Devitrification of Zr55Cu30Al15Ni5Bulk Metallic Glass under Heating and HPT Deformation
Abstract
:1. Introduction
2. Materials and Methods
3. Resultsand Discussion
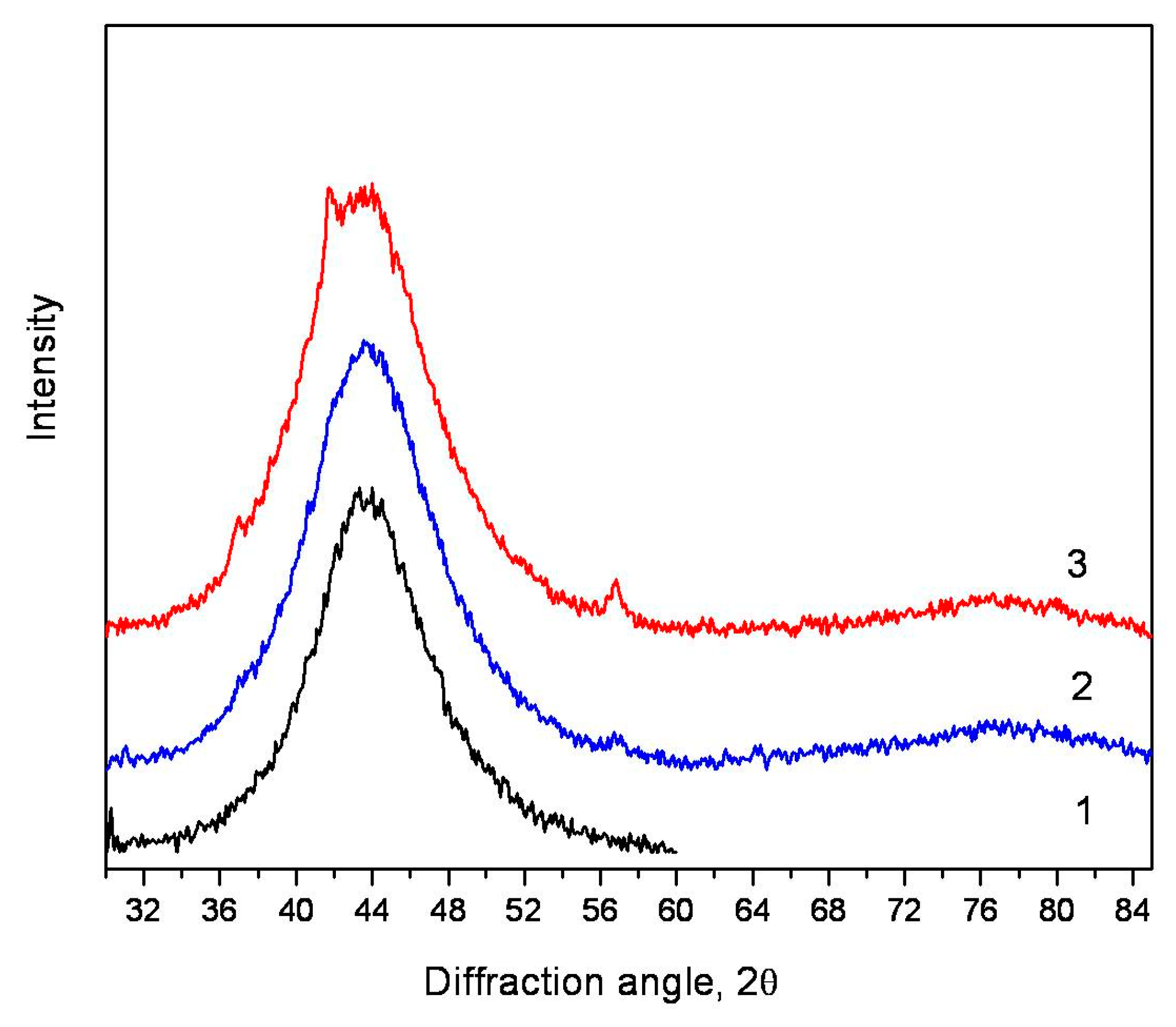
3.1. Analysis of Devitrification under Heating
3.2. Crystallization of the Alloy during Deformation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duwez, P.; Willens, R.H.; Klement, W. Continuous Series of Metastable Solid Solutions in Silver-Copper Alloys. J. Appl. Phys. 1960, 31, 1136. [Google Scholar] [CrossRef]
- Luo, Y.; Ke, H.; Zeng, R.; Liu, X.; Luo, J.; Zhang, P. Crystallization behavior of Zr60Cu20Fe10Al10 amorphous alloy. J. Non-Cryst. Solids 2020, 528, 119728. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y. Recent advances in bulk metallic glasses for biomedical applications. Acta Biomater. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Stoica, M.; Das, J.; Bednarcik, J.; Franz, H.; Mattern, N.; Wang, W.H.; Eckert, J. Strain distribution in Zr64.13Cu15.75Ni10.12Al10 bulk metallic glass investigated byin situtensile tests under synchrotron radiation. J. Appl. Phys. 2008, 104, 013522. [Google Scholar] [CrossRef] [Green Version]
- Stolpe, M.; Kruzic, J.; Busch, R. Evolution of shear bands, free volume and hardness during cold rolling of a Zr-based bulk metallic glass. Acta Mater. 2014, 64, 231–240. [Google Scholar] [CrossRef]
- Guo, W.; Shao, Y.; Saida, J.; Zhao, M.; Lü, S.; Wu, S. Rejuvenation and plasticization of Zr-based bulk metallic glass with various Ta content upon deep cryogenic cycling. J. Alloys Compd. 2019, 795, 314–318. [Google Scholar] [CrossRef]
- Abrosimova, G.E.; Aronin, A.S.; Kir’janov, Y.V.; Matveev, D.V.; Zver’kova, I.I.; Molokanov, V.V.; Pan, S.; Slipenyuk, A. The structure and mechanical properties of bulk Zr50Ti16.5Cu14Ni18.5 metallic glass. J. Mater. Sci. 2001, 36, 3933–3939. [Google Scholar] [CrossRef]
- Abrosimova, G.; Aronin, A.; Matveev, D.; Molokanov, V. Formation and structure of nanocrystals in bulk cтекле Zr50Ti16Cu15Ni19 metallic glass. Phys. Solid State 2004, 46, 2191–2195. [Google Scholar] [CrossRef]
- Saida, J.; Kato, H.; Inoue, A. Primary precipitation of icosahedral quasicrystal with rearrangement of constitutional elements in Zr65Al7.5Cu27.5 glassy alloy with low oxygen impurity. J. Mater. Res. 2005, 20, 303–306. [Google Scholar] [CrossRef]
- Zhao, X.; Pang, S.; Ma, C.; Zhang, T. Precipitation of Icosahedral Phase in Zr-Ni-Nb-Cu-Al Metallic Glasses. Mater. Trans. 2009, 50, 1838–1842. [Google Scholar] [CrossRef] [Green Version]
- Wilde, G.; Rösner, H. Nanocrystallization in a shear band: An in situ investigation. Appl. Phys. Lett. 2011, 98, 251904. [Google Scholar] [CrossRef]
- Abrosimova, G.; Aronin, A.; Barkalov, O.; Matveev, D.; Rybchenko, O.; Maslov, V.; Tkach, V. Structural transformations in the Al85Ni6.1Co2Gd6Si0.9 amorphous alloy during multiple rolling. Phys. Solid State 2011, 53, 229–233. [Google Scholar] [CrossRef]
- Aronin, A.; Louzguine-Luzgin, D.V. On nanovoids formation in shear bands of an amorphous Al-based alloy. Mech. Mater. 2017, 113, 19–23. [Google Scholar] [CrossRef]
- Glezer, A.M.; Permyakova, I.E.; Manaenkov, S.E. Plasticizing effect in the transition from an amorphous state to a nanocrystalline state. Dokl. Phys. 2008, 53, 8–10. [Google Scholar] [CrossRef]
- Abrosimova, G.; Matveev, D.; Pershina, E.; Aronin, A. Effect of treatment conditions on parameters of nanocrystalline structure in Al-based alloys. Mater. Lett. 2016, 183, 131–134. [Google Scholar] [CrossRef]
- Aronin, A.; Matveev, D.; Pershina, E.; Tkatch, V.; Abrosimova, G. The effect of changes in Al-based amorphous phase structure on structure forming upon crystallization. J. Alloys Compd. 2017, 715, 176–183. [Google Scholar] [CrossRef]
- Zhilyaev, A.; Langdon, T. Using high-pressure torsion for metal processing: Fundamentals and applications. Prog. Mater. Sci. 2008, 53, 893–979. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Z.; Zhang, J. Crystallization kinetics of Zr55Cu30Al10Ni5 bulk amorphous alloy. J. Alloys Compd. 2002, 339, 90–95. [Google Scholar] [CrossRef]
- Tariq, N.; Iqbal, M.; Shaikh, M.; Akhter, J.I.; Ahmad, M.; Ali, G.; Xu, M. Evolution of microstructure and non-equilibrium phases in electron beam treated Zr55Cu30Al10Ni5 bulk amorphous alloy. J. Alloys Compd. 2008, 460, 258–262. [Google Scholar] [CrossRef]
- Tao, P.J.; Yang, Y.Z.; Bai, X.J.; Mu, Z.X.; Li, G. Non-Isothermal Crystallization Behavior in Zr55Cu30Ni5Al10 Bulk Metallic Glass. Adv. Mater. Res. 2010, 146, 560–564. [Google Scholar] [CrossRef]
- Gao, Y.-L.; Shen, J.; Sun, J.-F.; Wang, G.; Xing, D.-W.; Xian, H.-Z.; Zhou, B.-D. Crystallization behavior of ZrAlNiCu bulk metallic glass with wide supercooled liquid region. Mater. Lett. 2003, 57, 1894–1898. [Google Scholar] [CrossRef]
- Yavari, A.; Le Moulec, A.; Botta, W.J.; Inoue, A.; Rejmankova, P.; Kvick, A. In Situ crystallization of Zr55Cu30Al10Ni5 bulk glass forming from the glassy and undercooled liquid states using synchrotron radiation. J. Non-Cryst. Solids 1999, 247, 31–34. [Google Scholar] [CrossRef]
- Yavari, A.; Le Moulec, A.; Inoue, A.; Botta, W.J.; Vaughan, G.; Kvick, A. Metastable phases in Zr-based bulk glass-forming alloys detected using a synchrotron beam in transmission. Mater. Sci. Eng. A 2001, 304–306, 34–38. [Google Scholar] [CrossRef]
- Aronin, A.; Abrosimova, G.; Matveev, D.; Rybchenko, O. Structure and properties of nanocrystalline alloys prepared by high pressure torsion. Rev. Adv. Mater. Sci. 2010, 25, 52–57. [Google Scholar]
- Ubyivovk, E.V.; Boltynjuk, E.; Gunderov, D.; Churakova, A.A.; Kilmametov, A.; Valiev, R. HPT-induced shear banding and nanoclustering in a TiNiCu amorphous alloy. Mater. Lett. 2017, 209, 327–329. [Google Scholar] [CrossRef]
- Abrosimova, G.; Aronin, A. Nanocrystal formation in Al- and Ti-based amorphous alloys at deformation. J. Alloys Compd. 2018, 747, 26–30. [Google Scholar] [CrossRef]
- Trudeau, M.L. Deformation induced crystallization due ti instability in amorphous FeZr alloys. Appl. Phys. Lett. 1994, 64, 3661–3663, doiorg/101063/1111953. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Saida, J.; Inoue, A. Room-temperature mechanical induced solid-state devitrification od glassy Zr65Al7.5Ni10Cu12.5Pd5 alloy. Acta. Mater. 2003, 51, 4519–4532. [Google Scholar] [CrossRef]
- Yavari, A.R.; Georgarakis, K.; Antonowicz, J.; Stoica, V.; Nishiyama, N.; Vaughan, G.; Chen, M.; Pons, M. Crystallization during Bending of a Pd-based metallic glass detected by X-ray microscopy. Phys. Rev. Lett. 2012, 109, 085501. [Google Scholar] [CrossRef]
- Yan, Z.H.; Song, K.; Hu, Y.; Dai, F.; Chu, Z.; Eckert, J. Localized crystallization in shear bands of a metallic glass. Sci. Rep. 2016, 6, 19358. [Google Scholar] [CrossRef]
- Abrosimova, G.; Aronin, A.; Fokin, D.; Orlova, N.; Postnova, E. The decrase of Young’s modulus in shear bands of amorphous Al87Ni8Gd5 alloy. Mater. Lett. 2019, 252, 114–116. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Greer, A.L. Temperature rise at shear bands in metallic glasses. Nat. Mater. 2006, 5, 15–18. [Google Scholar] [CrossRef]
- Georgarakis, K.; Aljerf, M.; Li, Y.; LeMoulec, A.; Charlot, F.; Yavari, A.R.; Chornokhvostenko, K.; Tabachnikova, E.; Evangelakis, G.A.; Miracle, D.B.; et al. Shear band melting and serrated flow in metallic glasses. App. Phys. Lett. 2008, 93, 031907. [Google Scholar] [CrossRef]
- Schmidt, V.; Rösner, H.; Peterlechler, M.; Wilde, G. Quantitative Measurement of Density in a Shear Band of Metallic Glass Monitored Along its Propagation Direction. Phys. Rev. Lett. 2015, 115, 035501. [Google Scholar] [CrossRef]
- Greer, A.L.; Cheng, Y.Q.; Ma, E. Shear bands in metallic glasses. Mater. Sci. Eng. 2013, 74, 71–132. [Google Scholar] [CrossRef]
- Flores, K.M.; Suh, D.; Dauskardt, R.H. Characterization of free volume in a bulk metallic glass using positron annihilation spectroscopy. J. Mater. Res. 2002, 17, 1153–1161. [Google Scholar] [CrossRef]
- Kanungo, B.P.; Gladeb, S.C.; Asoka-Kumarb, P.; Floresa, K.M. Characterization of free volume changes associated with shear band formation in Zr- and Cu-based bulk metallic glasses. Intermetallics 2004, 12, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Huo, L.S.; Ma, J.; Ke, H.B.; Bai, H.Y.; Zhao, D.Q.; Wang, W.H. The deformation units in metallic glasses revealed by stress-induced localized glass transition. J. Appl. Phys. 2012, 111, 113522. [Google Scholar] [CrossRef]
- Tang, X.P.; Geyer, U.; Busch, R.; Johnson, W.L.; Wu, Y. Diffusion mechanisms in metallic supercooled liquids and glasses. Nature 1999, 402, 160–162. [Google Scholar] [CrossRef]
- Bokeloh, J.; Divinski, S.V.; Reglitz, G.; Wilde, G. Tracer measurements of atomic diffusion inside shear bands of a bulk metallic glass. Phys. Rev. Lett. 2011, 107, 235503. [Google Scholar] [CrossRef]
- Foley, J.C.; Allen, D.R.; Perepezko, J.H. Analysis of nanocrystal development in Al-Y-Fe and Al-Sm glasses. Scr. Mater. 1996, 35, 655–669. [Google Scholar] [CrossRef]
- Abrosimova, G.E.; Aronin, A.S. Effect of the Concentration of a rare-earth component on the parameters of the nanocrystalline structure in aluminum-based alloys. Phys. Solid State 2009, 51, 1765–1771. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Inoue, A. Solid-state crystalline-glassy cyclic phase transformations of mechanically alloyed Cu33Zr67 powders. Met. Trans. A 2002, 33, 135. [Google Scholar] [CrossRef]










| Heating Rate β, K/min | Temperature of Crystallization Start Ts, K |
|---|---|
| 5 | 730 |
| 10 | 740 |
| 20 | 755 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrosimova, G.; Gnesin, B.; Gunderov, D.; Drozdenko, A.; Matveev, D.; Mironchuk, B.; Pershina, E.; Sholin, I.; Aronin, A. Devitrification of Zr55Cu30Al15Ni5Bulk Metallic Glass under Heating and HPT Deformation. Metals 2020, 10, 1329. https://doi.org/10.3390/met10101329
Abrosimova G, Gnesin B, Gunderov D, Drozdenko A, Matveev D, Mironchuk B, Pershina E, Sholin I, Aronin A. Devitrification of Zr55Cu30Al15Ni5Bulk Metallic Glass under Heating and HPT Deformation. Metals. 2020; 10(10):1329. https://doi.org/10.3390/met10101329
Chicago/Turabian StyleAbrosimova, Galina, Boris Gnesin, Dmitry Gunderov, Alexandra Drozdenko, Danila Matveev, Bogdan Mironchuk, Elena Pershina, Ilia Sholin, and Alexandr Aronin. 2020. "Devitrification of Zr55Cu30Al15Ni5Bulk Metallic Glass under Heating and HPT Deformation" Metals 10, no. 10: 1329. https://doi.org/10.3390/met10101329
APA StyleAbrosimova, G., Gnesin, B., Gunderov, D., Drozdenko, A., Matveev, D., Mironchuk, B., Pershina, E., Sholin, I., & Aronin, A. (2020). Devitrification of Zr55Cu30Al15Ni5Bulk Metallic Glass under Heating and HPT Deformation. Metals, 10(10), 1329. https://doi.org/10.3390/met10101329







