Effects of One Versus Two Doses of a Multi-Ingredient Pre-Workout Supplement on Metabolic Factors and Perceived Exertion during Moderate-Intensity Running in Females
Abstract
:1. Introduction
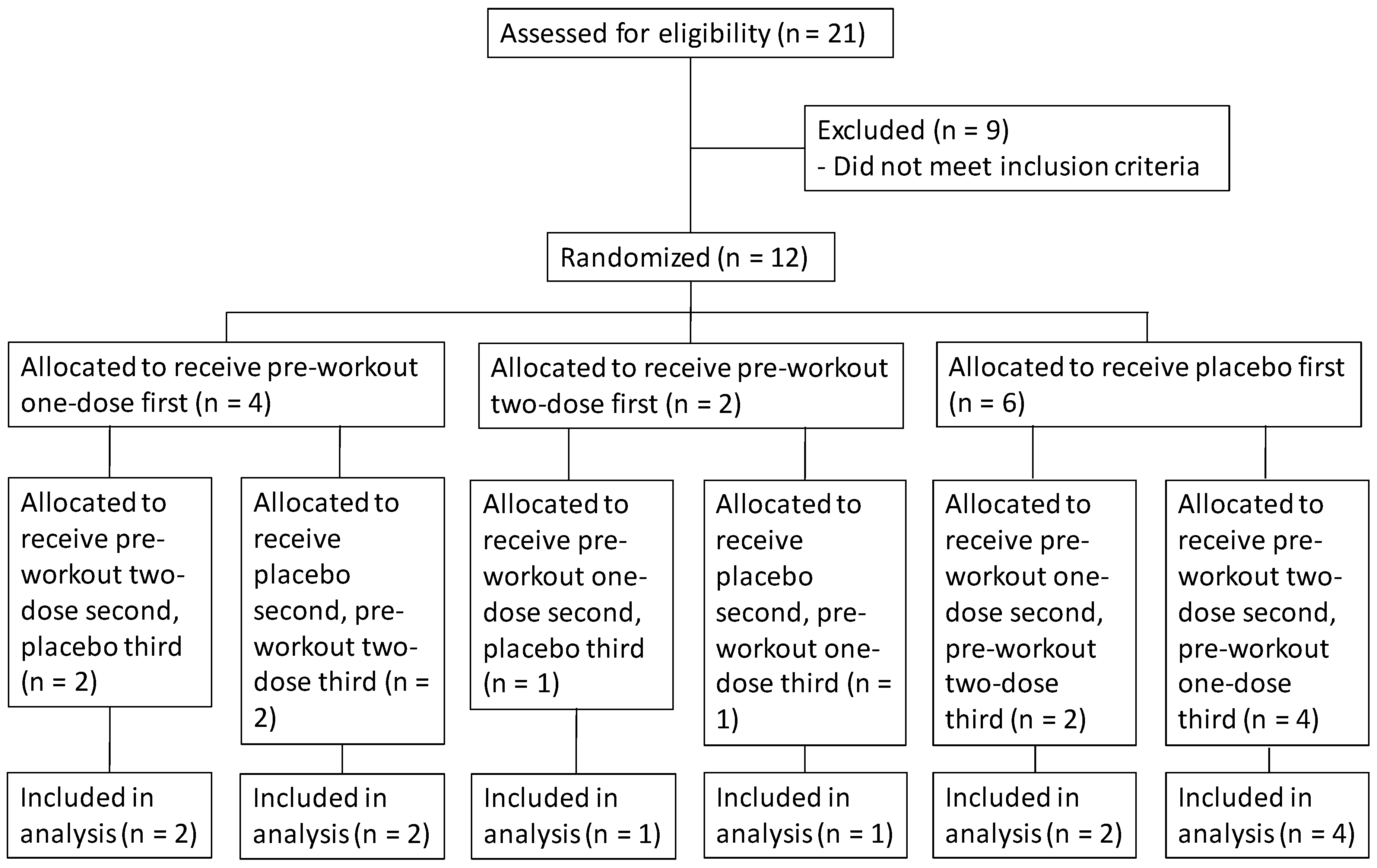
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Determination of VT and 2peak
2.4. Standardized Meal and Supplementation Protocol
2.5. Heart Rate and Blood Pressure
2.6. Constant Velocity Runs
2.7. Estimation of Substrate Utilization
2.8. Statistical Analyses
3. Results
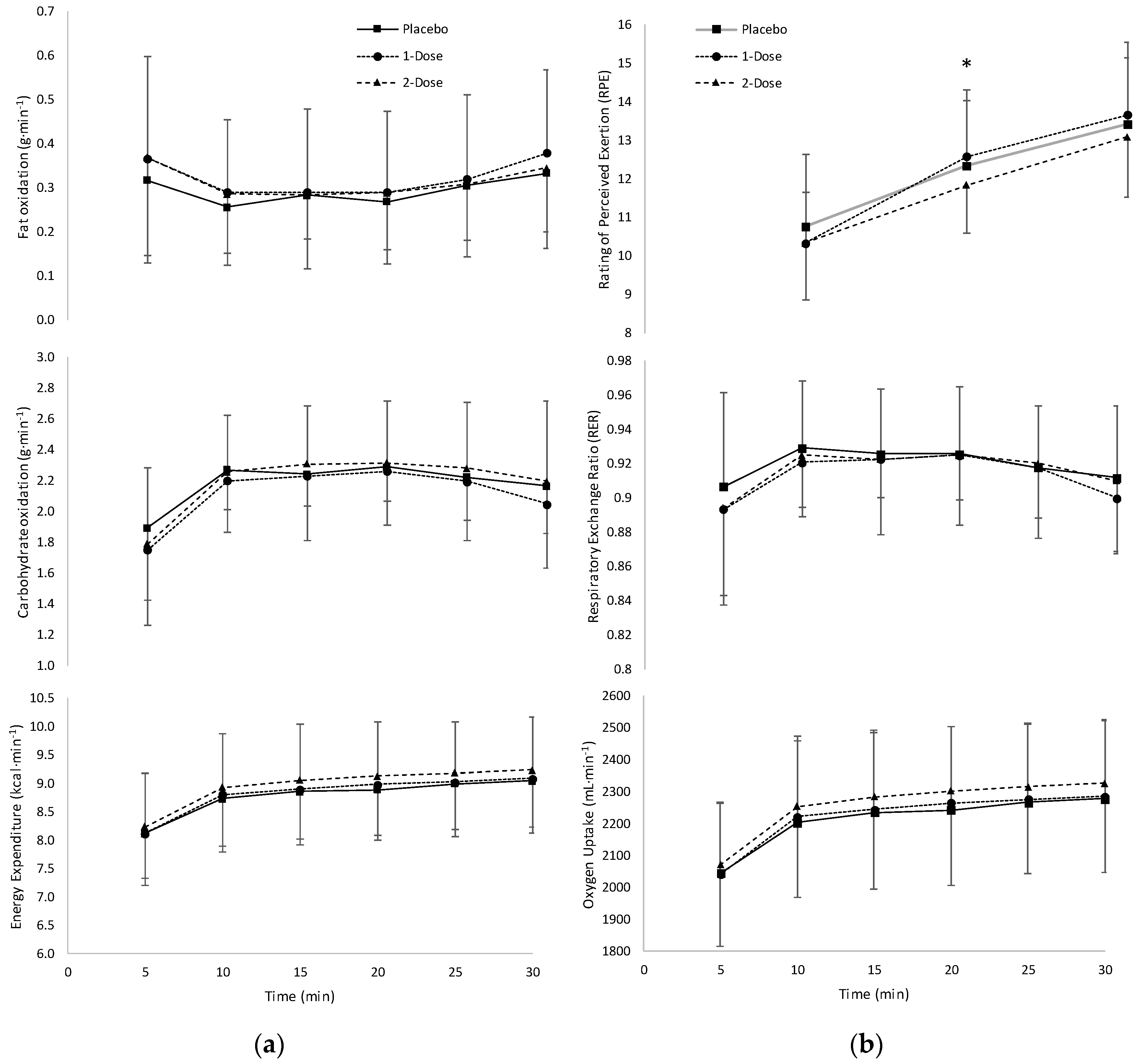
3.1. Macronutrient Data
3.2. Metabolic Variables
3.3. Cardiovascular Variables
3.4. Rating of Perceived Exertion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jagim, A.R.; Harty, P.S.; Camic, C.L. Common ingredient profiles of multi-ingrendient pre-workout supplements. Nutrients 2019, 11, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergstrom, H.C.; Byrd, M.T.; Wallace, B.J.; Clasey, J.L. Examination of a multi-ingredient preworkout supplement on total volume of resistance exercise and subsequent strength and power performance. J. Strength Cond. Res. 2018, 32, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, H.C.; Housh, T.J.; Traylor, D.A.; Lewis, R.W.; Cochrane, K.C.; Jenkins, N.D.; Schmidt, R.J.; Johnson, G.O.; Housh, D.J.; Cramer, J.T. Metabolic, cardiovascular, and perceptual responses to a thermogenic nutritional supplement at rest, during exercise, and recovery in men. J. Strength Cond. Res. 2014, 28, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.; Camic, C.L.; Doberstein, S.; Erickson, J.L.; Jagim, A.R. The acute effects of a multi-ingredient preworkout supplement on resting energy expenditure and exercise performance in recreationally active females. J. Int. Soc. Sports Nutr. 2018, 15, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joy, J.M.; Lowery, R.P.; Falcone, P.H.; Vogel, R.M.; Mosman, M.M.; Tai, C.Y.; Carson, L.R.; Kimber, D.; Choate, D.; Kim, M.P.; et al. A multi-ingredient, pre-workout supplement is apparently safe in healthy males and females. Food Nutr. Res. 2015, 59, 27470. [Google Scholar] [CrossRef] [Green Version]
- Vogel, R.M.; Joy, J.M.; Falcone, P.H.; Mosman, M.M.; Kim, M.P.; Moon, J.R. Consuming a multi-ingredient thermogenic supplement for 28 days is apparently safe in healthy adults. Food Nutr. Res. 2015, 59, 279299. [Google Scholar] [CrossRef] [Green Version]
- Stohs, S.J.; Badmaev, V. A review of natural stimulant and non-stimulant thermogenic agents. Phytother. Res. 2016, 30, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Daniels, J.W.; Molé, P.A.; Saffrath, J.D.; Stebbins, C.L. Effects of caffeine on blood pressure, heart rate, and forearm blood flow during dynamic leg exercise. J. Appl. Physiol. 1998, 85, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Robertson, D.; Erölich, J.C.; Carr, R.K.; Watson, J.T.; Hollifield, J.W.; Shand, D.G.; Oates, J.A. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N. Engl. J. Med. 1978, 298, 181–186. [Google Scholar] [CrossRef]
- Schubert, M.M.; Hall, S.; Leveritt, M.; Grant, G.; Sabapathy, S.; Desbrow, B. Caffeine consumption around an exercise bout: Effects on energy expenditure, energy intake, and exercise enjoyment. J. Appl. Physiol. 2014, 117, 745–754. [Google Scholar] [CrossRef]
- Ahrens, J.N.; Crixell, S.H.; Lloyd, L.K.; Walker, J.L. The physiological effects of caffeine in women during treadmill walking. J. Strength Cond. Res. 2007, 21, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Wallman, K.E.; Goh, J.W.; Guelfi, K.J. Effects of caffeine on exercise performance in sedentary females. J. Sport Sci. Med. 2010, 9, 183–189. [Google Scholar]
- Duncan, M.J.; Dobell, A.P.; Caygill, C.L.; Eyre, E.; Tallis, J. The effect of acute caffeine ingestion on upper body anaerobic exercise and cognitive performance. Eur. J. Sport Sci. 2019, 19, 103–111. [Google Scholar] [CrossRef]
- McClaran, S.R.; Wetter, T.J. Low doses of caffeine reduce heart rate during submaximal cycle ergometry. J. Int. Soc. Sports Nutr. 2007, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denadai, B.S.; Denadai, M.L. Effects of caffeine on time to exhaustion in exercise performed below and above the anaerobic threshold. Braz. J. Med. Bio. Res. 1998, 31, 581–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belza, A.; Toubro, S.; Astrup, A. The effect of caffeine, green tea and tyrosine on thermogenesis and energy intake. Eur. J. Clin. Nutr. 2009, 63, 57–64. [Google Scholar] [CrossRef]
- Lejeune, M.P.; Kovacs, E.M.; Westerterp-Plantenga, M.S. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br. J. Nutr. 2003, 90, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Snitker, S.; Fujishima, Y.; Shen, H.; Ott, S.; Pi-Sunyer, X.; Furuhata, Y.; Sato, H.; Takahashi, M. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: Possible pharmacogenetic implications. Am. J. Clin. Nutr. 2009, 89, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, V.; Harding, I.H.; Palombo, E.A. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycemia. BMC Complement. Altern. Med. 2012, 12, 77. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, C.; Thangamani, C. Amazing bean “Mucuna pruriens”: A comprehensive review. J. Med. Plants Res. 2014, 8, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.; Terry, R.; Ernst, E. The use of green coffee extract as a weight loss supplement: A systematic review and meta-analysis of randomised clinical trials. Gastroenterol. Res. Pract. 2011, 2011, 382852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Loftus, H.L.; Astell, K.J.; Mathai, M.L.; Su, X.Q. Coleus forskohlii extract supplementation in conjunction with a hypocaloric diet reduces the risk factors of metabolic syndrome in overweight and obese subjects: A randomized controlled trial. Nutrients 2015, 7, 9508–9522. [Google Scholar] [CrossRef] [Green Version]
- Broad, E.M.; Maughan, R.J.; Galloway, S. Effects of exercise intensity and altered substrate availability on cardiovascular and metabolic responses to exercise after oral carnitine supplementation in athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Jagim, A.R.; Camic, C.L.; Harty, P.S. Common habits, adverse events, and opinions regarding pre-workout supplement use among regular consumers. Nutrients 2019, 11, 855. [Google Scholar] [CrossRef] [Green Version]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef]
- Péronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport. Sci. 1991, 16, 23–29. [Google Scholar]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26 (Suppl 1), 28–37. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Kang, J.; Ratamess, N.A.; Rashti, S.L.; Tranchina, C.P.; Faigenbaum, A.D. Thermogenic effect of an acute ingestion of a weight loss supplement. J. Int. Soc. Sports Nutr. 2009, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Bergstrom, H.C.; Housh, T.J.; Traylor, D.A.; Lewis, R.W.; Jenkins, N.D.; Cochrane, K.C.; Schmidt, R.J.; Johnson, G.O.; Housh, D.J. Physiologic responses to a thermogenic nutritional supplement at rest, during low-intensity exercise, and during recovery from exercise in college-aged women. Appl. Physiol. Nutr. Metab. 2013, 38, 988–995. [Google Scholar] [CrossRef]
- Henderson, S.; Magu, B.; Rasmussen, M.; Lancaster, S.; Kerksick, C.; Smith, P.; Melton, C.; Cowan, P.; Greenwood, M.; Earnest, C.; et al. Effects of coleus forskohlii supplementation on body composition and hematological profiles in mildly overweight women. J. Int. Soc. Sports Nutr. 2005, 2, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Inoue, N.; Matsunaga, Y.; Satoh, H.; Takahashi, M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues. Biosci. Biotechnol. Biochem. 2007, 71, 380–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.P.; Earnest, C.P.; Koozehchian, M.; Galvan, E.; Dalton, R.; Walker, D.; Rasmussen, C.; Murano, P.S.; Greenwood, M.; Kreider, R.B. Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance. J. Int. Soc. Sports Nutr. 2017, 14, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Babu, K.M. Caffeine reduces myocardial blood flow during exercise. Am. J. Med. 2013, 126, 730. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.R.; Ziegenfuss, T.; Kalman, D.; Kreider, R.; Campbell, B.; Wilborn, C.; Taylor, L.; Willoughby, D.; Stout, J.; Graves, B.S.; et al. International Society of Sports Nutrition Position Stand: Caffeine and Performance. J. Int. Soc. Sports Nutr. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Ingredient | Amount Per Serving |
|---|---|
| Vitamin C (as Ascorbic Acid) | 250 mg |
| Niacin (as Niacinamide) | 30 mg |
| Vitamin B6 (as Pyridoxal-5-Phosphate) | 500 mcg |
| Folic Acid | 250 mcg |
| Vitamin B12 (as Methylcobalamin) | 35 mcg |
| Calcium | 22 mg |
| Caffeine Anhydrous | 150 mg |
| Beta Alanine | 1.6 g |
| Arginine AKG | 1.0 g |
| Explosive Energy Blend | 221 mg |
| N-Acetyl-l-Tyrosine | |
| Velvet Bean (Mucuna pruriens) seed | |
| extract (standardized for l-Dopa) | |
| Ripped Blend | 1.0 g |
| l-Carnitine Tartrate | |
| Green Coffee bean extract (standardized for Chlorogenic Acids) | |
| Capsimax® Cayenne (Capsicum annuum) | |
| fruit extract | |
| Coleus forskohlii root extract |
| Variable | Placebo | 1-Dose | 2-Dose |
|---|---|---|---|
| Total calories (kcals·d−1) | 1604 ± 423 | 1833 ± 569 | 1636 ± 446 |
| Carbohydrates (g·d−1) | 189 ± 47 | 204 ± 88 | 180 ± 65 |
| Fat (g·d−1) | 67 ± 29 | 77 ± 28 | 68 ± 31 |
| Protein (g·d−1) | 68 ± 17 | 83 ± 33 | 80 ± 29 |
| Time (min) | Energy Expenditure (kcal·min−1) | ||
|---|---|---|---|
| Placebo | 1-Dose | 2-Dose | |
| 5 | 8.12 ± 0.80 | 8.13 ± 0.93 | 8.24 ± 0.94 |
| 10 | 8.72 ± 0.83 | 8.81 ± 1.02 | 8.93 ± 0.95 |
| 15 | 8.86 ± 0.83 | 8.90 ± 0.98 | 9.05 ± 0.98 |
| 20 | 8.89 ± 0.80 | 8.98 ± 0.97 | 9.13 ± 0.95 |
| 25 | 8.99 ± 0.80 | 9.03 ± 0.96 | 9.19 ± 0.90 |
| 30 | 9.04 ± 0.81 | 9.09 ± 0.97 | 9.24 ± 0.93 |
| Time | Heart Rate (bpm) | |||
|---|---|---|---|---|
| Placebo | 1-Dose | 2-Dose | ||
| Rest | Baseline | 67 ± 12 | 68 ± 12 | 65 ± 11 |
| Post-15 | 66 ± 12 | 64 ± 9 | 64 ± 8 | |
| Post-30 | 69 ± 10 | 64 ± 12 | 66 ± 14 | |
| Exercise | 5 | 150 ± 10 | 147 ± 12 | 144 ± 16 |
| 10 | 159 ± 15 | 159 ± 16 | 157 ± 17 | |
| 15 | 164 ± 17 | 164 ± 17 | 162 ± 19 | |
| 20 | 167 ± 17 | 167 ± 18 | 165 ± 19 | |
| 25 | 169 ± 18 | 171 ± 18 | 169 ± 20 | |
| 30 | 172 ± 18 | 174 ± 19 | 170 ± 20 | |
| Time | Systolic Blood Pressure (mmHg) | Diastolic Blood Pressure (mmHg) | |||||
|---|---|---|---|---|---|---|---|
| Placebo | 1-Dose | 2-Dose * | Placebo | 1-Dose | 2-Dose | ||
| Rest | Baseline | 104 ± 12 | 102 ± 11 | 107 ± 11 | 64 ± 7 | 61 ± 7 | 66 ± 5 |
| Post-15 | 101 ± 9 | 104 ± 11 | 111 ± 12 | 67 ± 6 | 67 ± 7 | 74 ± 6 | |
| Post-30 | 105 ± 10 | 110 ± 9 | 112 ± 10 | 67 ± 6 | 72 ± 6† | 75 ± 5† | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erickson, J.R.; Camic, C.L.; Jagim, A.R.; Pellersels, P.M.; Wright, G.A.; Henert, S.E.; Foster, C. Effects of One Versus Two Doses of a Multi-Ingredient Pre-Workout Supplement on Metabolic Factors and Perceived Exertion during Moderate-Intensity Running in Females. Sports 2020, 8, 52. https://doi.org/10.3390/sports8040052
Erickson JR, Camic CL, Jagim AR, Pellersels PM, Wright GA, Henert SE, Foster C. Effects of One Versus Two Doses of a Multi-Ingredient Pre-Workout Supplement on Metabolic Factors and Perceived Exertion during Moderate-Intensity Running in Females. Sports. 2020; 8(4):52. https://doi.org/10.3390/sports8040052
Chicago/Turabian StyleErickson, Jamie R., Clayton L. Camic, Andrew R. Jagim, Paige M. Pellersels, Glenn A. Wright, Shaine E. Henert, and Carl Foster. 2020. "Effects of One Versus Two Doses of a Multi-Ingredient Pre-Workout Supplement on Metabolic Factors and Perceived Exertion during Moderate-Intensity Running in Females" Sports 8, no. 4: 52. https://doi.org/10.3390/sports8040052







