1. Introduction
Ore-based ironmaking via the blast furnace (BF) dominates the metal supply for steel making [
1]. For every ton of steel produced, on average 1.83 tons of CO
2 was emitted in 2017. According to the World Steel Association, the iron and steel industry accounts for approximately 7% to 9% of total world CO
2 emission [
2]. Coke and coal as main reducing agents in the BF are the main contributors to CO
2 emitted during iron and steel making. The European Union (EU) has set a target to cut 80% of the CO
2 of fossil carbon, by 2050 [
3]. In several studies the possible decrease in fossil CO
2 emissions by using biomass is reported [
4,
5,
6].
The use of raw biomass as a reducing agent in the BF is difficult due to high moisture content, low content of fixed carbon (C
fix) as well as a high content of volatile matter (VM) and oxygen [
7,
8]. Different pretreatment technologies like pyrolysis [
9], torrefaction [
10], etc. can convert biomass into products with properties suitable for metallurgical applications. Pretreated biomass (bio-coals) has higher content of C
fix, lower contents of VM and oxygen, higher calorific value and can be pulverized without the formation of high ratio of fibers, properties which overall correspond more to the ones of injection coal [
10,
11]. It was reported that 70% of mass yield is retained as a solid product (char) during torrefaction process while 30% of mass is converted to gases [
12]. The opposite is found for highly pretreated biomass, which is characterized by low char yield (~38% mass yield) as a big part of VM was removed during process [
13]. It can be an advantage to use torrefied product as reducing agent in composites containing iron oxide if remaining volatile contribute to the reduction. To select suitable bio-coals for use in composites with iron oxide an improved knowledge on the effect from bio-coal properties is required.
Swedish industry aims to reduce the CO
2 emission by different means and in the short term, this includes, e.g., improving the energy efficiency of the process, replacing fossil coal with reactive carbonaceous material like bio-coal (pretreated biomass) and in the longer term to use hydrogen. The use of biomass resources is a possible alternative in Sweden, as there are biomass resources available from forestland, areas estimated to be 28.1 million hectares [
14]. The use of bio-coal as part of top charged briquettes also containing iron oxide has the potential to lower the thermal reserve zone temperature (TRZ) of the BF and thereby give a high replacement ratio to coke due to improved gas efficiency [
15].
Partial replacement of coke by bio-coals and raw biomass in self-reducing mixtures or composites is reported in the literature [
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. The effect of the C/O molar ratio on the reduction behavior of iron ore has been studied earlier [
16,
24,
25]. Najmi et al. [
16] found that the reduction of iron oxide was enhanced when using pyrolized bio-coal in a composite tested isothermally at 1550 °C when compared to composites containing only coke. Liu et al. [
24] studied the reduction behavior of bio-coal containing iron oxide composite pellets. The reduction was done isothermally at 1000–1300 °C in samples with different C/O molar ratio, it was found that as the C/O ratio increased the reduction rate also increased. Zuo et al. [
25] compared the reduction behavior of iron oxide by using bio-coal, coal and coke. Self-reducing mixtures were reduced in a non-isothermal test procedure having a constant heating rate up to 1200 °C. It was found that bio-coal enhanced the reduction of hematite compared to coal and coke. Ubando et al. [
27] found enhanced reduction using torrefied biomass in comparison to graphite in samples containing hematite to carbonaceous material in ratios of 2:1 and 1:1 heated non- isothermally up to 1200 °C.
Some of the recent research concerns bio-coals produced from similar original raw biomass pretreated at different temperatures and thereby getting different VM and ash content but having similar ash composition [
19,
23]. In isothermal reduction of iron ore containing composite in inert atmosphere at temperatures in the range of 800–1000 °C Hirokazu et al. [
23] found that bio-coal having higher content of VM will enhance the reduction of iron ore more compared to bio-coal with lower VM content and the reduction rate of iron oxide was higher when bio-coal containing 18% VM was used in agglomerates compared to when coke was used. Ueda et al. [
19] compared the reduction of composites containing carbon in CO/CO
2 and in Ar atmosphere up to 1200 °C but the impact from VM was not evaluated. It was stated that bio-coals, in general, enhance the reduction in comparison to coke.
Except for the work by Hirokazu et al. [
23], there is no study on the influences of properties (C
fix, VM and ash composition) for different types of pre-treated bio-coal on the reduction of iron ore. In the current study, different pre-treated biomasses with different properties, e.g., in terms of VM and ash content as well as ash composition was used as a reducing agent in iron ore containing composite prepared as briquettes, with the aim to understand their impact on the reduction of iron ore. VM present in bio-coals contained in the composites should preferably contribute to the reduction and not being released and lost with the BF top gas. Composites containing only coke breeze are used as reference.
4. Discussion
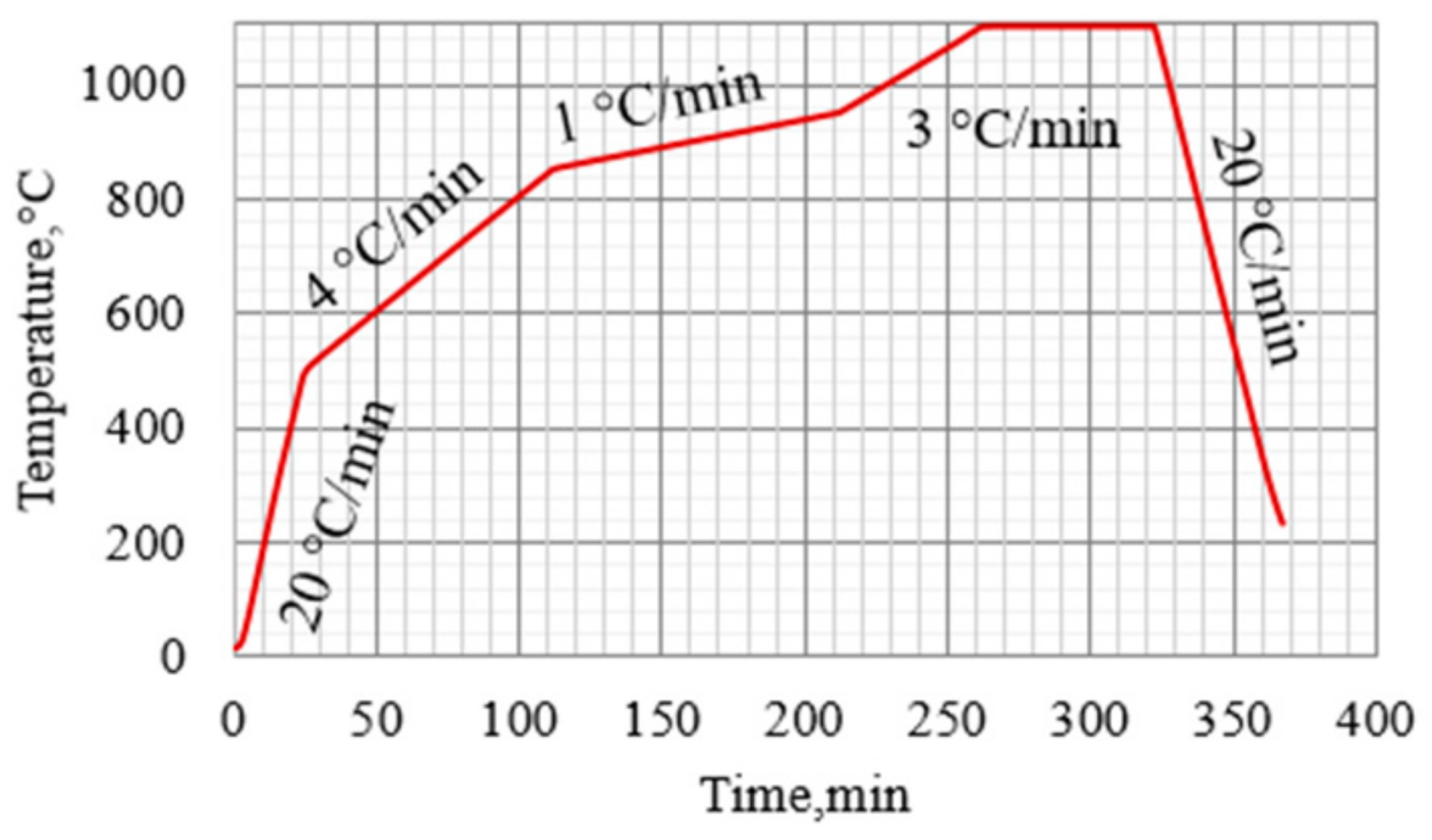
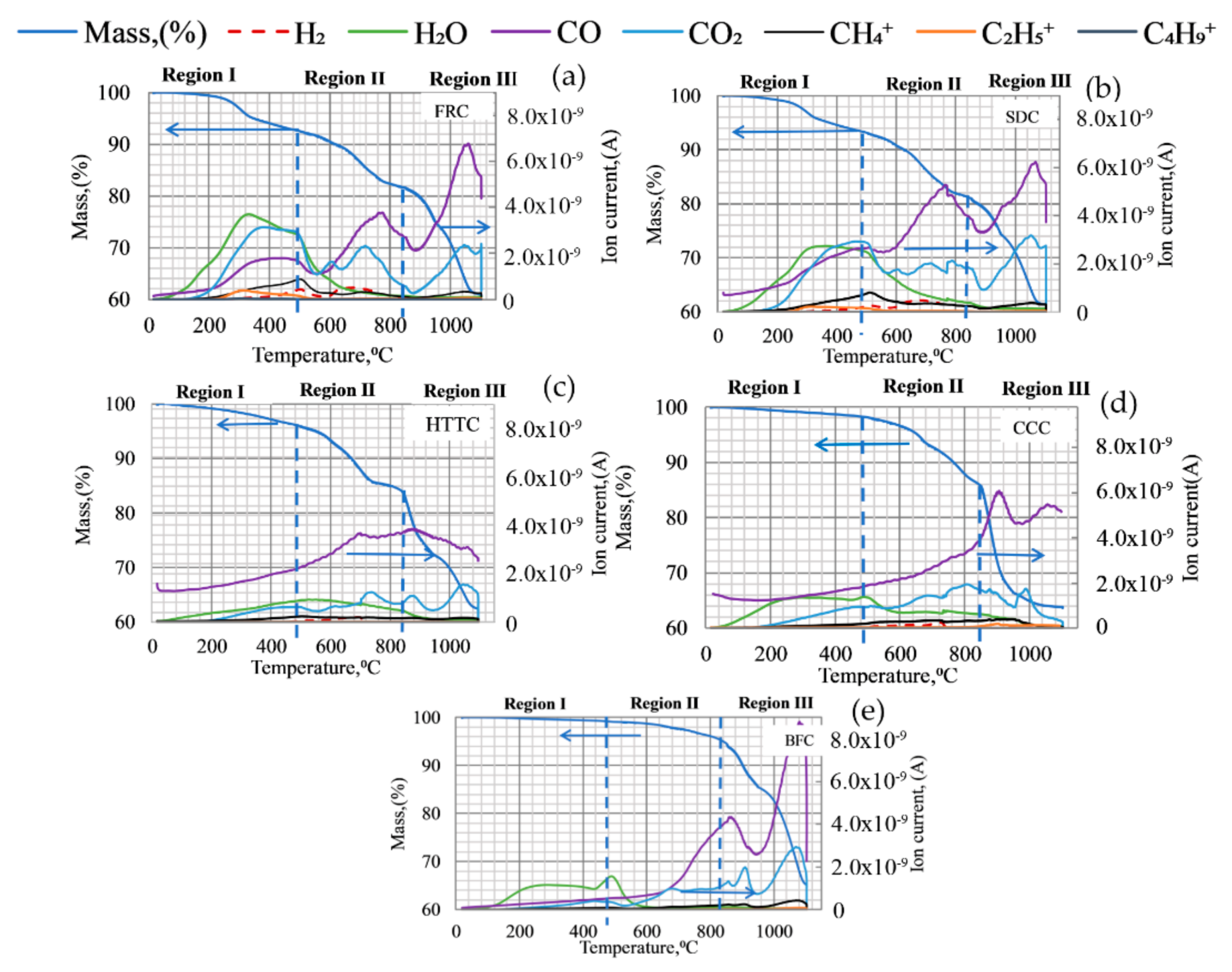
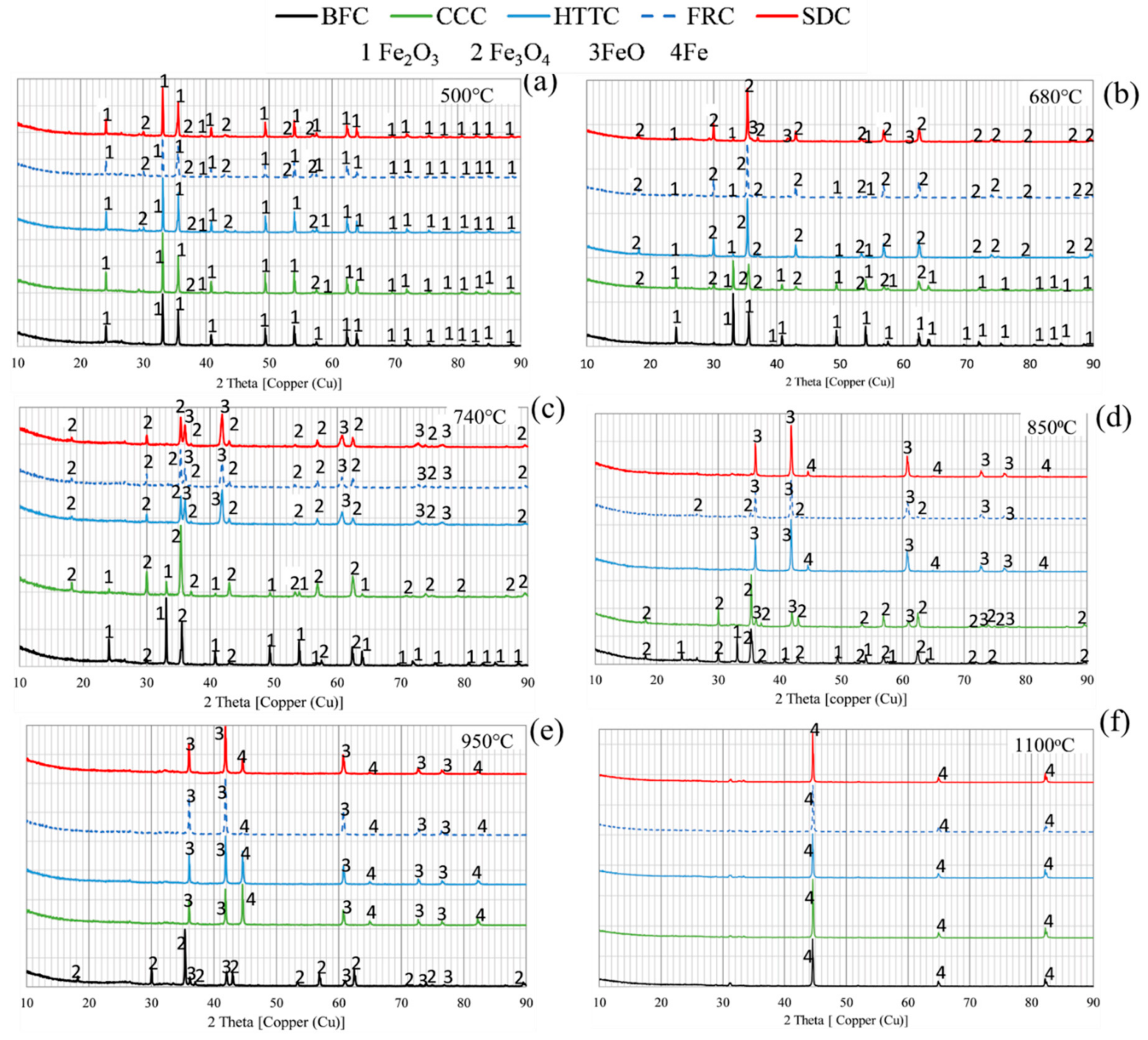
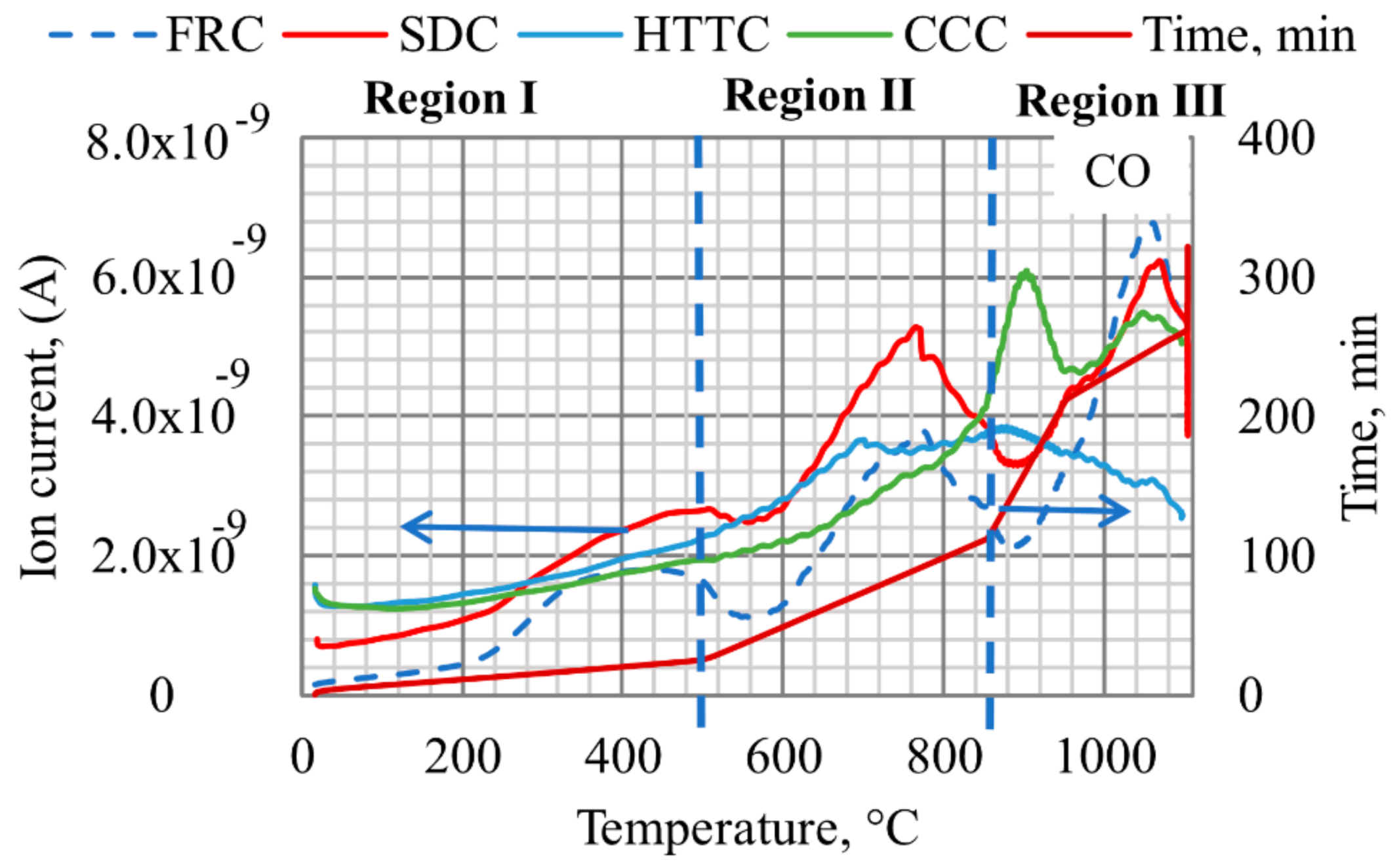
The effect of different pre-treated biomass on the self-reduction of agglomerates containing iron oxide and coke breeze has been investigated. Composites were analyzed in TGA and treated in vertical tube furnace up to pre-defined temperatures. To evaluate the impact of bio-coal on reduction, samples from interrupted tests were investigated by XRD, LOM and SEM. The mass loss occurring in TGA is assumed to depend on partial devolatilization of VM (, gasification , and reduction of iron oxides (. CO formed during devolatilization contributed to the reduction of iron oxides.
The TGA results and interrupted test up to 500 °C showed that FRC has higher mass loss than all other composites, and a higher mass-loss rate was clear from DTG analysis at ~300 °C. Although TSD and TFR have quite similar content of VM, the higher mass loss detected at low temperature for FRC could be due to the presence of higher contents of catalyzing components such as CaO and K
2O in TFR than in TSD. This may affect the release of H
2O during reaction as the ion current for H
2O is highest for FRC although that the H-content is similar as for SDC. The impact of ash composition on the devolatilization behavior of bio-coals was indicated in a previous study [
28] where bio-coal with a higher content of catalyzing components released the VM at lower temperature, and this may restrict its contribution to the reduction. High intensity of H
2O followed by CO
2 and H
2 detected in QMS analyses for FRC indicates that some of the CO is consumed in the reaction. It is possible that this affects the contribution to reduction of iron oxide in FRC. From XRD analysis, it was seen that FeO had been formed in samples interrupted at 740 °C for FRC while it was detected in samples interrupted at 680 °C for SDC.
QMS analysis showed that the intensity of CO gas in regions I and II is highest for SDC, reaching a maximum at ~760 °C, as shown in
Figure 8. Part of this CO gas might originate from thermal decomposition of VM as well as from the Boudouard reaction in which CO
2 reacts with remaining carbon above 700 °C as reported by Butterman [
33]. Furthermore, it is observed that FeO is formed in SDC at lower temperatures than for other composites and SDC has the highest mass loss rate in region II (up to 850 °C).
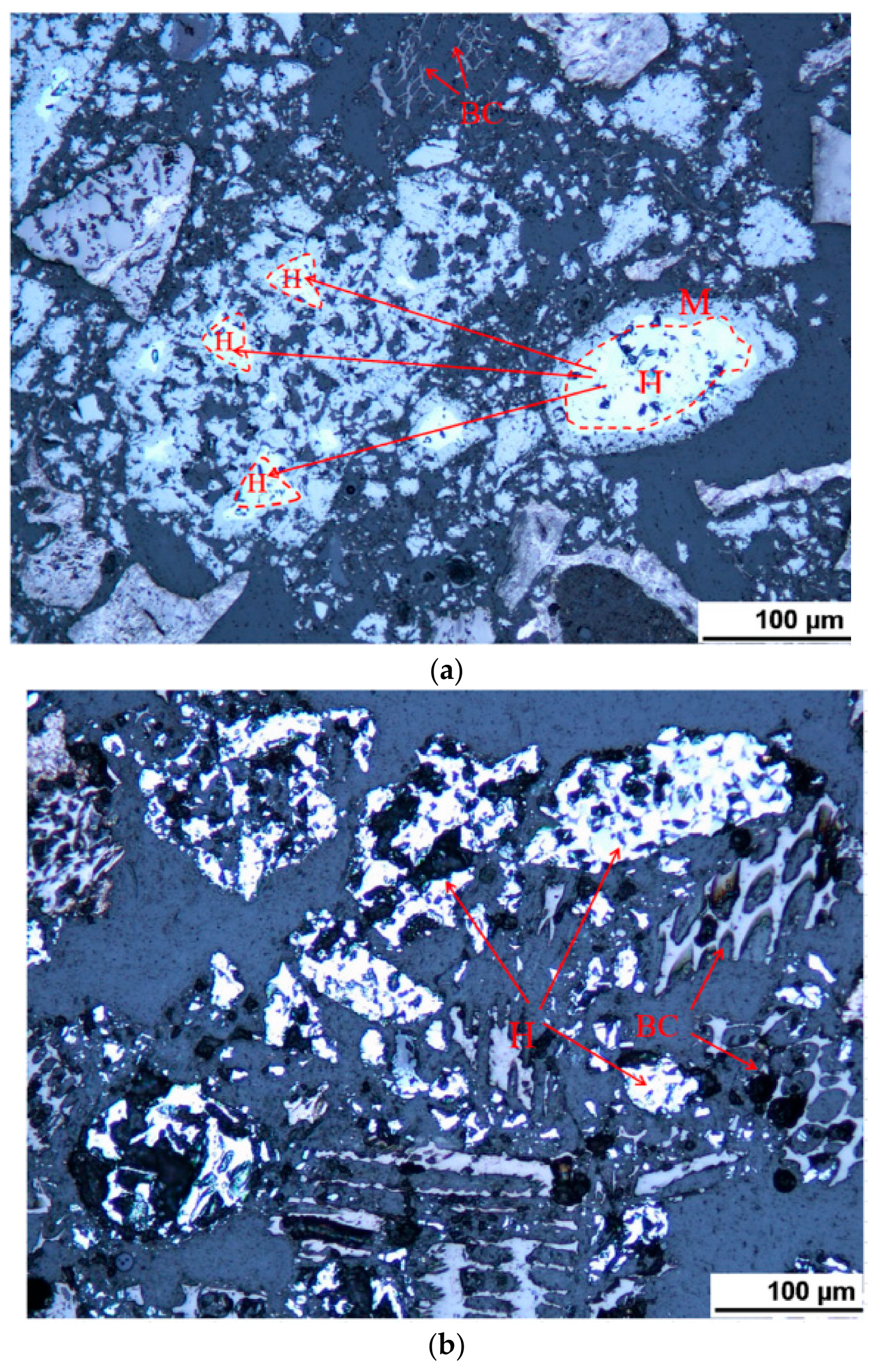
The progress of the reduction is indicated in LOM by more cracks in SDC samples interrupted at 740 °C while it was detected only in the outer layer for HTTC at the same temperature. Wustite was not detected in CCC samples interrupted below 850 °C. CO generation from CCC samples occurred mainly above 850 °C, likely due to C
fix starting to react, c.f.
Figure 8, simultaneously with a higher mass loss rate for CCC. This can be compared with BFC in which wustite and iron were first detected by XRD in samples interrupted at 950 °C and in the final sample at 1100 °C, respectively. The higher intensity for CO seen in QMS results for BFC at temperatures above 950 °C is likely due to the transformation step from FeO to Fe
met and solution loss reactions taking place above 950 °C.
XRD analysis shows also that magnetite is formed in all BCC samples at 500 °C, but not in BFC composites. CO formed in Region I in all BCC, but not in BFC, may have contributed to the earlier start of reduction in BCC. It is shown that FeO is formed at a lower temperature in the composites containing more VM, indicating either that more reactive char is formed or that volatiles still existing above 500 °C, as in SDC, also contributes to the reduction. It is also shown that BFC containing coke breeze demand higher temperature for gasification reaction and reduction compared to BCC, in agreement with Ueda et al. [
19].
The results show that if bio-coal with high volatile content is selected for use in a composite, a low content of catalyzing components is preferable as volatiles are released at higher temperatures and thereby could contribute to the reduction.
The investigated composites are potential innovative materials to be added to the BF together with coke. By energy consuming reactions, the thermal reserve zone temperature is reduced and the overall reduction efficiency of the BF improved. When selecting carbon source for the composites, the results show that coke breeze demands higher temperature for reaction compared to bio-coals. TSD could be the most suitable material to be utilized in composites in the BF. This material has higher content of volatile matter content with a lower content of catalyzing components, which means that the volatiles can be released at higher temperatures and thereby can contribute to the reduction. Additionally, outside the scope of this paper, it is known that the yield when producing bio-coal is high for products as TSD.