Effect of Processing Conditions on the Microstructure, Mechanical Properties, and Corrosion Behavior of Two Austenitic Stainless Steels for Bioimplant Applications
Abstract
:1. Introduction
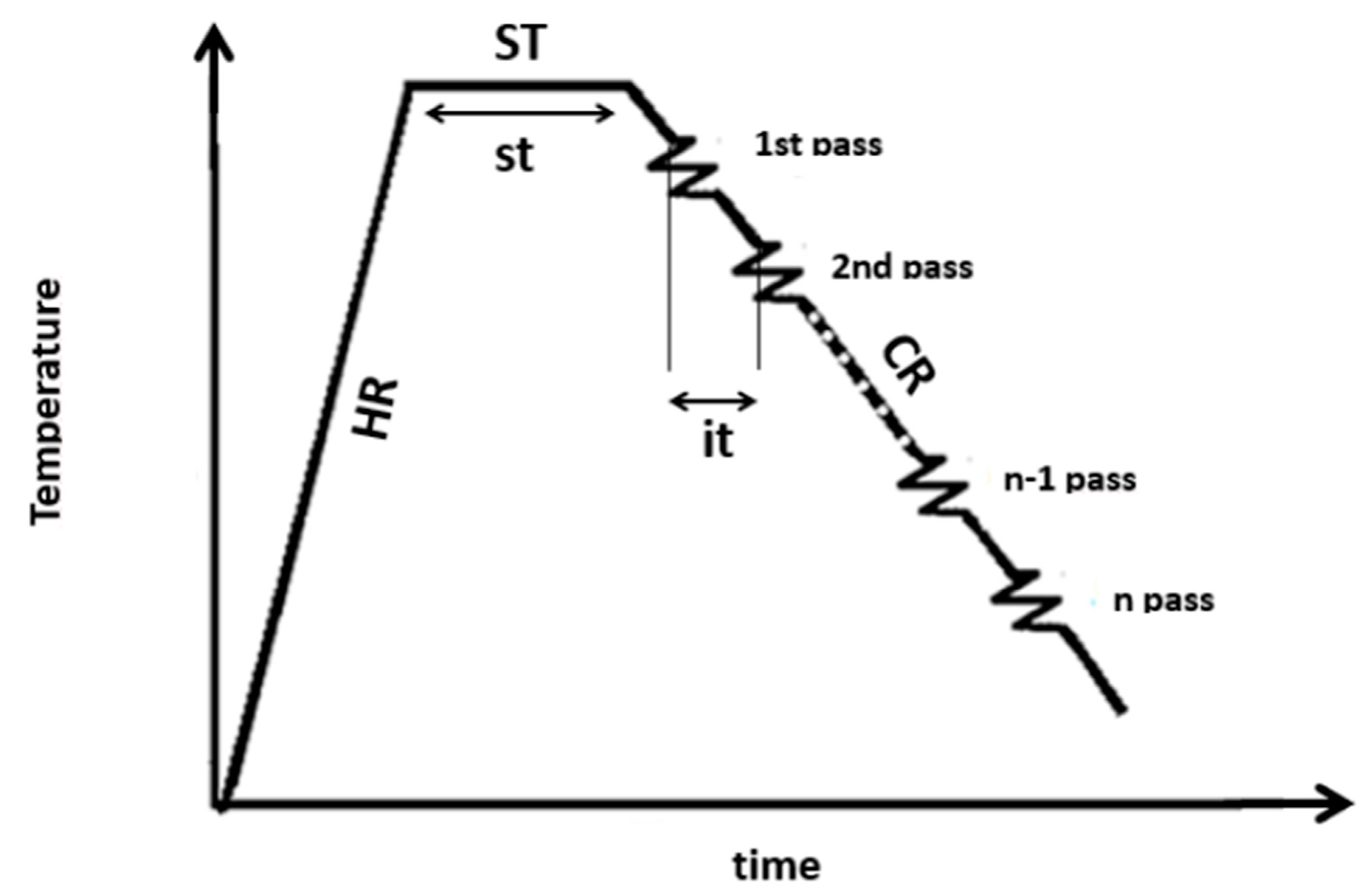
2. Materials and Methods
3. Results
3.1. Flow Stress Curves
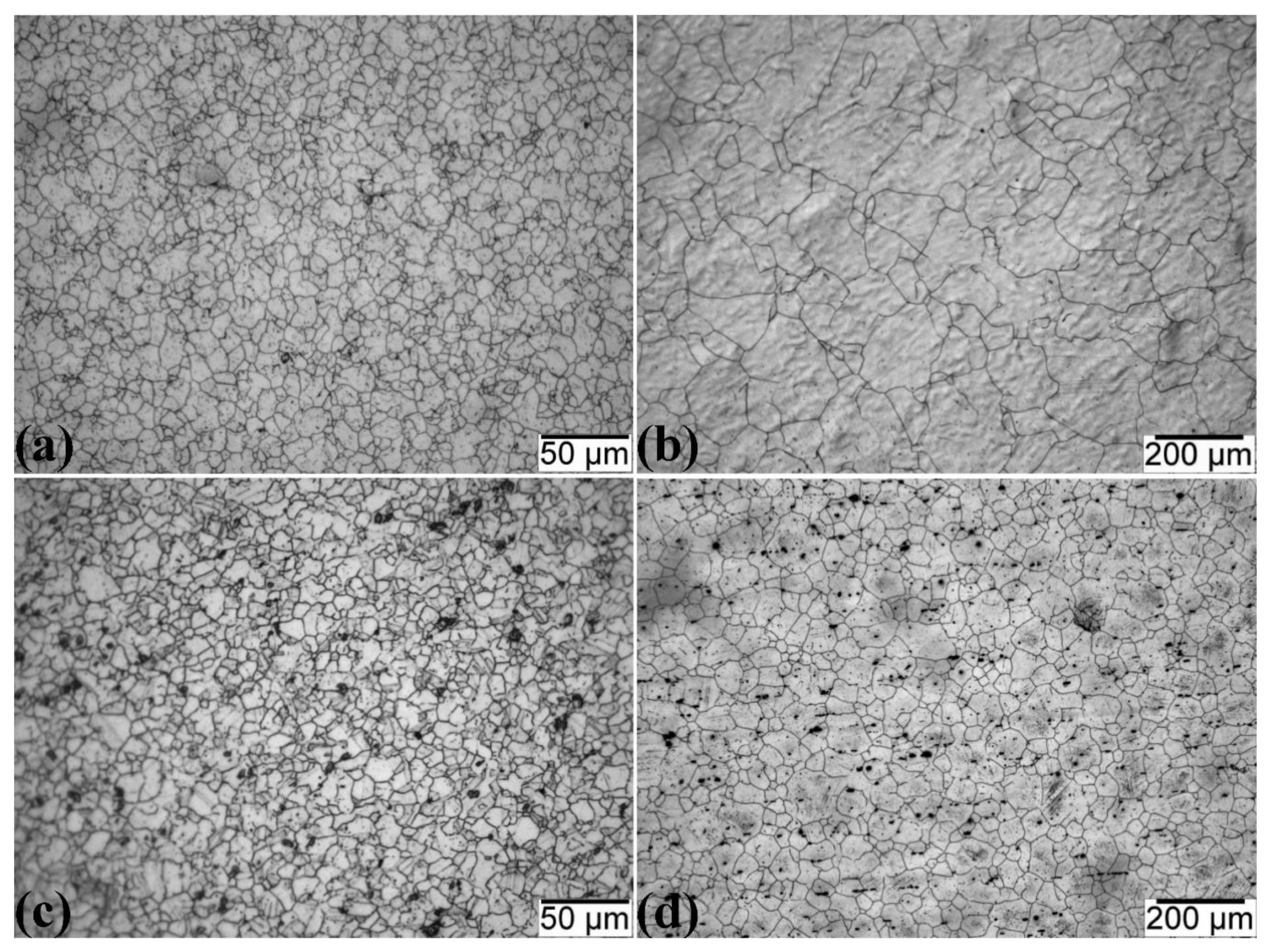
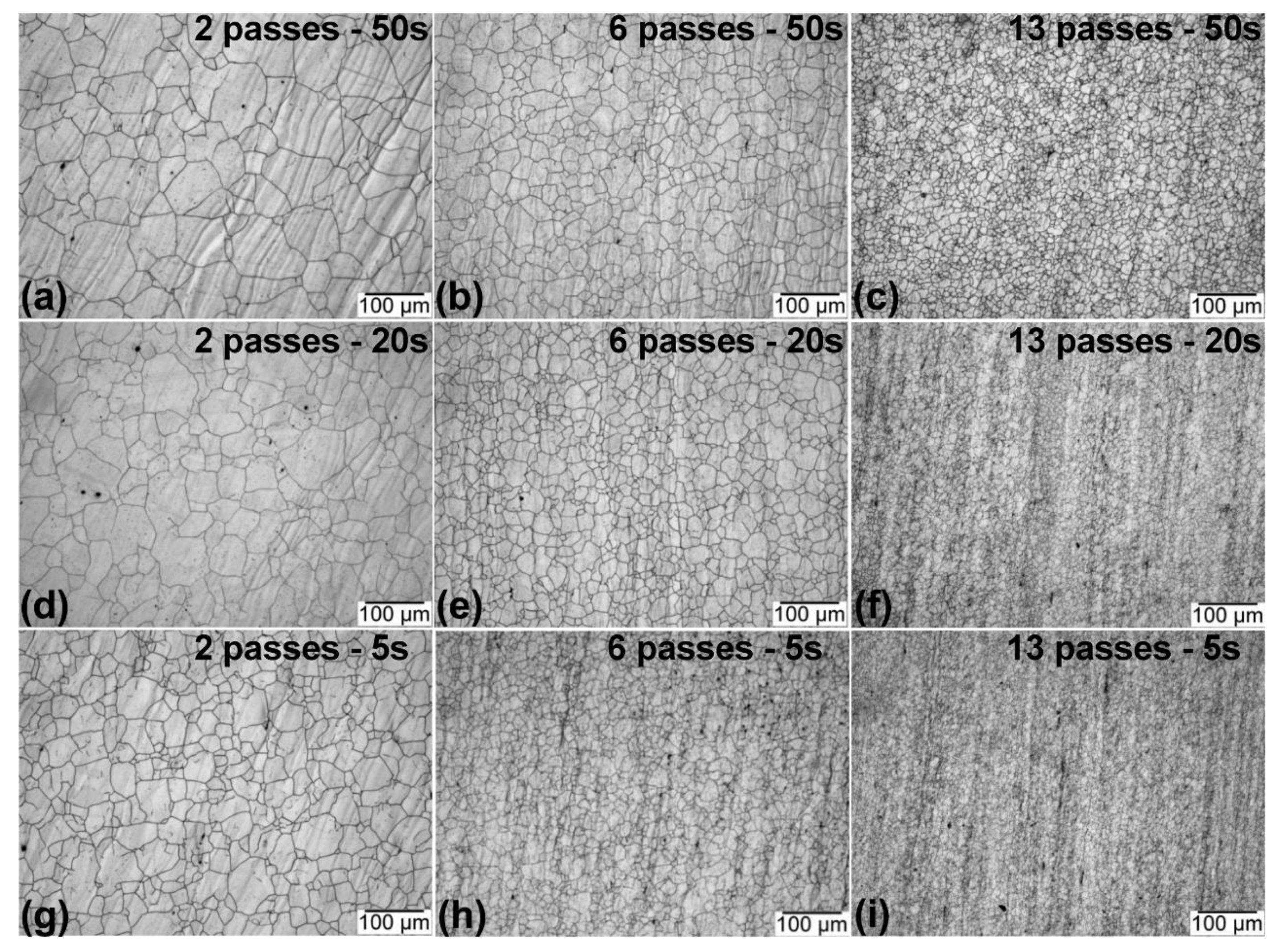
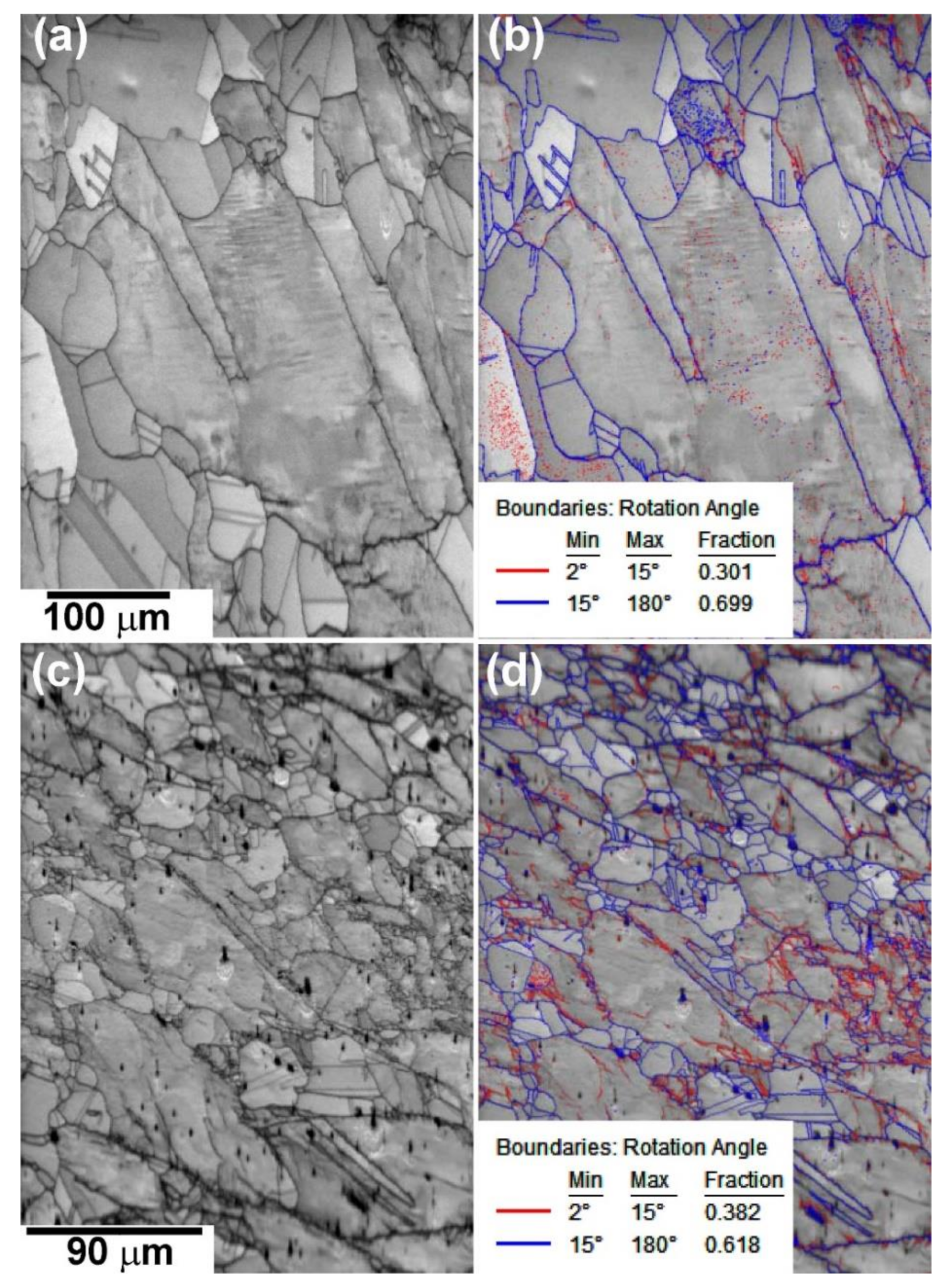
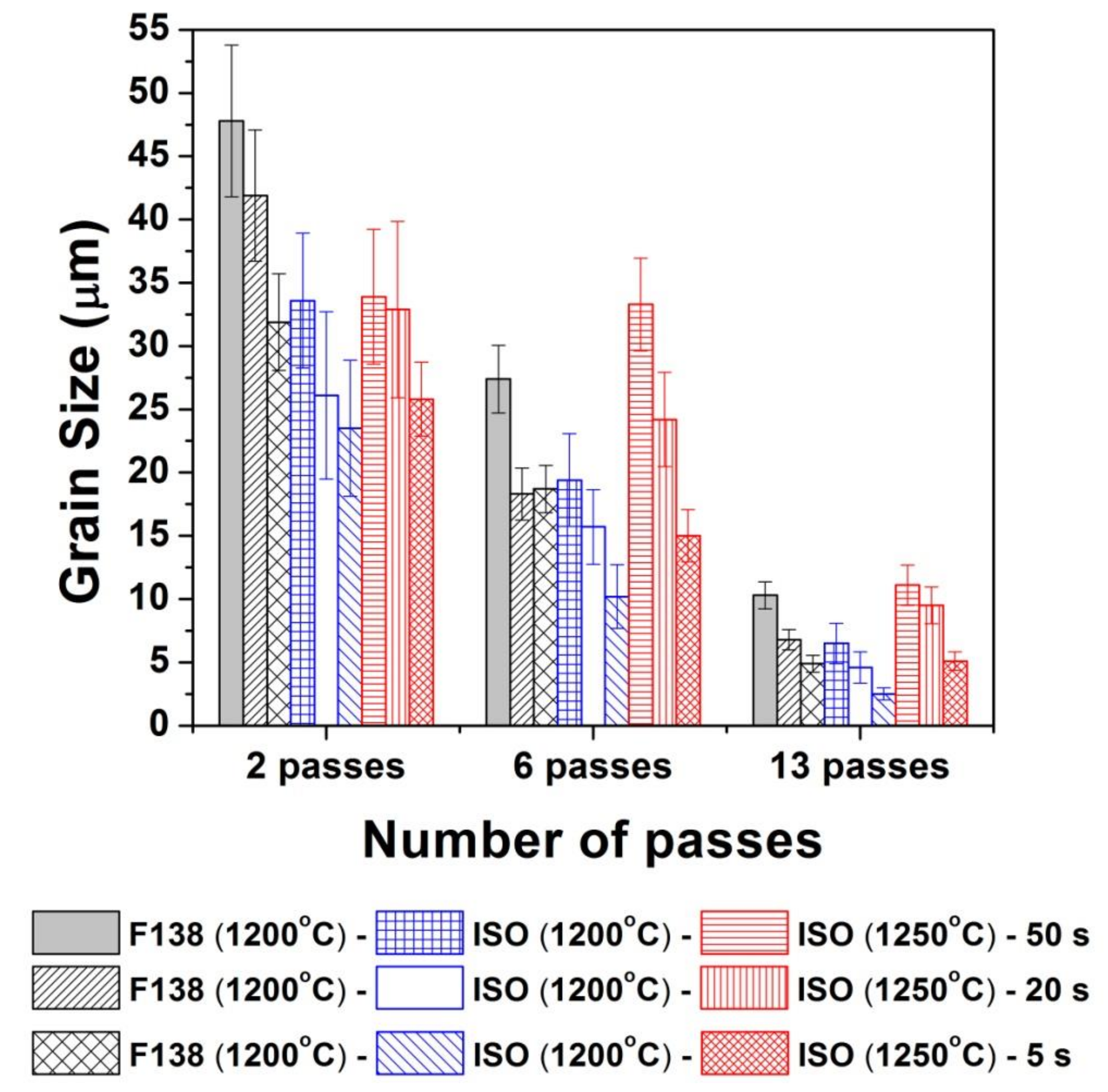
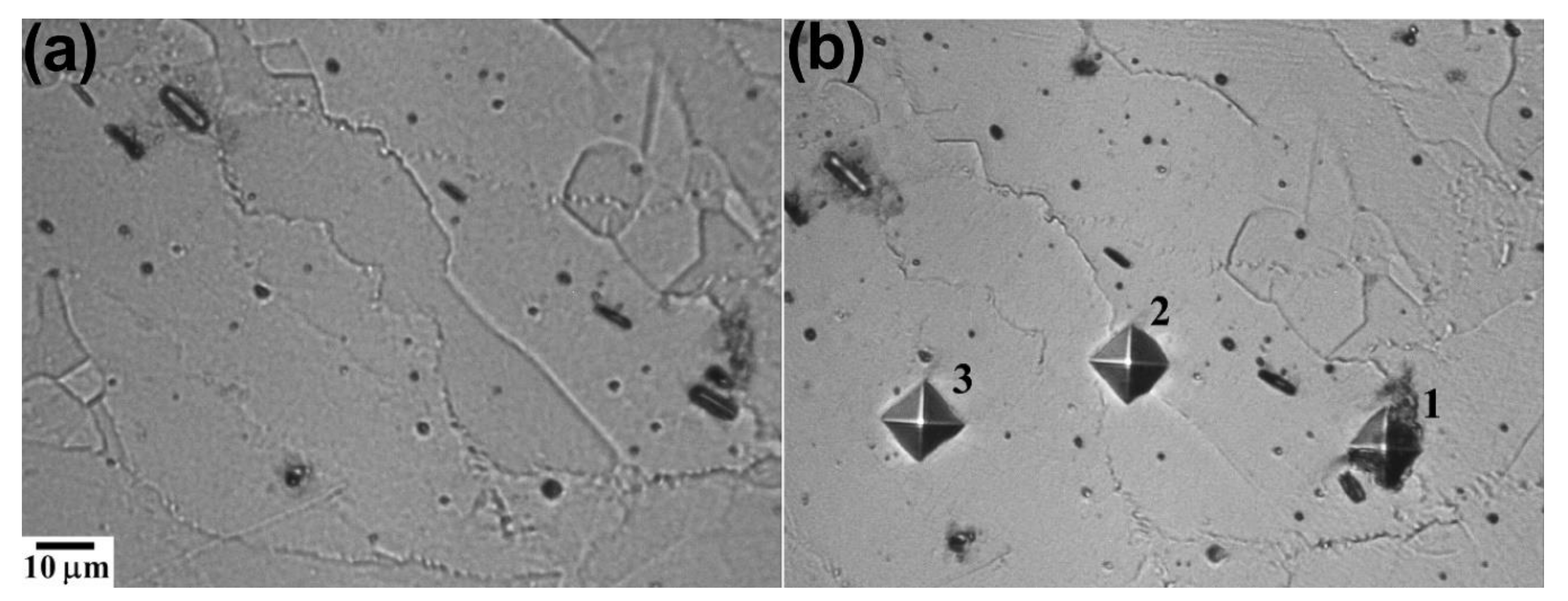
3.2. Microstructural Evolution
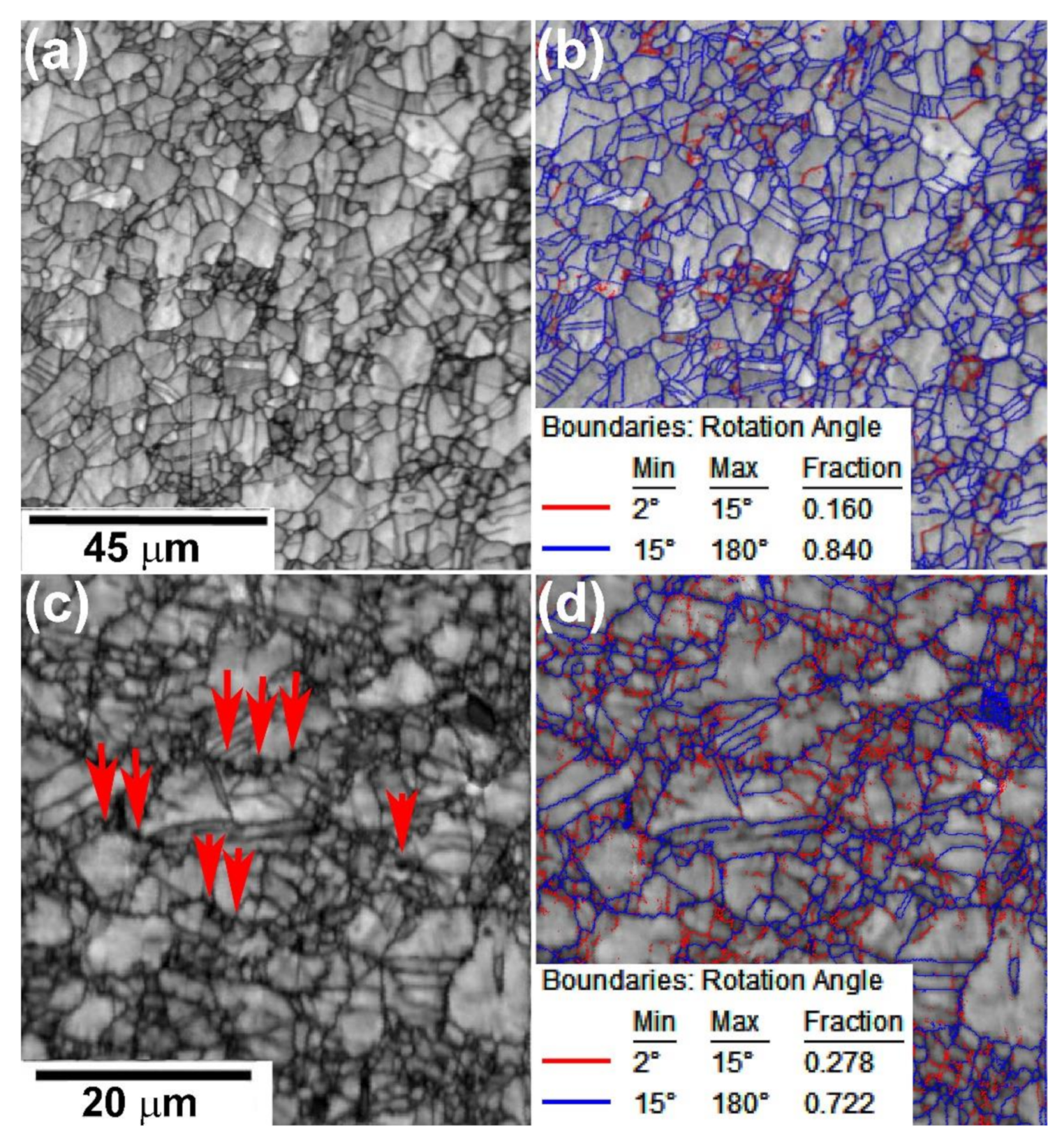
3.2.1. Optical Microscopy
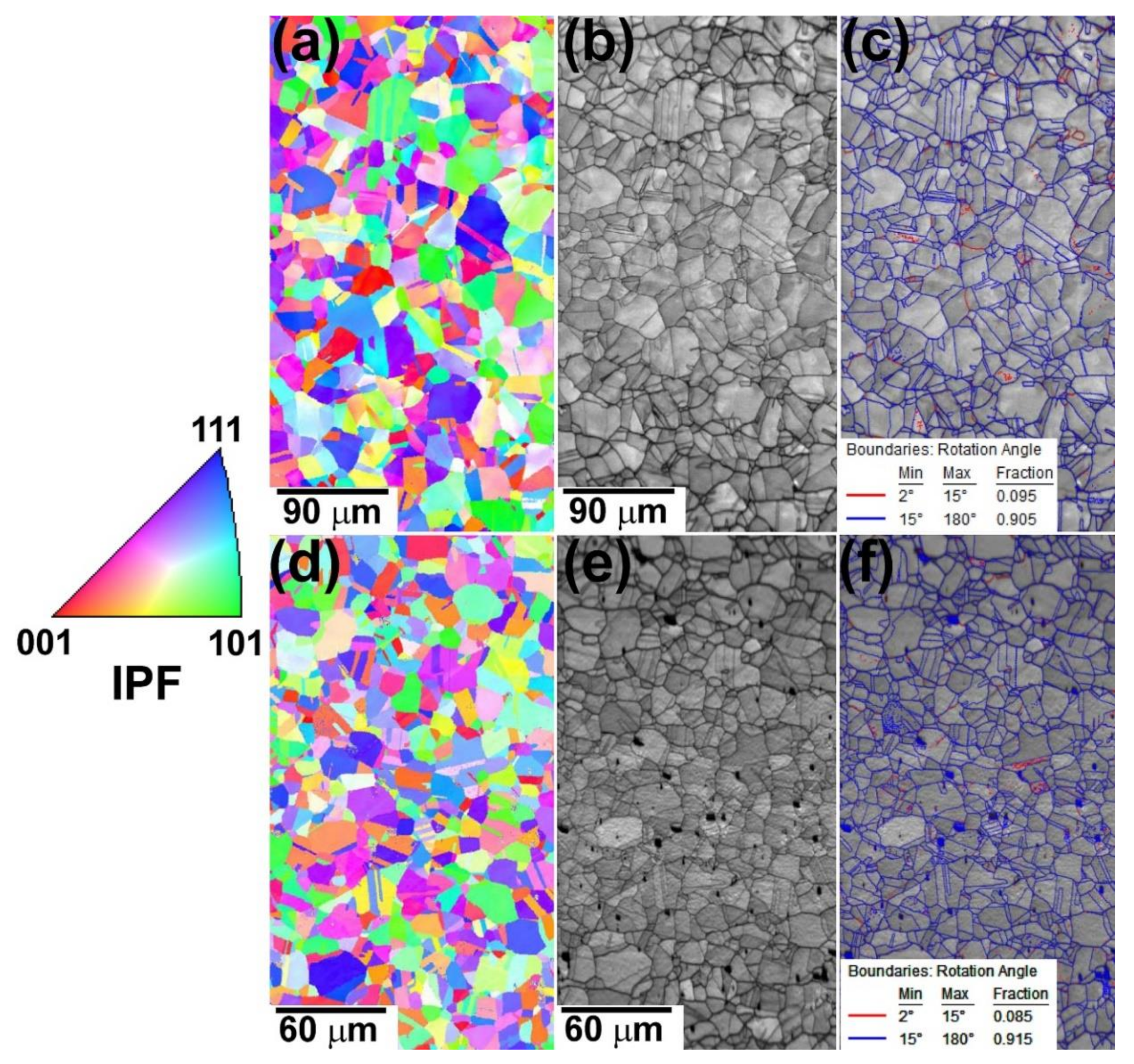
3.2.2. EBSD Analysis
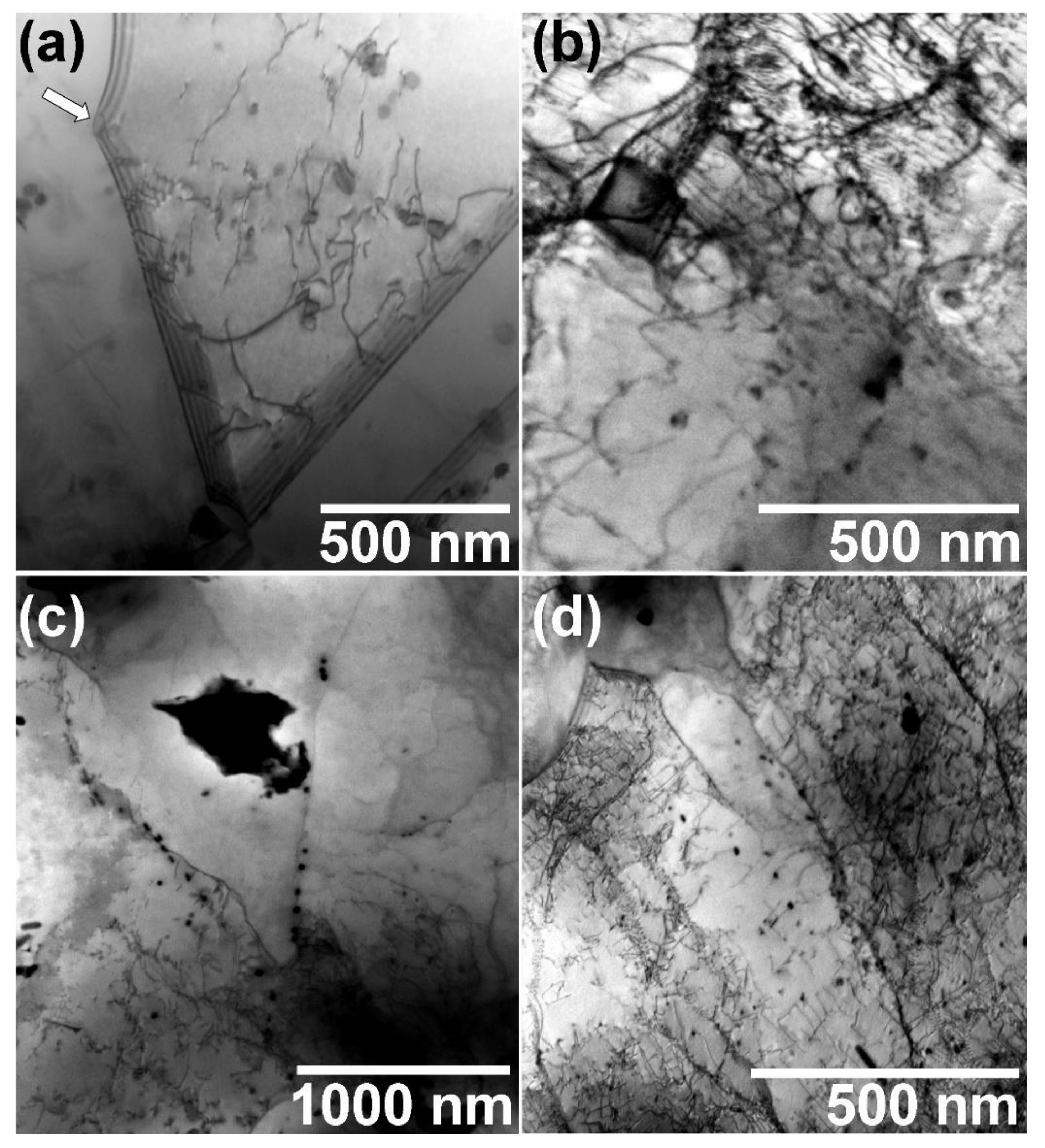
3.2.3. TEM Observations
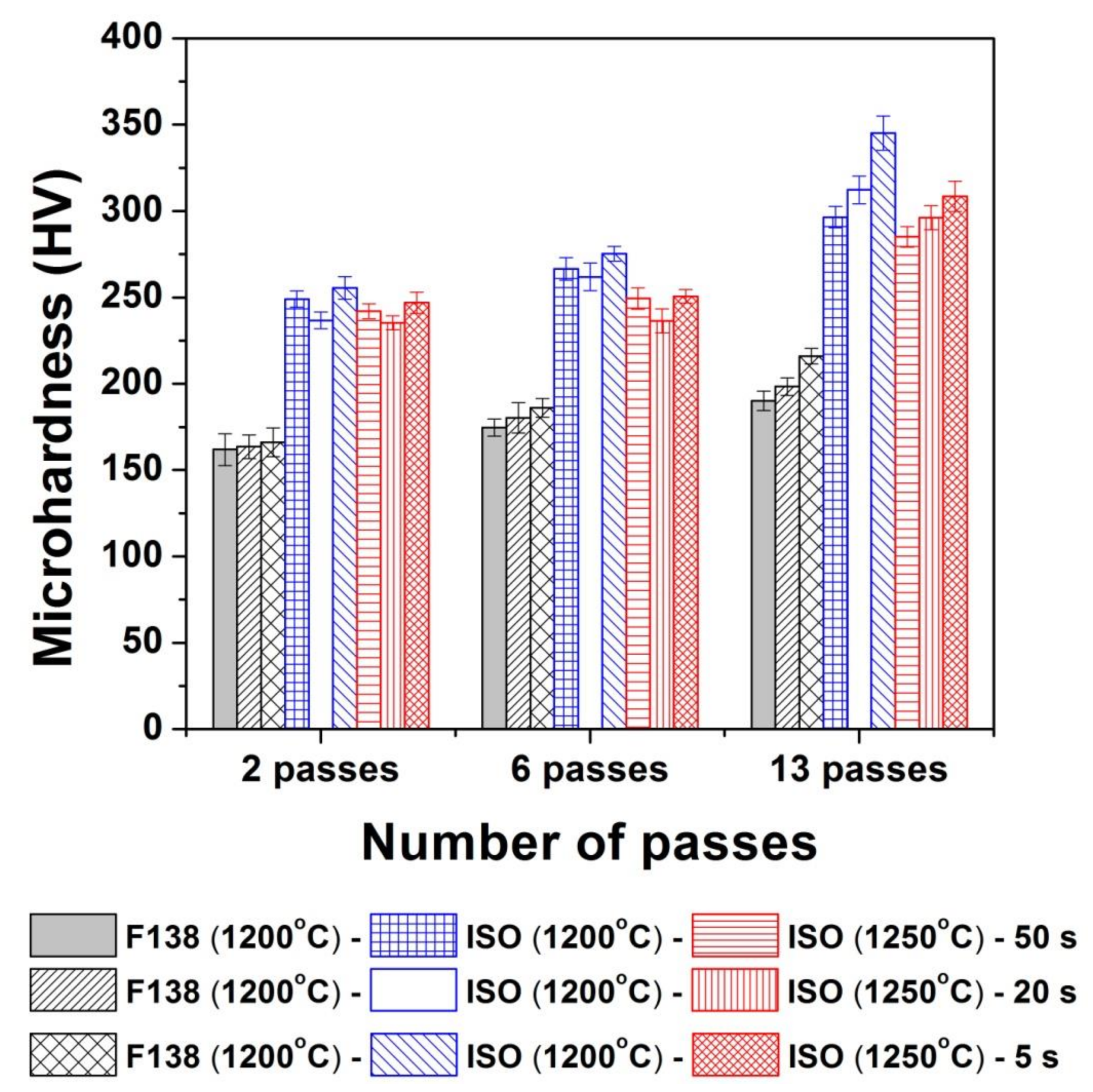
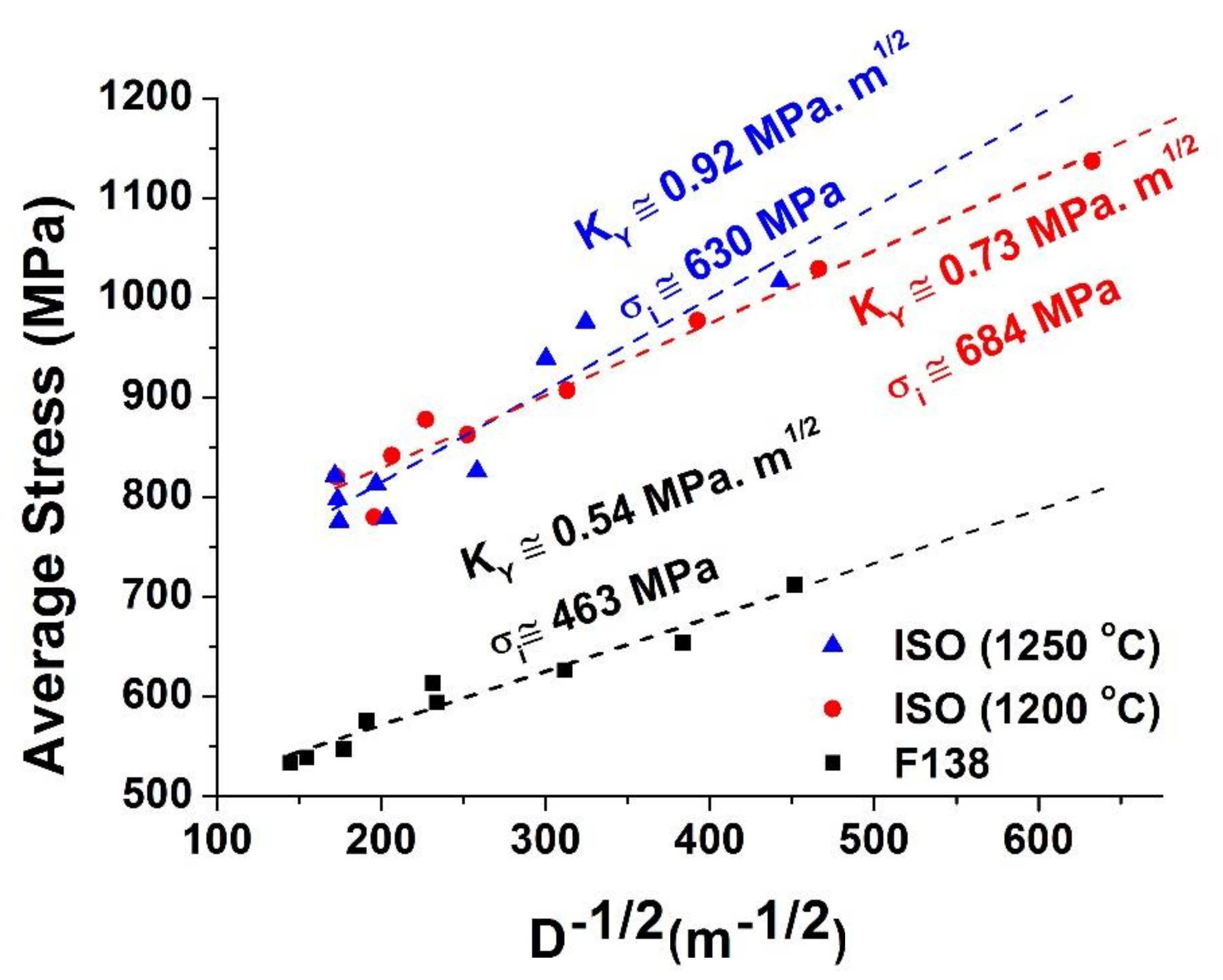
3.3. Mechanical Properties (Microhardness)
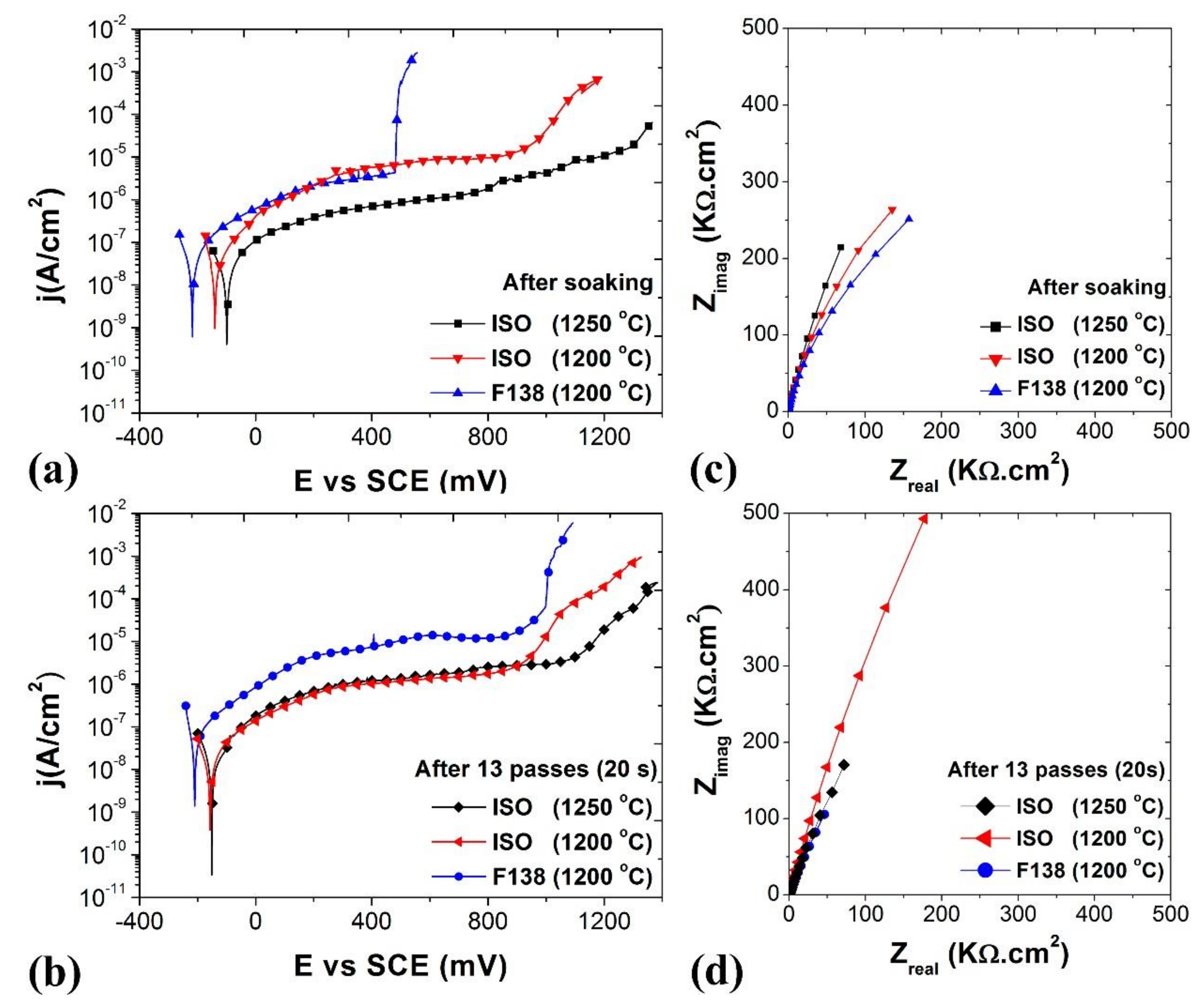
3.4. Corrosion Behavior
4. Discussion
4.1. Flow Stress Behavior
4.2. Microstructural Evolution
4.3. Mechanical Properties
4.4. Corrosion Behavior
5. Conclusions
- The reduction of reheating temperature to 1200 °C led the steel ISO 5832-9 to have better mechanical and corrosion properties than when soaked at 1250 °C.
- Processing loads were not significantly increased with the reduction of the reheating temperature. This fact indicates that existing processing equipment can continue to be used.
- The decrease of interpass times and reduction of reheating temperature shorten the processing time, causing production costs to be reduced, which would help to reduce the price of prosthesis’ implantation greatly.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gong, P.; Palmiere, E.J.; Rainforth, W.M. Dissolution and precipitation behaviour in steels microalloyed with niobium during thermomechanical processing. Acta Mater. 2015, 97, 392–403. [Google Scholar] [CrossRef]
- Lu, J.; Omotoso, O.; Wiskel, J.B.; Ivey, D.G.; Henein, H. Strengthening mechanisms and their relative contributions to the yield strength of microalloyed Steels. Metall. Mater. Trans. A 2012, 43, 3043–3061. [Google Scholar] [CrossRef]
- Taca, M.; Constantinescu, D.M.; Baciu, F.; Geanta, V.; Daisa, D.; Stefanoiu, R. Influence of technological parameters on the toughness improvement of microalloyed steels. Mater. Today Proc. 2016, 3, 1177–1182. [Google Scholar] [CrossRef]
- Yuan, S.Q.; Liang, G.L.; Zhang, X.J. Interaction between elements Nb and Mo during precipitation in microalloyed austenite. J. Iron Steel Res. 2010, 17, 60–63. [Google Scholar] [CrossRef]
- Elwazri, A.M.; Yue, S.; Wanjara, P. Effect of prior-austenite grain size and transformation temperature on nodule size of microalloyed hypereutectoid steels. Metall. Mater. Trans. A 2005, 36, 2297–2305. [Google Scholar] [CrossRef]
- Phaniraj, M.P.; Behera, B.B.; Lahiri, A.K. Thermo-mechanical modeling of two phase rolling and microstructure evolution in the hot strip mill: Part I. Prediction of rolling loads and finish rolling temperature. J. Mater. Process. Technol. 2005, 170, 323–335. [Google Scholar] [CrossRef]
- Craven, A.J.; He, K.; Garvie, L.A.J.; Baker, T.N. Complex heterogeneous precipitation in titanium–niobium microalloyed Al-killed HSLA steels-I. (Ti,Nb)(C, N) particles. Acta Mater. 2000, 48, 3857–3868. [Google Scholar] [CrossRef]
- Show, B.K.; Veerababu, R.; Balamuralikrishnan, R.; Malakondaiah, G. Effect of vanadium and titanium modification on the microstructure and mechanical properties of a microalloyed HSLA steel. Mater. Sci. Eng. A 2010, 527, 1595–1604. [Google Scholar] [CrossRef]
- Karmakar, A.; Biswas, S.; Mukherjee, S.; Chakrabarti, D.; Kumar, V. Effect of composition and thermo-mechanical processing schedule on the microstructure, precipitation and strengthening of Nb-microalloyed steel. Mater. Sci. Eng. A 2017, 690, 158–169. [Google Scholar] [CrossRef]
- Souza, R.C.; Silva, E.S.; Jorge, A.M., Jr.; Cabrera, J.M.; Balancin, O. Dynamic recovery and dynamic recrystallization competition on a Nb- and N-bearing austenitic stainless steel biomaterial: Influence of strain rate and temperature. Mater. Sci. Eng. A 2013, 582, 96–107. [Google Scholar] [CrossRef]
- Mandal, G.K.; Stanford, N.; Hodgson, P.; Beynon, J.H. Static recrystallisation study of as-cast austenitic stainless steel. Mater. Sci. Eng. A 2013, 576, 118–125. [Google Scholar] [CrossRef]
- Silva, E.S.; Sousa, R.C.; Jorge, A.M.; Balancin, O. Hot deformation behavior of an Nb- and N-bearing austenitic stainless steel biomaterial. Mater. Sci. Eng. A 2012, 543, 69–75. [Google Scholar] [CrossRef]
- Okazaki, Y. Effects of solution treatment and cold rolling on microstructure and mechanical properties of stainless steel for surgical implants. Mater. Trans. 2008, 49, 1423–1427. [Google Scholar] [CrossRef]
- Giordano, E.J.; Guimarães, V.A.; Pinto, T.B.; Ferreira, I. Effect of precipitates on the corrosion–fatigue crack initiation of ISO 5832-9 stainless steel biomaterial. Int. J. Fatigue 2004, 26, 1129–1136. [Google Scholar] [CrossRef]
- Giordano, E.J.; Alonso-Falleiros, N.; Ferreira, I.; Balancin, O. Electrochemical behavior of two austenitic stainless steel biomaterials. REM: R. Esc. Minas Ouro Preto 2010, 63, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.B.R.; Gallego, J.; Cabrera, J.M.; Balancin, O.; Jorge, A.M. Interaction between recrystallization and strain-induced precipitation in a high Nb- and N-bearing austenitic stainless steel: Influence of the interpass time. Mater. Sci. Eng. A 2015, 637, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.B.R.; Gallego, J.; Cabrera, J.M.; Balancin, O.; Jorge, A.M. Thermomechanical controlled processing to achieve very fine grains in the ISO 5832-9 austenitic stainless steel biomaterial. Mater. Charact. 2017, 127, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Giordano, E.J.; Jorge, A.M., Jr.; Balancin, O. Evidence of strain induced precipitation on a Nb- and N-bearing austenitic stainless steel biomaterial. Mater. Sci. Forum 2005, 500, 179–186. [Google Scholar] [CrossRef]
- Cuddy, L.J.; Raley, J.C. Austenite grain coarsening in microalloyed steels. Met. Trans. A 1983, 14, 1989–1995. [Google Scholar] [CrossRef]
- Altuna, M.A.; Iza-Mendia, A.; Gutiérrez, I. Precipitation of Nb in ferrite after austenite conditioning. Part II: Strengthening contribution in high-strength low-alloy (HSLA) steels. Met. Mater. Trans. A 2012, 43, 4571–4586. [Google Scholar] [CrossRef] [Green Version]
- Gorni, A.A.; Mei, P.R. Development of alternative as-rolled alloys to replace quenched and tempered steels with tensile strength in the range of 600–800 MPa. J. Mater. Proc. Tech. 2005, 162, 298–303. [Google Scholar] [CrossRef]
- Jansto, S.G. MicroNiobium alloy approach in medium and high carbon steel bar, plate and sheet products. Metall. Mater. Trans. B 2014, 45, 438–444. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Boratto, F.; Barbosa, R.; Yue, S.; Jonas, J.J. Effect of chemical composition on the critical temperatures of microalloyed steels. In Proceedings of the International Conference on Physical Metallurgy of Thermomechanical Processing of Steels and Other Metals (Thermec-88), ISIJ, Tokyo, Japan, 6–10 June 1988; pp. 383–390. [Google Scholar]
- Bandy, R.; Cahoon, J.R. Effect of composition on the electrochemical behaviour of austenitic stainless steel in Ringer’s solution. Corrosion 1977, 33, 204–208. [Google Scholar] [CrossRef]
- Levey, P.R.; Van Bennekom, A.A. A mechanistic study of the effects of nitrogen on the corrosion properties on stainless steels. Corrosion 1995, 51, 911–921. [Google Scholar] [CrossRef]
- Varela-Castro, G.; Cabrera, J.M.; Prado, J.M. Critical strain for dynamic recrystallization. The particular case of steels. Metals 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Hall, E.O. Variation of hardness of metals with grain size. Nature 1954, 173, 948–949. [Google Scholar] [CrossRef]
- Hall, E.O. The deformation and ageing of mild steel: III Discussion of results. Proc. Phys. Soc. B 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Petch, N.J. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Pavlina, E.; Van Tyne, C. Correlation of yield strength and tensile strength with hardness for steels. J. Mater. Eng. Perform. 2008, 17, 888–893. [Google Scholar] [CrossRef]
- Anderson, E.; King, D.W.W.; Spreadborough, J. The relationship between lower yield stress and grain size in Armco iron. Trans. TMS-AIME 1968, 242, 115–119. [Google Scholar]
- Morrison, W.B. The effect of grain size on the stress-strain relationship in low-carbon steel. Trans. Am. Soc. Met. 1966, 59, 824–846. [Google Scholar]
- Kashyap, B.P.; Tangri, K. On the Hall-Petch relationship in type 316L stainless steel at room temperature. Scr. Metall. Mater. 1990, 24, 1777–1782. [Google Scholar] [CrossRef]
- Takeda, K.; Nakada, N.; Tsuchiyama, T.; Takaki, S. Effect of interstitial elements on Hall–Petch coefficient of ferritic iron. ISIJ Int. 2008, 48, 1122–1125. [Google Scholar] [CrossRef] [Green Version]



| Alloy | C | Si | Mn | Ni | Cr | Mo | S | P | Cu | N | Nb | V | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ISO | 0.031 | 0.38 | 3.98 | 10.43 | 20.43 | 2.46 | 0.0018 | 0.022 | 0.12 | 0.35 | 0.28 | 0.12 | 0.005 | bal. |
| F138 | 0.011 | 0.19 | 1.75 | 14.30 | 17.30 | 2.77 | <0.001 | 0.019 | 0.09 | 0.079 | - | - | - | bal. |
| Potentiodynamic Values | After Soaking | After 13 Passes, 20 s | ||||
|---|---|---|---|---|---|---|
| F138 | ISO (1200 °C) | ISO (1250 °C) | F138 | ISO (1200 °C) | ISO (1250 °C) | |
| Icorr (A/cm2) | 9.5 × 10−8 | 4.3 × 10−8 | 3.7 × 10−8 | 9.5 × 10−8 | 3.7 × 10−8 | 4.5 × 10−8 |
| Ecorr (mV) | −217 | −140 | −100 | −210 | −157 | −151 |
| Epit (mV) | 480 | 879 | - | 864 | 902 | 1056 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.-B.R.; Roche, V.; Blanco, T.M.; Viet, N.H.; Balancin, O.; Cabrera, J.-M.; Jorge, A.M., Jr. Effect of Processing Conditions on the Microstructure, Mechanical Properties, and Corrosion Behavior of Two Austenitic Stainless Steels for Bioimplant Applications. Metals 2020, 10, 1311. https://doi.org/10.3390/met10101311
Silva M-BR, Roche V, Blanco TM, Viet NH, Balancin O, Cabrera J-M, Jorge AM Jr. Effect of Processing Conditions on the Microstructure, Mechanical Properties, and Corrosion Behavior of Two Austenitic Stainless Steels for Bioimplant Applications. Metals. 2020; 10(10):1311. https://doi.org/10.3390/met10101311
Chicago/Turabian StyleSilva, Mariana-Beatriz R., Virgine Roche, Telma M. Blanco, Nguyen Hoang Viet, Oscar Balancin, José-María Cabrera, and Alberto Moreira Jorge, Jr. 2020. "Effect of Processing Conditions on the Microstructure, Mechanical Properties, and Corrosion Behavior of Two Austenitic Stainless Steels for Bioimplant Applications" Metals 10, no. 10: 1311. https://doi.org/10.3390/met10101311
APA StyleSilva, M.-B. R., Roche, V., Blanco, T. M., Viet, N. H., Balancin, O., Cabrera, J.-M., & Jorge, A. M., Jr. (2020). Effect of Processing Conditions on the Microstructure, Mechanical Properties, and Corrosion Behavior of Two Austenitic Stainless Steels for Bioimplant Applications. Metals, 10(10), 1311. https://doi.org/10.3390/met10101311










