The authors wish to make the following corrections to this paper [1]:
In the results presented in Section 3.2. The Effect of Temperature on CuFeS2 Dissolution; Figure 4a,b are repeated, simply presenting the results for the extraction of Fe, and not that of Mn. Because of this, readers cannot observe the behavior of pH with respect to Mn extractions in the manuscript.
The correct Figure 4 is:
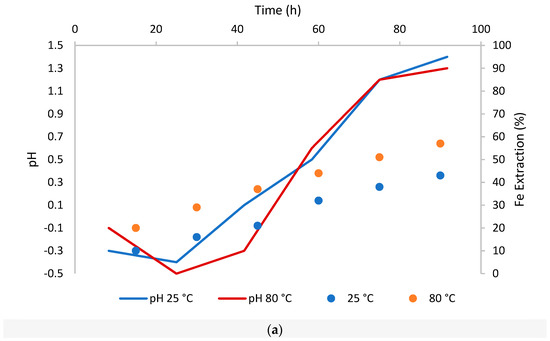
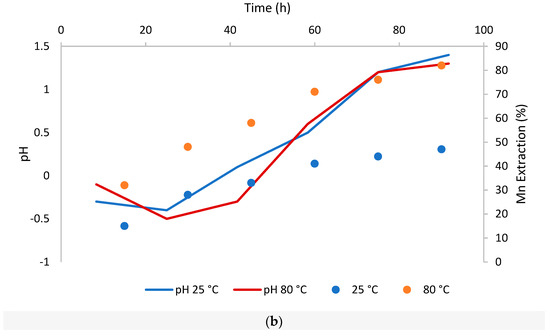
Figure 4.
Dissolution of Fe and Mn over time from CuFeS2 at room temperature (25 °C) and high temperature (80 °C) (a): dissolution of Fe and its behavior at changes in pH; (b): dissolution of Mn and its behavior at changes in pH).
Figure 4.
Dissolution of Fe and Mn over time from CuFeS2 at room temperature (25 °C) and high temperature (80 °C) (a): dissolution of Fe and its behavior at changes in pH; (b): dissolution of Mn and its behavior at changes in pH).
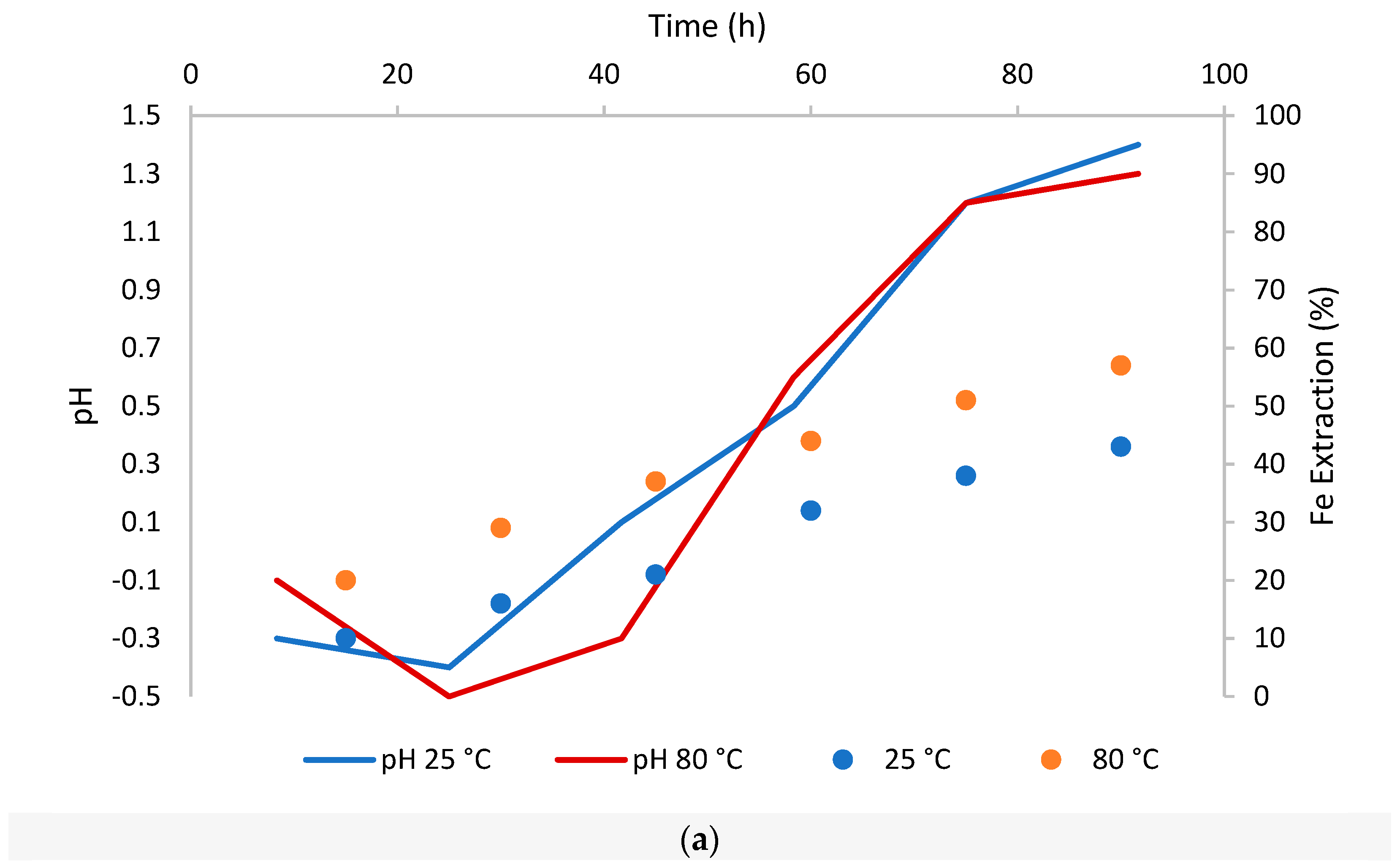
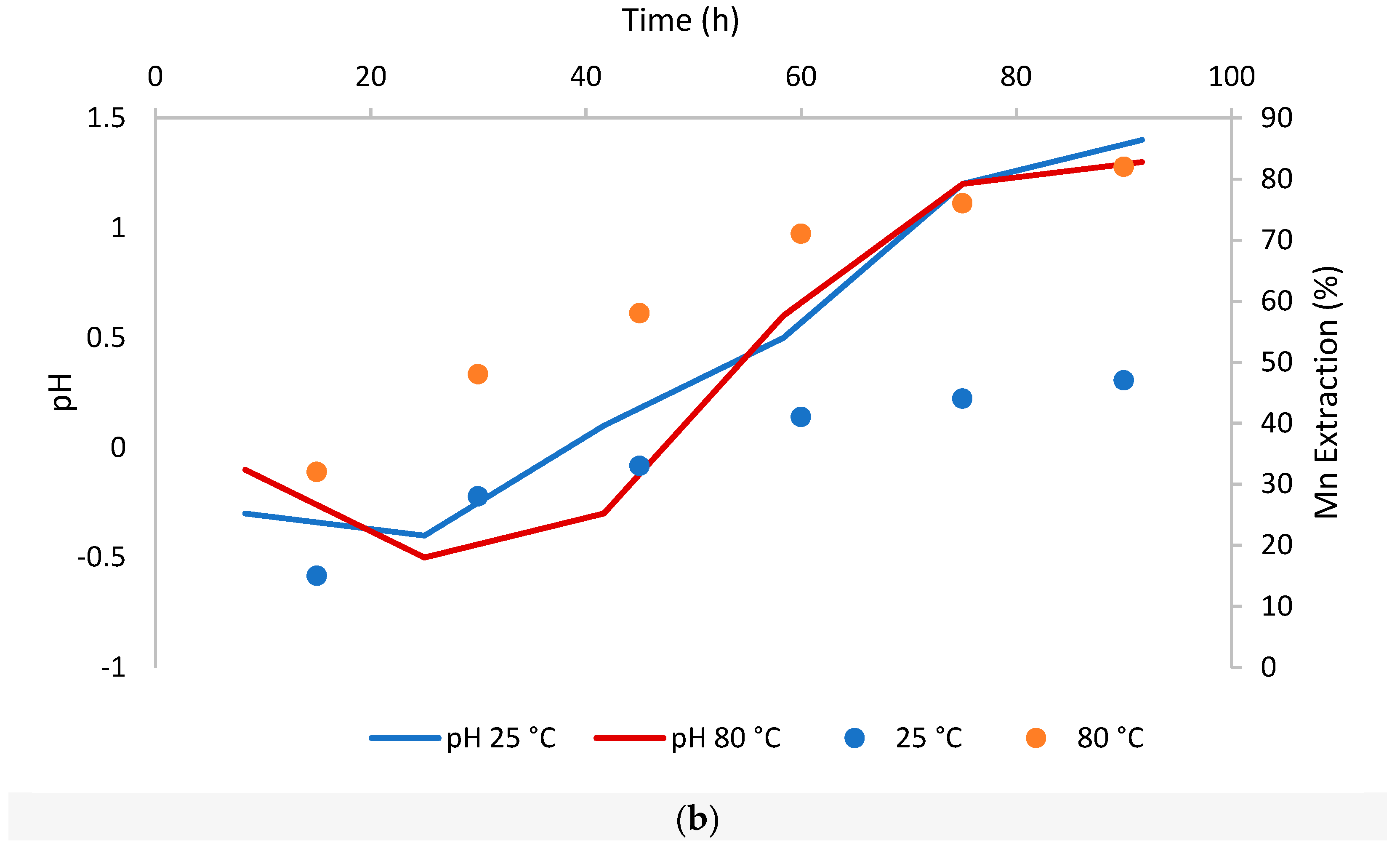
We will update the article and the original version will remain available on the article webpage.
Author Contributions
All of the authors contributed to analyzing the results and writing the paper All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Reference
- Torres, D.; Ayala, L.; Jeldres, R.I.; Cerecedo-Sáenz, E.; Salinas-Rodríguez, E.; Robles, P.; Toro, N. Leaching Chalcopyrite with High MnO2 and Chloride Concentrations. Metals 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
