Production of Ferronickel Concentrate from Low-Grade Nickel Laterite Ore by Non-Melting Reduction Magnetic Separation Process
Abstract
1. Introduction
2. Materials and Methods
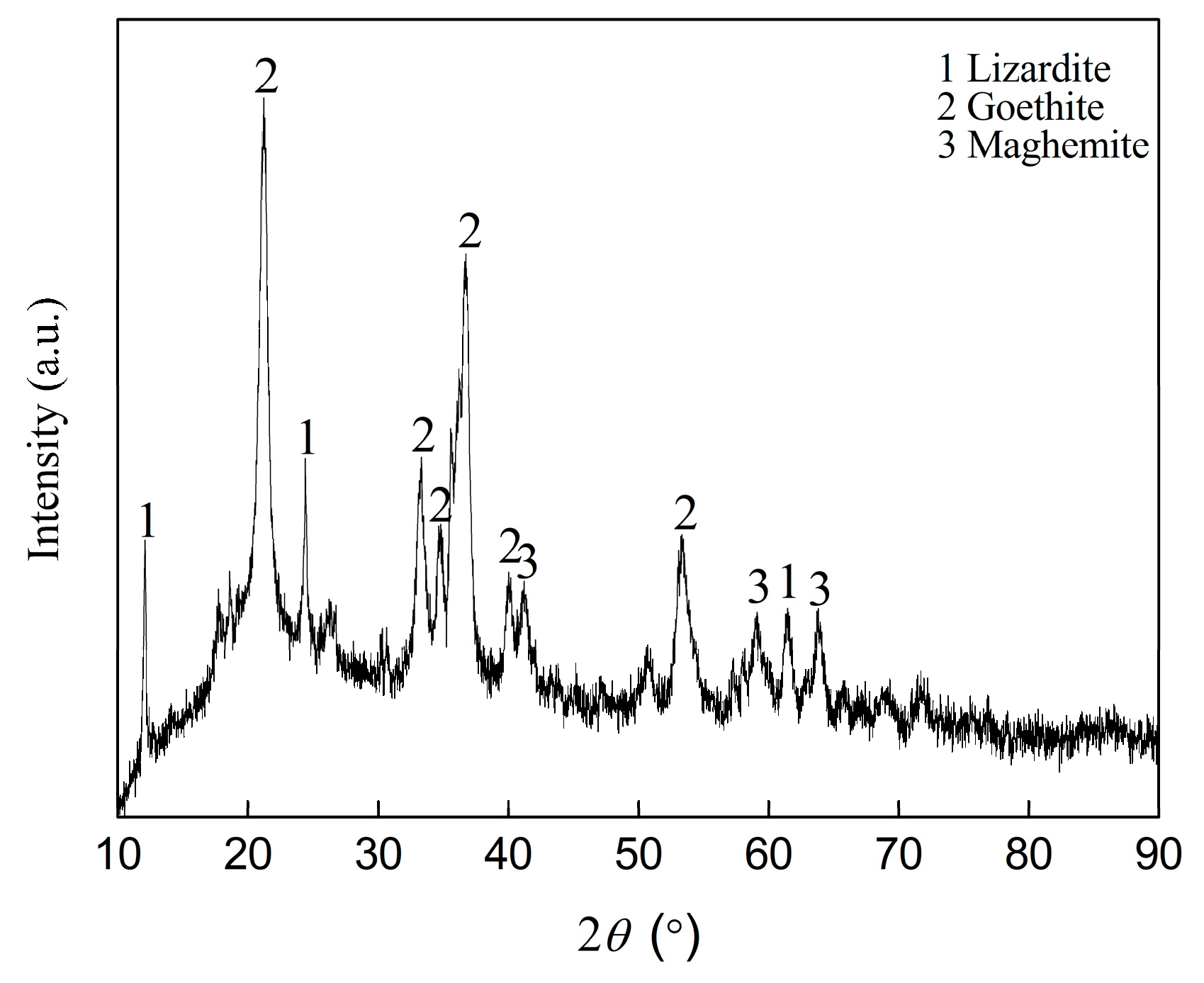
2.1. Analysis of Nickel Laterite
2.2. Reductants and Additives
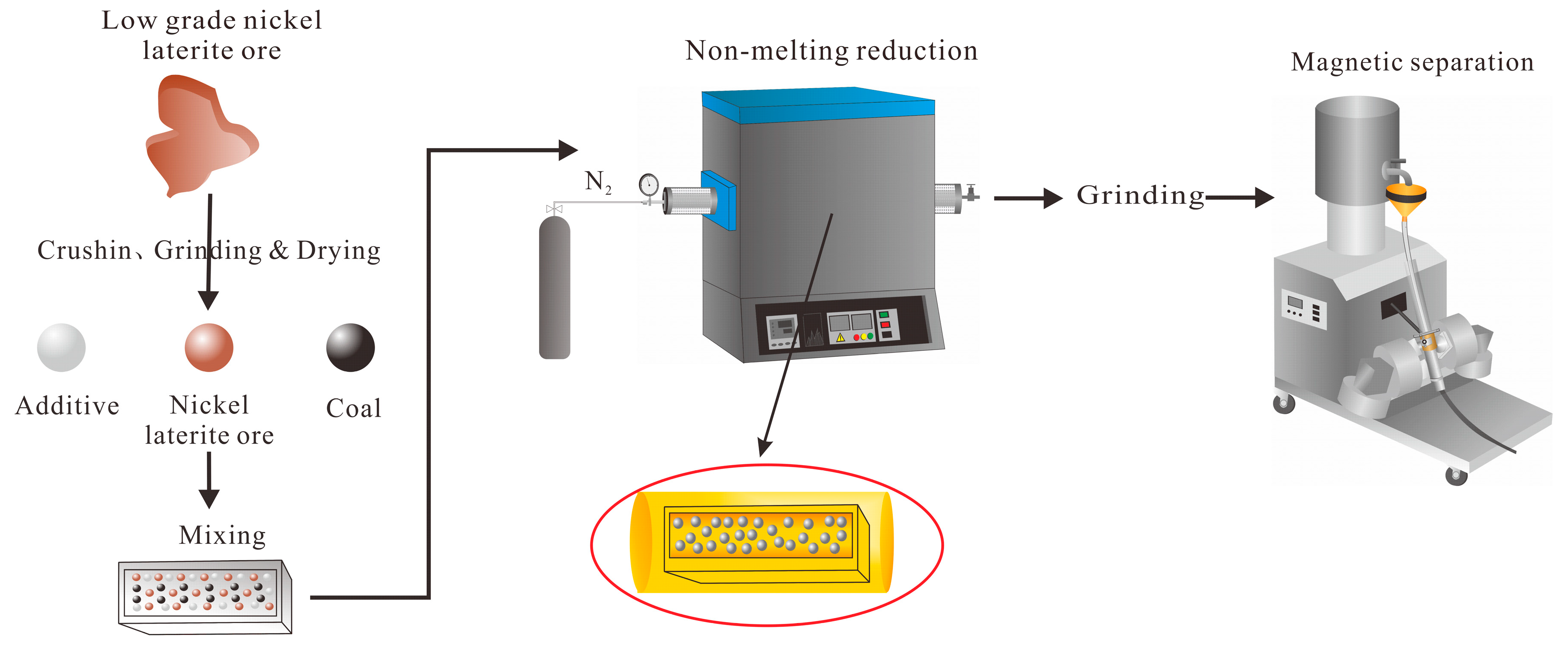
2.3. Methods
3. Results and Discussion
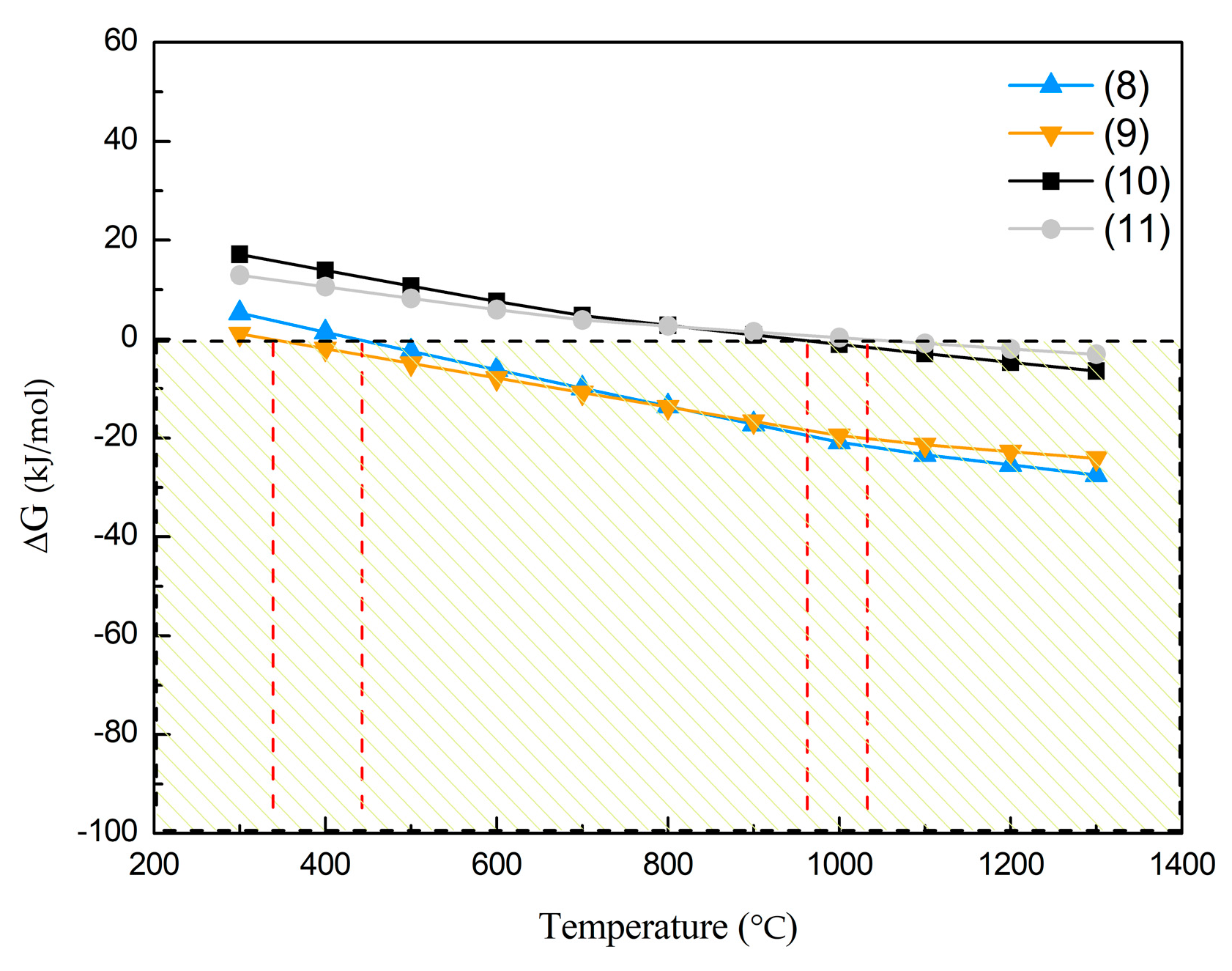
3.1. Thermodynamic Analysis of Selective Reduction
3.2. Effect of Different Influencing Factors on the Grade and Recovery of Ni and Fe
3.2.1. Effect of Reduction Roasting Temperature
3.2.2. Effect of Roasting Duration
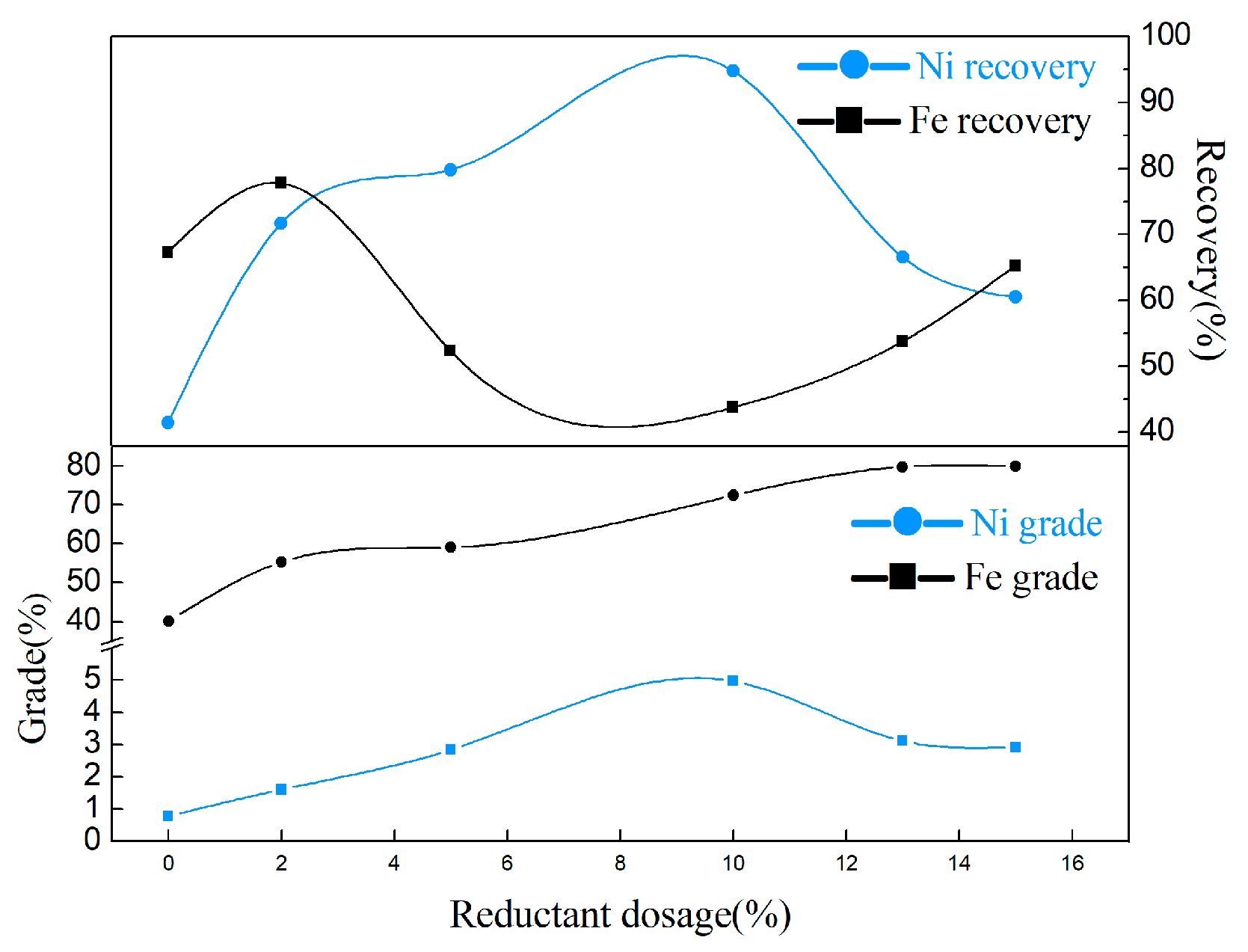
3.2.3. Effect of Reductant Dosage
3.2.4. Effect of Additive Dosage
3.2.5. Effect of Grinding Time
4. Conclusions
- (1)
- The addition of sodium chloride as an additive during the reduction process can significantly improve the Ni and Fe grades and recovery in the concentrate. Under the conditions of a roasting temperature of 1250 °C, roasting duration of 80 min, reductant dosage of 10%, additive dosage of 5%, and a grinding time of 12 min, the grade of concentrate Ni and Fe was increased from 1.13% and 51.12% without additives to 8.15% and 64.28%, and the recovery of Ni was increased from 75.40% to 97.76%;
- (2)
- The addition of additives promotes the transformation of the lizardite phase to the forsterite phase, facilitates the dissociation of nickel from the mineral, and improves the reduction effect of nickel. At the same time, the aggregation and growth behavior of ferronickel particles is improved, and the efficiency of magnetic separation is improved.
Author Contributions
Funding
Conflicts of Interest
References
- Sattar, R.; Ilyas, S.; Bhatti, H.N.; Ghaffar, A. Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep. Purif. Technol. 2019, 209, 725–733. [Google Scholar] [CrossRef]
- Mudd, G.M. Global trends and environmental issues in nickel mining: Sulfides versus laterites. Ore Geol. Rev. 2010, 38, 9–26. [Google Scholar] [CrossRef]
- Zappala, L.C.; Balucan, R.D.; Vaughan, J.; Steel, K.M. Analysis of a reactive distillation process to recover tertiary amines and acid for use in a combined nickel extraction-mineral carbonation process. Environ. Prog. Sustain. Energy 2019, 38. [Google Scholar] [CrossRef]
- Mudd, G.M. Nickel sulfide versus laterite: The hard sustainability challenge remains. In Proceedings of the 48th Annual Conference of Metallurgists, Sudbury, ON, Canada, 23–26 August 2009. [Google Scholar]
- Zhu, D.Q.; Cui, Y.; Vining, K.; Hapugoda, S.; Douglas, J.; Pan, J.; Zheng, G.L. Upgrading low nickel content laterite ores using selective reduction followed by magnetic separation. Int. J. Miner. Process. 2012, 106, 1–7. [Google Scholar] [CrossRef]
- Rao, M.; Li, G.; Jiang, T.; Luo, J.; Zhang, Y.; Fan, X. Carbothermic reduction of nickeliferous laterite ores for nickel pig iron production in China: A review. JOM 2013, 65, 1573–1583. [Google Scholar] [CrossRef]
- Whittington, B.I.; Muir, D. Pressure acid leaching of nickel laterites: A review. Miner. Process. Extr. Metull. Rev. 2000, 21, 527–599. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Keskinkilic, E. Nickel laterite smelting processes and some examples of recent possible modifications to the conventional route. Metals 2019, 9, 974. [Google Scholar] [CrossRef]
- Valix, M.; Cheung, W.H. Effect of sulfur on the mineral phases of laterite ores at high temperature reduction. Miner. Eng. 2002, 15, 523–530. [Google Scholar] [CrossRef]
- Harris, C.T.; Peacey, J.G.; Pickles, C.A. Selective sulphidation of a nickeliferous lateritic ore. Miner. Eng. 2011, 24, 651–660. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, L.; Fang, L.; Yang, R.; Fu, Y.; Zhao, H. Measurement and analyses of molten nickel-cobalt alloy surface tension. Rare Met. Mater. Eng. 2008, 7, 255–258. [Google Scholar] [CrossRef]
- Shen, S.B.; Hao, X.F.; Yang, G.W. Kinetics of selective removal of iron from chromite by carbochlorination in the presence of sodium chloride. J. Alloy. Compd. 2009, 476, 653–661. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Gao, Y.; Zhang, Y.; Chen, Z. Chlorination roasting of laterite using salt chloride. Int. J. Miner. Process. 2016, 148, 23–31. [Google Scholar] [CrossRef]
- Okamoto, K.; Ueda, Y.; Noguchi, F. Extraction of nickel from garnierite ore by the segregation-magnetic separation process. Mem. Kyushu Inst. Technol. Eng. 1971, 1, 41–61. [Google Scholar]
- Ilic, I.; Krstev, B.; Stopic, S.; Cerovic, K. The study of chlorination of nickel oxide by chlorine and calcium chloride in the presence of active additives. Scand. J. Metall. 1997, 26, 14–19. [Google Scholar]
- Ilić, I.; Stopić, S.; Cerović, K.; Kamberović, Ž. Study of chlorination of nickel ferrite by gaseous chlorine and calcium chloride in the presence of active additives. Scand. J. Metall. 2000, 29, 1–8. [Google Scholar] [CrossRef]
- Li, X.H.; Zhang, L.X.; Hu, Q.Y.; Wang, Z.X. Effect of phase transformation on chloridizing segregation of laterite ores. Chin. J. Nonferr. Met. 2011, 7, 238–243. (in Chinese). [Google Scholar]
- Fan, C.; Zhai, X.; Fu, Y.; Chang, Y.; Li, B.; Zhang, T.A. Leaching behavior of metals from chlorinated limonitic nickel laterite. Int. J. Miner. Process. 2012, 110, 117–120. [Google Scholar] [CrossRef]
- Li, B.; Wei, Y.G.; Wang, H. Action mechanism and phase transformation characteristics of garnierite in drying process. Chin. J. Nonferr. Met. 2013, 5, 1440–1446. (in Chinese). [Google Scholar]
- Rhamdhani, M.A.; Hayes, P.C.; Jak, E. Nickel laterite Part 1—Microstructure and phase characterisations during reduction roasting and leaching. Miner. Process. Extr. Metall. 2009, 118, 129–145. [Google Scholar] [CrossRef]
- Metallurgical Laboratory of Central-South Institute of Mining and Metallurgy. Chloridizing Metallurgy; Metallurgical Industry Press: Beijing, China, 1978; p. 180. (in Chinese) [Google Scholar]
- Kanungo, S.B.; Mishra, S.K. Kinetics of chloridization of nickel oxide with gaseous hydrogen chloride. Metall. Mater. Trans. B 1997, 28, 371–387. [Google Scholar] [CrossRef]
- Pickles, C.A.; Forster, J.; Elliott, R. Thermodynamic analysis of the carbothermic reduction roasting of a nickeliferous limonitic laterite ore. Miner. Eng. 2014, 65, 33–40. [Google Scholar] [CrossRef]
- Iwasaki, I. A thermodynamic interpretation of the segregation process for copper and nickel ores. Miner. Sci. Eng. 1972, 4, 14–23. [Google Scholar]




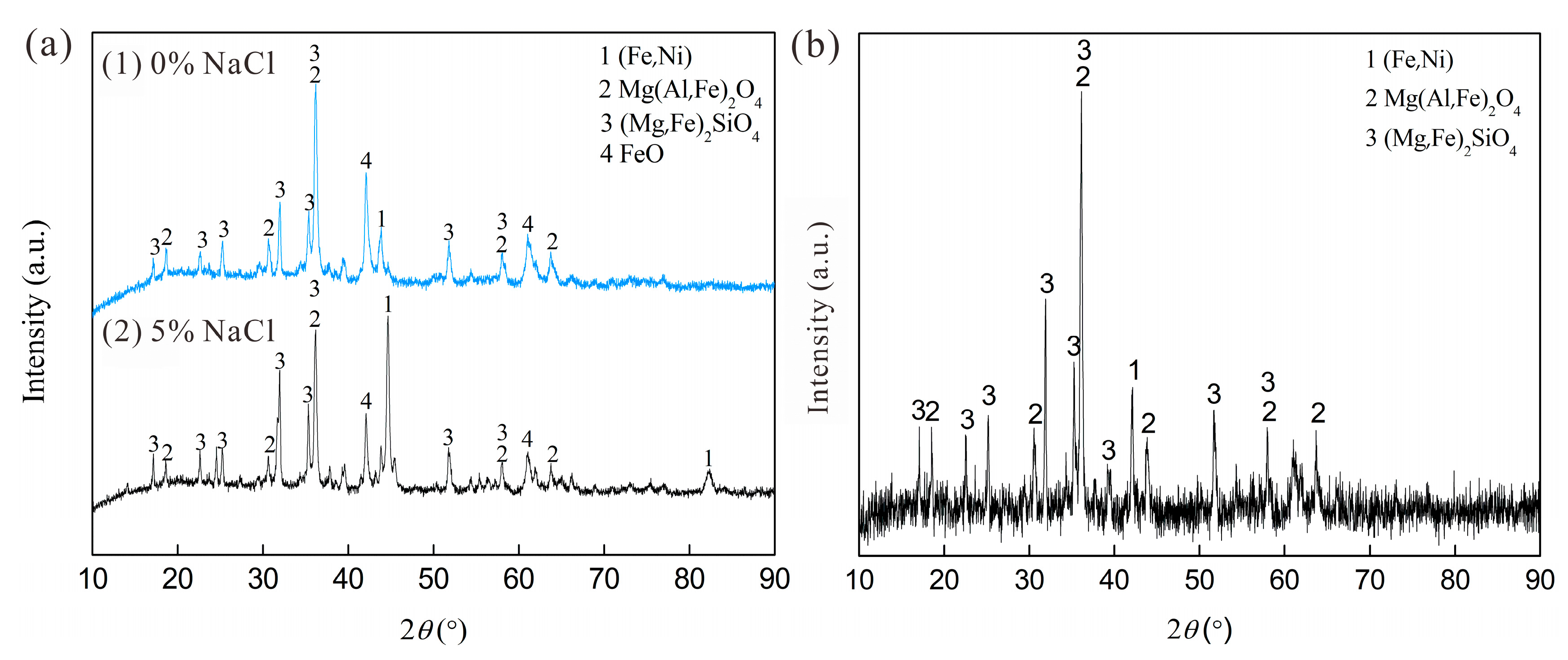
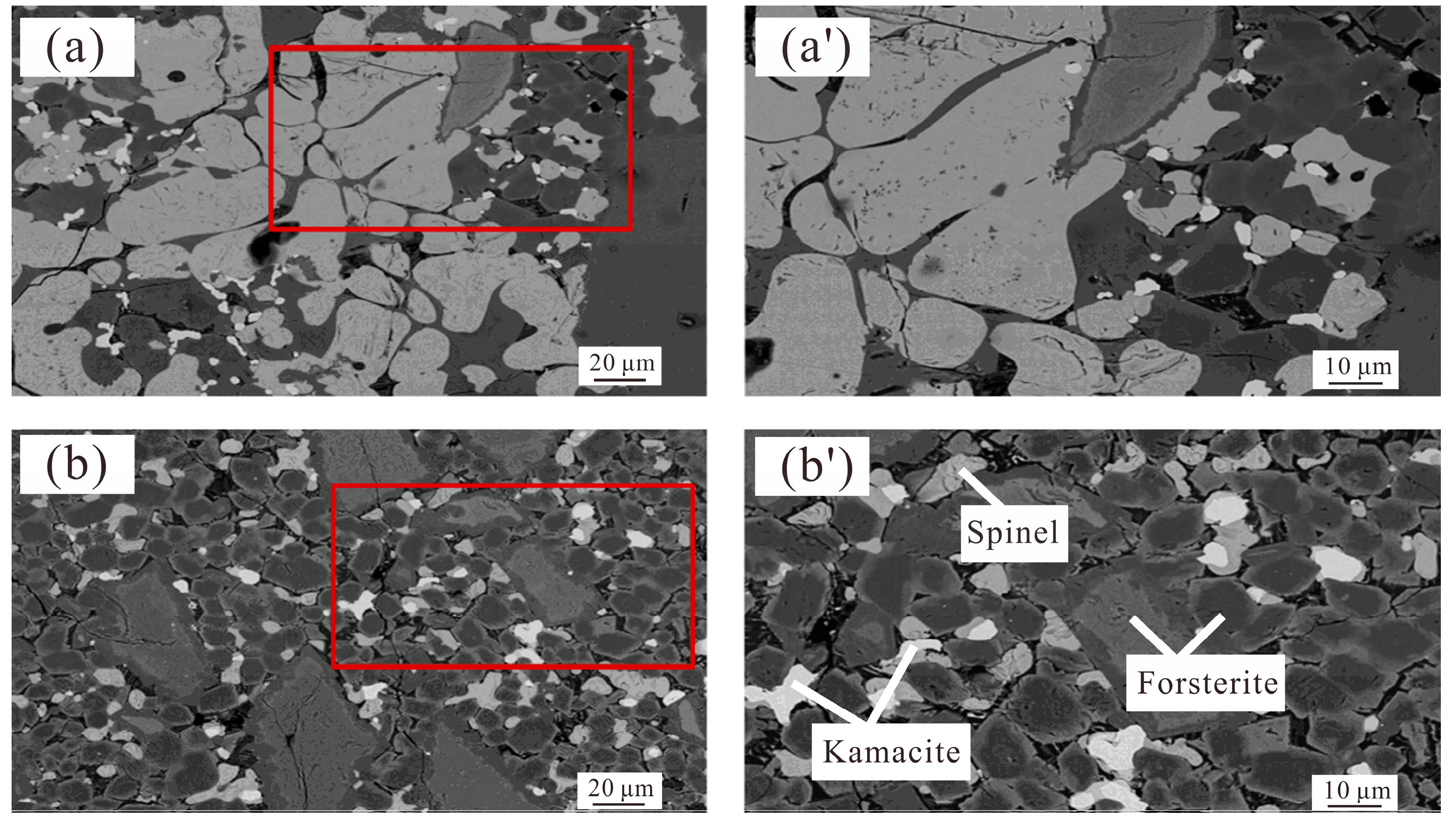

| Component | TFe(total) | Ni | Co | MgO | SiO2 | Al2O3 |
|---|---|---|---|---|---|---|
| Content weight (wt. %) | 35.79 | 1.13 | 0.097 | 3.65 | 10.38 | 10.31 |
| Component | Fixed Carbon | Volatile Matter | Ash | Moisture |
|---|---|---|---|---|
| Content wt. % | 76.43 | 7.78 | 15.29 | 1.02 |
| Studied Parameters | Range |
|---|---|
| Roasting temperature/°C | 900, 1000, 1100, 1200, 1250, 1300 |
| Roasting duration/min | 10, 30, 60, 80, 100 |
| Reductant dosage/wt. % | 2, 5, 10, 13, 15 |
| NaCl dosage/wt. % | 0, 5, 8, 10, 15 |
| Grinding time/min | 2, 4, 8, 12, 16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, G.; Zhou, S.; Wang, H.; Li, B.; Wei, Y. Production of Ferronickel Concentrate from Low-Grade Nickel Laterite Ore by Non-Melting Reduction Magnetic Separation Process. Metals 2019, 9, 1340. https://doi.org/10.3390/met9121340
Qu G, Zhou S, Wang H, Li B, Wei Y. Production of Ferronickel Concentrate from Low-Grade Nickel Laterite Ore by Non-Melting Reduction Magnetic Separation Process. Metals. 2019; 9(12):1340. https://doi.org/10.3390/met9121340
Chicago/Turabian StyleQu, Guorui, Shiwei Zhou, Huiyao Wang, Bo Li, and Yonggang Wei. 2019. "Production of Ferronickel Concentrate from Low-Grade Nickel Laterite Ore by Non-Melting Reduction Magnetic Separation Process" Metals 9, no. 12: 1340. https://doi.org/10.3390/met9121340
APA StyleQu, G., Zhou, S., Wang, H., Li, B., & Wei, Y. (2019). Production of Ferronickel Concentrate from Low-Grade Nickel Laterite Ore by Non-Melting Reduction Magnetic Separation Process. Metals, 9(12), 1340. https://doi.org/10.3390/met9121340





