Leaching Chalcopyrite with High MnO2 and Chloride Concentrations
Abstract
1. Introduction
2. Methodology
2.1. Chalcopyrite Sample
2.2. MnO2 (Manganese Nodules)
2.2.1. Reagent and Leaching Test
2.2.2. Effect of Particle Size
2.2.3. Effect of Temperature
3. Results
3.1. The Effect of Particle Size on CuFeS2 Dissolution
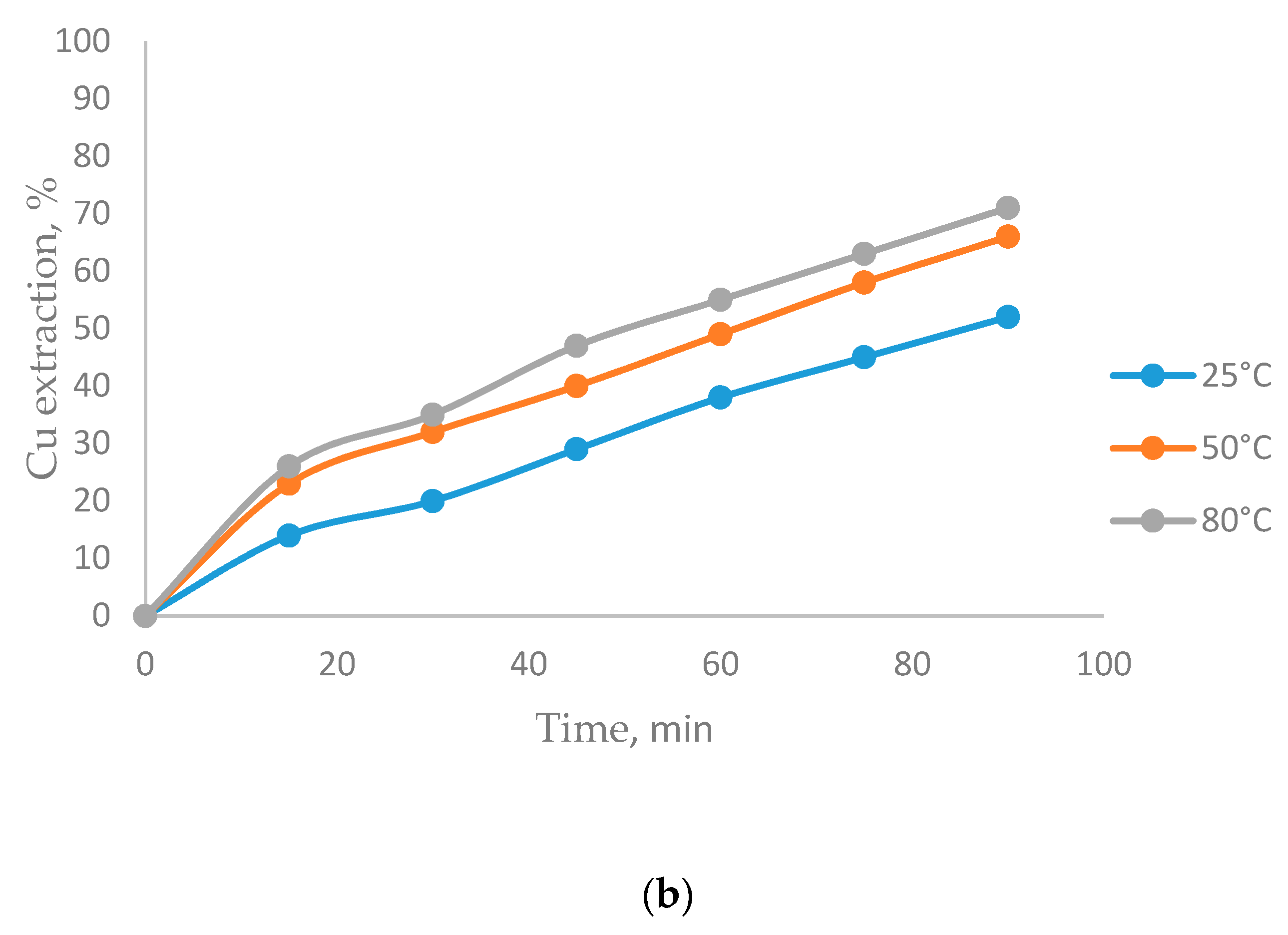
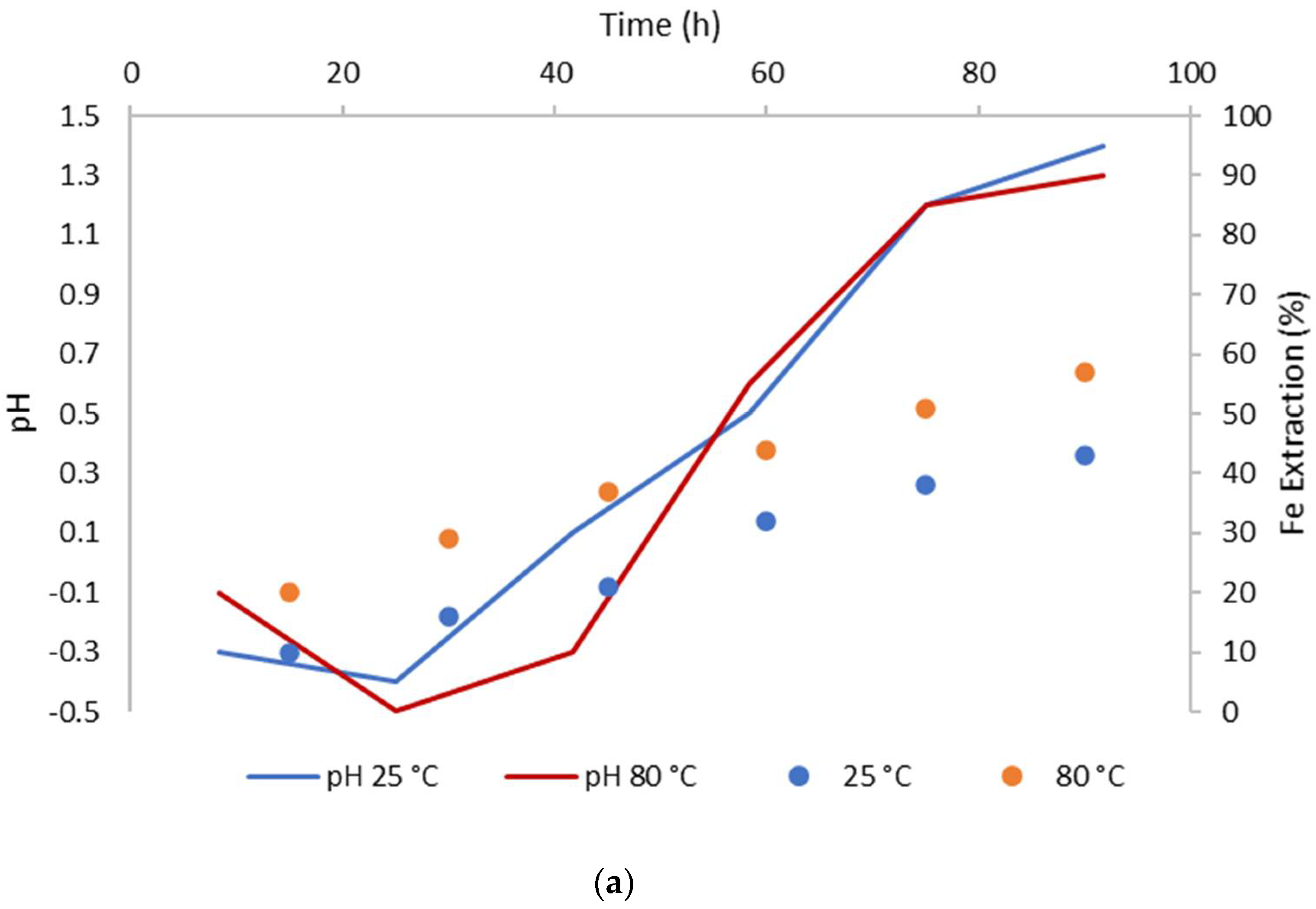
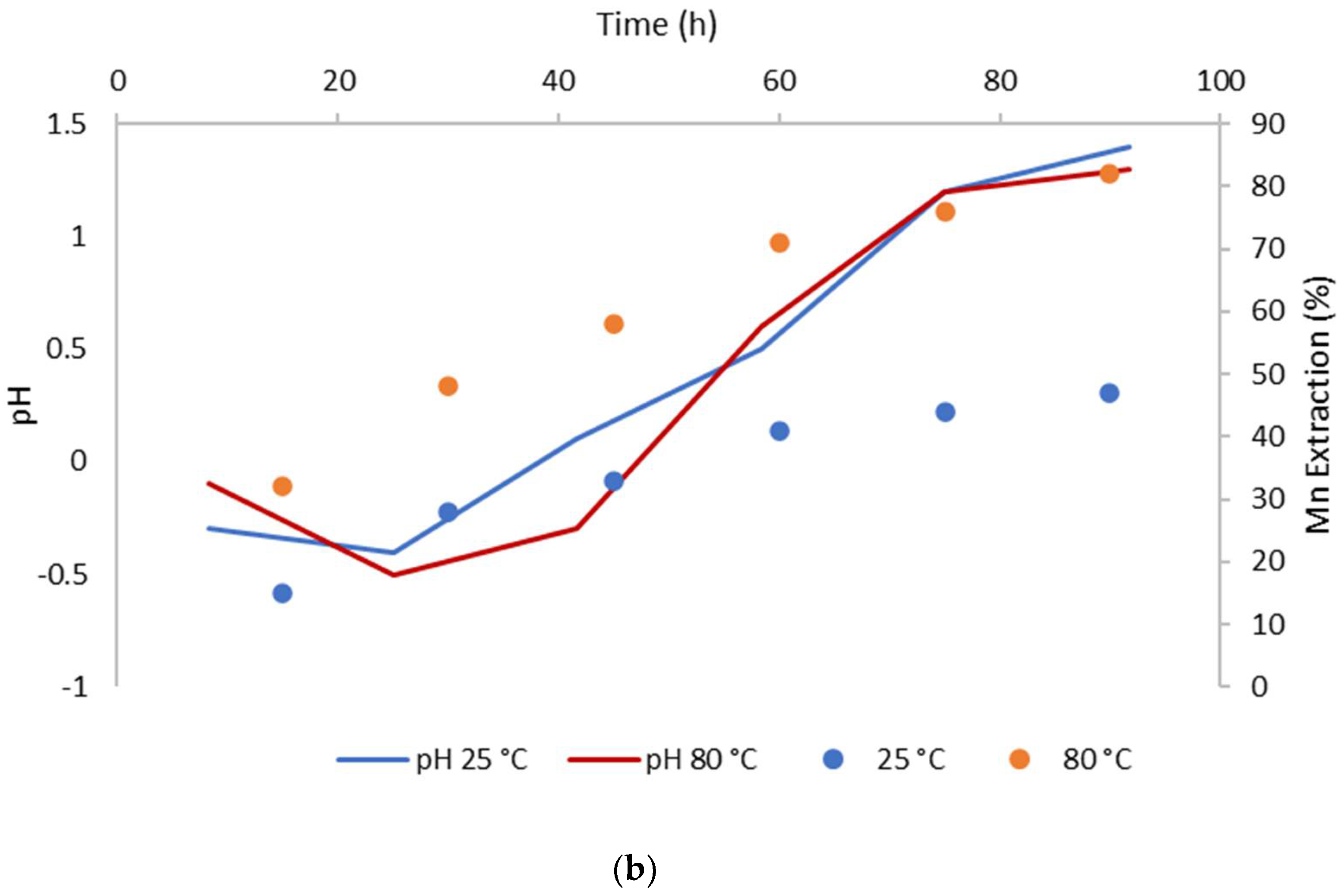
3.2. The Effect of Temperature on CuFeS2 Dissolution
4. Conclusions
- There were no differences in copper dissolution rates at particle sizes between −75 + 53 and −47 + 38 µm. at different H2SO4 concentrations.
- Small particle size (−20 µm) increases CuFeS2 dissolution kinetics, due to the mechanical-chemical activation of the mineral.
- Temperatures of 80 °C positively affect CuFeS2 dissolution, while the MnO2 concentration did not have a significant effect in the system.
- The biggest copper extractions in this research (71%) was obtained working at 80 °C, a particle size of −47 + 38 µm, a 5/1 MnO2/CuFeS2 ratio, and 1 mol/L of H2SO4.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aguirre, C.L.; Toro, N.; Carvajal, N.; Watling, H.; Aguirre, C. Leaching of chalcopyrite (CuFeS2) with an imidazolium-based ionic liquid in the presence of chloride. Miner. Eng. 2016, 99, 60–66. [Google Scholar] [CrossRef]
- Cerda, C.P.; Taboada, M.E.; Jamett, N.E.; Ghorbani, Y.; Hernández, P.C. Effect of pretreatment on leaching primary copper sulfide in acid-chloride media. Minerals 2018, 8, 1. [Google Scholar] [CrossRef]
- Lu, D.; Wang, W.; Chang, Y.; Xie, F.; Jiang, K. Thermodynamic analysis of possible chalcopyrite dissolution mechanism in sulfuric acidic aqueous solution. Metals 2016, 6, 303. [Google Scholar] [CrossRef]
- Beiza, L.; Quezada, V.; Melo, E.; Valenzuela, G. Electrochemical behaviour of chalcopyrite in chloride solutions. Metals 2019, 9, 67. [Google Scholar] [CrossRef]
- Sokić, M.; Marković, B.; Kamberović, Ž.; Stanković, S. Leaching of chalcopyrite concentrate by hydrogen peroxide in sulphuric acid solution. Tehnika 2019, 74, 66–70. [Google Scholar] [CrossRef]
- Choubey, P.K.; Lee, J.C.; Kim, M.S.; Kim, H.S. Conversion of chalcopyrite to copper oxide in hypochlorite solution for selective leaching of copper in dilute sulfuric acid solution. Hydrometallurgy 2018, 178, 224–230. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Coll. Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef]
- International Copper Study Group. The World Copper Factbook 2017; International Copper Study Group: Lisbon, Portugal, 2017. [Google Scholar]
- Hiroyoshi, N.; Arai, M.; Miki, H.; Tsunekawa, M.; Hirajima, T. A new reaction model for the catalytic effect of silver ions on chalcopyrite leaching in sulfuric acid solutions. Hydrometallurgy 2002, 63, 257–267. [Google Scholar] [CrossRef]
- Velásquez-yévenes, L.; Torres, D.; Toro, N. Hydrometallurgy Leaching of chalcopyrite ore agglomerated with high chloride concentration and high curing periods. Hydrometallurgy 2018, 181, 215–220. [Google Scholar] [CrossRef]
- Baba, A.A.; Ayinla, K.I.; Adekola, F.A.; Ghosh, M.K.; Ayanda, O.S. A Review on Novel Techniques for Chalcopyrite Ore Processing. Int. J. Min. Eng. Miner. Process. 2012, 1, 1–16. [Google Scholar] [CrossRef]
- Padilla, R.; Pavez, P.; Ruiz, M.C. Kinetics of copper dissolution from sulfidized chalcopyrite at high pressures in H2SO4-O2. Hydrometallurgy 2008, 91, 113–120. [Google Scholar] [CrossRef]
- Dutrizac, J.E. Elemental sulphur formation during the ferric chloride leaching of chalcopyrite. Hydrometallurgy 1990, 23, 153–176. [Google Scholar] [CrossRef]
- Hackl, R.P.; Dreisinger, D.B.; Peters, E.; King, J.A. Passivation of chalcopyrite during oxidative leaching in sulfate media. Hydrometallurgy 1995, 39, 25–48. [Google Scholar] [CrossRef]
- Torres, C.M.; Ghorbani, Y.; Hernández, P.C.; Justel, F.J.; Aravena, M.I.; Herreros, O.O. Cupric and Chloride Ions: Leaching of Chalcopyrite Concentrate with Low Chloride Concentration Media. Minerals 2019, 9, 639. [Google Scholar] [CrossRef]
- Carneiro, M.F.C.; Leão, V.A. The role of sodium chloride on surface properties of chalcopyrite leached with ferric sulphate. Hydrometallurgy 2007, 87, 73–82. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Montes, K.S.; Padilla, R. Chalcopyrite leaching in sulfate-chloride media at ambient pressure. Hydrometallurgy 2011, 109, 37–42. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D. Copper leaching from chalcopyrite concentrate in Cu (II)/Fe (III) chloride system. Miner. Eng. 2013, 45, 185–190. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A.; Connor, G.M.O. The kinetics of chalcopyrite leaching in alkaline glycine/glycinate solutions. Miner. Eng. 2019, 135, 118–128. [Google Scholar] [CrossRef]
- Toro, N.; Herrera, N.; Castillo, J.; Torres, C.; Sepúlveda, R. Initial Investigation into the Leaching of Manganese from Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Foundry Slag—Part I. Minerals 2018, 8, 565. [Google Scholar] [CrossRef]
- Toro, N.; Saldaña, M.; Gálvez, E.; Cánovas, M.; Trigueros, E.; Castillo, J.; Hernández, P.C. Optimization of Parameters for the Dissolution of Mn from Manganese Nodules with the Use of Tailings in An Acid Medium. Minerals 2019, 9, 387. [Google Scholar] [CrossRef]
- Saldaña, M.; Toro, N.; Castillo, J.; Hernández, P.; Trigueros, E.; Navarra, A. Development of an Analytical Model for the Extraction of Manganese from Marine Nodules. Metals 2019, 9, 903. [Google Scholar] [CrossRef]
- Cronan, D.S. Handbook of Marine Mineral Deposits; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Usui, A.; Nishi, K.; Sato, H.; Nakasato, Y.; Thornton, B.; Kashiwabara, T. Continuous growth of hydrogenetic ferromanganese crusts since 17 Myr ago on Takuyo-Daigo Seamount, NW Paci fi c, at water depths of 800–5500 m. Ore Geol. Rev. 2017, 87, 71–87. [Google Scholar] [CrossRef]
- Toro, N.; Saldaña, M.; Castillo, J.; Higuera, F.; Acosta, R. Leaching of Manganese from Marine Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Tailings. Minerals 2019, 9, 289. [Google Scholar] [CrossRef]
- Devi, N.B.; Madhuchhanda, M.; Rao, K.S.; Rath, P.C.; Paramguru, R.K. Oxidation of chalcopyrite in the presence of manganese dioxide in hydrochloric acid medium. Hydrometallurgy 2000, 57, 57–76. [Google Scholar] [CrossRef]
- Devi, N.B.; Madhuchhanda, M.; Rath, P.C.; Rao, K.S.; Paramguru, R.K. Simultaneous Leaching of a Deep-Sea Manganese Nodule and Chalcopyrite in Hydrochloric Acid. Metall. Mater. Trans. B 2001, 32, 777–784. [Google Scholar] [CrossRef]
- Havlik, T.; Laubertova, M.; Miskufova, A.; Kondas, J.; Vranka, F. Extraction of copper, zinc, nickel and cobalt in acid oxidative leaching of chalcopyrite at the presence of deep-sea manganese nodules as oxidant. Hydrometallurgy 2005, 77, 51–59. [Google Scholar] [CrossRef]
- Toro, N.; Pérez, K.; Saldaña, M.; Jeldres, R.; Jeldres, M.; Cánovas, M. Dissolution of pure chalcopyrite with manganese nodules and waste water. J. Mater. Res. Technol. 2019, 9. [Google Scholar] [CrossRef]
- Lundstrom, M.; Aromaa, J.; Forsén, O.; Hyvarinen, O.; Barker, M. Leaching of chalcopyrite in cupric chloride solution. Hydrometallurgy 2005, 77, 89–95. [Google Scholar] [CrossRef]
- Bobadilla-Fazzini, R.A.; Pérez, A.; Gautier, V.; Jordan, H.; Parada, P. Primary copper sulfides bioleaching vs. chloride leaching: Advantages and drawbacks. Hydrometallurgy 2017, 168, 26–31. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Miki, H.; Nicol, M. The dissolution of chalcopyrite in chloride solutions. Part 2: Effect of various parameters on the rate. Hydrometallurgy 2010, 103, 80–85. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Nicol, M.; Miki, H. The dissolution of chalcopyrite in chloride solutions: Part 1. The effect of solution potential. Hydrometallurgy 2010, 103, 108–113. [Google Scholar] [CrossRef]
- Nicol, M.; Miki, H.; Velásquez-yévenes, L. Hydrometallurgy The dissolution of chalcopyrite in chloride solutions Part 3. Mechanisms. Hydrometallurgy 2010, 103, 86–95. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The Dissolution of Chalcopyrite in Ferric Sulfate and Ferric Chloride Media. Metall. Trans. B 1981, 12, 371–378. [Google Scholar] [CrossRef]
- Skrobian, M.; Havlik, T.; Ukasik, M. Effect of NaCl concentration and particle size on chalcopyrite leaching in cupric chloride solution. Hydrometallurgy 2005, 77, 109–114. [Google Scholar] [CrossRef]
- Yévenes, L.V. The Kinetics of the Dissolution of Chalcopyrite in Chloride Media; Murdoch University: Perth, Australia, 2009. [Google Scholar]
- Hernández, P.C.; Taboada, M.E.; Herreros, O.O.; Graber, T.A.; Ghorbani, Y. Leaching of chalcopyrite in acidified nitrate using seawater-based media. Minerals 2018, 8, 238. [Google Scholar] [CrossRef]
- Tundisi, J.G. Water resources in the future: Problems and solutions. Estudos Avançados 2008, 22, 7–16. [Google Scholar] [CrossRef]
- Toro, N.; Briceño, W.; Pérez, K.; Cánovas, M.; Trigueros, E.; Sepúlveda, R.; Hernández, P. Leaching of Pure Chalcocite in a Chloride Media Using Sea Water and Waste Water. Metals (Basel) 2019, 9, 780. [Google Scholar] [CrossRef]
- Peters, N.E.; Meybeck, M. Water quality degradation effects on freshwater availability: Impacts of human activities. Water Int. 2000, 25, 185–193. [Google Scholar] [CrossRef]
- Ridoutt, B.G.; Pfister, S. A revised approach to water footprinting to make transparent the impacts of consumption and production on global freshwater scarcity. Glob. Environ. Chang. 2010, 20, 113–120. [Google Scholar] [CrossRef]
- Cruz, C.; Reyes, A.; Jeldres, R.I.; Cisternas, L.A.; Kraslawski, A. Using Partial Desalination Treatment To Improve the Recovery of Copper and Molybdenum Minerals in the Chilean Mining Industry. Ind. Eng. Chem. Res. 2019, 58, 8915–8922. [Google Scholar] [CrossRef]
- MCH. Agua en la Minería. 2018. Available online: https://www.mch.cl/columnas/agua-la-mineria/# (accessed on 3 June 2019).
- Cisternas, L.A.; Gálvez, E.D. The use of seawater in mining. Miner. Process. Extr. Metall. Rev. 2018, 39, 18–33. [Google Scholar] [CrossRef]
- Juhász, A.; Opoczky, L. Mechanical Activation of Minerals by Grinding Pulverizing and Morphology of Particles; Akademia Kiado: Budapest, Hungary, 1990. [Google Scholar]
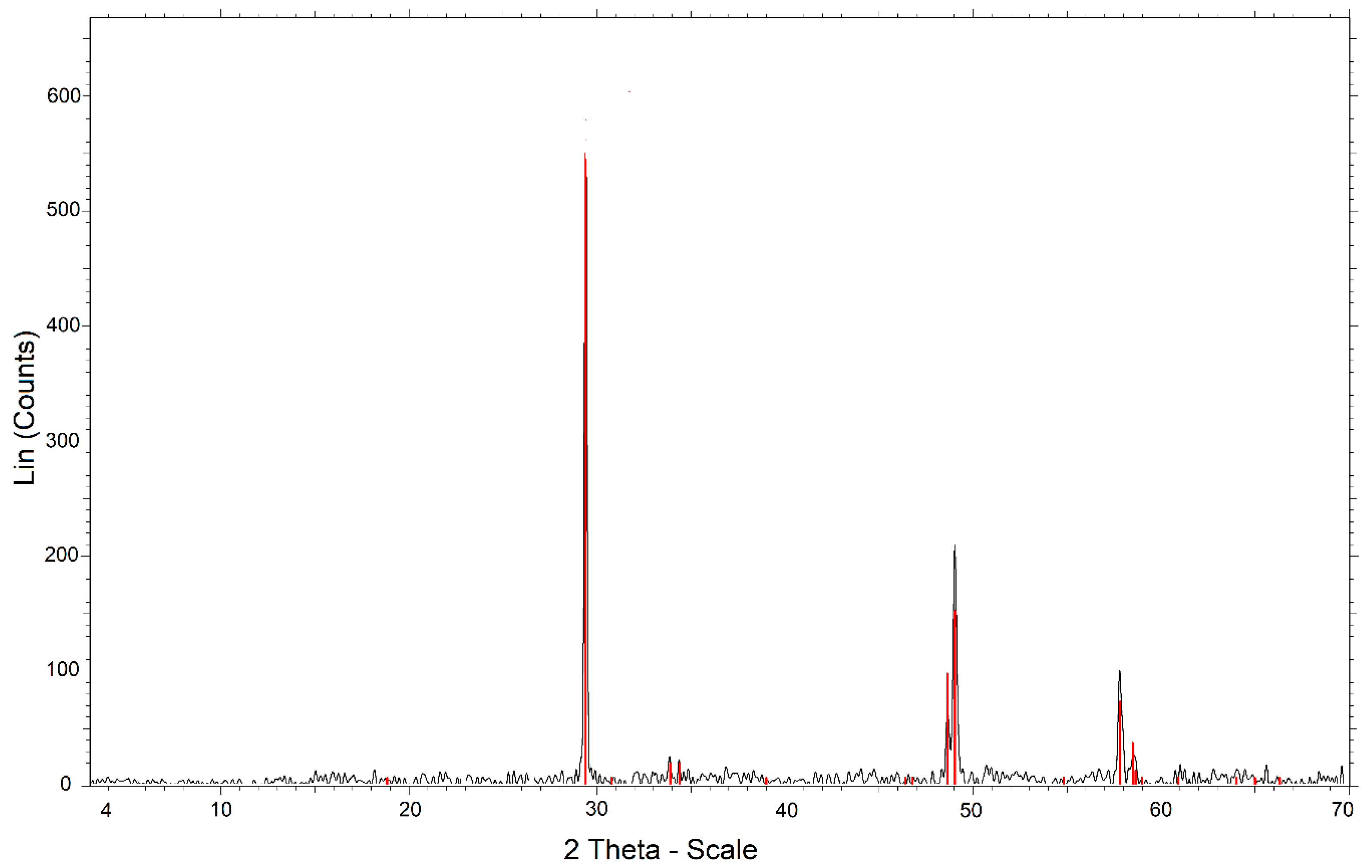
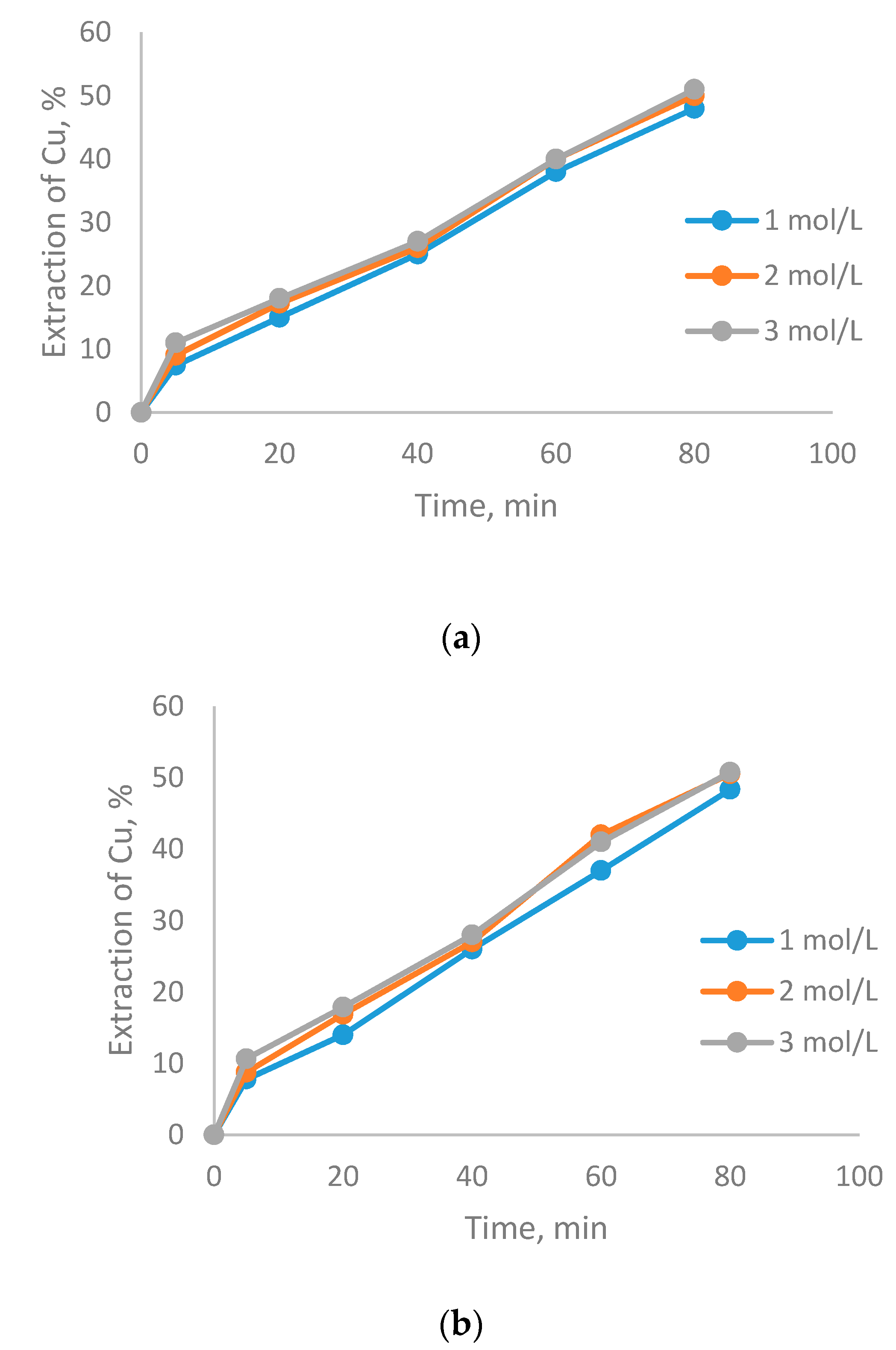
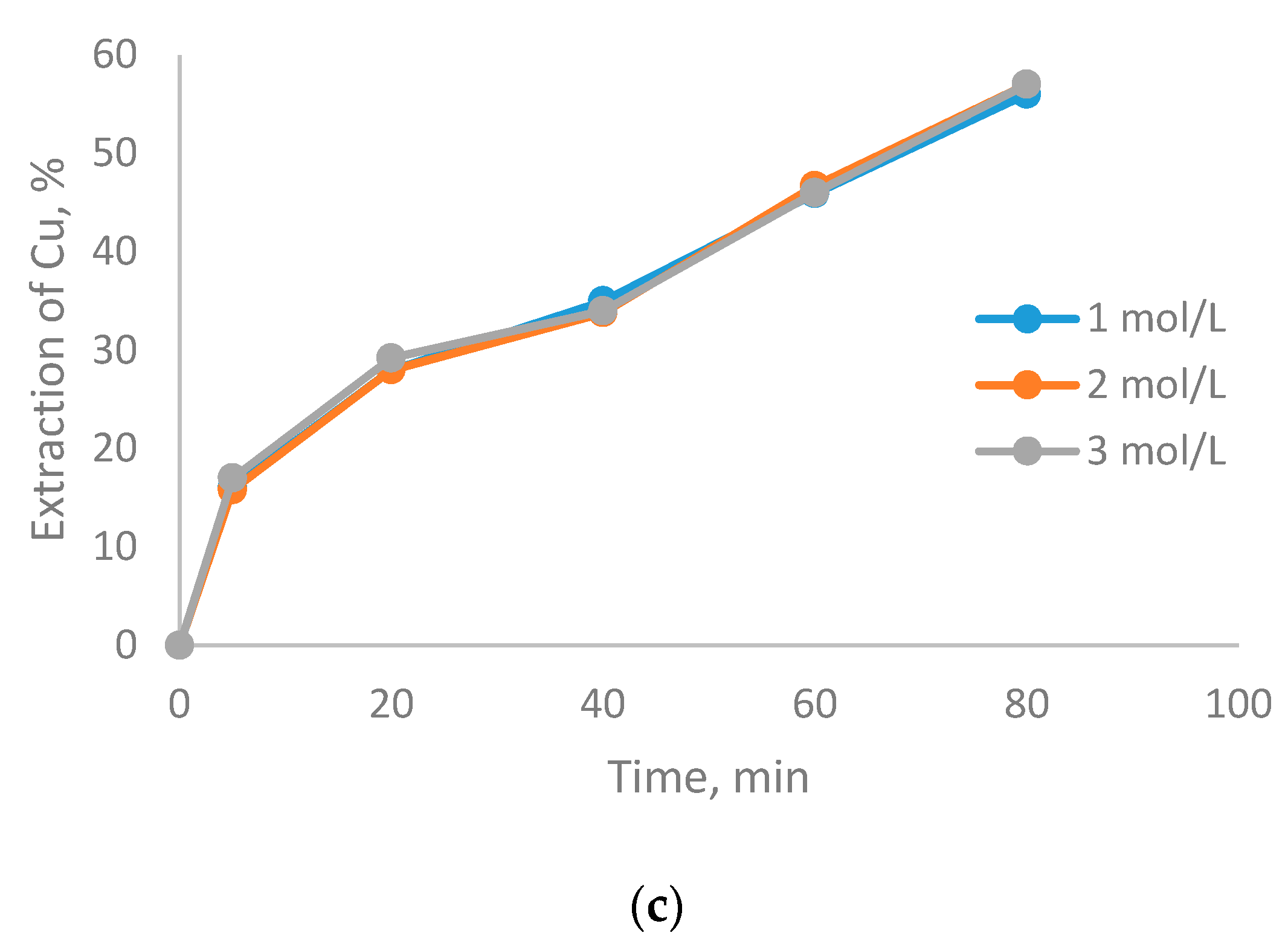
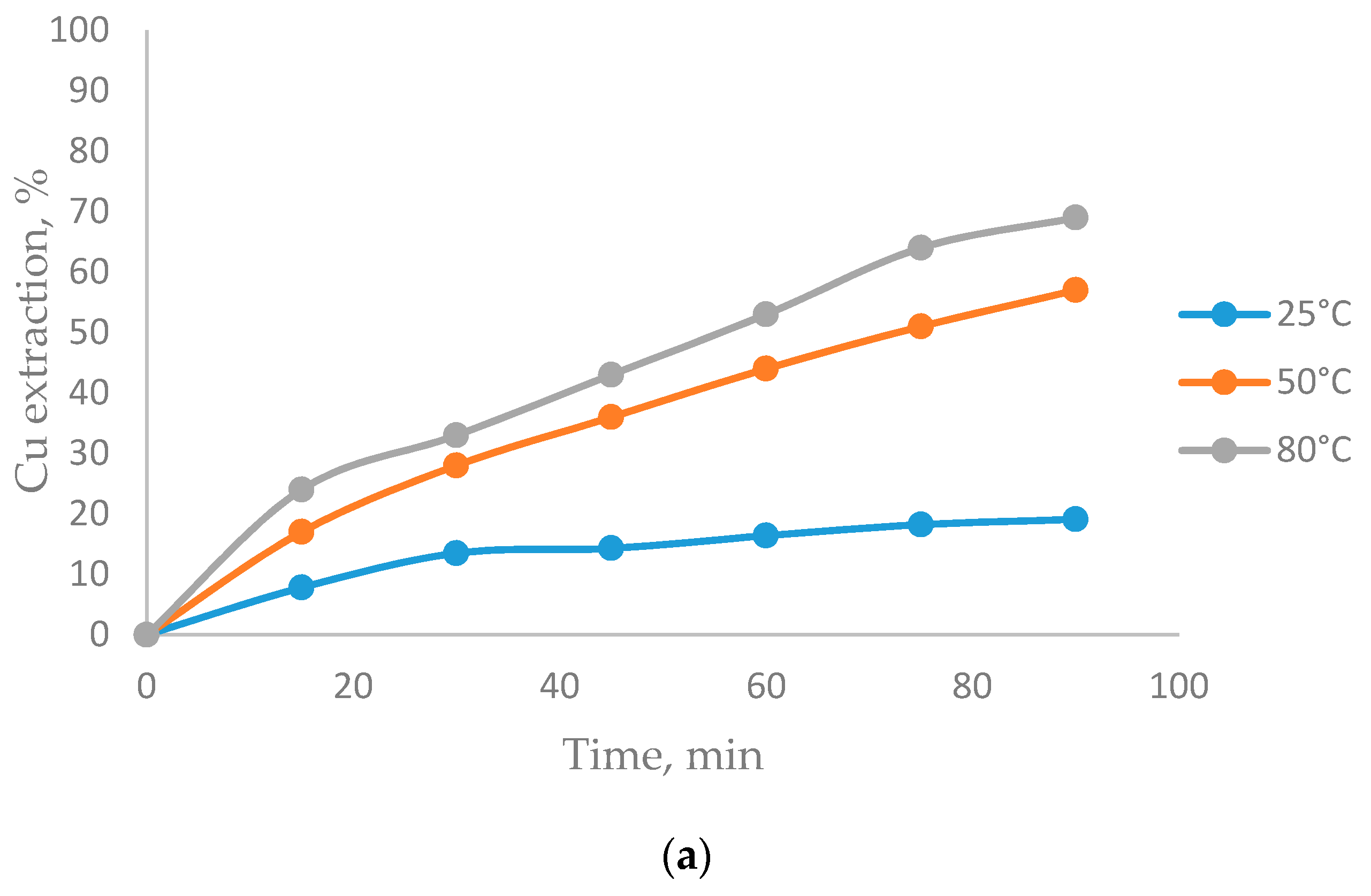
| Component | Cu | Fe | S |
|---|---|---|---|
| Mass (%) | 33.89 | 30.62 | 35.49 |
| Component | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | TiO2 | MnO2 | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Mass (%) | 3.54 | 3.69 | 2.97 | 7.20 | 1.17 | 0.33 | 22.48 | 1.07 | 29.85 | 26.02 |
| Compound | Concentration (g/L) |
|---|---|
| Fluoride (F−) | 0.002 |
| Calcium (Ca2+) | 0.8 |
| Magnesium (Mg2+) | 2.65 |
| Bicarbonate (HCO3−) | 1.1 |
| Chloride (Cl−) | 39.16 |
| Calcium carbonate (CaCO3) | 13 |
| Solute | g/kg of solution |
|---|---|
| Na+ | 10.781 |
| Mg2+ | 1.283 |
| Ca2+ | 0.412 |
| K+ | 0.399 |
| Cl− | 19.353 |
| SO42- | 2.712 |
| HCO3- | 0.105 |
| Br− | 0.067 |
| CO32− | 0.014 |
| Total | 35.146 |
| Parameters | Values |
|---|---|
| Particle size (µm) | −75 + 53, −47 + 38, −20 |
| Time (min) | 5, 20, 40, 60, 80 |
| H2SO4 (mol/L) | 1, 2, 3 |
| MnO2/CuFeS2 ratio | 5/1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, D.; Ayala, L.; Jeldres, R.I.; Cerecedo-Sáenz, E.; Salinas-Rodríguez, E.; Robles, P.; Toro, N. Leaching Chalcopyrite with High MnO2 and Chloride Concentrations. Metals 2020, 10, 107. https://doi.org/10.3390/met10010107
Torres D, Ayala L, Jeldres RI, Cerecedo-Sáenz E, Salinas-Rodríguez E, Robles P, Toro N. Leaching Chalcopyrite with High MnO2 and Chloride Concentrations. Metals. 2020; 10(1):107. https://doi.org/10.3390/met10010107
Chicago/Turabian StyleTorres, David, Luís Ayala, Ricardo I. Jeldres, Eduardo Cerecedo-Sáenz, Eleazar Salinas-Rodríguez, Pedro Robles, and Norman Toro. 2020. "Leaching Chalcopyrite with High MnO2 and Chloride Concentrations" Metals 10, no. 1: 107. https://doi.org/10.3390/met10010107
APA StyleTorres, D., Ayala, L., Jeldres, R. I., Cerecedo-Sáenz, E., Salinas-Rodríguez, E., Robles, P., & Toro, N. (2020). Leaching Chalcopyrite with High MnO2 and Chloride Concentrations. Metals, 10(1), 107. https://doi.org/10.3390/met10010107








