Combustion of Metals in Oxygen-Enriched Atmospheres
Abstract
1. Introduction
2. Test Methods
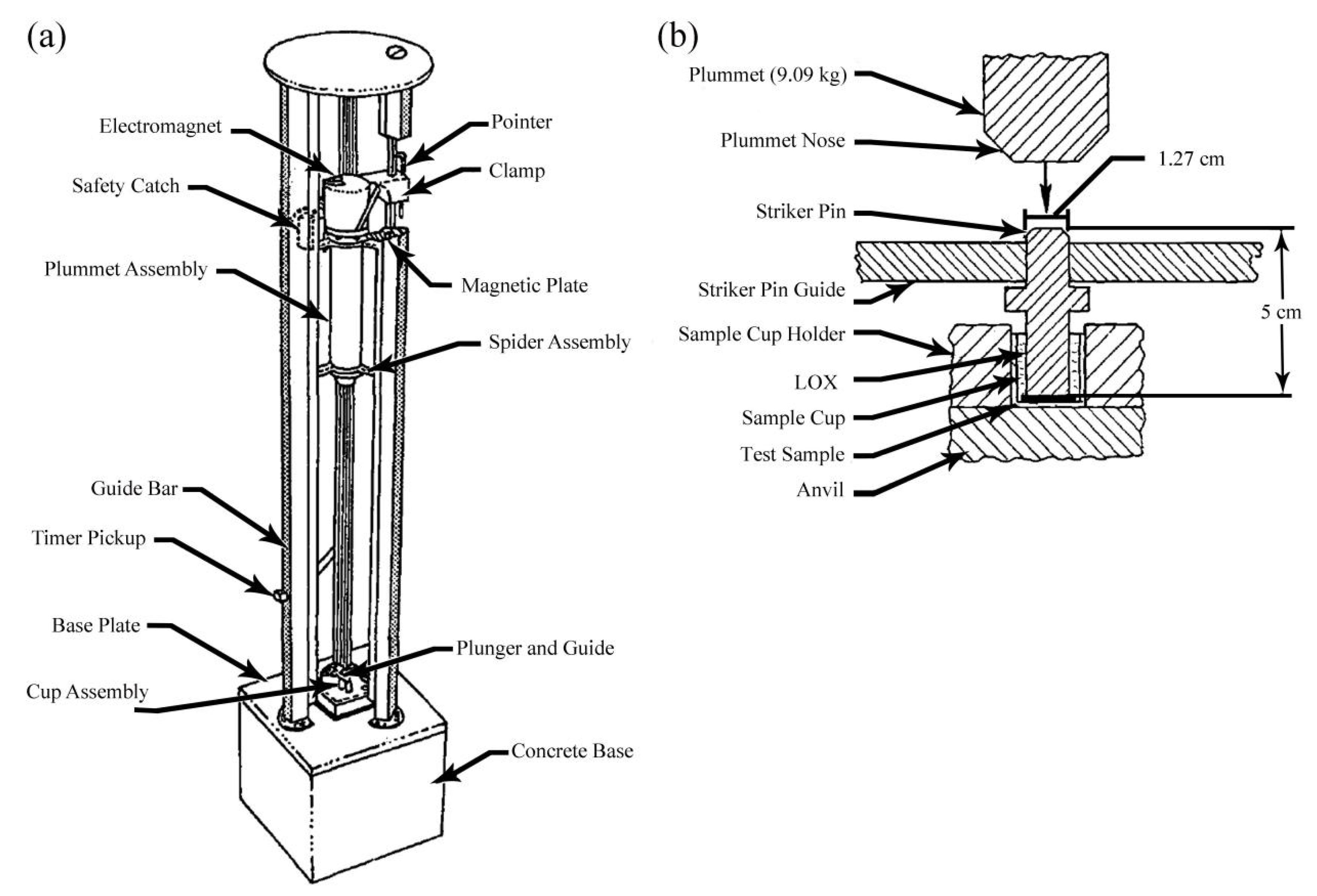
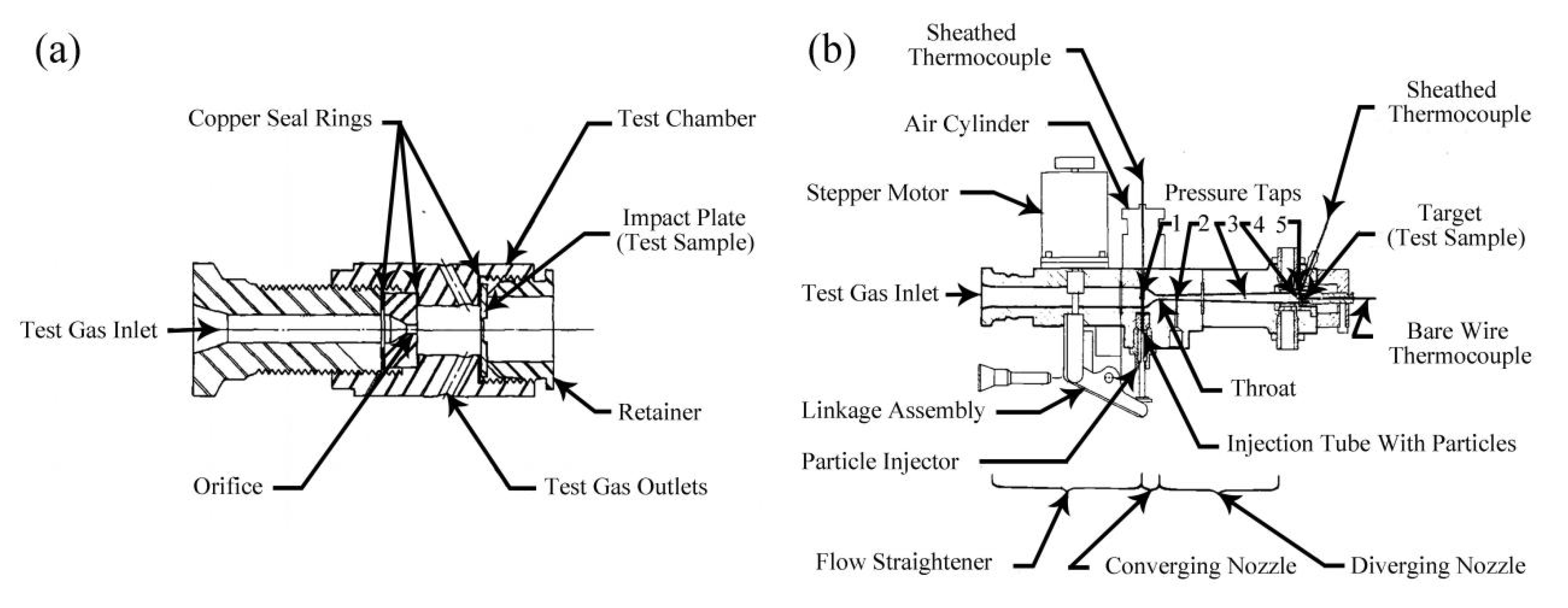
2.1. NASA Mechanical Impact Tests
2.1.1. Ambient Pressure Liquid Oxygen Mechanical Impact Tests [41,42,43,44]
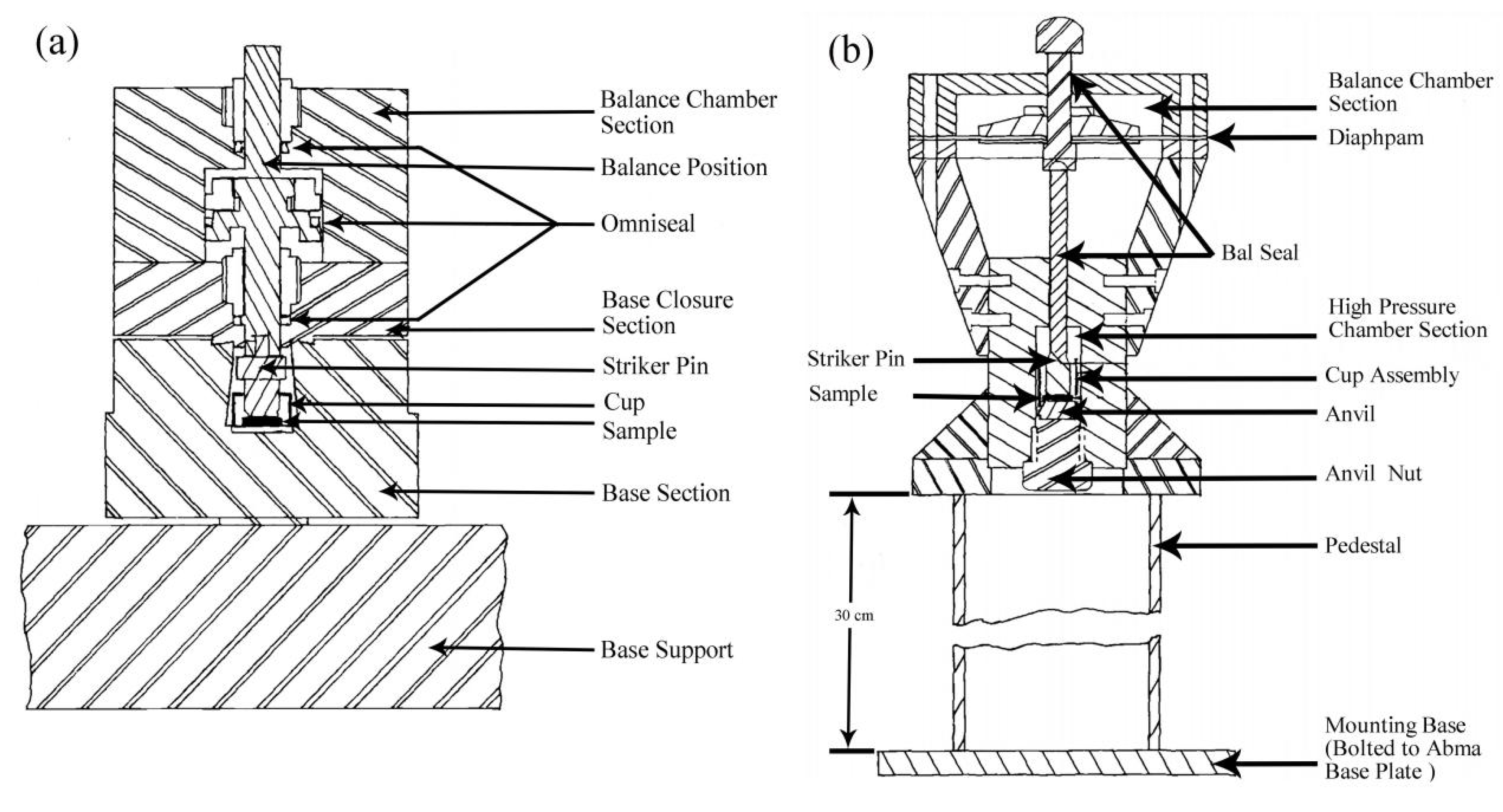
2.1.2. High-Pressure Oxygen Mechanical Impact Tests [41,43,45]
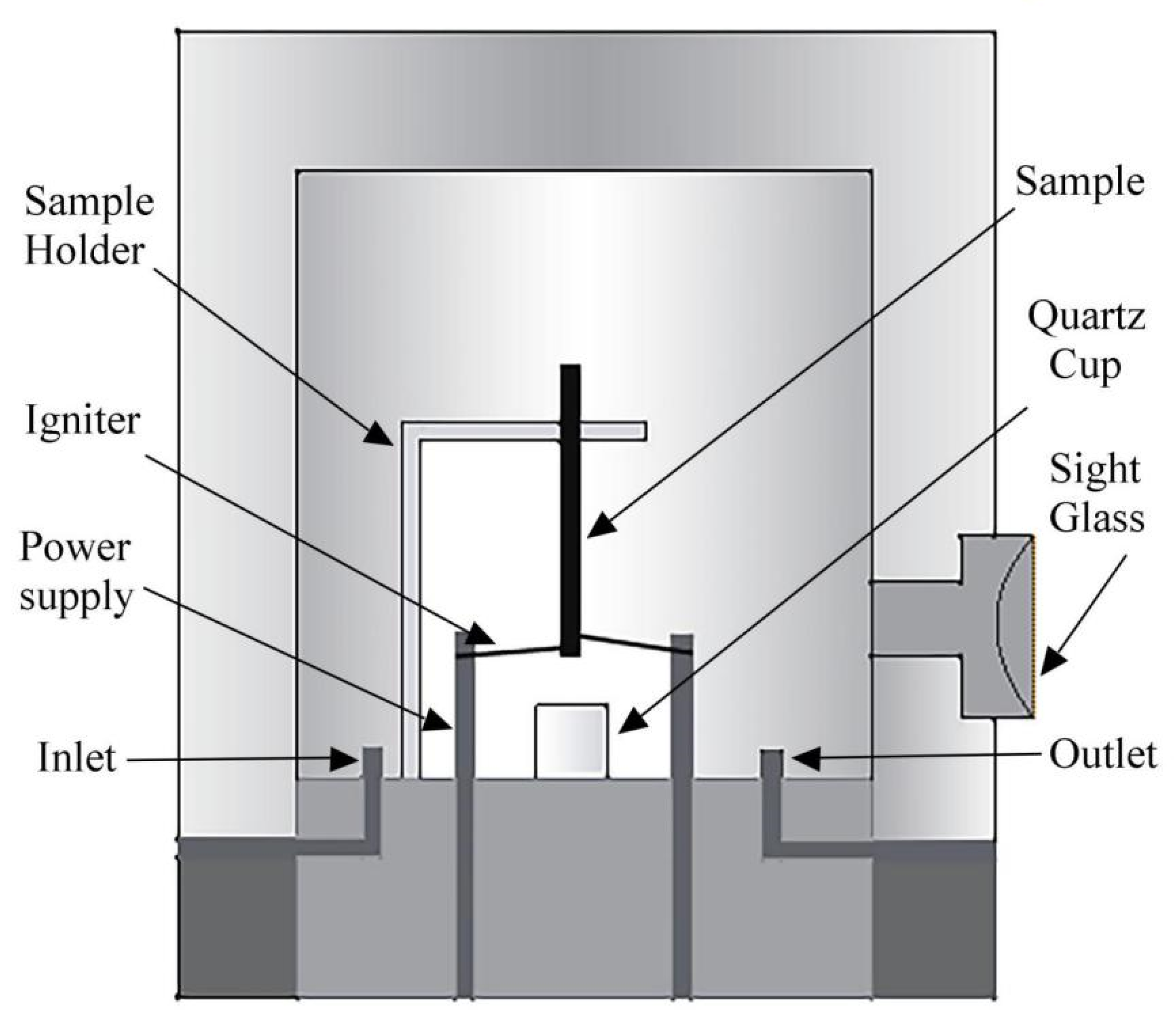
2.2. Promoted Combustion Tests [46,47]
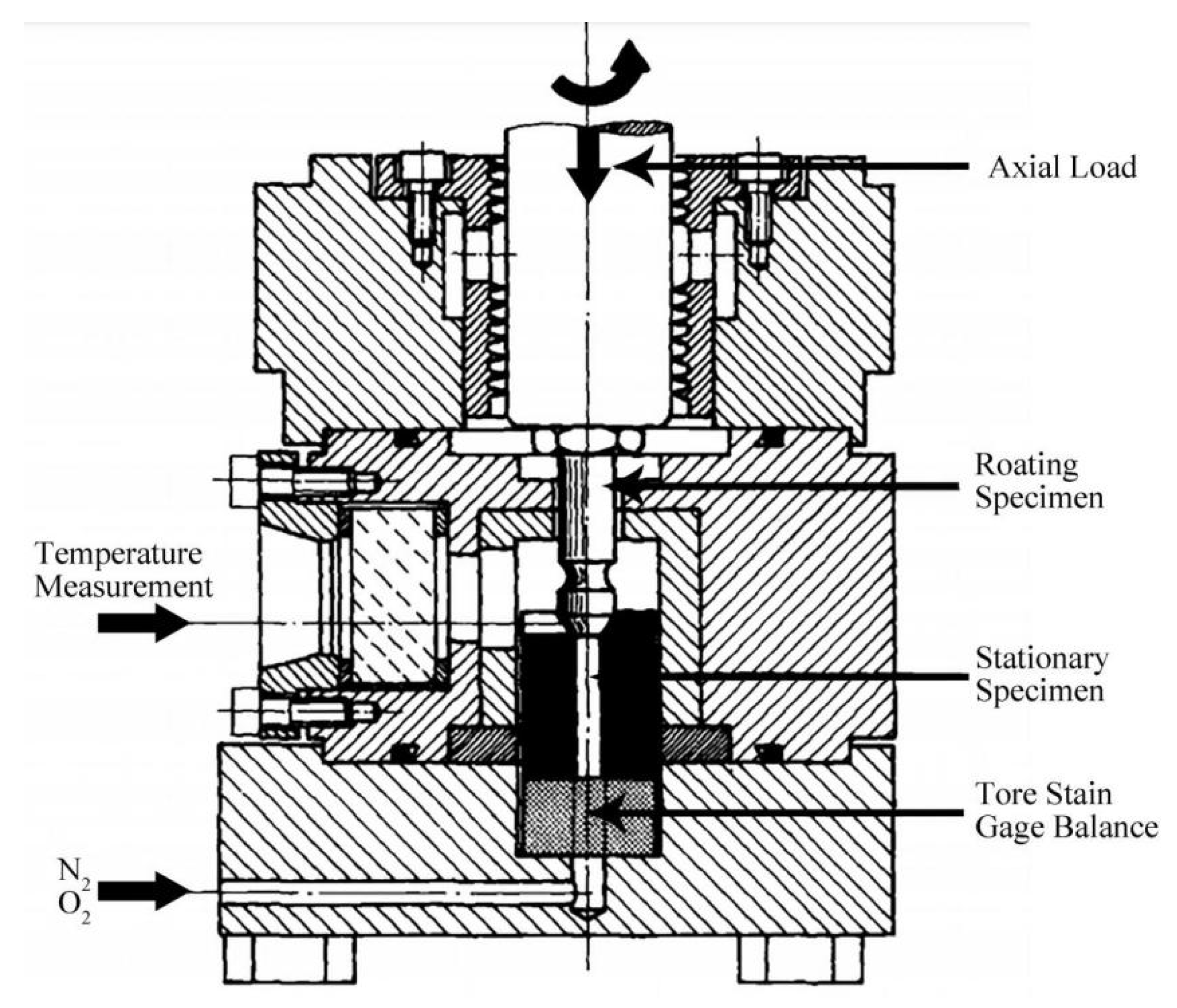
2.3. Friction-Induced Ignition Tests [48,49]
2.4. High-Velocity Particles Impact Tests [50,51]
3. Combustion Behaviors of Stainless Steels
4. Combustion Behaviors of Nickel Superalloys
5. Combustion Behaviors of Titanium Alloys
6. Summary
- (1)
- The mechanism of metals combustion in oxygen-enriched atmospheres is still unclear. Metals combustion is a complex problem touching upon many subjects such as material science, hydromechanics and physics, and the ignition mechanism is complicated. Works on metals combustion in oxygen-enriched atmospheres have only recently begun, consisting of only exploratory researches.
- (2)
- The repeatability of the test results is poor. There are so many factors that influence the flammability, such as configuration, size, temperature, gravity, and oxygen pressure.
- (3)
- There is a major gap between the experimental study and field application. Researchers used some application principles and sorted the materials according to their burn-resistant properties only. However, there are still some abnormal phenomena.
- (1)
- Establishing the test standards of metals combustion in oxygen-enriched atmospheres.
- (2)
- Studying the mechanism of metals combustion in oxygen-enriched atmospheres and building the combustion model.
- (3)
- Designing more burn-resistant alloys to use in oxygen-enriched atmospheres.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.L.; Huang, J.F.; Lian, Y.; Cheng, Z.; Zhou, Q.M.; Li, S.K.; Xuan, T. Combustion behavior of GH4169 and GH4202 superalloys in oxygen-enrichedatmosphere. Chin. J. Eng. 2016, 9, 1288–1295. [Google Scholar]
- Neary, R.M. ASTM G63: A Milestone in a 60-year Safety Effort. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Phoenix, AZ, USA, 1983. [Google Scholar]
- Bryan, C.J. NASA mechanical impact testing in high-pressure oxygen. Can. Med. Assoc. J. 1983, 88, 642. [Google Scholar]
- Clark, A.F.; Hust, J.G. A Review of the Compatibility of Structural Materials with Oxygen. AIAA J. 1974, 12, 441–454. [Google Scholar] [CrossRef]
- Monroe, R.W.; Bates, C.E.; Pears, C.D. Metal Combustion in High-Pressure Flowing Oxygen. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Phoenix, AZ, USA, 1983. [Google Scholar]
- Sato, J.I.; Ohtani, H.; Hirano, T. Ignition Process of a Heated Iron Block in High-Pressure Oxygen Atmosphere. Combust. Flame 1995, 100, 376–383. [Google Scholar] [CrossRef]
- Blandin, J.; Grosjean, E.; Suery, M.; Kumar, N.R.; Mebarki, N. Ignition Resistance of Various Magnesium Alloys. In Magnesium Technology; Springer: Columbus, OH, USA, 2004. [Google Scholar]
- Shih, T.S.; Wang, J.H.; Chong, K.Z. Combustion of magnesium alloys in air. Mater. Chem. Phys. 2004, 85, 302–309. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yim, C.D.; Kim, H.S. Key factor influencing the ignition resistance of magnesium alloys at elevated temperatures. Scr. Mater. 2011, 65, 958–961. [Google Scholar] [CrossRef]
- Czerwinski, F. Controlling the ignition and flammability of magnesium for aerospace applications. Corros. Sci. 2014, 86, 1–16. [Google Scholar] [CrossRef]
- Lin, P.; Zhou, H.; Li, W.; Sun, N.; Yang, R. Interactive effect of cerium and aluminum on the ignition point and the oxidation resistance of magnesium alloys. Corros. Sci. 2008, 50, 2669–2675. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, H.; Zhou, T.; Zhang, Z.; Lin, P.; Ren, L. The oxidation resistance and ignition temperature of AZ31 magnesium alloy with additions of La2O3 and La. Corros. Sci. 2013, 67, 75–81. [Google Scholar] [CrossRef]
- Liu, C.; Lu, S.; Fu, Y. Flammability and the oxidation kinetics of the magnesium alloys AZ31, WE43, and ZE10. Corros. Sci. 2015, 100, 177–185. [Google Scholar] [CrossRef]
- Kumar, N.V.; Blandin, J.J.; Suery, M. Effect of alloying elements on the ignition resistance of magnesium alloys. Scr. Mater. 2003, 49, 225–230. [Google Scholar] [CrossRef]
- Fan, J.F.; Chen, Z.Y.; Yang, W.D.; Fang, S.; Xu, B.S. Effect of yttrium, calcium and zirconium on ignition-proof principle and mechanical properties of magnesium alloys. J. Rare Earths 2012, 30, 74–78. [Google Scholar] [CrossRef]
- Prasad, A.; Shi, Z.; Atrens, A. Influence of Al and Y on the ignition and flammability of Mg alloys. Corros. Sci. 2012, 55, 153–163. [Google Scholar] [CrossRef]
- Zhao, H.J.; Zhang, Y.H.; Kang, Y.L. Effect of cerium on ignition point of AZ91D magnesium alloy. China Foundry 2008, 5, 32–35. [Google Scholar]
- Zeng, X.Q.; Wang, Q.D.; Lu, Y.Z.; Ding, W.J.; Lu, C.; Zhu, Y.P.; Zhai, C.Q.; Xu, X.P. Study on ignition proof magnesium alloy with beryllium and rare earth additions. Scr. Mater. 2000, 43, 403–409. [Google Scholar] [CrossRef]
- Kasprzak, W.; Sokolowski, J.; Sahoo, M.; Dobrzanski, L. Thermal and structural characteristics of the AM50 magnesum alloy. J. Achievments Mater. Manuf. Eng. 2008, 28, 131–138. [Google Scholar]
- Fassell, W.; Gulbransen, L.; Lewis, J.; Hamilton, J. Ignition temperature of magnesium and magnesium alloys. JOM 1951, 3, 522–528. [Google Scholar] [CrossRef]
- Mebarki, N.; Kumar, R.; Blandin, J.; Sery, M.; Pelloux, F.; Khelifati, G. Correlation between ignition and oxidation behaviors of AZ91 magnesium alloy. Mater. Sci. Technol. 2005, 21, 1145–1151. [Google Scholar] [CrossRef]
- Xu, S.; Edet-Ikpi, M.; Dong, J.; Wei, J.; Ke, W.; Chen, N. Effect of cadmium on the corrosion and mechanical properties of magnesium. Int. J. Electrochem. Sci. 2012, 7, 4735–4755. [Google Scholar]
- Moser, Z.; Gasior, W.; Wypartowicz, J.; Zabdyr, L. The Cd-Mg (Cadmium–Magnesium) system. Bull. Alloy Phase Diagr. 1984, 5, 23–30. [Google Scholar] [CrossRef]
- Dreizin, E.L. On the Mechanism of Asymmetric Aluminum Particle Combustion. Combust. Flame 1999, 117, 841–850. [Google Scholar] [CrossRef]
- Tayal, M.; Wilson, D.B.; Stoltzfus, J.M. Influence of Alloying Additions on the Flammability of Ni-Based Alloys in an Oxygen Environment. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Denver, CO, USA, 1997. [Google Scholar]
- Slusser, J.W.; Miller, K.A. Selection of Metals for Gaseous Oxygen Service. In ASTM Symposium on Flammability and Sensitivity of Materials in Oxygen Enriched Atmospheres; ASTM: Phoenix, AZ, USA, 1982. [Google Scholar]
- Kirschfeld, L. Combustion of Non-Iron Heavy Metal Wires in Oxgen. Metall 1960, 14, 792–796. [Google Scholar]
- Slusser, J.M.; Miller, K.A. Selection of Metals for Gaseous Oxygen Service. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Phoenix, AZ, USA, 1983. [Google Scholar]
- Borisova, Y.A.; Sklyarov, N.M. Fireproof titanium alloys. Phys. Metallogr. 1993, 6, 21–24. [Google Scholar]
- Zhao, Y.Q.; Zhou, L.; Deng, J. Effects of the alloying element Cr on the burning behavior of titanium alloys. J. Alloys Compd. 1999, 284, 190–193. [Google Scholar]
- Chen, Y.N.; Huo, Y.Z.; Song, X.D.; Bi, Z.Z.; Gao, Y.; Zhao, Y.Q. Burn-resistant behavior and mechanism of Ti14 alloy. Int. J. Miner. Metall. Mater. 2016, 23, 215–221. [Google Scholar] [CrossRef]
- Wang, B.; Tian, W. Combustion Morphology and Mechanism Analysis of Titanium Alloy TC4. Gas Turbine Exp. Res. 2013, 3, 50–52. [Google Scholar]
- Dreizin, E.L. Phase changes in metal combustion. Prog. Energy Combust. Sci. 2000, 26, 57–78. [Google Scholar] [CrossRef]
- Yu, J.L.; Zhang, X.Y.; Zhang, Q.; Wang, L.B.; Ji, K.; Peng, L.; Gao, W. Combustion behaviors and flame microstructures of micro-and nano-titanium dust explosions. Fuel 2016, 181, 785–792. [Google Scholar] [CrossRef]
- Mi, G.B.; Cao, C.X.; Huang, X.; Cao, J.X.; Wan, B. Ignition Resistance Performance and Its Mechanism of TC11 Titanium Alloy for Aero-Engine. J. Aeronaut. Mater. 2014, 34, 83–91. [Google Scholar]
- Tian, W.; Wang, B. Combustion Characteristics of Titanium Alloy TC11. Gas Turbine Exp. Res. 2012, 3, 40–43. [Google Scholar]
- Mi, G.B.; Huang, X.; Cao, J.X. Frictional ignition of Ti40 fireproof titanium alloys for aero-engine in oxygen-containing media. Trans. Nonferr. Met. Soc. China 2013, 23, 2270–2275. [Google Scholar] [CrossRef]
- Chen, Y.N.; Yang, W.Q.; Arixin, B.; Zhan, H.F.; Zhang, F.Y.; Zhao, Y.Q.; Zhao, Q.Y.; Wan, M.P.; Gu, Y.T. Underlying burning resistant mechanisms for titanium alloy. Mater. Des. 2018, 156, 588–595. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Zhao, Y.Q.; Qu, H.L. Microstructure and properties of burn-resistant Ti-Al-Cu alloys. J. Mater. Sci. 2000, 35, 5609–5612. [Google Scholar] [CrossRef]
- Wilson, D.B.; Stoltzfus, J.M. Metals Flammability: Review and Model Analysis. ASTM Spec. Tech. Publ. 2000, 9, 28–35. [Google Scholar]
- Bryan, C.J. NASA Mechanical Impact Testing in High-Pressure Oxygen. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Phoenix, AZ, USA, 1983. [Google Scholar]
- Moffett, G.E.; Schmidt, N.E.; Pedley, M.D. An evaluation of the liquid oxygen mechanical impact test. ASTM Spec. Tech. Publ. 1989, 14, 11–22. [Google Scholar]
- Lockhart, B.J.; Bryan, C.J.; Hampton, M.D. The oxygen sensitivity compatibility ranking of several materials by different test methods. ASTM Spec. Tech. Publ. 1989, 14, 93–105. [Google Scholar]
- Hirsch, D.; Hshieh, F.Y.; Beeson, H.; Bryan, C. Ignitibility in Air, Gaseous Oxygen and Oxygen-Enriched Environments of Polymers Used in Breathing-Air Devices. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Phoenix, AZ, USA, 1997. [Google Scholar]
- Bryan, C.J. A Review of Test Methods Used in the Selection of Materials for Oxygen Service: Keynote Address. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: West Conshohocken, PA, USA, 2000. [Google Scholar]
- ASTM. ASTM G124 Standard Test Method for Determining the Burning Behavior of Metallic Materials in Oxygen-Enriched Atmospheres, Annual Book of ASTM Standards; ASTM Int.: West Conshohocken, PA, USA, 2010; Volume 14.04, pp. 1–10. [Google Scholar]
- Sircar, S.; Stoltzfus, J.; Bryan, C.; Kazaroff, J. Promoted Combustion of Pure Metals in Oxygen-Enriched Atmospheres. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Philadelphia, PA, USA, 1995. [Google Scholar]
- Benz, F.J.; Stoltzfus, J.M. Ignition of Metals and Alloys in Gaseous Oxygen by Fractional Heating. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Washington, DC, USA, 1986. [Google Scholar]
- Jenny, R.; Wyssmann, H. Friction-Induced Ignition in Oxygen. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Phoenix, AZ, USA, 1983. [Google Scholar]
- Benz, F.J.; Williams, R.E.; Armstriong, D. Ignition of metals and alloys by high-velocity particals. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Washington, DC, USA, 1986. [Google Scholar]
- Dees, J.; Forsyth, E.; Gunaji, M.V. An Evaluation of Polymers as Ignition Sources During Particle Impact in Oxygen. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Philadelphia, PA, USA, 1995. [Google Scholar]
- Wit, J.D.; Steinberg, T.A.; Haas, J.P. ASTM G 124 test data for selected Al-Si alloys, Al-composites, binary alloys and stainless steels. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Steinberg, T.A.; Rucker, M.A.; Beeson, H.D. Promoted combustion of nine structural metals in high-pressure gaseous oxygen; a comparison of ranking methods. ASTM Spec. Tech. Publ. 1989, 14, 54–75. [Google Scholar]
- Benz, F.J.; Shaw, R.C.; Homa, J.M. Burn propagation rates of metals and alloys in gaseous oxygen. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: Washington, DC, USA, 1986. [Google Scholar]
- Slockers, M.J.; Robles, C.R. Ignition of Metals at High Temperatures in Oxygen. J. ASTM Int. 2006, 3, 1–18. [Google Scholar] [CrossRef]
- Mcilroy, K.; Zawierucha, R.; Million, J.F. Promoted Ignition-Combustion Behavior of Alternative High Performance Engineering Alloys in Oxygen-Enriched Atmospheres. ASTM Spec. Tech. Publ. 1997, 18, 157–169. [Google Scholar]
- Million, J.F.; Samant, A.V. Zawierucha, R Promoted Ignition-Combustion Behavior of Cobalt and Nickel Alloys in Oxygen-Enriched Atmospheres. J. ASTM Int. 2009, 6, 1–8. [Google Scholar] [CrossRef]
- Mcllroy, K.; Zawierucha, R. The Effects of Testing Methodology on the Promoted Ignition-Combustion Behavior of Carbon Steel and 316L Stainless Steel in Oxygen Gas Mixtures. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmospheres; ASTM: Philadelphia, PA, USA, 1989. [Google Scholar]
- Wilson, D.B.; Steinberg, T.A.; Dewit, J.R. The presence of excess oxygen in burning metallic materials. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmosphere; ASTM: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Zhao, Y.Q.; Xi, Z.P.; Qu, H. Current situation of titanium alloy materials used for national aviation. J. Aeronaut. Mater. 2003, 23, 215–219. [Google Scholar]
- Qian, J.H. Application and developement of new titanium alloys for aerospace. Chin. J. Rare Met. 2000, 24, 218–223. [Google Scholar]
- Millogo, M.; Bernard, S.; Gillard, P.; Frascati, F. Combustion properties of titanium alloy powder in ALM processes: Ti6Al4V. J. Loss Prev. Process Ind. 2018, 56, 254–261. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhang, P.Z.; Huang, G.Q.; Peng, X.; Zheng, S.Q. Study on the burn-resistant properties of Titanium alloy Ti-6Al-4V surface by diffusing copper. Rare Metal Mater. Eng. 2011, 40, 286–289. [Google Scholar]
- Shao, L.; Xie, G.L.; Liu, X.H.; Wu, Y.; Yu, J.B.; Wang, Y.Y. Combustion behaviour and mechanism of a Cu-Ni-Mn alloy in an oxygen enriched atmosphere. Corros. Sci. 2019, 108253. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhao, X.M.; Zhu, K.Y. Effect of major alloying elements on microstructure and mechanical properties of a highly stabilized titanium alloy. Alloy. Compd. 2009, 481, 190–194. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhu, K.Y.; Qu, H.L.; Wu, H. Microstructures of a burn resistant highly stabilized titanium alloy. Mater. Sci. Eng. A 2000, 282, 153–157. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhou, L.; Deng, J. Study on the burning behavior of Ti-Cr binary. alloys and their burning products. J. Aeronaut. Mater. 2001, 1, 6–9. [Google Scholar]
- Xin, S.W.; Zhao, Y.Q.; Zeng, W.D.; Wu, H. Research on thermal stability of Ti40 alloy at 550 °C. Mater. Sci. Eng. A 2008, 477, 372–378. [Google Scholar] [CrossRef]
- Shao, L.; Wang, Y.; Xie, G. Combustion Mechanism of Alloying Elements Cr in Ti-Cr-V Alloys. Materials 2019, 12, 3206. [Google Scholar] [CrossRef]
- Mi, G.B.; Huang, X.; Cao, J.X.; Cao, C.X. Ignition resistance performance and its theoretical analysis of Ti-V-Cr type fireproof titanium alloy. Acta Metall. Sin. 2014, 50, 575–586. [Google Scholar]
- Zhao, Y.Q.; Zhou, L.; Zhou, Y.G.; Qu, H.L.; Wu, H.; Yang, H.Y. Research on basic theories of Ti40 burn resistant titanium alloy. J. Aeronaut. Mater. 2006, 26, 233–237. [Google Scholar]
- Hayama, A.O.; Andrade, P.N.; Cremasco, A. Effects of composition and heat treatment on the mechanical behavior of Ti–Cu alloys. Mater. Des. 2014, 55, 1006–1013. [Google Scholar] [CrossRef]
- Park, S.H.; Lim, K.R.; Na, M.Y. Oxidation behavior of Ti–Cu binary metallic glass. Corros. Sci. 2015, 99, 304–312. [Google Scholar] [CrossRef]
- Souza, S.A.; Afonso, C.R.; Ferrandini, P.L. Effect of cooling rate on Ti–Cu eutectoid alloy microstructure. Mater. Sci. Eng. C 2009, 29, 1023–1028. [Google Scholar] [CrossRef]
- Cardoso, F.F.; Cremasco, A.; Contieri, R.J. Hexagonal martensite decomposition and phase precipitation in Ti–Cu alloys. Mater. Des. 2011, 32, 4608–4613. [Google Scholar] [CrossRef]
- Chen, Y.N.; Yang, W.Q.; Bo, A.X.; Zhan, H.F.; Huo, Y.Z. Tailorable Burning Behavior of Ti14 Alloy by Controlling Semi-Solid Forging Temperature. Materials 2016, 9, 697. [Google Scholar] [CrossRef]
- Kikuchi, M.; Takada, Y.; Kiyosue, S.; Yoda, M.; Woldu, M.; Cai, Z.; Okuno, O. Mechanical properties and microstructures of cast Ti-Cu alloys. Dent. Mater. 2003, 19, 174–181. [Google Scholar] [CrossRef]
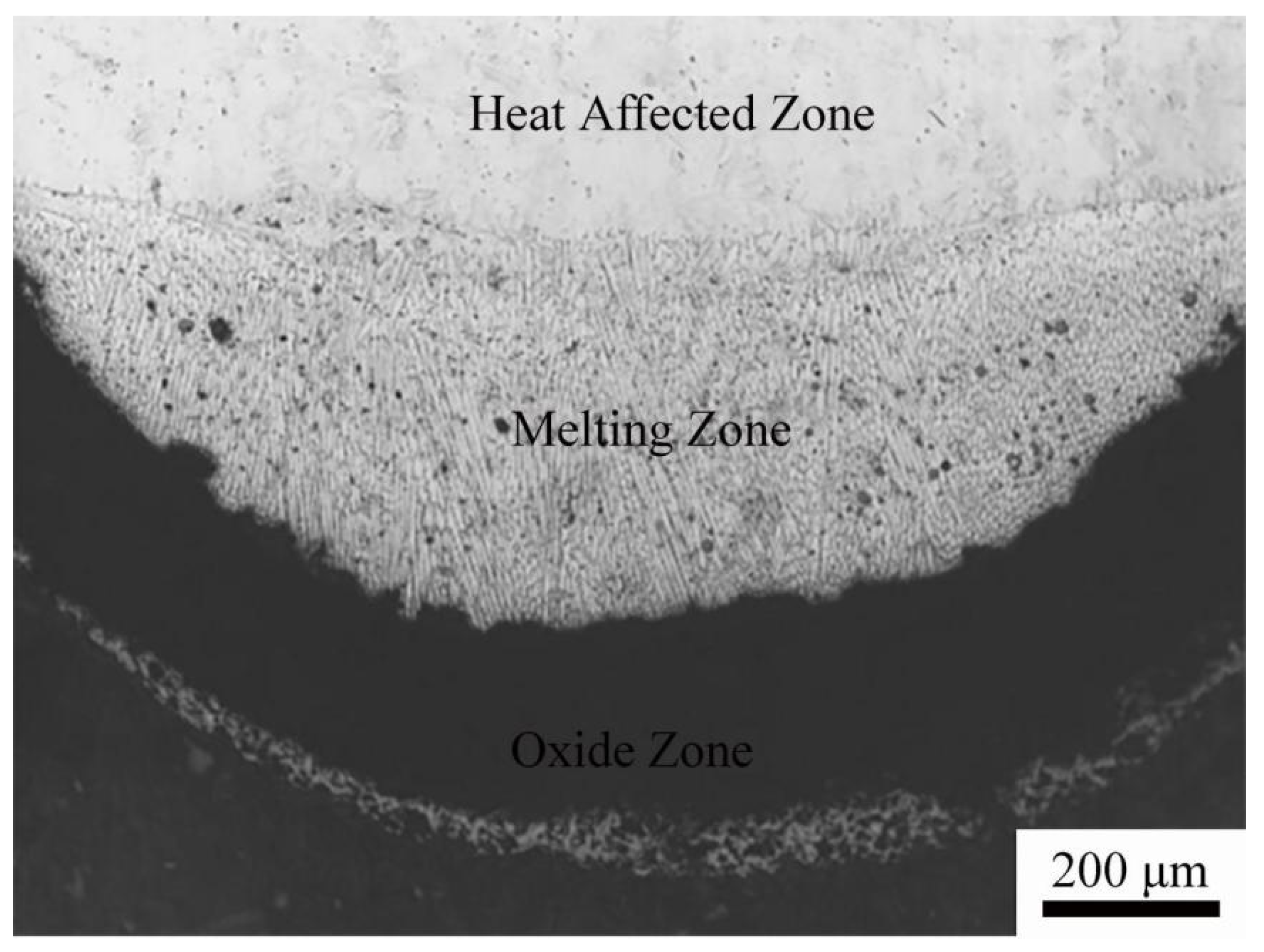
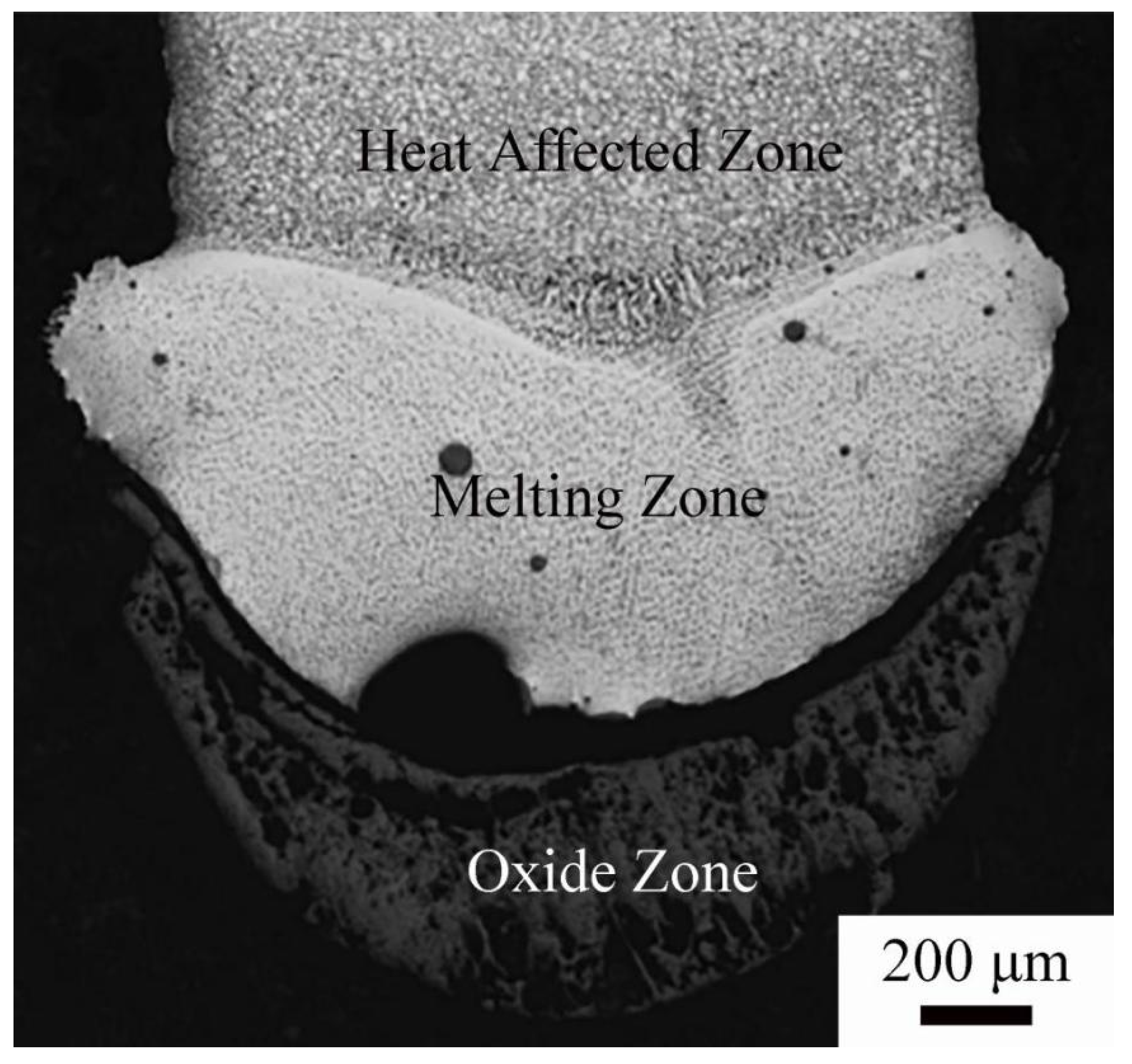
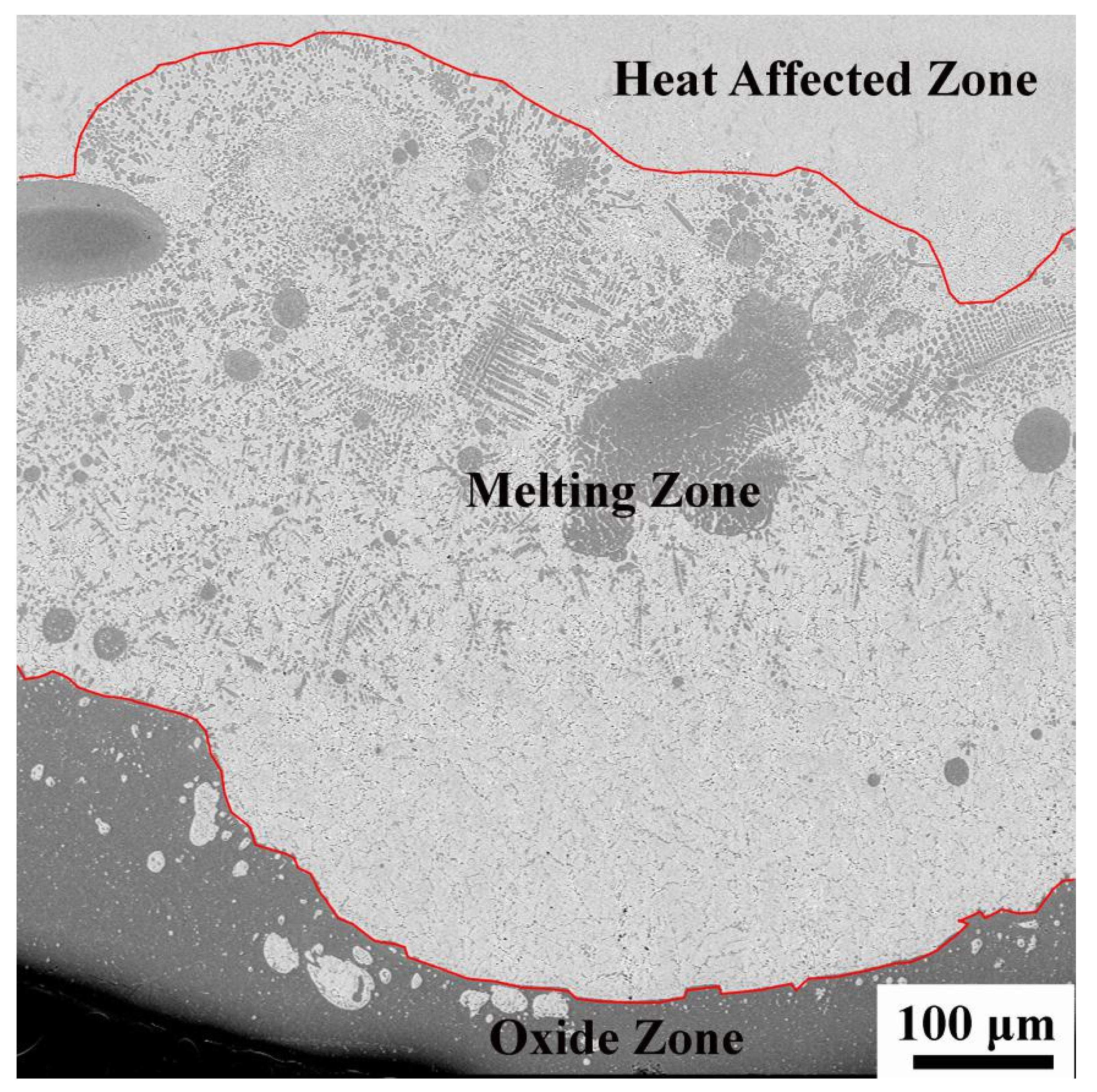
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, L.; Xie, G.; Zhang, C.; Liu, X.; Lu, W.; He, G.; Huang, J. Combustion of Metals in Oxygen-Enriched Atmospheres. Metals 2020, 10, 128. https://doi.org/10.3390/met10010128
Shao L, Xie G, Zhang C, Liu X, Lu W, He G, Huang J. Combustion of Metals in Oxygen-Enriched Atmospheres. Metals. 2020; 10(1):128. https://doi.org/10.3390/met10010128
Chicago/Turabian StyleShao, Lei, Guoliang Xie, Cheng Zhang, Xiao Liu, Wanran Lu, Guangyu He, and Jinfeng Huang. 2020. "Combustion of Metals in Oxygen-Enriched Atmospheres" Metals 10, no. 1: 128. https://doi.org/10.3390/met10010128
APA StyleShao, L., Xie, G., Zhang, C., Liu, X., Lu, W., He, G., & Huang, J. (2020). Combustion of Metals in Oxygen-Enriched Atmospheres. Metals, 10(1), 128. https://doi.org/10.3390/met10010128





