Mechanical Properties and Corrosion Resistance of Magnesium–Hydroxyapatite Composites Fabricated by Spark Plasma Sintering
Abstract
:1. Introduction
2. Experimental Details
2.1. Sample Fabrication
2.2. Characterization
2.3. Mechanical Properties
2.4. Corrosion Properties
3. Results and Discussion
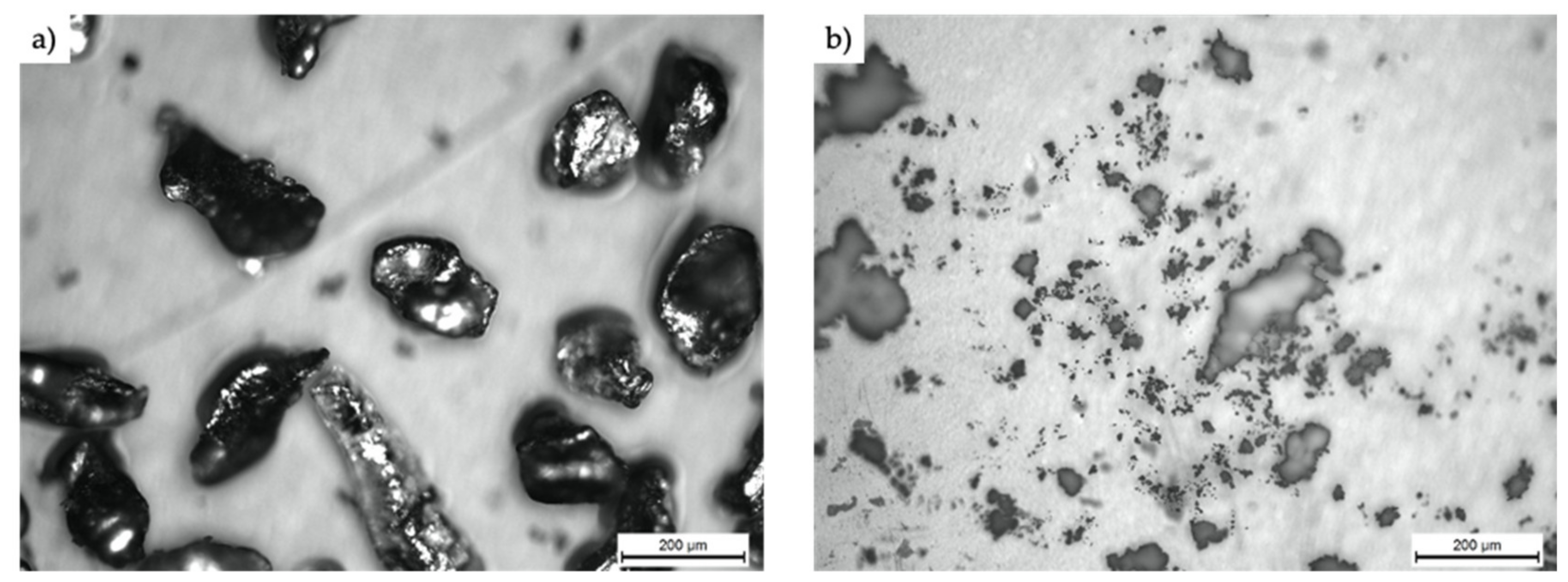
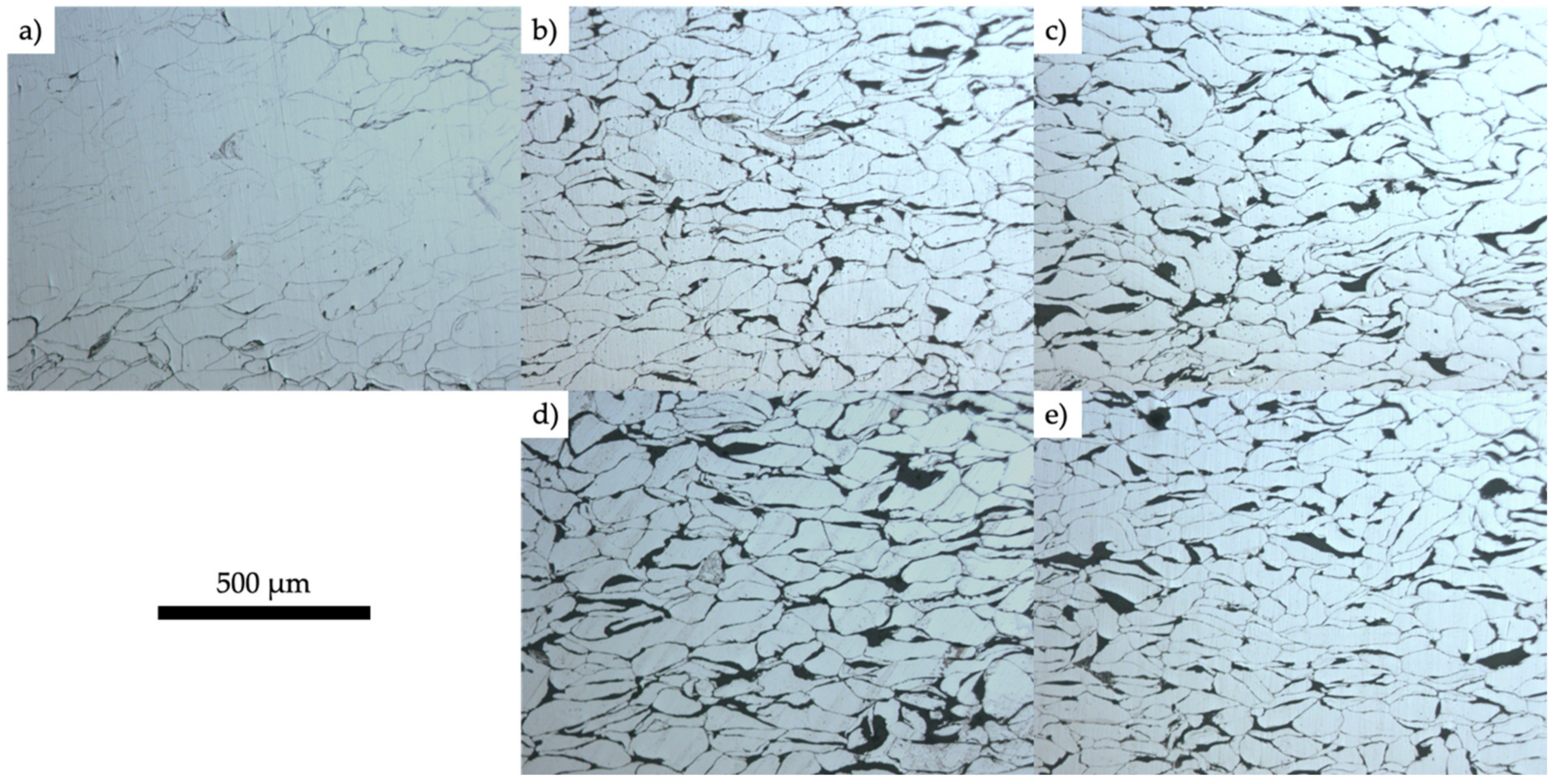
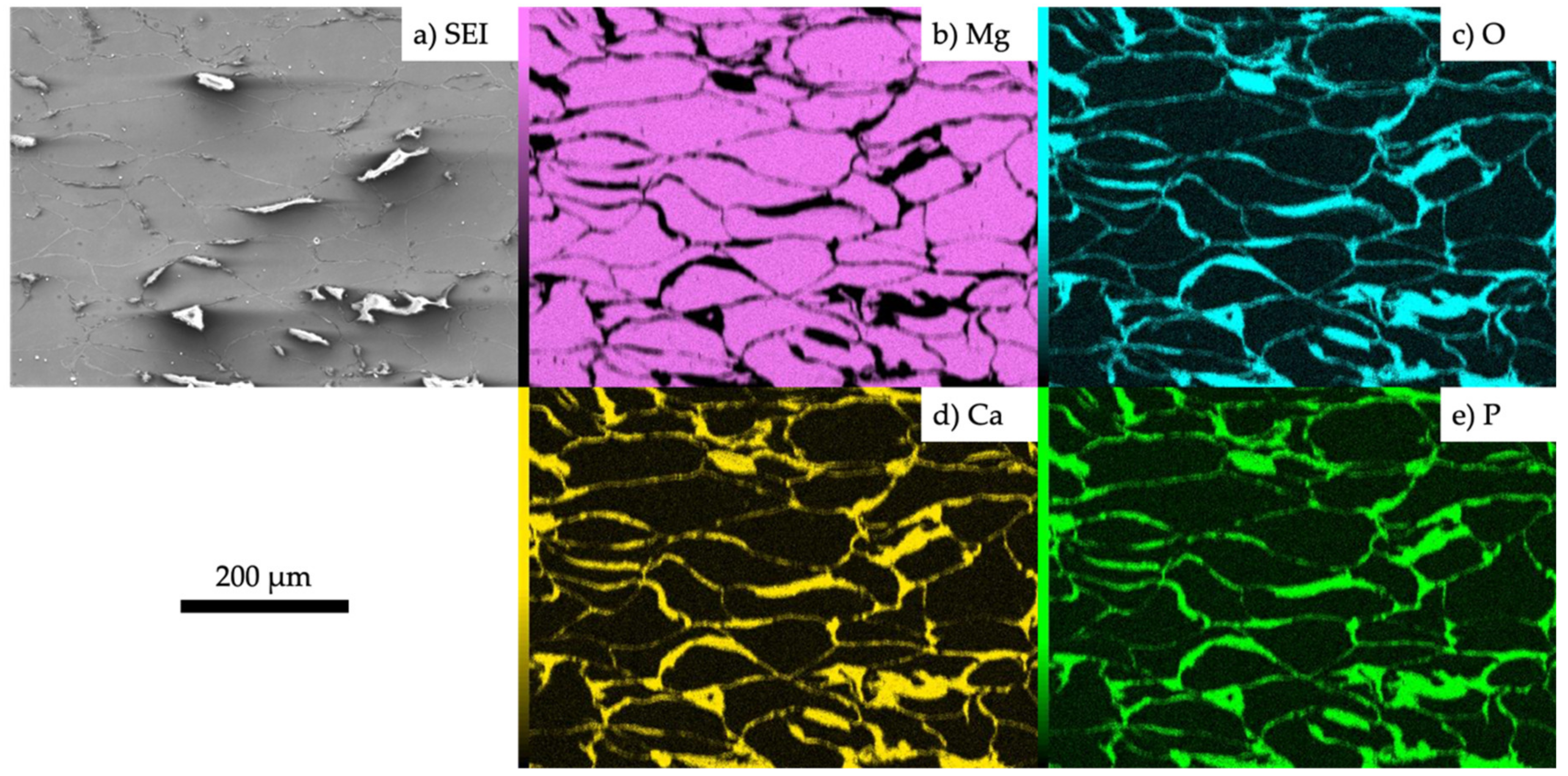
3.1. Characterization of the Fabricated Samples
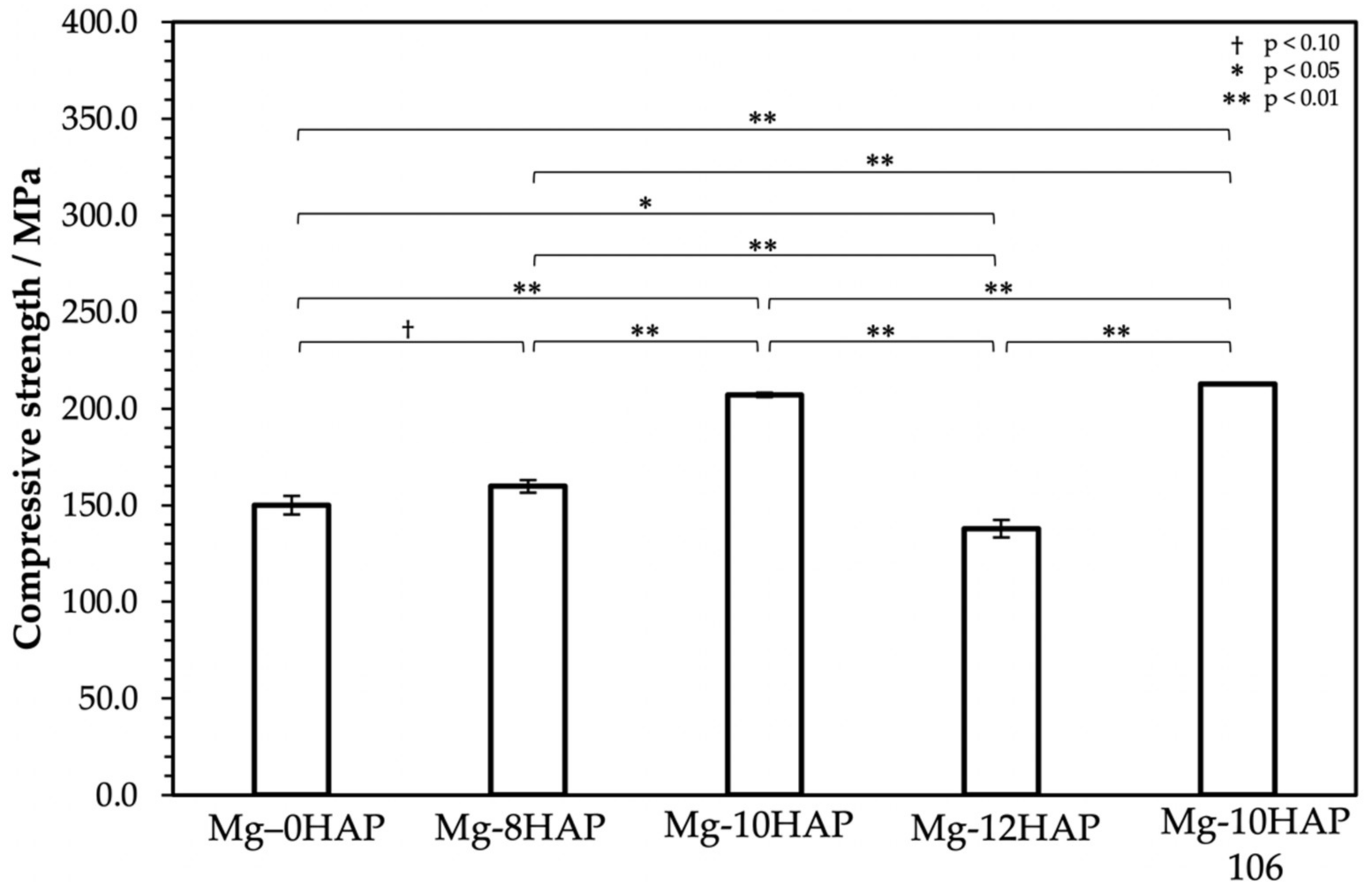
3.2. Mechanical Properties
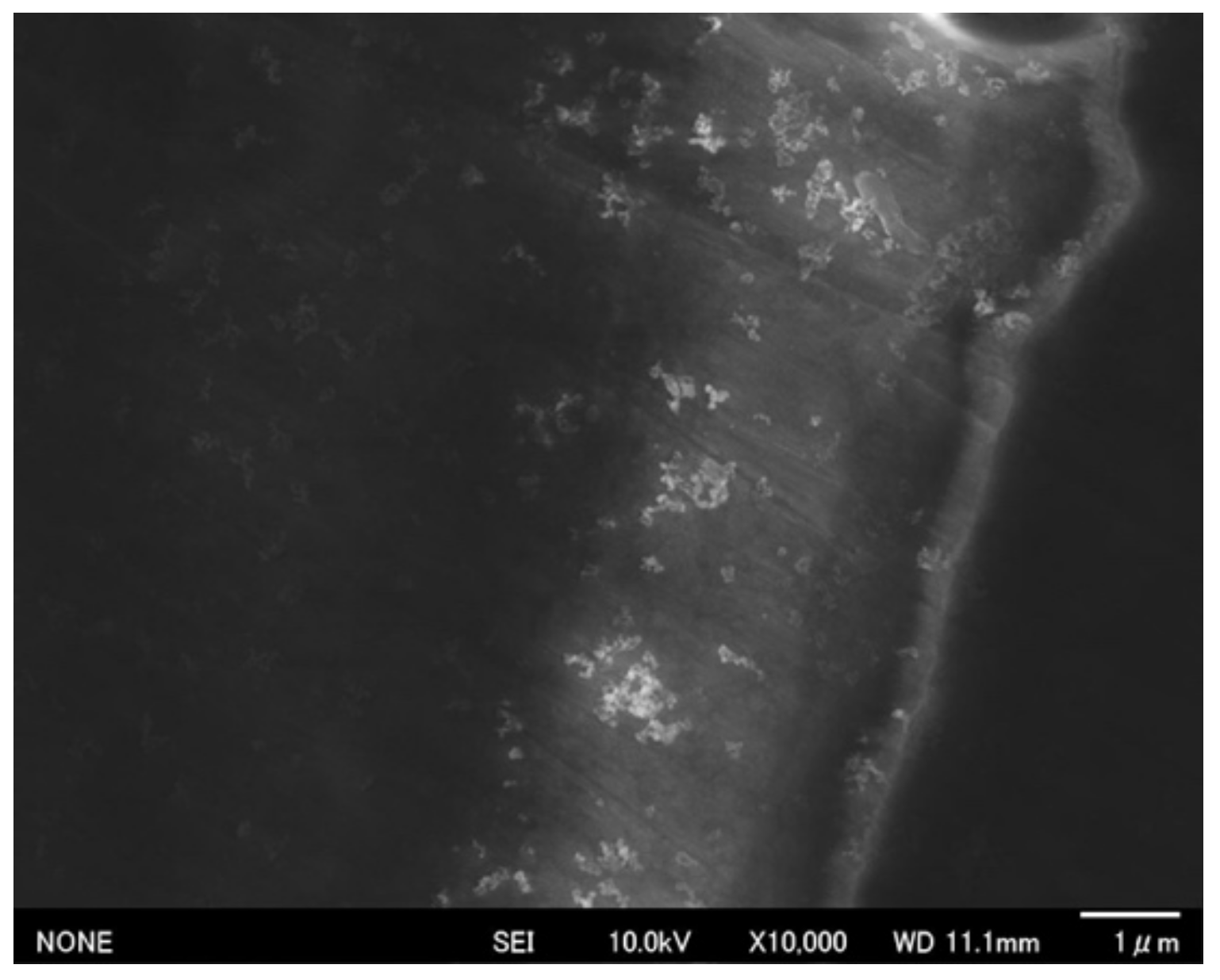
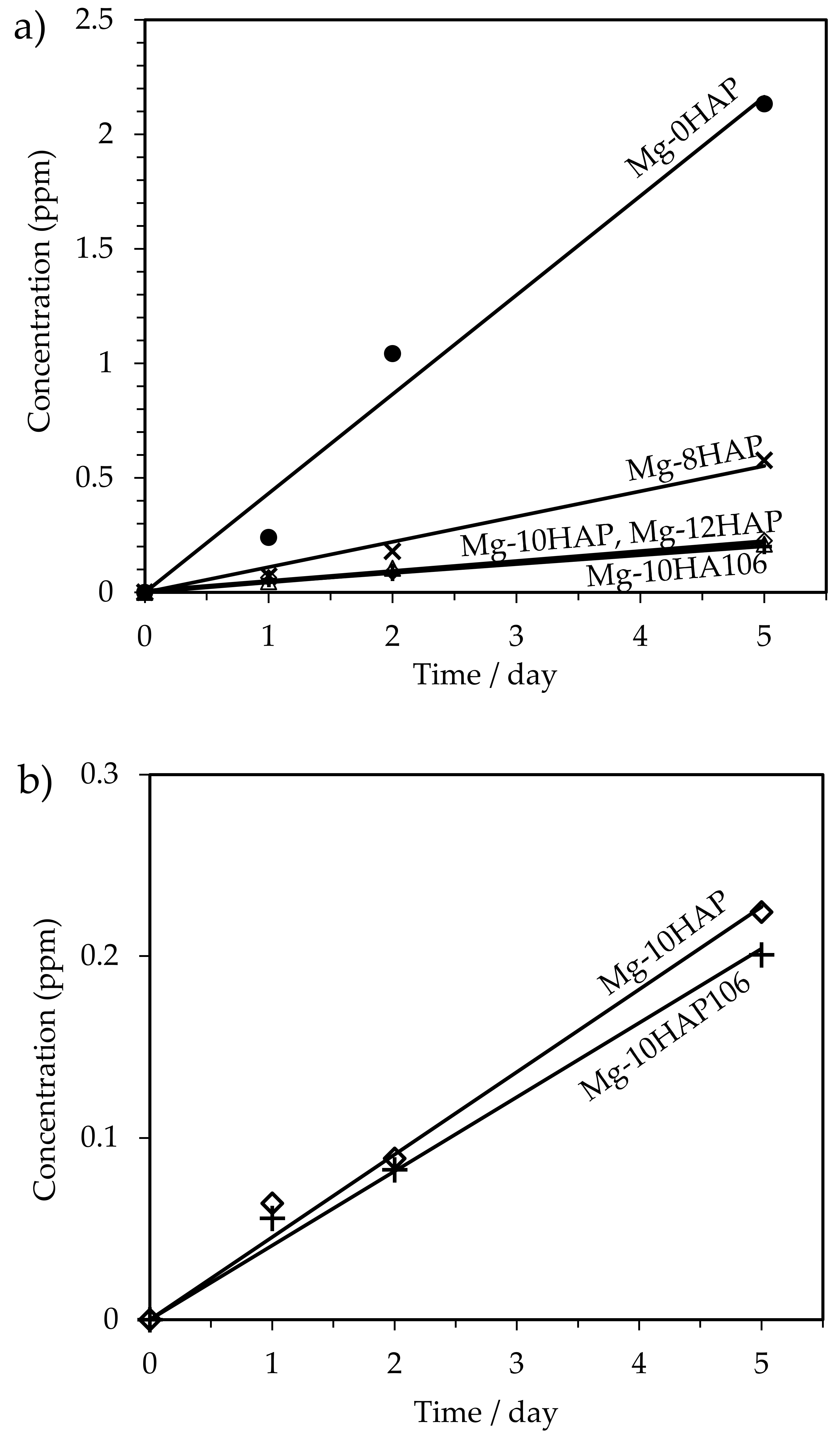
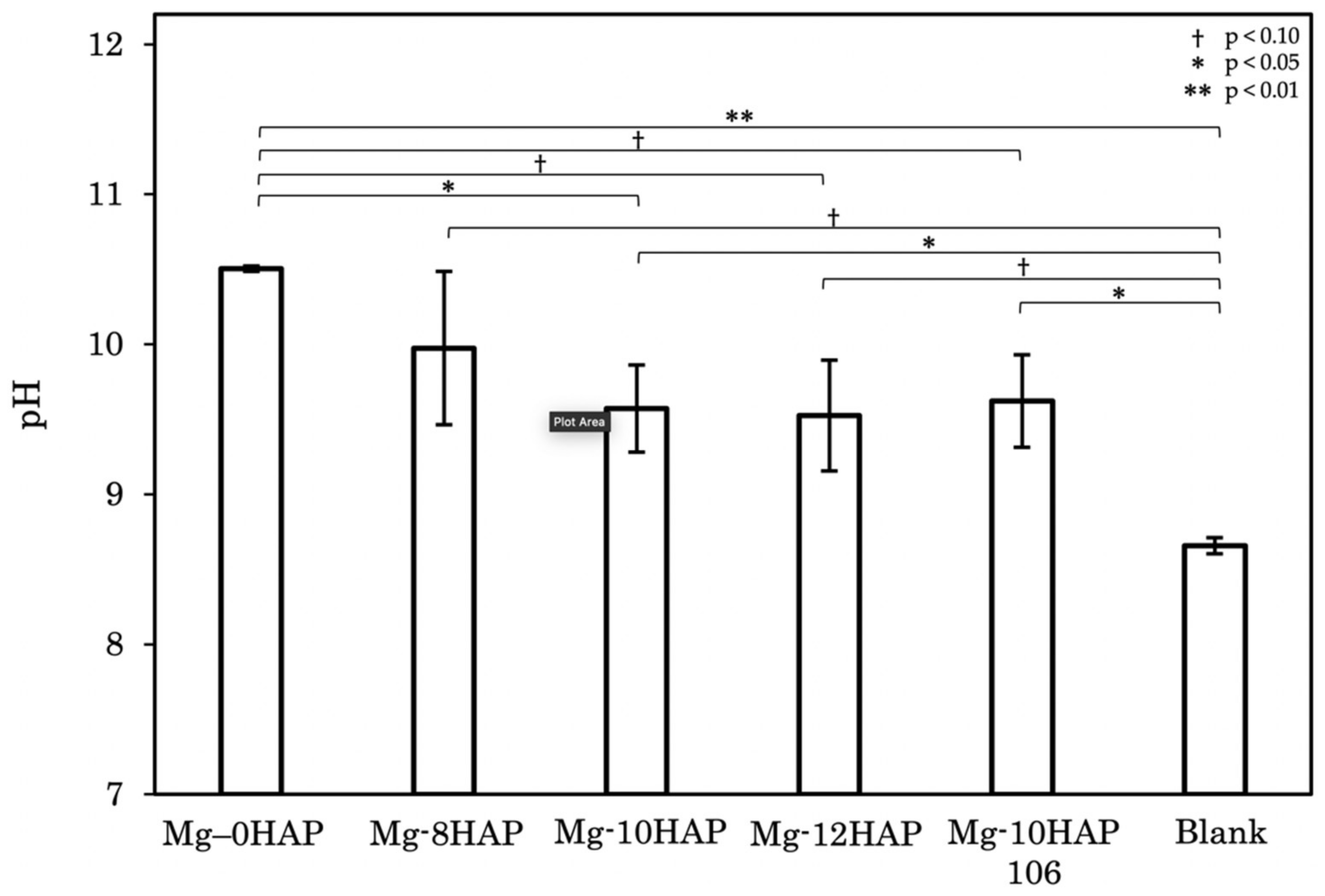
3.3. Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Narita, K.; Kobayashi, E.; Sato, T. Sintering behavior and mechanical properties of magnesium/β-tricalcium phosphate composites sintered by spark plasma sintering. Mater. Trans. 2016, 57, 1620–1627. [Google Scholar] [CrossRef] [Green Version]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Ahangarani, S.; Esmailian, M.; Shanaghi, A. Investigation on the corrosion behavior and biocompatibility of Ti-6Al-4V implant coated with HA/TiN dual layer for medical applications. Surf. Coatings Technol. 2020, 397, 126044. [Google Scholar] [CrossRef]
- Dogan, H.; Findik, F.; Morgul, O. Friction and wear behaviour of implanted AISI 316L SS and comparison with a substrate. Mater. Des. 2002, 23, 605–610. [Google Scholar] [CrossRef]
- Shenhar, A.; Gotman, I.; Radin, S.; Ducheyne, P. Microstructure and fretting behavior of hard TiN-based coatings on surgical titanium alloys. Ceram. Int. 2000, 26, 709–713. [Google Scholar] [CrossRef]
- Zhong, Z.; Ma, J. Fabrication, characterization, and in vitro study of zinc substituted hydroxyapatite/silk fibroin composite coatings on titanium for biomedical applications. J. Biomater. Appl. 2017, 32, 399–409. [Google Scholar] [CrossRef]
- Ersen, O.; Tuzun, H.Y.; Ozsezen, A.M.; Bilekli, A.B.; Koca, K.; Kurklu, M. The procedure with less interest than it is done in orthopedic practice: Implant removal. Acta Medica Mediterr. 2019, 35, 825–828. [Google Scholar]
- Shibata, Y.; Tanimoto, Y.; Maruyama, N.; Nagakura, M. A review of improved fixation methods for dental implants. Part II: Biomechanical integrity at bone-implant interface. J. Prosthodont. 2015, 59, 84–95. [Google Scholar] [CrossRef]
- Hanson, B.; van der Werken, C.; Stengel, D. Surgeons’ beliefs and perceptions about removal of orthopaedic implants. BMC Musculoskelet. Disord. 2008, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Onche, I.I.; Osagie, O.E.; INuhu, S. Removal of orthopaedic implants: Indications, outcome and economic implications. J. West Afr. Coll. Surg. 2011, 1, 101–112. [Google Scholar] [PubMed]
- Cao, N.Q.; Pham, D.N.; Kai, N.; Dinh, H.V.; Hiromoto, S.; Kobayashi, E. In vitro corrosion properties of mg matrix in situ composites fabricated by spark plasma sintering. Metals 2017, 7, 358. [Google Scholar] [CrossRef] [Green Version]
- Cao, N.Q.; Le, H.M.; Pham, K.M.; Nguyen, N.V.; Hiromoto, S.; Kobayashi, E. In vitro corrosion and cell response of hydroxyapatite coated mg matrix in situ composites for biodegradable material applications. Materials 2019, 12, 3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Linford, J.; Chen, S.; Smith, C.; Sankar, J. Preparation and characterization of porous magnesium alloys in biomedical applications. ASME Int. Mech. Eng. Congr. Expo. Proc. 2009, 14, 37–41. [Google Scholar]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. CKJ Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canillas, M.; Pena, P.; Aza, A.H.D.; Rodríguez, M.A. Calcium phosphates for biomedical applications. Boletín la Soc. Española Cerámica y Vidr. 2017, 56, 91–112. [Google Scholar] [CrossRef]
- Corrosion behavior in hank’s solution of a magnesium—Hydroxyapatite composite processed by high-pressure torsion. Adv. Eng. Mater. 2020, 2000765. [CrossRef]
- Su, J.; Teng, J.; Xu, Z. Corrosion-wear behavior of a biocompatible magnesium matrix composite in simulated body fluid. Friction 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Pinc, J.; Capek, J.; Kubasek, J.; Prusa, F.; Hybasek, V.; Vertat, P.; Sedlarova, I.; Vojtech, D. Characterization of a Zn-Ca5(PO4)3(OH) composite with a high content of the hydroxyapatite particles prepared by the spark plasma sintering process. Metals 2020, 10, 372. [Google Scholar] [CrossRef] [Green Version]
- Pinc, J.; Capek, J.; Hybasek, V.; Prusa, F.; Hosova, K.; Manak, J.; Vojtech, D. Characterization of newly developed zinc composite with the content of 8 wt. % of hydroxyapatite particles processed by extrusion. Materials 2020, 13, 1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodaei, M.; Nejatidanesh, F.; Shirani, M.J.; Iyenger, S.; Sina, H.; Savabi, O. Magnesium/nano-hydroxyapatite composite for bone reconstruction: The effect of processing method. J. Bionic Eng. 2020, 17, 92–99. [Google Scholar] [CrossRef]
- Kubasek, J.; Vojtech, D.; Maixner, J.; Dvorsky, D. The effect of hydroxyapatite reinforcement and preparation methods on the structure and mechanical properties of Mg-HA composites. Sci. Eng. Compos. Mater. 2017, 24, 297–307. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwahara, H.; Al-Abdullat, Y.; Mazaki, N.; Tsutsumi, S.; Aizawa, T. Preparation of magnesium apatite on pure magnesium surface during immersing in hank’s solution. Mater. Trans. 2001, 42, 1317–1321. [Google Scholar] [CrossRef] [Green Version]
- Munir, Z.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Huang, J.L.; Nayak, P.K. Strengthening alumina ceramic matrix nanocomposites using spark plasma sintering. In Advances in Ceramic Matrix Composites; Low, I.M., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 218–234. [Google Scholar]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Göttingen 1912, 26, 98–100. [Google Scholar]
- Langford, J.I.; Wilson, A.J.C. Seherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Cryst. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Alvarez-Lopez, M.; Pereda, M.D.; Del Valle, J.A.; Fernandez-Lorenzo, M.; Garcia-Alonso, M.C.; Ruano, O.A.; Escudero, M.L. Corrosion behaviour of AZ31 magnesium alloy with different grain sizes in simulated biological fluids. Acta Biomater. 2010, 6, 1763–1771. [Google Scholar] [CrossRef] [Green Version]
- Ge, Q.; Dellasega, D.; Demir, A.G.; Vedani, M. The processing of ultrafine-grained Mg tubes for biodegradable stents. Acta Biomater. 2013, 9, 8604–8610. [Google Scholar] [CrossRef]
- Argade, G.R.; Panigrahi, S.K.; Mishra, R.S. Effects of grain size on the corrosion resistance of wrought magnesium alloys containing neodymium. Corros. Sci. 2012, 58, 145–151. [Google Scholar] [CrossRef]




| Sample Name | Mg–0HAP | Mg–8HAP | Mg–10HAP | Mg–12HAP | Mg–10HAP106 |
|---|---|---|---|---|---|
| HAP composition | 0 wt% | 8 wt% | 10 wt% | 12 wt% | 10 wt% |
| Mg powder size | ~180 µm | ~180 µm | ~180 µm | ~180 µm | ≤106 µm |
| Reagents | NaCl | KCl | Na2HPO4·2H2O | KH2PO4 | MgSO4·7H2O | NaHCO3 | CaCl2 |
|---|---|---|---|---|---|---|---|
| Concentration (g/L) | 8.00 | 0.40 | 0.06 | 0.06 | 0.20 | 0.35 | 0.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakahata, I.; Tsutsumi, Y.; Kobayashi, E. Mechanical Properties and Corrosion Resistance of Magnesium–Hydroxyapatite Composites Fabricated by Spark Plasma Sintering. Metals 2020, 10, 1314. https://doi.org/10.3390/met10101314
Nakahata I, Tsutsumi Y, Kobayashi E. Mechanical Properties and Corrosion Resistance of Magnesium–Hydroxyapatite Composites Fabricated by Spark Plasma Sintering. Metals. 2020; 10(10):1314. https://doi.org/10.3390/met10101314
Chicago/Turabian StyleNakahata, Ikuho, Yusuke Tsutsumi, and Equo Kobayashi. 2020. "Mechanical Properties and Corrosion Resistance of Magnesium–Hydroxyapatite Composites Fabricated by Spark Plasma Sintering" Metals 10, no. 10: 1314. https://doi.org/10.3390/met10101314
APA StyleNakahata, I., Tsutsumi, Y., & Kobayashi, E. (2020). Mechanical Properties and Corrosion Resistance of Magnesium–Hydroxyapatite Composites Fabricated by Spark Plasma Sintering. Metals, 10(10), 1314. https://doi.org/10.3390/met10101314




