Mechanism of CaF2 under Vacuum Carbothermal Conditions for Recovering Nickel, Iron, and Magnesium from Garnierite
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Method and Equipment
2.3. Analysis Methods
3. Results and Discussion
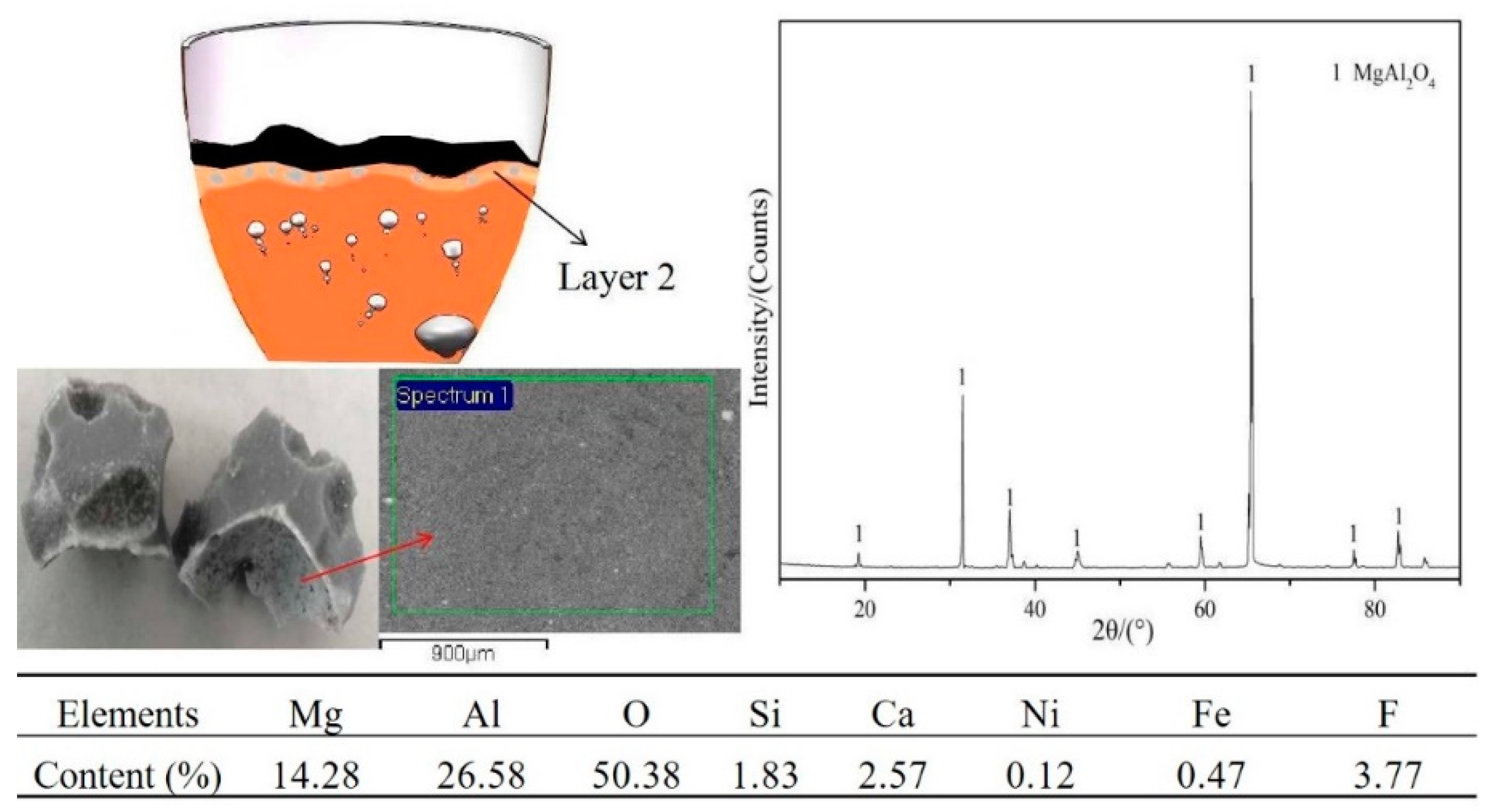
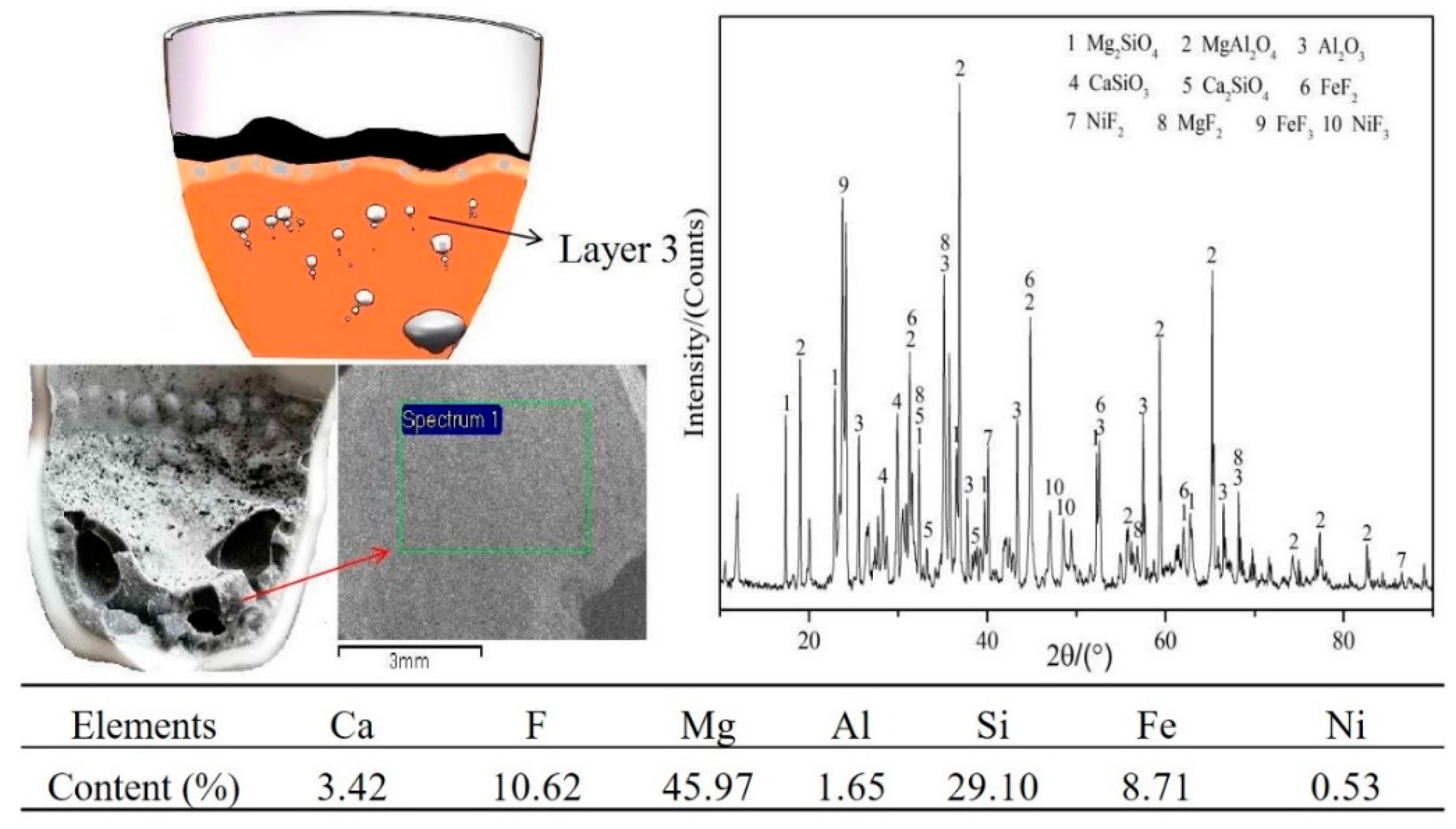
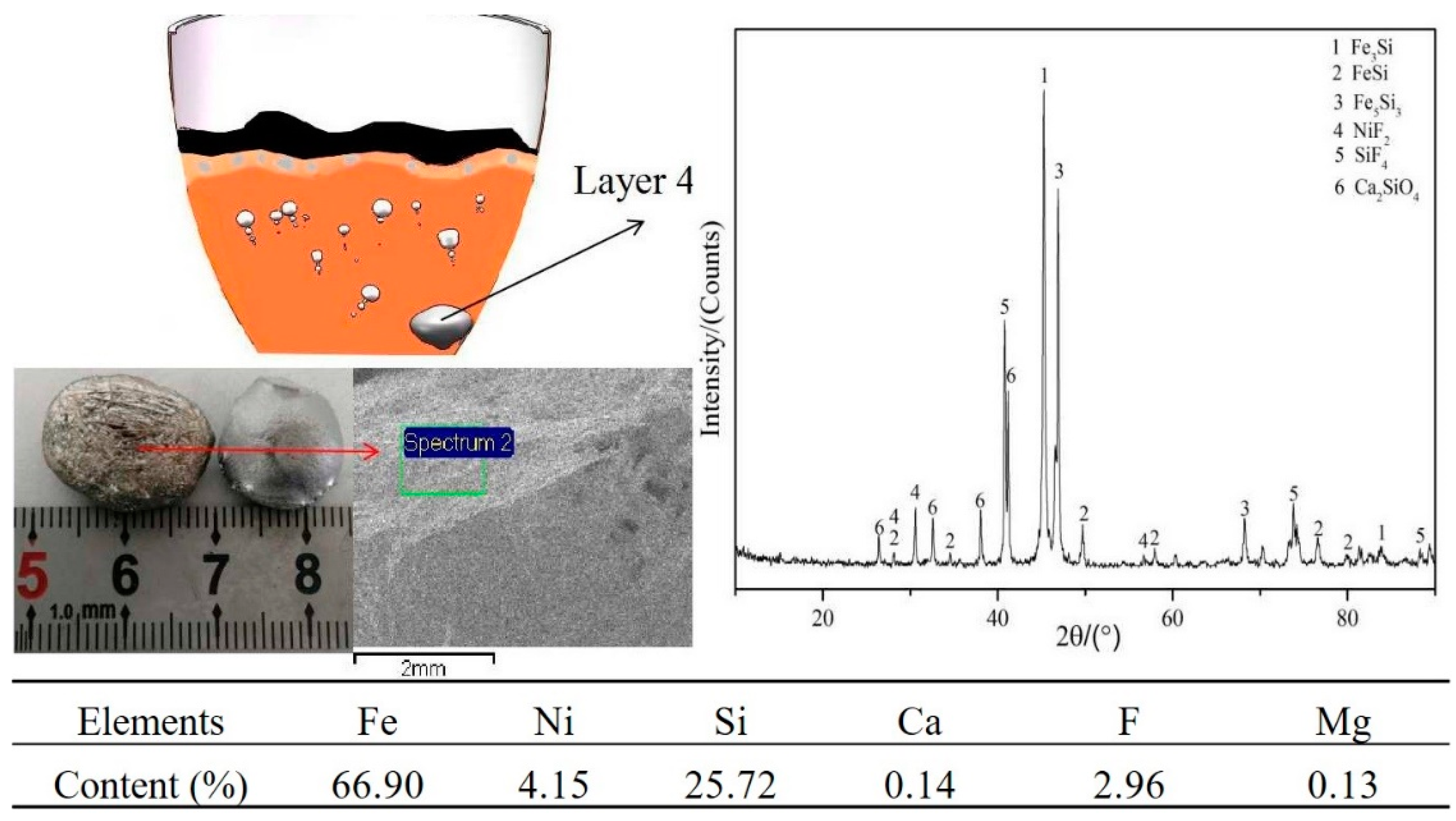
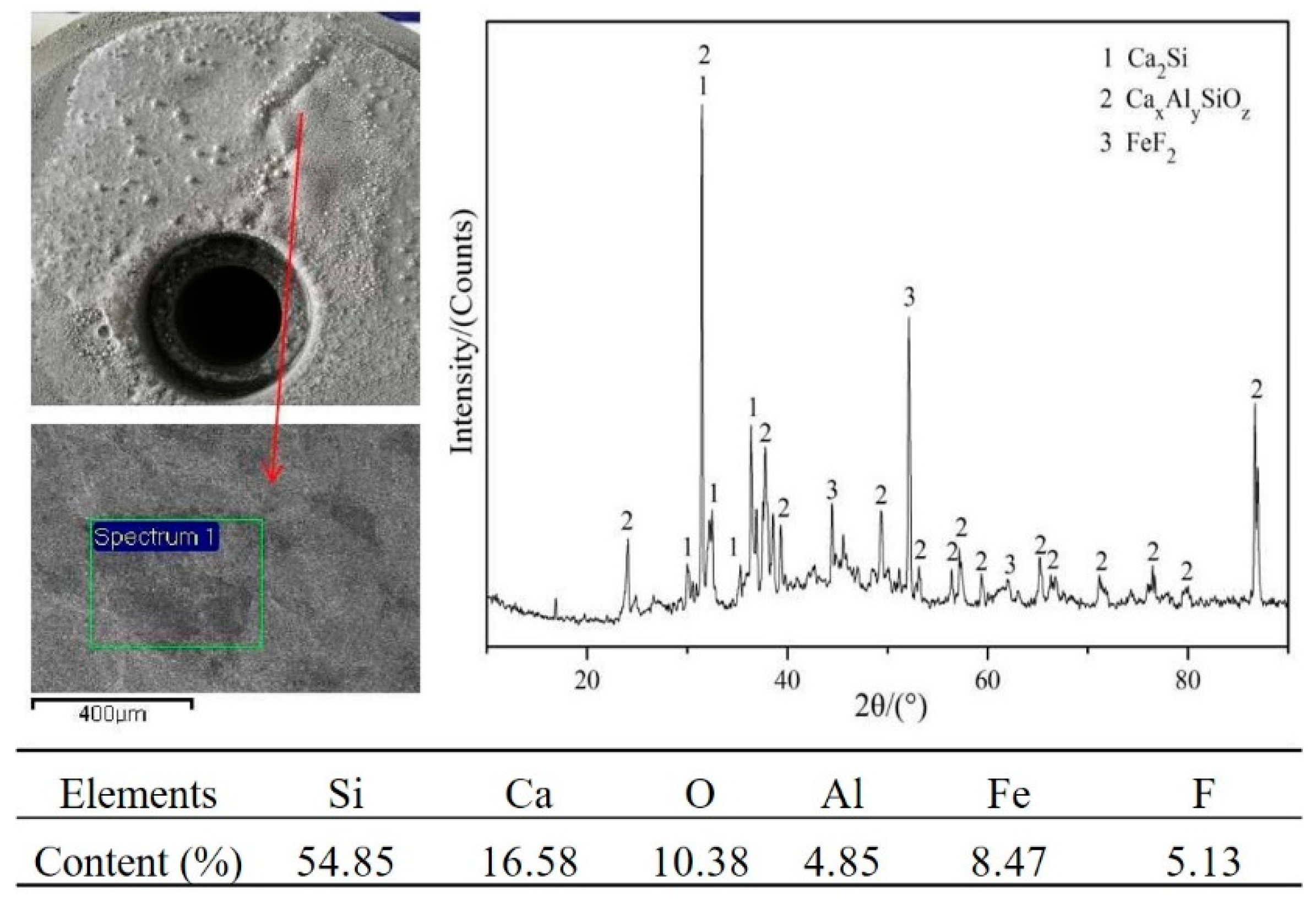
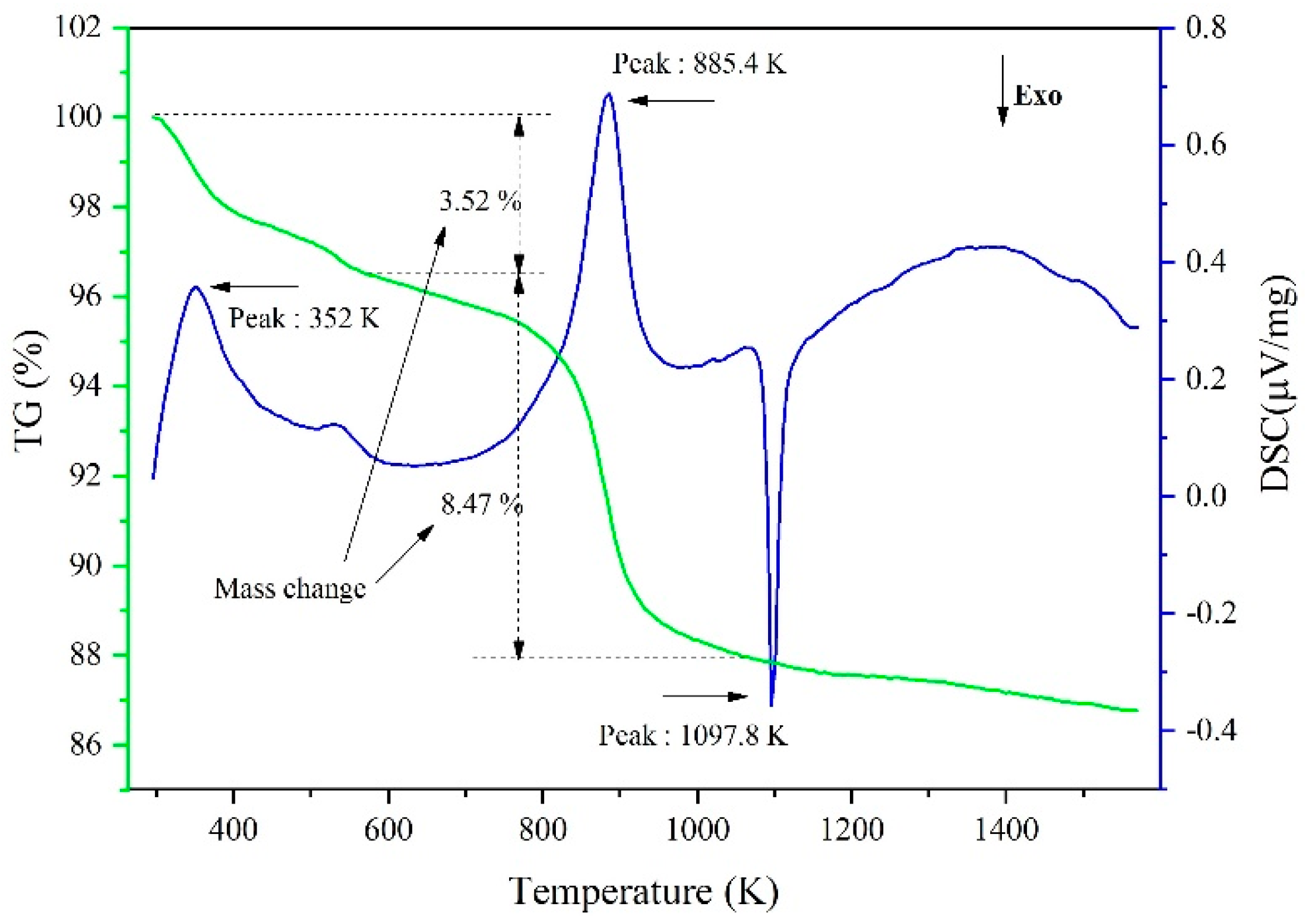
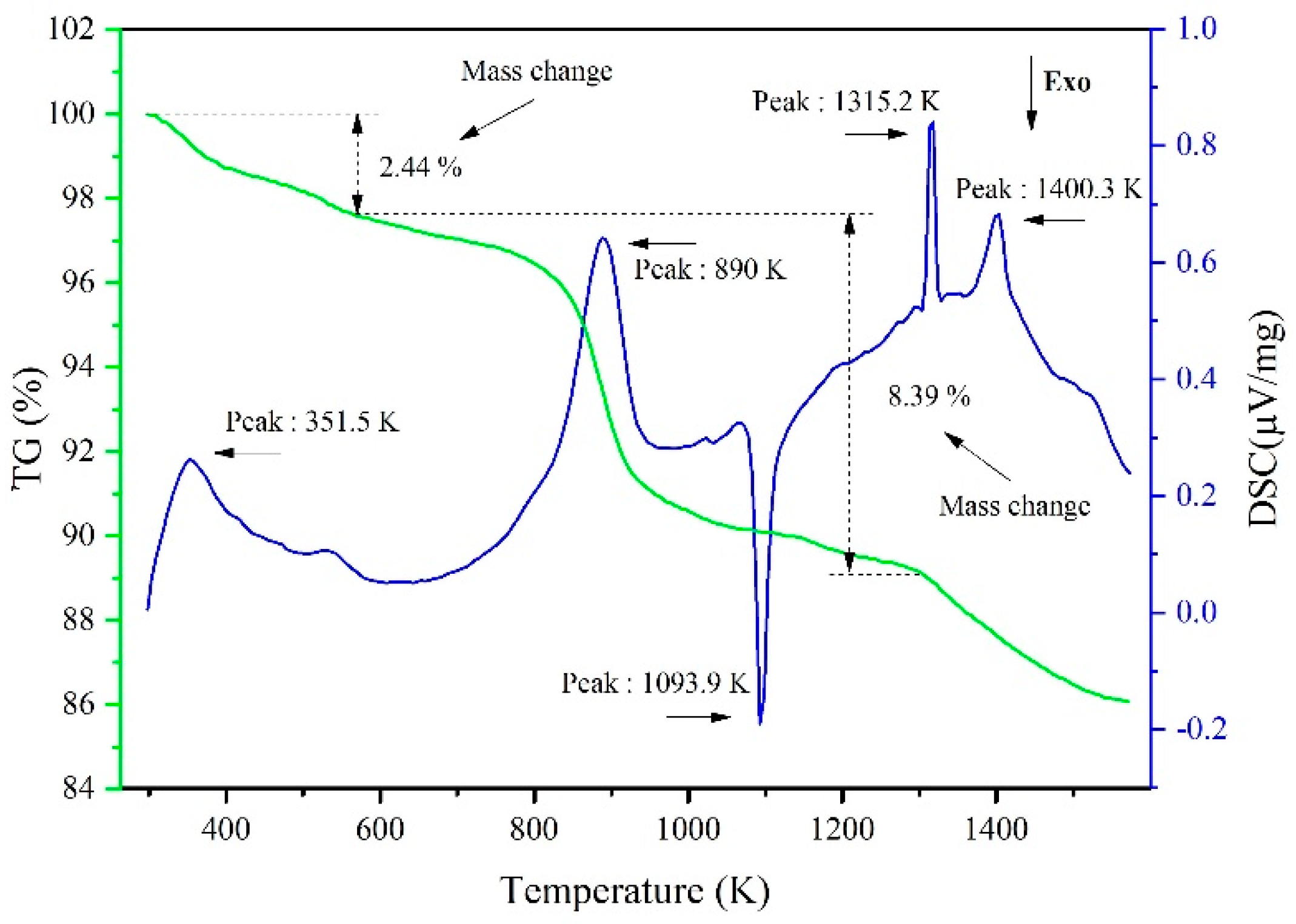
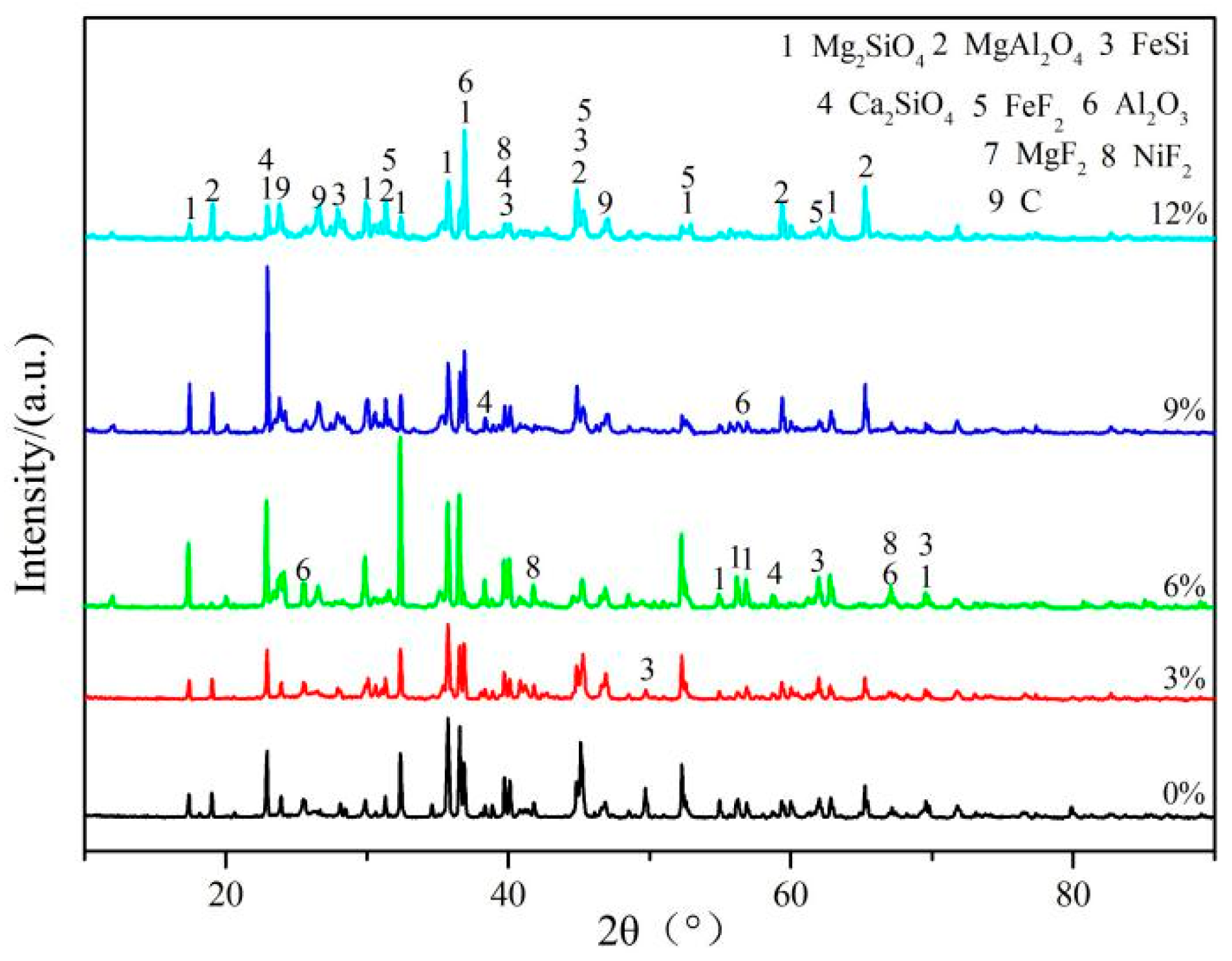
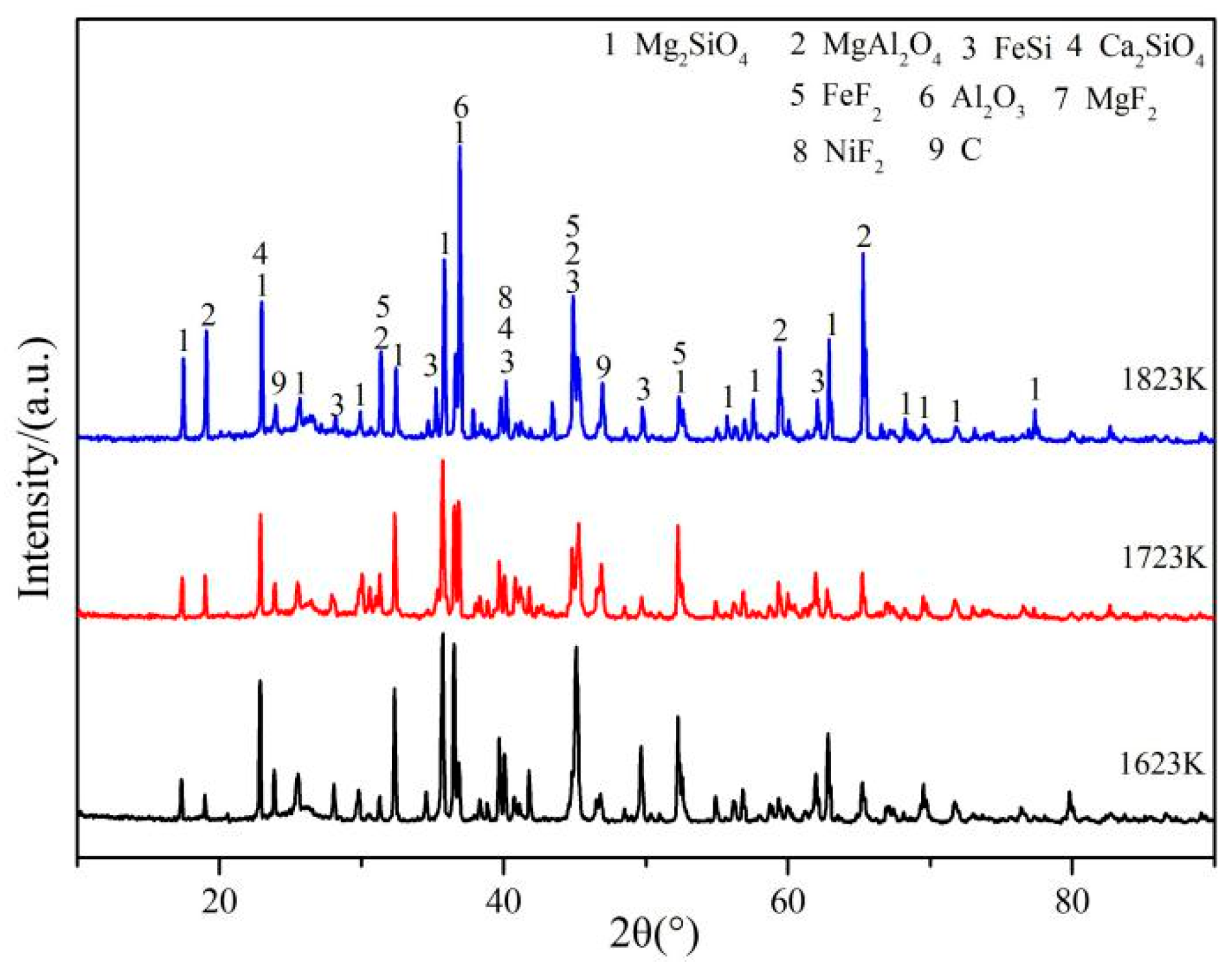
3.1. Experimental Phenomena and XRD/EDS Analysis
ΔGT = 1731.126 − 1.238T kJ/mol.
ΔGT = 19.255 − 0.022T kJ/mol,
ΔGT = 1344.640 − 0.795T kJ/mol.
ΔGT = 4297.884 − 2.920T kJ/mol.
ΔGT = 1336.421 − 1.653T kJ/mol,
ΔGT = 1110.829 − 1.797T kJ/mol,
ΔGT = 1384.267 − 1.055T kJ/mol.
ΔGT = 1044.890 − 0.962T kJ/mol,
ΔGT = 968.751 − 1.008T kJ/mol,
ΔGT = 697.671 − 0.487T kJ/mol.
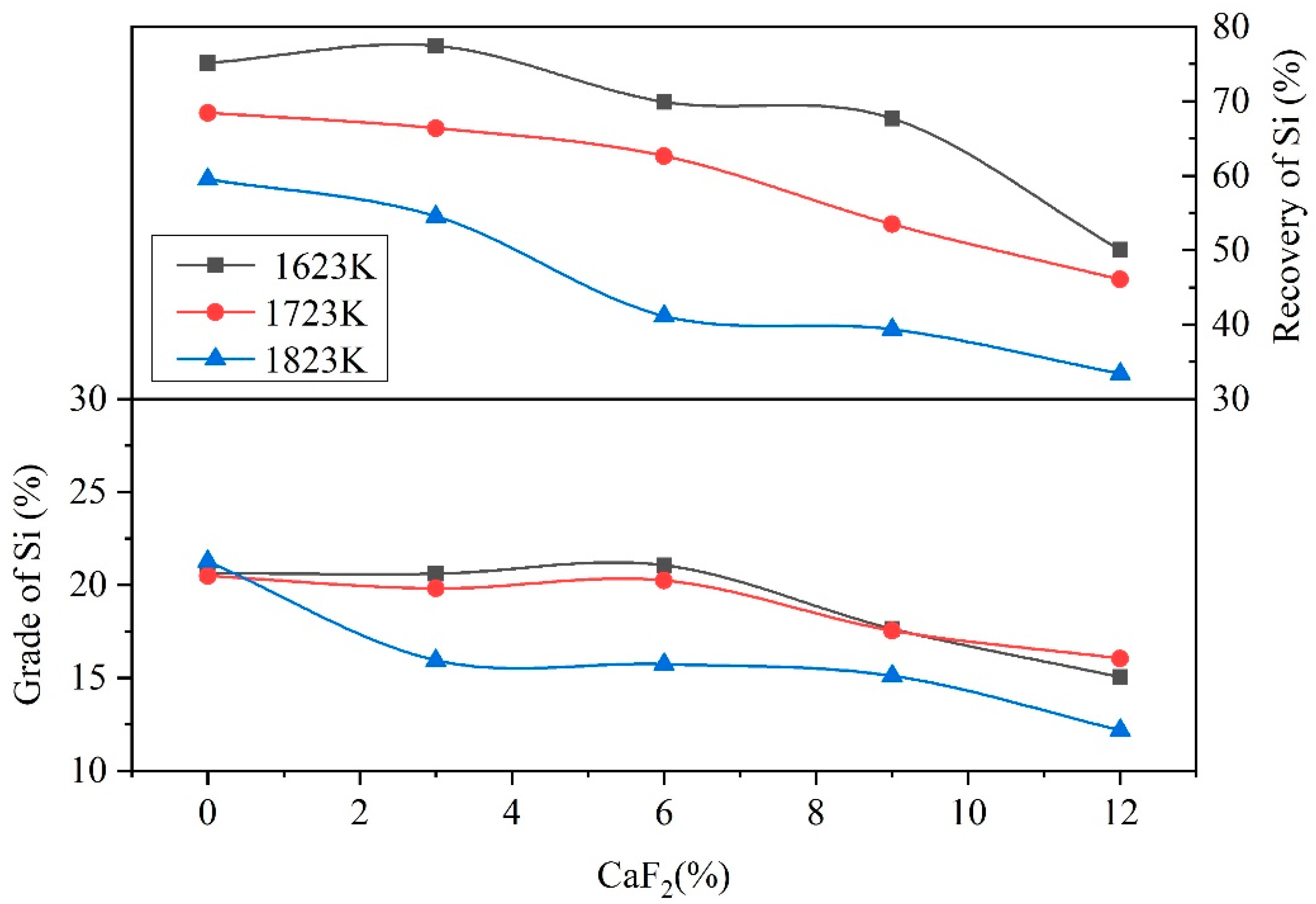
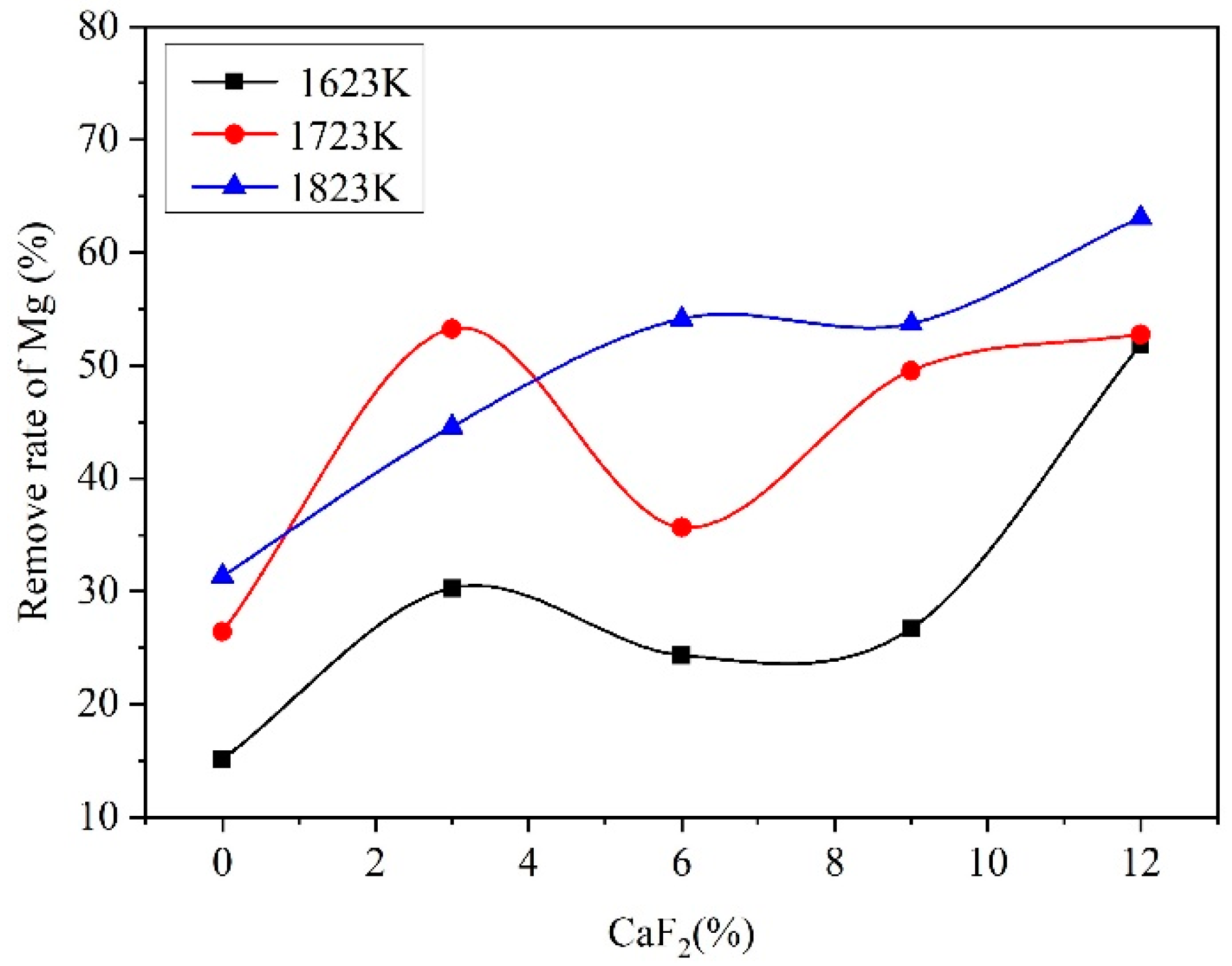
3.2. Behavior of CaF2
ΔGT = −271.244 + 0.221T kJ/mol.
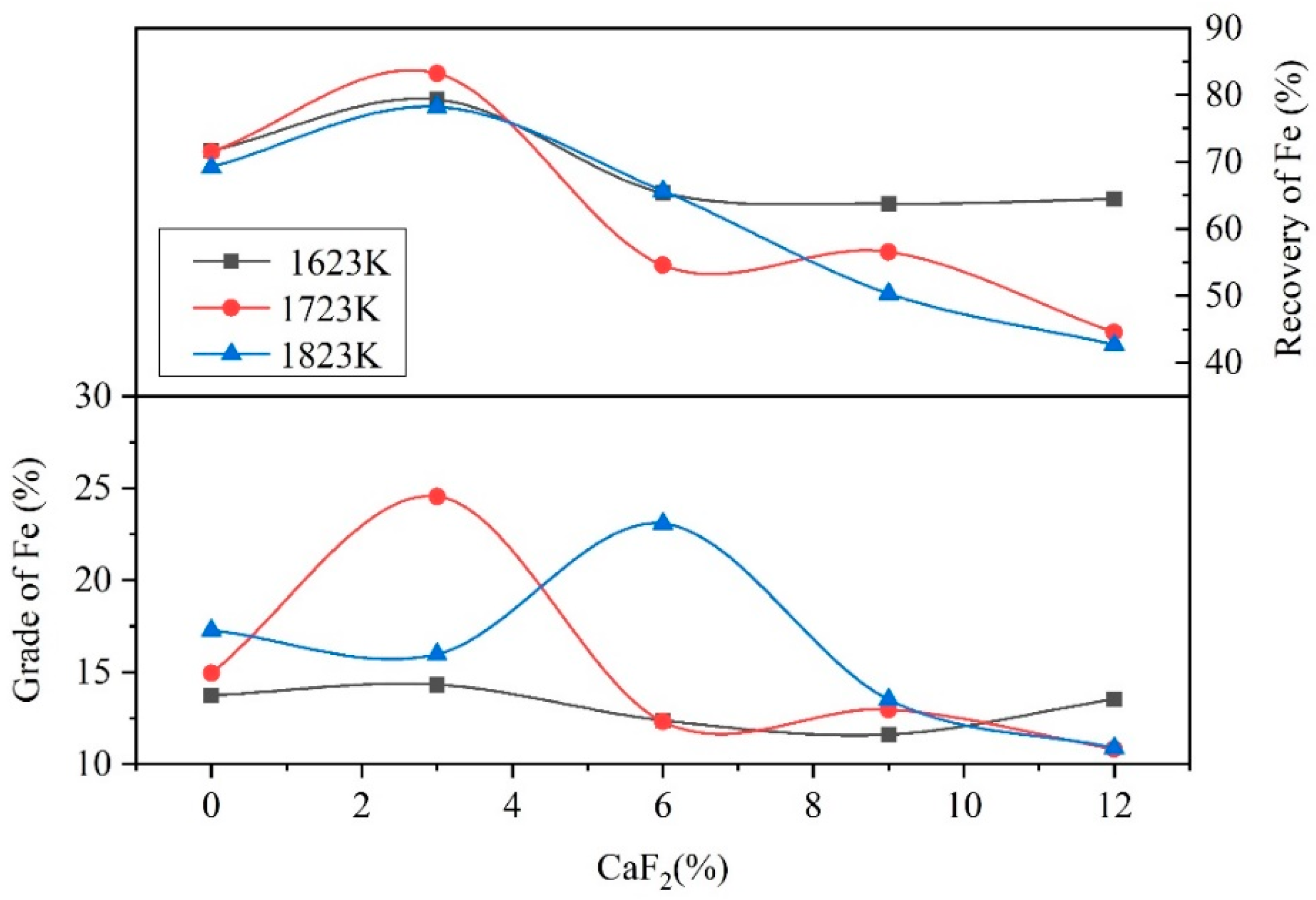
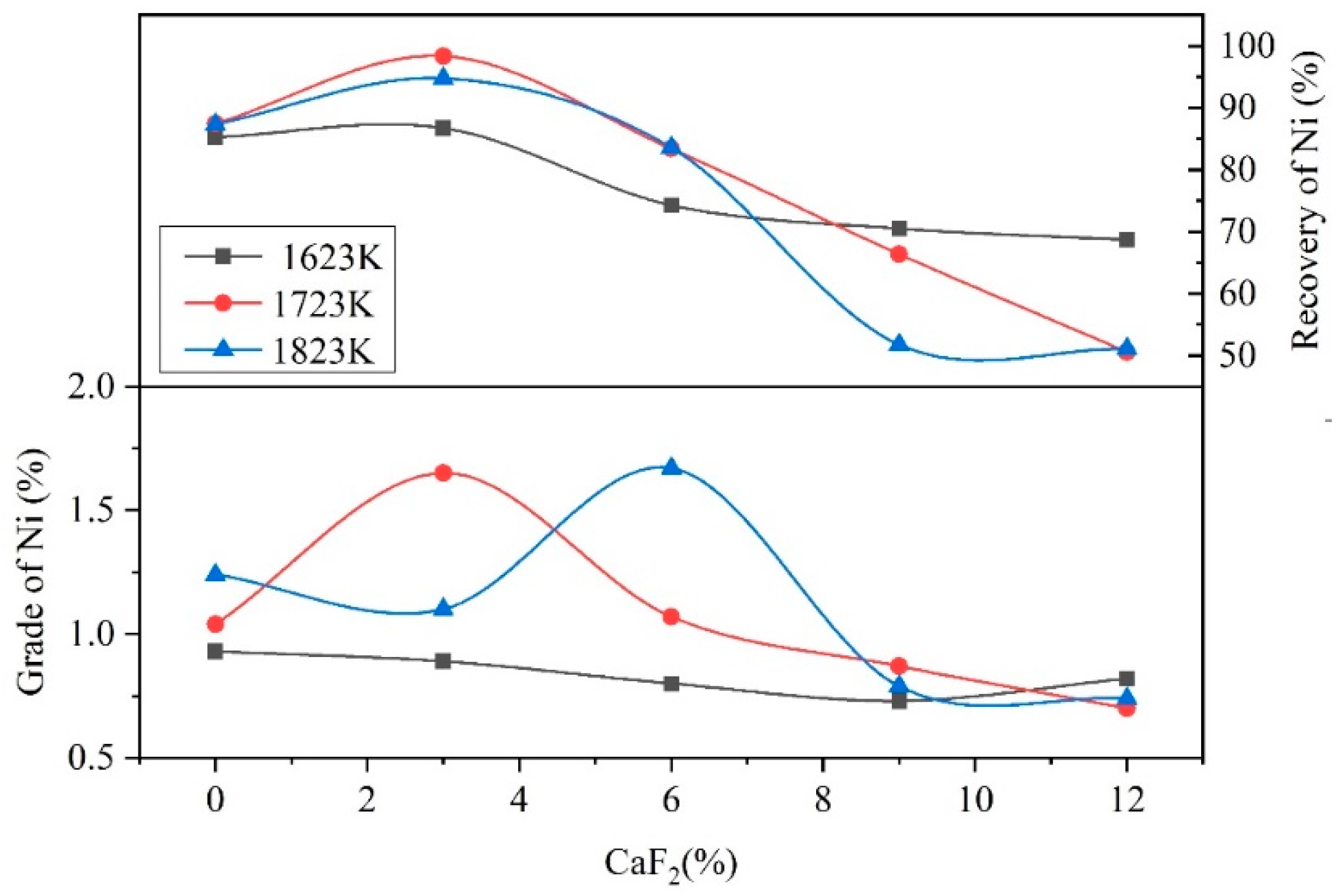
3.3. Analysis of the Reduction Process
- (1)
- When the addition of CaF2 was 0%, low-melting-point compounds did not form in the raw materials, and the reactions struggle to occur in the solid state in the reduction process, which caused the low grades and direct recovery of Fe and Ni.
- (2)
- When the addition of CaF2 was 3%, the CaF2 could react with the raw materials to form low-melting-point compounds, such as FeF2, NiF2, and MgF2. The reduction process transformed from a solid–solid reaction to a solid–liquid reaction, which accelerated the heat transfer and the mass transfer of the materials. The Fe and Ni aggregated and grew easily in the molten materials and formed small visible Fe–Ni particles on the surface of the Fe–Ni-rich residue. The raw materials were not completely melted in the reduction process due to the low addition of CaF2. Therefore, Fe and Ni were sufficiently reduced in the raw materials, which results in high grades and direct recovery of Fe and Ni.
- (3)
- When the addition of CaF2 was more than 3%, a large amount of the low-melting-point compounds formed in the raw materials, which caused the raw materials to change from a solid–solid reaction to a liquid–liquid reaction. This in turn caused the reduction of Fe and Ni to aggregate and grow into large metal particles with diameters of 20 mm. However, the coking coal would not melt at the experimental temperature due to its high melting point, and the density of the coking coal was low, which caused stratification of the molten materials and floating in the upper layer. This resulted in the reducing agent being unable to fully contact the raw materials, preventing the reduction process from occurring, which drastically reduced the grades and direct recovery of Fe and Ni.
- (4)
- Due to excessively high temperatures during the reduction process, the raw materials melted completely at temperature of 1823 K with 3% CaF2, hindering the reduction process, which reduced the grades and the direct recovery of Fe and Ni. Therefore, the grades and direct recovery of Fe and Ni were lower than 1723 K with 3% CaF2.
3.4. Phase Analysis of Fe–Ni-Rich Residue
3.5. Mechanism of CaF2 in the Reduction Process
3.6. Magnetic Separation Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Esmaeili, N.; Ojo, O.A. Analysis of Brazing Effect on Hot Corrosion Behavior of a Nickel-Based Aerospace Superalloy. Metall. Mater. Trans. B 2018, 49, 912–918. [Google Scholar] [CrossRef]
- Zhang, L.N.; Szpunar, J.A.; Dong, J.X.; Ojo, O.A.; Wang, X. Dependence of Crystallographic Orientation on Pitting Corrosion Behavior of Ni-Fe-Cr Alloy 028. Metall. Mater. Trans. B 2018, 49, 919–925. [Google Scholar] [CrossRef]
- Saad, E.A.; Hassanien, M.M.; Ellban, F.W. Nickel(II) diacetyl monoxime-2-pyridyl hydrazone complex can inhibit Ehrlich solid tumor growth in mice: A potential new antitumor drug. Biochem. Biophys. Res. Commun. 2017, 484, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Nasab, M.B.; Hassan, M.R. Metallic Biomaterials of Knee and Hip—A Review. Trends Biomater. Artif. Organs 2010, 24, 69–82. [Google Scholar]
- Babić, M.M.; Božić, B.Đ.; Božić, B.Đ.; Filipović, J.M.; Ušćumlić, G.S.; Tomić, S.L. Evaluation of novel antiproliferative controlled drug delivery system based on poly(2-hydroxypropyl acrylate/itaconic acid) hydrogels and nickel complex with Oxaprozin. Mater. Lett. 2016, 163, 214–217. [Google Scholar] [CrossRef]
- Setcos, J.C.; Mahani, A.B.; Silvio, L.D.; Mjor, I.A.; Wilson, N.H.F. The safety of nickel containing dental alloys. Dent. Mater. 2006, 22, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, H.P.; Cao, F.; Feng, A.S.; Zhao, J.W. Study on Current Situation and Analysis of Supply and Demand of Global Nickel Resource. Conser. Utilizat. Miner. Resour. 2016, 1, 64–69. [Google Scholar]
- Zong, K.; Zhou, J.Y.; Fu, S.X. Study on global market allocation of Chinese nickel metal resources. Miner. Explor. 2015, 6, 86–91. [Google Scholar]
- Valix, M.; Tang, J.Y.; Cheung, W.H. The effects of mineralogy on the biological leaching of nickel laterite ores. Miner. Eng. 2001, 14, 1629–1635. [Google Scholar] [CrossRef]
- Myagkiy, A.; Truche, L.; Cathelineau, M.; Golfier, F. Revealing the conditions of Ni mineralization in the laterite profiles of New Caledonia: Insights from reactive geochemical transport modelling. Chem. Geol. 2017, 466, 274–284. [Google Scholar] [CrossRef]
- Alfantazi, A.M.; Moskalyk, R.R. Nickel laterite processing and electrowinning practice. Miner. Eng. 2002, 15, 593–605. [Google Scholar]
- Ma, B.Z.; Wang, C.Y.; Yang, W.J.; Chen, Y.Q.; Yang, B. Comprehensive utilization of Philippine laterite ore, part 1: Design of technical route and classification of the initial ore based on mineralogical analysis. Int. J. Miner. Process. 2013, 124, 42–49. [Google Scholar] [CrossRef]
- Khoo, J.Z.; Haque, N.; Bhattacharya, S. Process simulation and exergy analysis of two nickel laterite processing technologies. Int. J. Miner. Process. 2017, 161, 83–93. [Google Scholar] [CrossRef]
- Elliott, R.; Pickles, C.A.; Forster, J. Thermodynamics of the Reduction Roasting of Nickeliferous Laterite Ores. J. Miner. Mater. Charact. Eng. 2016, 4, 320–346. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Ichlas, Z.T.; Kaya, M. Solvent extraction process for the recovery of nickel and cobalt from Caldag laterite leach solution: The first bench scale study. Hydrometallurgy 2017, 169, 135–141. [Google Scholar] [CrossRef]
- Leonardou, S.A.; Tsakiridis, P.E.; Oustadakis, P.; Karidakis, T.; Katsiapi, A. Hydrometallurgical process for the separation and recovery of nickel from sulphate heap leach liquor of nickeliferrous laterite ores. Miner. Eng. 2009, 22, 1181–1192. [Google Scholar] [CrossRef]
- Ma, B.Z.; Wang, C.Y.; Yang, W.J.; Yang, B.; Zhang, Y.L. Selective pressure leaching of Fe (II)-rich limonitic laterite ores from Indonesia using nitric acid. Miner. Eng. 2013, 45, 151–158. [Google Scholar] [CrossRef]
- Kaya, S.; Topkaya, Y.A. High pressure acid leaching of a refractory lateritic nickel ore. Miner. Eng. 2011, 24, 1188–1197. [Google Scholar] [CrossRef]
- Johnson, J.A.; Cashmore, B.C.; Hockridge, R.J. Optimisation of nickel extraction from laterite ores by high pressure acid leaching with addition of sodium sulphate. Miner. Eng. 2005, 18, 1297–1303. [Google Scholar] [CrossRef]
- Muir, B.I.; Whittington, D. Pressure acid leaching of nickel laterites: A review. Miner. Process. Extr. Metall. Rev. 2000, 21, 527–599. [Google Scholar]
- Dodbiba, G.; Kim, J.; Tanno, H.; Okaya, K.; Matsuo, S.; Fujita, T. Calcination of low-grade laterite for concentration of Ni by magnetic separation. Miner. Eng. 2010, 23, 282–288. [Google Scholar]
- Li, G.H.; Shi, T.M.; Rao, M.J.; Zhang, Y.B. Beneficiation of nickeliferous laterite by reduction roasting in the presence of sodium sulfate. Miner. Eng. 2012, 32, 19–26. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Cui, Y.; Vining, K.; Hapugoda, S.; Douglas, J.; Pan, J.; Zheng, G.L. Upgrading low nickel content laterite ores using selective reduction followed by magnetic separation. Int. J. Miner. Process. 2012, 106–109, 1–7. [Google Scholar] [CrossRef]
- Ma, B.Z.; Xing, P.; Yang, W.J.; Wang, C.Y.; Chen, Y.Q.; Wang, H. Solid-State Metalized Reduction of Magnesium-Rich Low-Nickel Oxide Ores Using Coal as the Reductant Based on Thermodynamic Analysis. Metall. Mater. Trans. B 2017, 48, 2037–2046. [Google Scholar] [CrossRef]
- Cao, C.; Xue, Z.L.; Duan, H.J. Making Ferronickel from Laterite Nickel Ore by Coal-Based Self-Reduction and High Temperature Melting Process. Int. J. Nonfer. Metall. 2016, 5, 9–15. [Google Scholar] [CrossRef]
- Li, J.H.; Chen, Z.F.; Shen, B.P.; Xu, Z.F.; Zhang, Y.F. The extraction of valuable metals and phase transformation and formation mechanism in roasting-water leaching process of laterite with ammonium sulfate. J. Clean. Prod. 2017, 140, 1148–1155. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, H.W.; Li, G.S.; Lu, C.Y.; Lu, X.G.; Zou, X.L.; Xu, Q. Extraction of metals from complex sulfide nickel concentrates by low-temperature chlorination roasting and water leaching. Int. J. Miner. Metall. 2017, 24, 377–385. [Google Scholar] [CrossRef]
- Zhou, S.W.; Wei, Y.G.; Li, B.; Ma, B.Z.; Wang, C.Y.; Wang, H. Kinetics study on the dehydroxylation and phase transformation of Mg3Si2O5(OH)4. J. Alloys Compd. 2017, 713, 180–186. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, T.; Gu, X.P.; Shi, L.; Yang, B.; Dai, Y.N. The effect of CaO on the recovery of Fe and Ni in a vacuum carbothermal reduction of garnierite. J. Min. Metall. Sect. B Metall. 2019, 55, 351–358. [Google Scholar] [CrossRef]

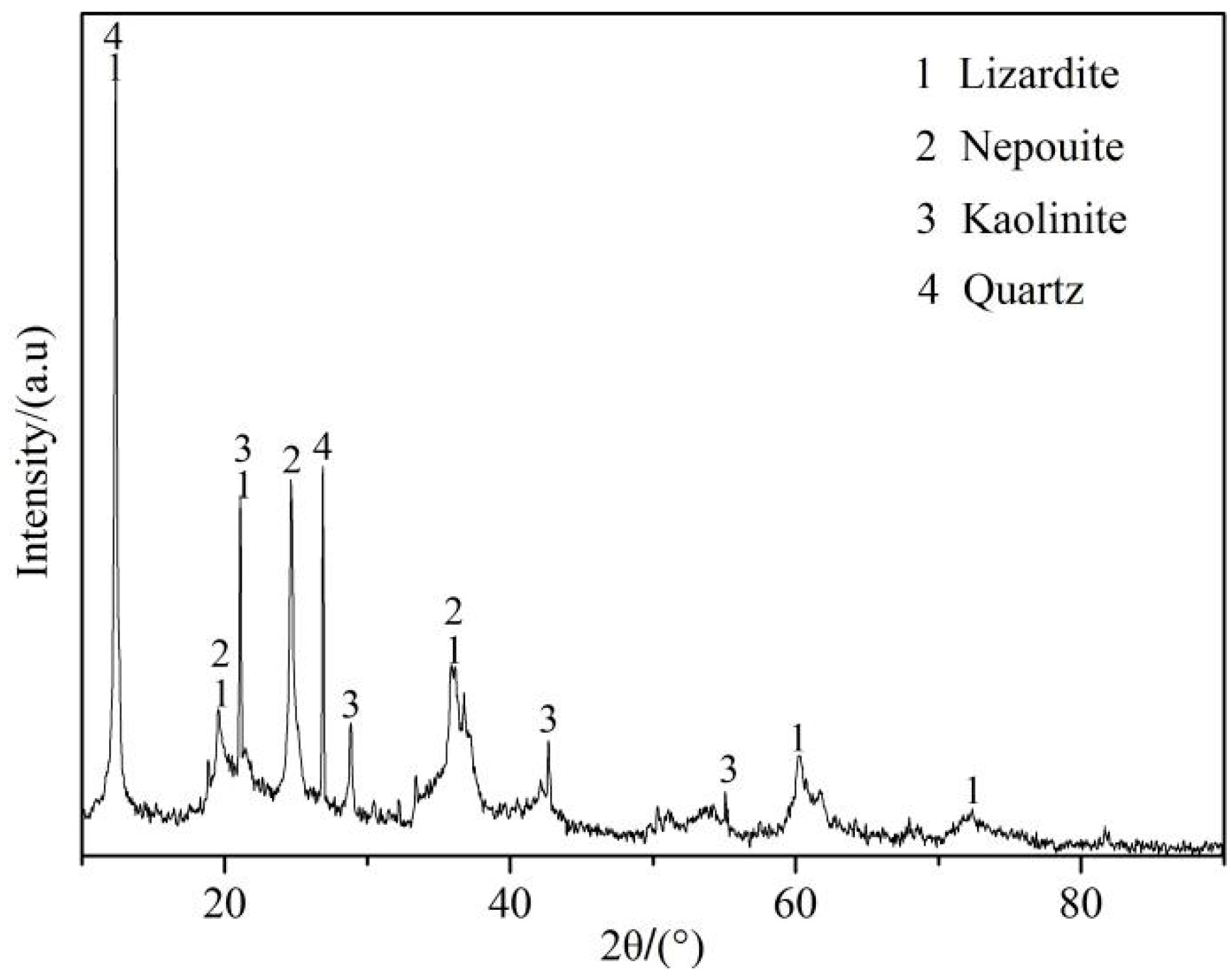
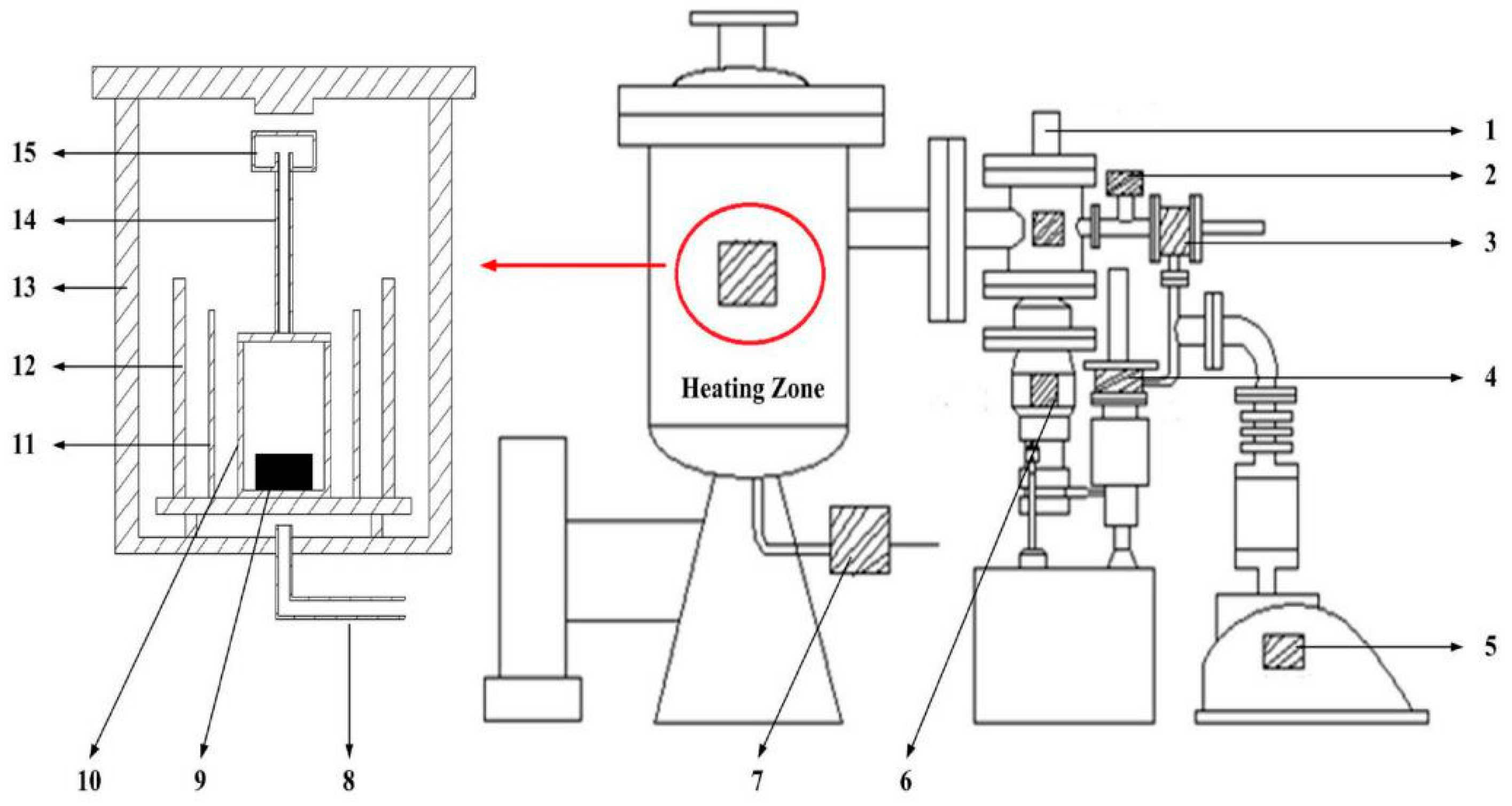

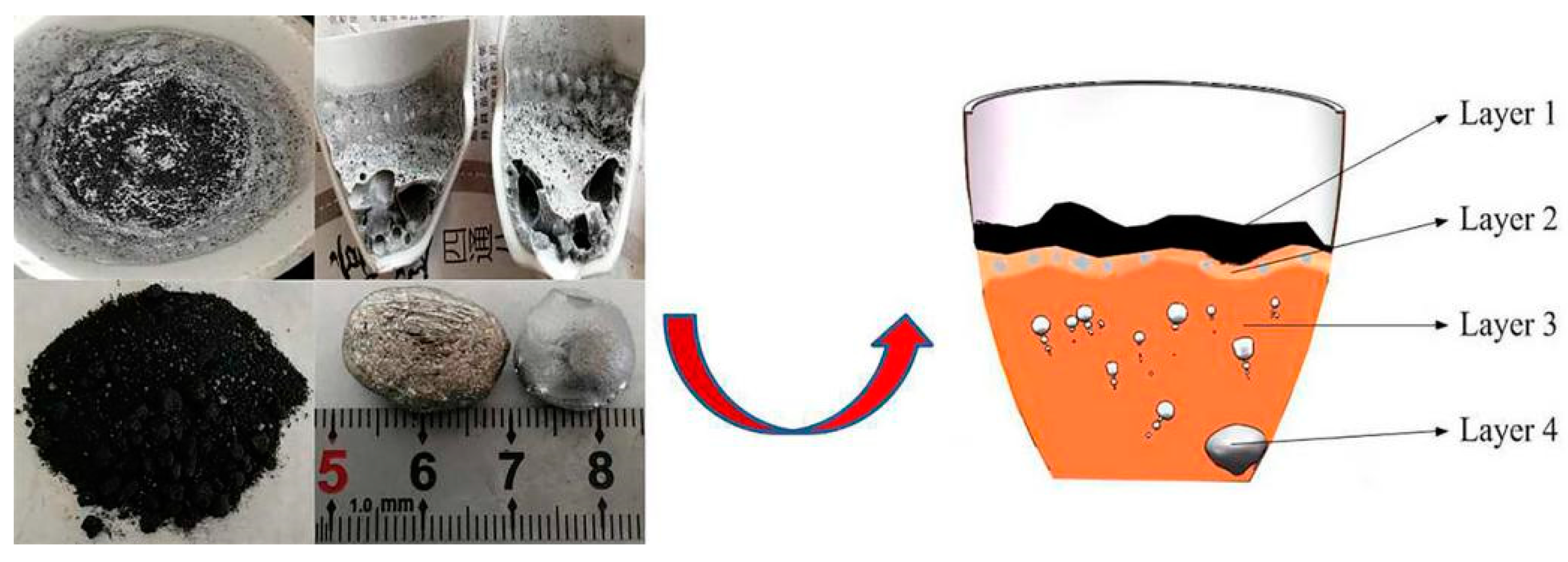
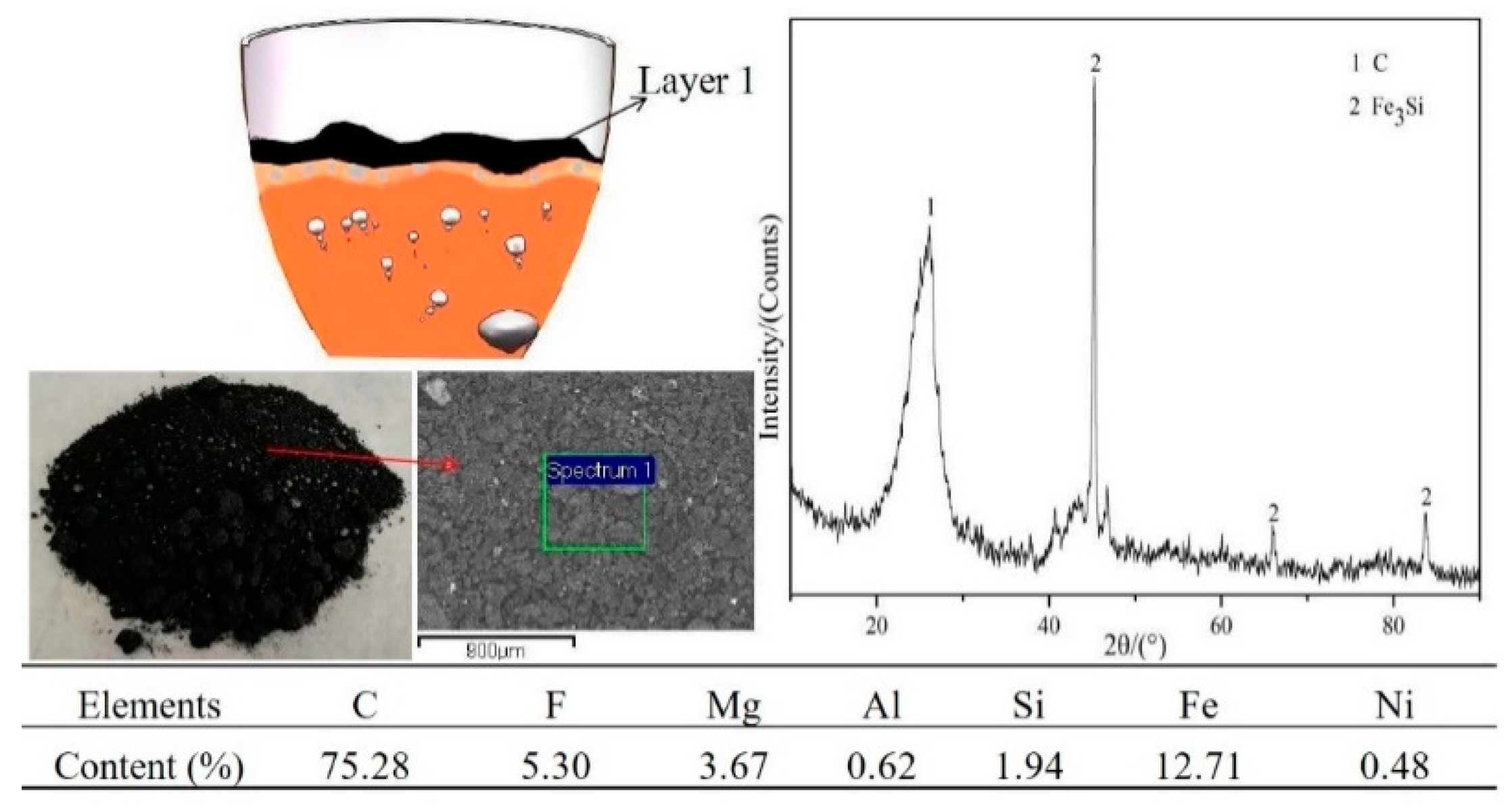

| Component | SiO2 | MgO | Fe | Ni | Co | Al2O3 | Others |
|---|---|---|---|---|---|---|---|
| Content | 38.82 | 22.83 | 12.66 | 0.72 | 0.03 | 4.57 | 20.37 |
| Component | CaF2 | Si | Fe | Heavy Metal | Chloride | Sulfate | Nitride |
|---|---|---|---|---|---|---|---|
| Content | ≥98.5 | ≤0.01 | ≤0.003 | ≤0.003 | ≤0.01 | ≤0.05 | ≤0.005 |
| Component | C | Ash | Moisture | Volatile | K (g) |
|---|---|---|---|---|---|
| Content | 85.83 | 12.02 | 0.14 | 2.01 | 7187.2 |
| Temperature (K) | CaF2 (wt. %) | Quality of Magnetic Material (g) | Chemical Analysis of Fe, Ni in Magnetic Materials (%) | Quality of Non-Magnetic Material (g) | Chemical Analysis of Fe, Ni in Non-Magnetic Materials (%) | ||
|---|---|---|---|---|---|---|---|
| Fe | Ni | Fe | Ni | ||||
| 1623 | 3 | 24.49 | 32.46 | 5.18 | 35.66 | 0.42 | 0.04 |
| 1823 | 3 | 30.29 | 38.17 | 5.99 | 21.71 | 0.33 | 0.05 |
| 1723 | 0 | 50.54 | 14.95 | 1.04 | 50.54 | 0.00 | 0.00 |
| 1723 | 3 | 11.16 | 40.20 | 6.73 | 21.75 | 0.18 | 0.03 |
| 1723 | 6 | 23.61 | 38.30 | 6.69 | 50.57 | 0.23 | 0.07 |
| 1723 | 9 | 23.06 | 37.30 | 6.22 | 49.73 | 0.26 | 0.06 |
| 1723 | 12 | 19.03 | 34.75 | 5.11 | 49.13 | 0.38 | 0.02 |
| Temperature (K) | 1623 | 1823 | 1723 | 1723 | 1723 | 1723 | 1723 |
|---|---|---|---|---|---|---|---|
| CaF2 (wt %) | 3 | 3 | 0 | 3 | 6 | 9 | 12 |
| Enrichment ratio of Fe (%) | 2.56 | 3.02 | 1.18 | 3.18 | 3.03 | 2.95 | 2.75 |
| Enrichment ratio of Ni (%) | 7.19 | 8.32 | 1.44 | 9.35 | 9.29 | 8.64 | 7.10 |
| Recovery of Fe (%) | 73.19 | 73.87 | 71.50 | 82.97 | 54.03 | 51.55 | 40.90 |
| Recovery of Ni (%) | 84.89 | 93.62 | 87.45 | 98.21 | 81.19 | 64.64 | 48.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Gu, X.; Qu, T.; Shi, L.; Luo, M.; Yang, B.; Dai, Y. Mechanism of CaF2 under Vacuum Carbothermal Conditions for Recovering Nickel, Iron, and Magnesium from Garnierite. Metals 2020, 10, 129. https://doi.org/10.3390/met10010129
Wang Q, Gu X, Qu T, Shi L, Luo M, Yang B, Dai Y. Mechanism of CaF2 under Vacuum Carbothermal Conditions for Recovering Nickel, Iron, and Magnesium from Garnierite. Metals. 2020; 10(1):129. https://doi.org/10.3390/met10010129
Chicago/Turabian StyleWang, Qiang, Xupeng Gu, Tao Qu, Lei Shi, Mingyang Luo, Bin Yang, and Yongnian Dai. 2020. "Mechanism of CaF2 under Vacuum Carbothermal Conditions for Recovering Nickel, Iron, and Magnesium from Garnierite" Metals 10, no. 1: 129. https://doi.org/10.3390/met10010129
APA StyleWang, Q., Gu, X., Qu, T., Shi, L., Luo, M., Yang, B., & Dai, Y. (2020). Mechanism of CaF2 under Vacuum Carbothermal Conditions for Recovering Nickel, Iron, and Magnesium from Garnierite. Metals, 10(1), 129. https://doi.org/10.3390/met10010129




