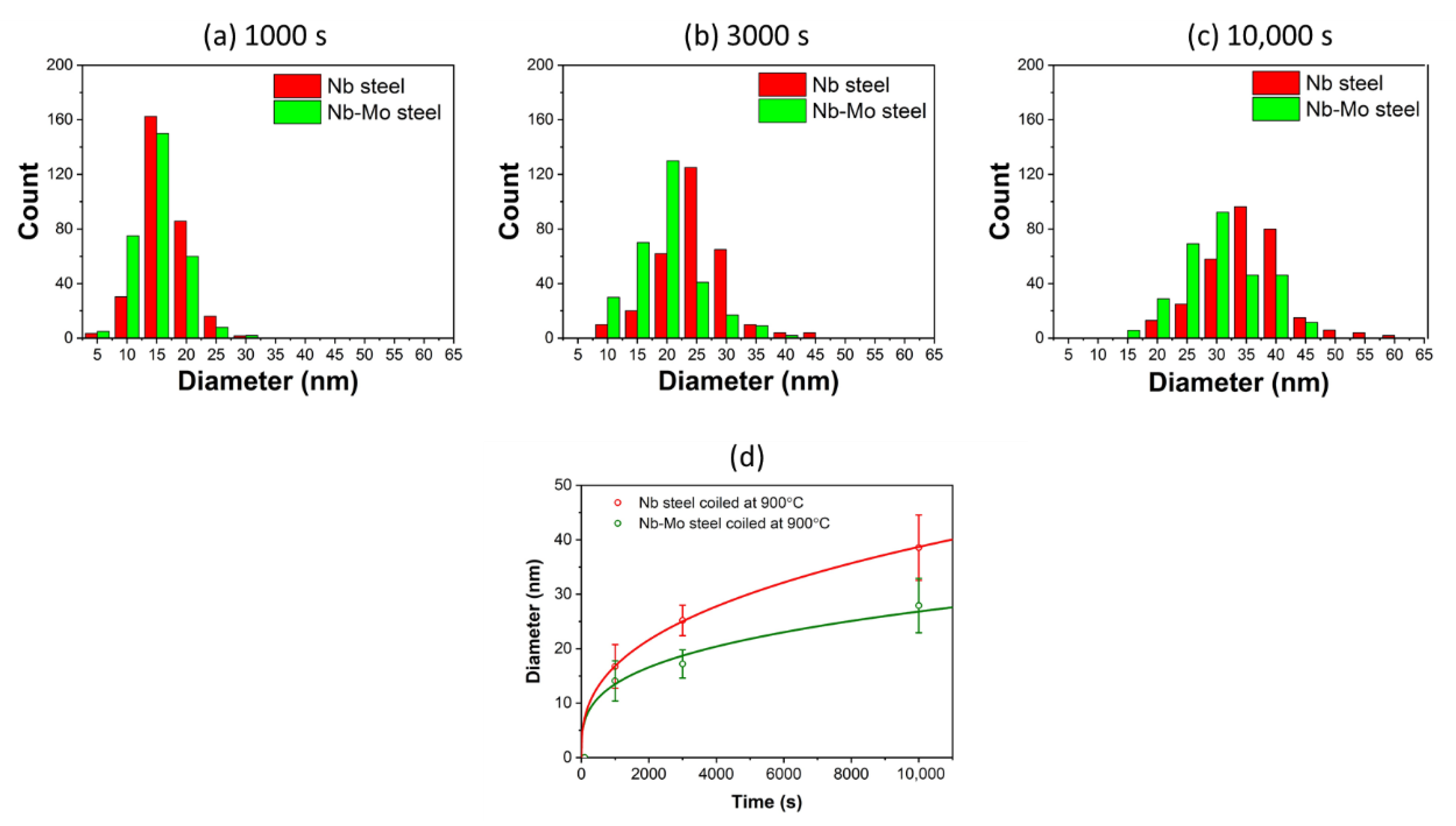
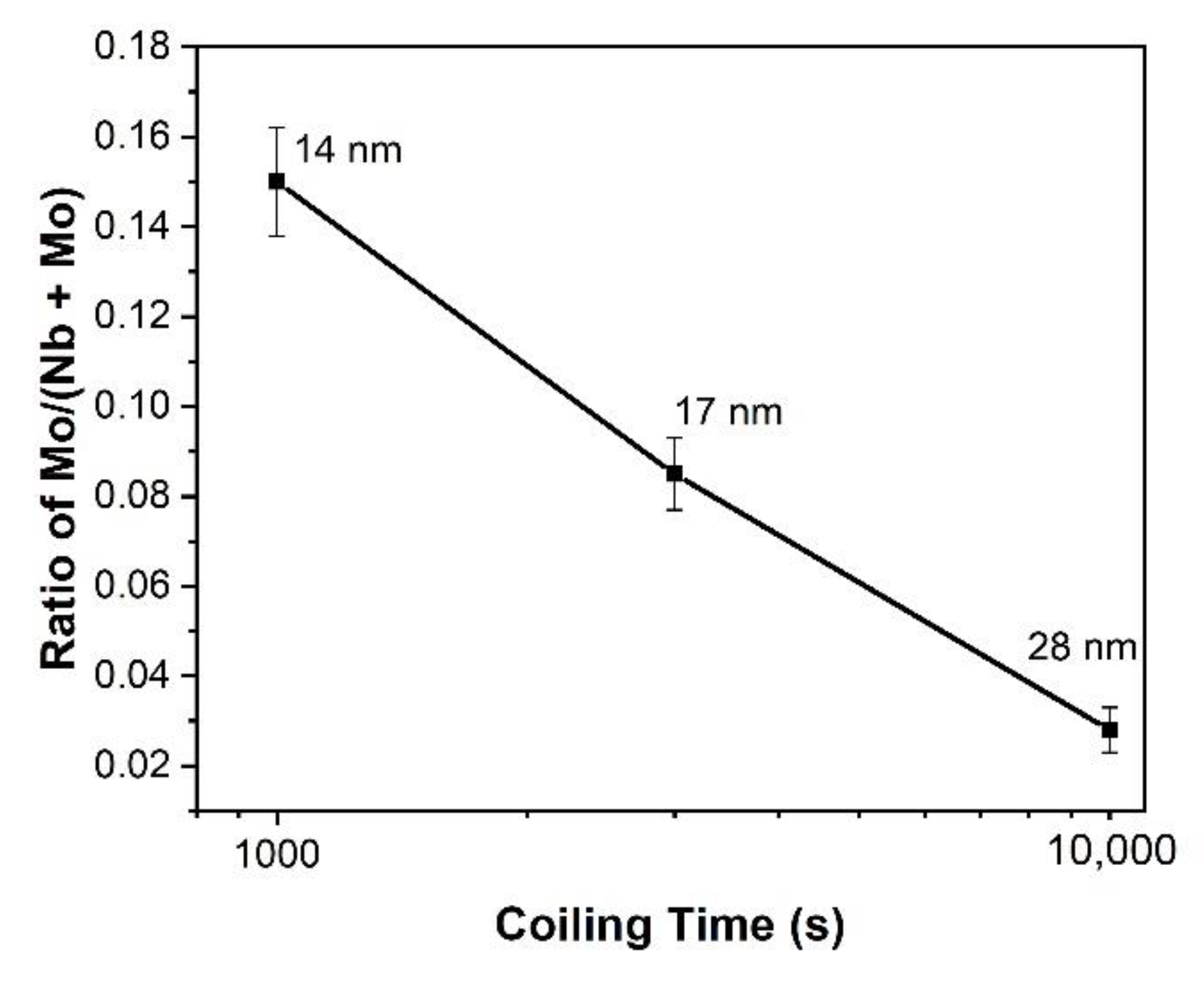
4.2. The Effect of Mo on the Coarsening Kinetics of Precipitation
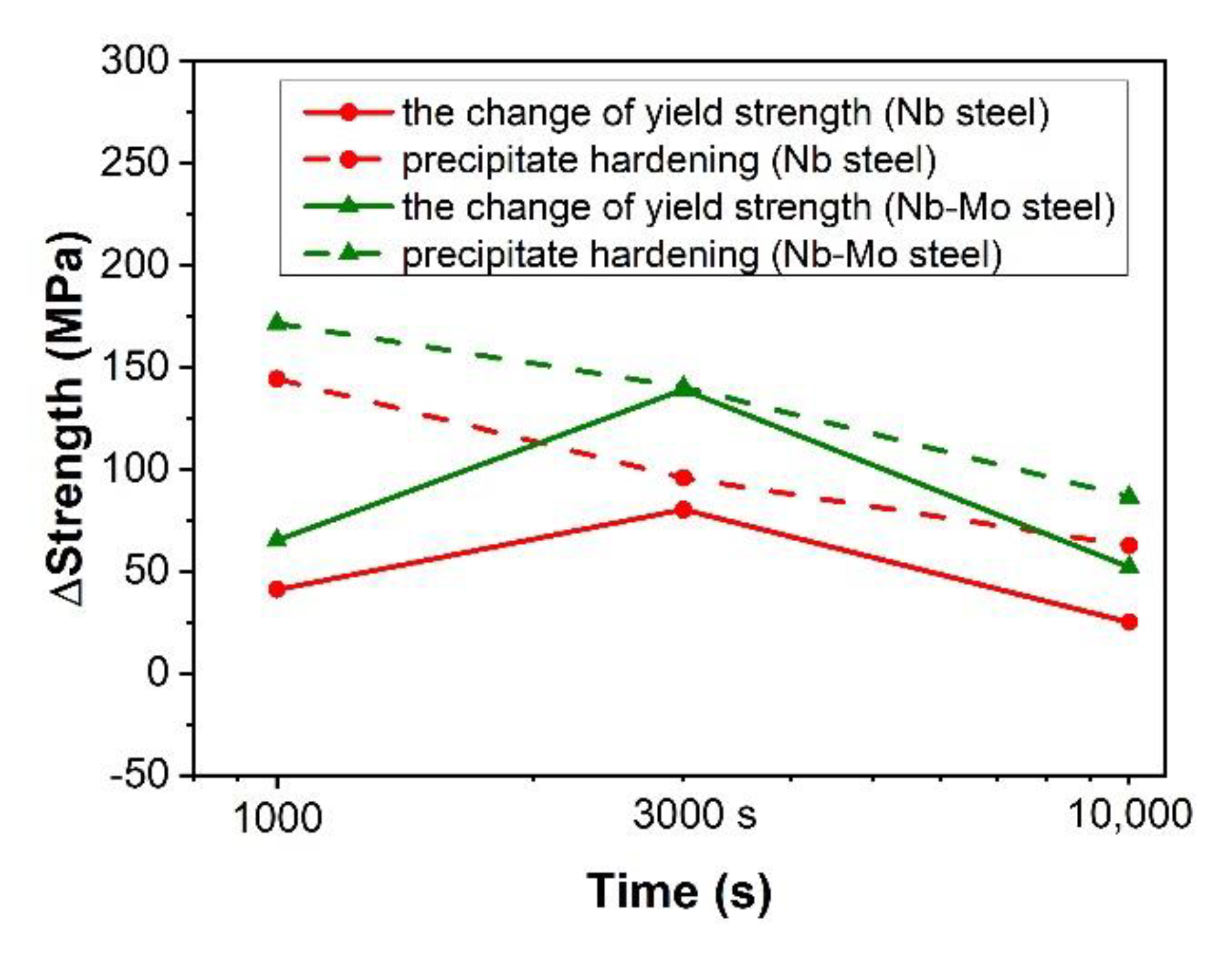
4.3. Precipitation Hardening
5. Conclusions
- (1)
- The hardness values of both as-cast steels were similar. During isothermal holding at 900 °C, the hardness of the two steels increased and reached the peak at 3000 s, then decreased after 10,000 s isothermal holding. Generally, the Nb-Mo steel had higher hardness than the Nb steel at all isothermal holding times.
- (2)
- The microstructures of both steels after isothermal holding at 900 °C were bainite, only some grain boundary allotriomorph (GBA) were formed in the Nb steels heat treated for 10,000 s at 900 °C.
- (3)
- Nb-carbonitrides precipitated in both steels after isothermal holding for 1000 s at 900 °C, and the size of the particles in the Nb-Mo steel was finer than that in the Nb steel. In the Mo-containing steel, Mo also participated in the precipitation, and the concentration of Mo in Nb-rich carbonitrides decreased with increasing particle size and isothermal holding time.
- (4)
- The enrichment of Mo in the Nb-rich carbonitrides reduces the interfacial energy between precipitates and the matrix, which lowers the nucleation energy barrier and the precipitate coarsening rate.
- (5)
- Strength modelling suggests that precipitation reached maximum volume fraction (0.0605%) after isothermal holding at 900 °C for 3000 s. The precipitates in the Nb-Mo steel imparted an increase in yield strength up to ~140 MPa, which was higher than that in the Nb steel, ~96 MPa. Further strengthening contributions of the precipitates in both steels decreased after 10,000 s isothermal holding at 900 °C due to the coarsening of the particles.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hong, S.; Kang, K.; Park, C.-G. Strain-induced precipitation of NbC in Nb and Nb–Ti microalloyed HSLA steels. Scr. Mater. 2002, 46, 163–168. [Google Scholar] [CrossRef]
- Dutta, B.; Sellars, C.M. Effect of composition and process variables on Nb (C, N) precipitation in niobium microalloyed austenite. Mater. Sci Tech. 1987, 3, 197–206. [Google Scholar] [CrossRef]
- Le Bon, A.; Rofes-Vernis, J.; Rossard, C. Recrystallization and Precipitation during Hot Working of a Nb-Bearing HSLA Steel. Met. Sci. 1975, 9, 36–40. [Google Scholar] [CrossRef]
- Lee, W.; Hong, S.; Park, C.; Kim, K.; Park, S. Influence of Mo on precipitation hardening in hot rolled HSLA steels containing Nb. Scr. Mater. 2000, 43, 319–324. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, M.-X.; Zhou, Y.-L.; Liu, Z.-Y.; Wang, G.-D. Effect of Mo on Nano-Precipitation Behavior and Microscopic Mechanical Characteristics of Ferrite. Steel Res. Int. 2015, 86, 1056–1062. [Google Scholar] [CrossRef]
- Kamikawa, N.; Abe, Y.; Miyamoto, G.; Funakawa, Y.; Furuhara, T. Tensile Behavior of Ti,Mo-added Low Carbon Steels with Interphase Precipitation. ISIJ Int. 2014, 54, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, H.; Guo, C.; Liu, W.; Yang, Z.; Sun, X.; Zhang, Z.; Jiang, F. Effect of molybdenum addition on the precipitation of carbides in the austenite matrix of titanium micro-alloyed steels. J. Mater. Sci. 2016, 51, 4996–5007. [Google Scholar] [CrossRef]
- Dhara, S.; Marceau, R.K.; Wood, K.; Dorin, T.; Timokhina, I.; Hodgson, P. Precipitation and clustering in a Ti-Mo steel investigated using atom probe tomography and small-angle neutron scattering. Mater. Sci. Eng. A 2018, 718, 74–86. [Google Scholar] [CrossRef]
- Jang, J.H.; Heo, Y.-U.; Lee, C.-H.; Bhadeshia, H.K.D.H.; Suh, D.-W. Interphase precipitation in Ti–Nb and Ti–Nb–Mo bearing steel. Mater. Sci. Technol. 2013, 29, 309–313. [Google Scholar] [CrossRef]
- Chen, C.; Yen, H.-W.; Kao, F.; Li, W.; Huang, C.; Yang, J.-R.; Wang, S. Precipitation hardening of high-strength low-alloy steels by nanometer-sized carbides. Mater. Sci. Eng. A 2009, 499, 162–166. [Google Scholar] [CrossRef]
- Akben, M.; Bacroix, B.; Jonas, J. Effect of vanadium and molybdenum addition on high temperature recovery, recrystallization and precipitation behavior of niobium-based microalloyed steels. Acta Metallurg. 1983, 31, 161–174. [Google Scholar] [CrossRef]
- Cao, J. Study on the Precipitation of Carbonitride in Nb-Mo-bearing Steel. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 2006. [Google Scholar]
- Zhang, Z.; Li, Z.; Yong, Q.; Sun, X.; Wang, Z.; Wang, G. Precipitation behavior of carbide during heating process in Nb and No-Mo micro-alloyed steels. Acta Metallurg. Sin. 2015, 51, 315–324. [Google Scholar]
- Dorin, T.; Wood, K.; Taylor, A.; Hodgson, P.; Stanford, N. Effect of coiling treatment on microstructural development and precipitate strengthening of a strip cast steel. Acta Materalia 2016, 115, 167–177. [Google Scholar] [CrossRef]
- Dorin, T.; Stanford, N.; Taylor, A.; Hodgson, P. Effect of Cooling Rate on Phase Transformations in a High-Strength Low-Alloy Steel Studied from the Liquid Phase. Met. Mater. Trans. A 2015, 46, 5561–5571. [Google Scholar] [CrossRef]
- Jiang, L.; Marceau, R.K.W.; Guan, B.; Dorin, T.; Wood, K.; Hodgson, P.D.; Stanford, N. The effect of molybdenum on clustering and precipitation behaviour of strip-cast steels containing niobium. Materialia 2019, 8, 100462. [Google Scholar] [CrossRef]
- Strezov, L.; Herbertson, J. Experimental Studies of Interfacial Heat Transfer and Initial Solidification Pertinent to Strip Casting. ISIJ Int. 1998, 38, 959–966. [Google Scholar] [CrossRef]
- Jiang, L.; Marceau, R.K.; Dorin, T.; Hodgson, P.D.; Stanford, N. Effect of molybdenum on phase transformation and microstructural evolution of strip cast steels containing niobium. J. Mater. Sci. 2018, 54, 1769–1784. [Google Scholar] [CrossRef]
- Jiang, L.; Marceau, R.K.; Dorin, T.; Wood, K.; Hodgson, P.D.; Stanford, N. The effect of molybdenum on interphase precipitation at 700 °C in a strip-cast low-carbon niobium steel. Mater. Charact. 2020, 166, 110444. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, C.-H.; Heo, Y.-U.; Suh, D.-W. Stability of (Ti,M)C (M=Nb, V, Mo and W) carbide in steels using first-principles calculations. Acta Materialia 2012, 60, 208–217. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Guo, C.; Leng, Z.; Yang, Z.; Sun, X.; Yao, C.; Zhang, Z.; Jiang, F. Evolution of (Ti, Mo)C particles in austenite of a Ti–Mo-bearing steel. Mater. Des. 2016, 109, 361–366. [Google Scholar] [CrossRef]
- Gale, F.W.; Totemeier, T.C. Smithells Metals Reference Book; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Geise, J.; Herzig, C. Lattice and grain boundary diffusion of niobium in iron. Z. Metallkd. 1985, 7, 622–626. [Google Scholar]
- Alberry, P.J.; Haworth, C.W. Interdiffusion of Cr, Mo, and W in Iron. Met. Sci. 1974, 8, 407–412. [Google Scholar] [CrossRef]
- Yong, Q. Secondary Phases in Steels; Metallurgical Industry Press: Beijing, China, 2006. [Google Scholar]
- Bhadeshia, H.; Honeycombe, R. Steels: Microstructure and Properties; Butterworth-Heinemann: Oxford, UK, 2011. [Google Scholar]
- Leslie, W.C. Iron and its dilute substitutional solid solutions. Met. Mater. Trans. A 1972, 3, 5–26. [Google Scholar] [CrossRef]
- Zhang, Z.; Yong, Q.; Sun, X.; Li, Z.; Wang, Z.; Zhou, S.; Wang, G. Effect of Mo Addition on the Precipitation Behavior of Carbide in Nb-Bearing HSLA Steel. In HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Hehemann, R. Phase Transformations; ASM: Metals Park, OH, USA, 1970; p. 397. [Google Scholar]
- Lifshitz, I.; Slyozov, V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Wagner, C. Theorie der alterung von niederschlägen durch umlösen (Ostwald-reifung). Berichte der Bunsengesellschaft für Physikalische Chemie 1961, 65, 581–591. [Google Scholar]
- Busby, J.T.; Hash, M.C.; Was, G.S. The relationship between hardness and yield stress in irradiated austenitic and ferritic steels. J. Nucl. Mater. 2005, 336, 267–278. [Google Scholar] [CrossRef]
- Deschamps, A.; Brechet, Y. Influence of predeformation and agEing of an Al–Zn–Mg alloy—II. Modeling of precipitation kinetics and yield stress. Acta Materialia 1998, 47, 293–305. [Google Scholar] [CrossRef]


| C | Mn | Si | Al | Nb | Mo | S | N | Fe | |
|---|---|---|---|---|---|---|---|---|---|
| Nb steel | 0.05 | 1.45 | 0.21 | 0.003 | 0.05 | - | <0.0005 | 0.01 | bal. |
| Nb-Mo steel | 0.05 | 1.50 | 0.23 | 0.004 | 0.05 | 0.33 | <0.0005 | 0.01 | bal. |
| Ageing Time | Nb Steel | Nb-Mo Steel |
|---|---|---|
| 1000 s | 144 | 171 |
| 3000 s | 96 | 140 |
| 10,000 s | 63 | 87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Marceau, R.K.W.; Dorin, T.; Yin, H.; Sun, X.; Hodgson, P.D.; Stanford, N. The Effect of Molybdenum on Precipitation Behaviour in Austenite of Strip-Cast Steels Containing Niobium. Metals 2020, 10, 1330. https://doi.org/10.3390/met10101330
Jiang L, Marceau RKW, Dorin T, Yin H, Sun X, Hodgson PD, Stanford N. The Effect of Molybdenum on Precipitation Behaviour in Austenite of Strip-Cast Steels Containing Niobium. Metals. 2020; 10(10):1330. https://doi.org/10.3390/met10101330
Chicago/Turabian StyleJiang, Lu, Ross K. W. Marceau, Thomas Dorin, Huaying Yin, Xinjun Sun, Peter D. Hodgson, and Nicole Stanford. 2020. "The Effect of Molybdenum on Precipitation Behaviour in Austenite of Strip-Cast Steels Containing Niobium" Metals 10, no. 10: 1330. https://doi.org/10.3390/met10101330
APA StyleJiang, L., Marceau, R. K. W., Dorin, T., Yin, H., Sun, X., Hodgson, P. D., & Stanford, N. (2020). The Effect of Molybdenum on Precipitation Behaviour in Austenite of Strip-Cast Steels Containing Niobium. Metals, 10(10), 1330. https://doi.org/10.3390/met10101330






