The Effect of Body Mass Index on Acute Cardiometabolic Responses to Graded Exercise Testing in Children: A Narrative Review
Abstract
1. Introduction
1.1. Nutritional Status and Body Mass Index
1.2. Exercise Mode, Intensity, and Protocols
1.3. Maximal Oxygen Uptake and Body Composition
2. Methods
3. Results and Discussion
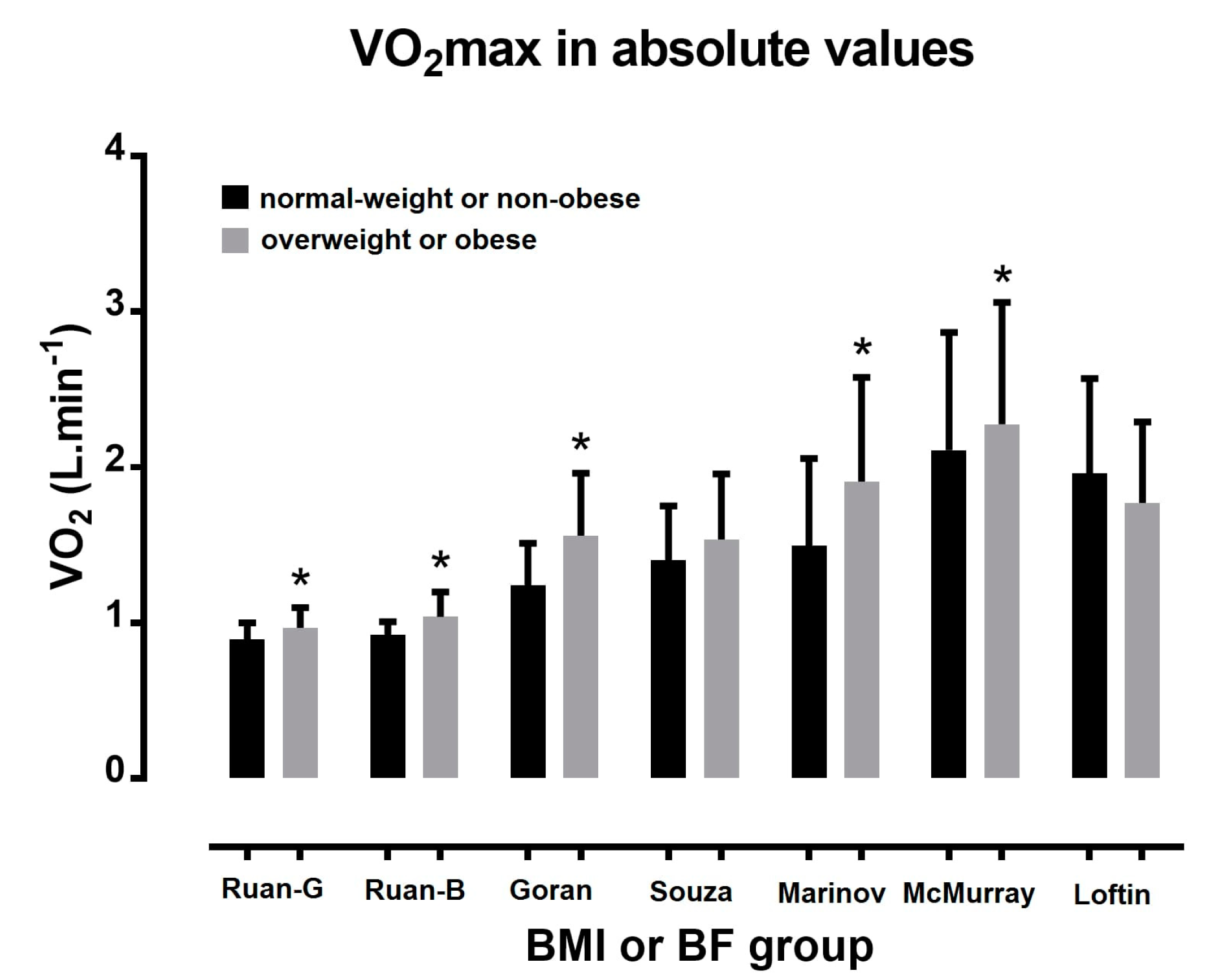
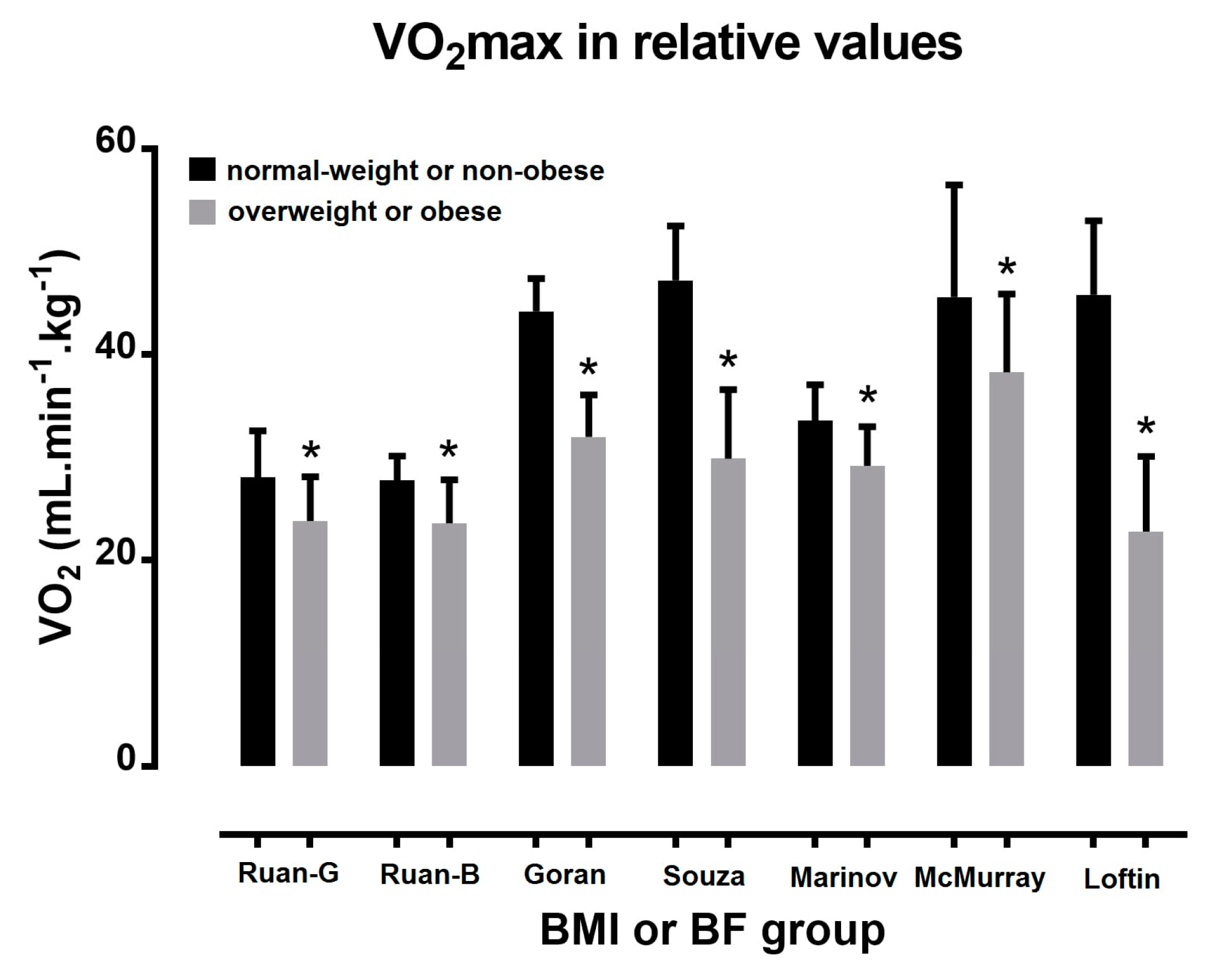
3.1. Acute Oxygen Uptake Responses to Exercise Testing
3.2. Relationship between Body Mass Index and Body Fat
3.3. Maximal Heart Rate and Heart Rate Acute Responses to Exercise Testing According to Body Mass Index
3.4. Heart Rate at Rest
3.5. Sport Populations
3.6. Resting Metabolic Rate
3.7. Respiratory Quotient
3.8. Limitations, Strengths, and Practical Applications
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| BF | body fat percentage |
| bpm | beats per minute |
| DBP | diastolic blood pressure |
| FFM | fat-free mass |
| GXT | graded exercise test |
| HR | heart rate |
| HRmax | maximal heart rate |
| HRrest | heart rate at rest |
| METs | metabolic equivalents |
| PE | Physical Education |
| PWC170 | physical working capacity test in HR 170 bpm |
| RMR | resting metabolic rate |
| RQ | respiratory quotient |
| RPE | rate of perceived exertion |
| SBP | systolic blood pressure |
| VO2 | oxygen uptake |
| VO2max | maximal oxygen uptake |
References
- Yanovski, S.Z. Overweight, obesity, and health risk: National task force on the prevention and treatment of obesity. Arch. Intern. Med. 2000, 160, 898–904. [Google Scholar]
- Rivera, J.A.; De Cossío, T.G.; Pedraza, L.S.; Aburto, T.C.; Sánchez, T.G.; Martorell, R. Childhood and adolescent overweight and obesity in latin america: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 321–332. [Google Scholar] [CrossRef]
- Ahrens, W.; Pigeot, I.; Pohlabeln, H.; De Henauw, S.; Lissner, L.; Molnár, D.; Moreno, L.A.; Tornaritis, M.; Veidebaum, T.; Siani, A.; et al. Prevalence of overweight and obesity in european children below the age of 10. Int. J. Obes. 2014, 38, S99–S107. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Graubard, B.I.; Williamson, D.F.; Gail, M.H. Excess deaths associated with underweight, overweight, and obesity. J. Am. Med. Assoc. 2005, 293, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Chrysohoou, C.; Tsiamis, E.; Kosyfa, H.; Kalogirou, L.; Filippou, A.; Iosifidis, S.; Aggelopoulos, P.; Pitsavos, C.; Tousoulis, D. The “overweight paradox” in the prognosis of acute coronary syndrome for patients with heart failure-a truth for all? A 10-year follow-up study. Maturitas 2017, 102, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, T.B. Health implications of overweight and obesity in the united states. Ann. Intern. Med. 1985, 103, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Hall, A.G. The financial burden of overweight and obesity among elderly americans: The dynamics of weight, longevity, and health care cost. Health Serv. Res. 2008, 43, 849–868. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.; Gennat, H.; O’Rourke, P.; Del Mar, C. Exercise for overweight or obesity. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef] [PubMed]
- Emerenziani, G.P.; Migliaccio, S.; Gallotta, M.C.; Lenzi, A.; Baldari, C.; Guidetti, L. Physical exercise intensity prescription to improve health and fitness in overweight and obese subjects: A review of the literature. Health 2013, 5, 113–121. [Google Scholar] [CrossRef]
- Karvonen, M.J.; Kentala, E.; Mustala, O. The effects of training on heart rate; a longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar] [PubMed]
- Buhendwa, R.A.; Roelants, M.; Thomis, M.; Nkiama, C.E. Nutritional status and height, weight and bmi centiles of school-aged children and adolescents of 6–18-years from kinshasa (DRC). Ann. Hum. Biol. 2017, 44, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Miguel, M.; Cavero-Redondo, I.; Alvarez-Bueno, C.; Rodriguez-Artalejo, F.; Moreno Aznar, L.; Ruiz, J.R.; Martinez-Vizcaino, V. Prevalence and trends of thinness, overweight and obesity among children and adolescents aged 3–18 years across europe: A protocol for a systematic review and meta-analysis. BMJ Open 2017, 7. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic. Report of a Who Consultation; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.H.; Robinson, T.N. Use of the body mass index (BMI) as a measure of overweight in children and adolescents. J. Pediatr. 1998, 132, 191–193. [Google Scholar] [PubMed]
- McMurray, R.G.; Ondrak, K.S. Effects of being overweight on ventilatory dynamics of youth at rest and during exercise. Eur. J. Appl. Physiol. 2011, 111, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Bovet, P.; Auguste, R.; Burdette, H. Strong inverse association between physical fitness and overweight in adolescents: A large school-based survey. Int. J. Behav. Nutr. Phys. Act. 2007, 4. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.C.; Drinkard, B.; McDuffie, J.R.; Ghorbani, S.; Yanoff, L.B.; Yanovski, J.A. Influence of excess adiposity on exercise fitness and performance in overweight children and adolescents. Pediatrics 2005, 115, e690–e696. [Google Scholar] [CrossRef] [PubMed]
- Pate, R.R.; Wang, C.Y.; Dowda, M.; Farrell, S.W.; O’Neill, J.R. Cardiorespiratory fitness levels among us youth 12 to 19 years of age: Findings from the 1999–2002 national health and nutrition examination survey. Arch. Pediatr. Adolesc. Med. 2006, 160, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.G.; Ribeiro, M.A.G.O.; Marson, F.A.L.; Schivinski, C.I.S.; Severino, S.D.; Ribeiro, J.D.; Barros Filho, A.A. Effect of exercise test on pulmonary function of obese adolescents. J. Pediatr. 2014, 90, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.S.F.; Cardoso, A.L.; Yasbek, P., Jr.; Faintuch, J. Aerobic endurance, energy expenditure, and serum leptin response in obese, sedentary, prepubertal children and adolescents participating in a short-term treadmill protocol. Nutrition 2004, 20, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Reybrouck, T.; Weymans, M.; Vinckx, J.; Stijns, H.; Vanderschueren-Lodeweyckx, M. Cardiorespiratory function during exercise in obese children. Acta Paediatr. Scand. 1987, 76, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, E.; Sigmundová, D.; Šnoblová, R.; Gecková, A.M. Actitrainer-determined segmented moderate-to-vigorous physical activity patterns among normal-weight and overweight-to-obese czech schoolchildren. Eur. J. Pediatr. 2014, 173, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Govindan, M.; Gurm, R.; Mohan, S.; Kline-Rogers, E.; Corriveau, N.; Goldberg, C.; Du Russel-Weston, J.; Eagle, K.A.; Jackson, E.A. Gender differences in physiologic markers and health behaviors associated with childhood obesity. Pediatrics 2013, 132, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Martin-Calvo, N.; Moreno-Galarraga, L.; Martinez-Gonzalez, M.A. Association between body mass index, waist-to-height ratio and adiposity in children: A systematic review and meta-analysis. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, O.G.; Douda, H.T.; Papazoglou, D.A.; Tokmakidis, S.P. The immediate adaptations to aerobic exercise of the cardiovascular function of overweight/obese pre-pubertal children. Arch. Hell. Med. 2014, 31, 477–486. [Google Scholar]
- Breithaupt, P.G.; Colley, R.C.; Adamo, K.B. Using the oxygen uptake efficiency slope as an indicator of cardiorespiratory fitness in the obese pediatric population. Pediatr. Exerc. Sci. 2012, 24, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Schutz, Y.; Schena, F.; Zaffanello, M.; Pinelli, L. Energy expenditure during walking and running in obese and nonobese prepubertal children. J. Pediatr. 1993, 123, 193–199. [Google Scholar] [CrossRef]
- Reybrouck, T.; Mertens, L.; Schepers, D.; Vinckx, J.; Gewillig, M. Assessment of cardiorespiratory exercise function in obese children and adolescents by body mass-independent parameters. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 478–483. [Google Scholar] [CrossRef] [PubMed]
- MacIejczyk, M.; Szymura, J.; Gradek, J.; Cempla, J.; Wiȩcek, M. Physiological response is similar in overweight and normoweight boys during cycling: A longitudinal study. Acta Physiol. Hung. 2014, 101, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T.; Bhargava, R.; Parslow, D.; Heptulla, R.A. Cardiac response to progressive cycle exercise in moderately obese adolescent females. J. Adoles. Health 2003, 32, 422–427. [Google Scholar] [CrossRef]
- Unnithan, V.B.; Baynard, T.; Potter, C.R.; Barker, P.; Heffernan, K.S.; Kelly, E.; Yates, G.; Fernhall, B. An exploratory study of cardiac function and oxygen uptake during cycle ergometry in overweight children. Obesity 2007, 15, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A. Cardiorespiratory fitness in obese girls. Indian J. Physiol. Pharmacol. 2012, 56, 393–395. [Google Scholar]
- Chatterjee, S.; Chatterjee, P.; Bandyopadhyay, A. Cardiorespiratory fitness of obese boys. Indian J. Physiol. Pharmacol. 2005, 49, 353–357. [Google Scholar] [PubMed]
- Gutin, B.; Yin, Z.; Johnson, M.; Barbeau, P. Preliminary findings of the effect of a 3-year after-school physical activity intervention on fitness and body fat: The medical college of georgia fitkid project. Int. J. Pediatr. Obes. 2008, 3, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; Beets, M.W.; Barr-Anderson, D.J.; Evenson, K.R. Sedentary time and vigorous physical activity are independently associated with cardiorespiratory fitness in middle school youth. J. Sports Sci. 2013, 31, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, M.A.; Chaloupka, E.C.; Rattigan, P. Cardiovascular fitness in obese versus nonobese 8–11-year-old boys and girls. Res. Q. Exerc. Sport 2008, 79, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Lambrick, D.; Mauger, A.R.; Woolley, B.; Faulkner, J. The effect of trial familiarisation on the validity and reproducibility of a field-based self-paced VO2max test. Biol. Sport 2016, 33, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.B.; Miyatake, N.; Aoyama, T.; Higuchi, M.; Tabata, I. Prediction of maximal oxygen uptake from a 3-minute walk based on gender, age, and body composition. J. Phys. Act. Health 2013, 10, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Penry, J.T.; Wilcox, A.R.; Yun, J. Validity and reliability analysis of cooper’s 12-minute run and the multistage shuttle run in healthy adults. J. Strength Cond. Res. 2011, 25, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Lintu, N.; Tompuri, T.; Viitasalo, A.; Soininen, S.; Laitinen, T.; Savonen, K.; Lindi, V.; Lakka, T.A. Cardiovascular fitness and haemodynamic responses to maximal cycle ergometer exercise test in children 6–8 years of age. J. Sports Sci. 2014, 32, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Potter, C.R.; Zakrzewski, J.K.; Draper, S.B.; Unnithan, V.B. The oxygen uptake kinetic response to moderate intensity exercise in overweight and non-overweight children. Int. J. Obes. 2013, 37, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lafortuna, C.L.; Lazzer, S.; Agosti, F.; Busti, C.; Galli, R.; Mazzilli, G.; Sartorio, A. Metabolic responses to submaximal treadmill walking and cycle ergometer pedalling in obese adolescents. Scand. J. Med. Sci. Sports 2010, 20, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Vrbik, I.; Sporis, G.; Stefan, L.; Madic, D.; Trajkovic, N.; Valantine, I.; Milanovic, Z. The influence of familiarization on physical fitness test results in primary school-aged children. Pediatr. Exerc. Sci 2017, 29, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bhati, P.; Bansal, V.; Moiz, J.A. Comparison of different volumes of high intensity interval training on cardiac autonomic function in sedentary young women. Int. J. Adoles. Med. Health 2017. [Google Scholar] [CrossRef] [PubMed]
- Dencker, M.; Bugge, A.; Hermansen, B.; Froberg, K.; Andersen, L.B. Aerobic fitness in prepubertal children according to level of body fat. Acta Paediatr. 2010, 99, 1854–1860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Pierson, R.N., Jr.; Heymsfield, S.B. The five-level model: A new approach to organizing body-composition research. Am. J. Clin. Nutr. 1992, 56, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Loftin, M.; Sothern, M.; Abe, T.; Bonis, M. Expression of VO2peak in children and youth, with special reference to allometric scaling. Sports Med. 2016, 46, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.G.; Butte, N.F.; Crouter, S.E.; Trost, S.G.; Pfeiffer, K.A.; Bassett, D.R.; Puyau, M.R.; Berrigan, D.; Watson, K.B.; Fulton, J.E. Exploring metrics to express energy expenditure of physical activity in youth. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R., Jr.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Dencker, M.; Wollmer, P.; Karlsson, M.K.; Linden, C.; Andersen, L.B.; Thorsson, O. Body fat, abdominal fat and body fat distribution related to VO(2peak) in young children. Int. J. Pediatr. Obes. 2011, 6, e597–e602. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Fields, D.A.; Hunter, G.R.; Herd, S.L.; Weinsier, R.L. Total body fat does not influence maximal aerobic capacity. Int. J. Obes. 2000, 24, 841–848. [Google Scholar] [CrossRef]
- Marinov, B.; Kostianev, S.; Turnovska, T. Ventilatory efficiency and rate of perceived exertion in obese and non-obese children performing standardized exercise. Clin. Physiol. Funct. Imaging 2002, 22, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Tang, Q.; Zhao, X.; Cai, W. Relationship between aerobic capacity and body composition in school-aged children. Chin. J. Clin. Nutr. 2014, 22, 234–238. [Google Scholar]
- Loftin, M.; Sothern, M.; Trosclair, L.; O’Hanlon, A.; Miller, J.; Udall, J. Scaling vo2 peak in obese and non-obese girls. Obes. Res. 2001, 9, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Byrne, N.M.; Kagawa, M.; Ma, G.; Poh, B.K.; Ismail, M.N.; Kijboonchoo, K.; Nasreddine, L.; Trinidad, T.P.; Hills, A.P. Ethnic differences in the relationship between body mass index and percentage body fat among asian children from different backgrounds. Br. J. Nutr. 2011, 106, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Srdić, B.; Obradović, B.; Dimitrić, G.; Stokić, E.; Babović, S.S. Relationship between body mass index and body fat in children—Age and gender differences. Obes. Res. Clin. Pract. 2012, 6, e167–e173. [Google Scholar] [CrossRef] [PubMed]
- Aeberli, I.; Gut-Knabenhans, M.; Kusche-Ammann, R.S.; Molinari, L.; Zimmermann, M.B. A composite score combining waist circumference and body mass index more accurately predicts body fat percentage in 6- to 13-year-old children. Eur. J. Nutr. 2013, 52, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, Y. Body mass index (BMI) predicts percent body fat better than body adiposity index (BAI) in school children. Anthropol. Anz. Ber. Über Die Biol. Anthropol. Lit. 2015, 72, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Birch, S.L.; Duncan, M.J.; Franklin, C. Overweight and reduced heart rate variability in british children: An exploratory study. Prev. Med. 2012, 55, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Charakida, M.; Jones, A.; Falaschetti, E.; Khan, T.; Finer, N.; Sattar, N.; Hingorani, A.; Lawlor, D.A.; Smith, G.D.; Deanfield, J.E. Childhood obesity and vascular phenotypes: A population study. J. Am. Coll. Cardiol. 2012, 60, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.F.; Bianchini, J.A.A.; Antonini, V.D.S.; Hermoso, D.A.M.; Lopera, C.A.; Pagan, B.G.M.; McNeil, J.; Nardo, N., Jr. Parasympathetic cardiac activity is associated with cardiorespiratory fitness in overweight and obese adolescents. Pediatr. Cardiol. 2014, 35, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Z.; Li, Y.; Zhou, X.H.; Guo, X.F.; Zhang, X.G.; Zheng, L.Q.; Li, Y.; Jiao, Y.D.; Sun, Y.X. Association between obesity and ecg variables in children and adolescents: A cross-sectional study. Exp. Ther. Med. 2013, 6, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Ravisankar, P.; Madanmohan; Udupa, K.; Prakash, E.S. Correlation between body mass index and blood pressure indices, handgrip strength and handgrip endurance in underweight, normal weight and overweight adolescents. Indian J. Physiol. Pharmacol. 2005, 49, 455–461. [Google Scholar] [PubMed]
- Nikolaidis, P.T. Elevated body mass index and body fat percentage are associated with decreased physical fitness in soccer players aged 12–14 years. Asian J. Sports Med. 2012, 3, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, P.T. Body mass index and body fat percentage are associated with decreased physical fitness in adolescent and adult female volleyball players. J. Res. Med. Sci. 2013, 18, 22–26. [Google Scholar] [PubMed]
- Nikolaidis, P.T. Prévalence du surpoids, et rapport entre l’indice de masse corporelle, le pourcentage de graisse corporelle et la condition physique chez les footballeurs masculins âgés de 14 à 16 ans. Sci. Sports 2013, 28, 125–132. [Google Scholar] [CrossRef]
- Nikolaïdis, P.T. Cardiorespiratory power across adolescence in male soccer players. Hum. Physiol. 2011, 37, 636–641. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Ingebrigtsen, J. The relationship between body mass index and physical fitness in adolescent and adult male team handball players. Indian J. Physiol. Pharmacol. 2013, 57, 361–371. [Google Scholar] [PubMed]
- Nikolaïdis, P.T. Physical fitness is inversely related with body mass index and body fat percentage in soccer players aged 16–18 years. Med. Pregl. 2012, 65, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ishikawa-Takata, K.; Lee, S.; Kim, E.; Lim, K.; Kim, H.; Lee, I.S.; Tanaka, S. Comparison of daily physical activity parameters using objective methods between overweight and normal-weight children. J. Sport Health Sci. 2016, 7, 210–217. [Google Scholar] [CrossRef]
- Redinger, R.N. Is enhanced energy utilization the answer to prevention of excessive adiposity? J. Ky. Med. Assoc. 2009, 107, 211–217. [Google Scholar] [PubMed]
- Clevenger, K.A.; Howe, C.A. Energy cost and enjoyment of active videogames in children and teens: Xbox 360 kinect. Games Health J. 2015, 4, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Schutz, Y.; Micciolo, R.; Zoccante, L.; Pinelli, L. Resting metabolic rate in six- to ten-year-old obese and nonobese children. J. Pediatr. 1993, 122, 556–562. [Google Scholar] [CrossRef]
- Lee, S.K.; Nam, S.Y.; Hoffman, D.J. Growth retardation at early life and metabolic adaptation among north korean children. J. Dev. Orig. Health Dis. 2015, 6, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, G.; O’Loughlin, J.; Sabiston, C.; Tremblay, A.; Mathieu, M.E. Increased lipid oxidation during exercise in obese pubertal girls: A quality study. Obesity 2014, 22, E85–E90. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.C.; Plank, L.D.; Davies, P.S.; Watson, P.; Wall, C.R. Body composition and physical activity in new zealand maori, pacific and european children aged 5–14 years. Br. J. Nutr. 2003, 90, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.; Tautolo el, S.; Paterson, J.; Obolonkin, V. Pacific islands families study: Signs of puberty are associated with physical growth at ages 9 and 11 years. N. Z. Med. J. 2015, 128, 24–33. [Google Scholar] [PubMed]
- Biro, F.M.; Kiess, W. Contemporary trends in onset and completion of puberty, gain in height and adiposity. Endocr. Dev. 2016, 29, 122–133. [Google Scholar] [PubMed]
- Barría, M.A.C.; Valdebenito, M.A.; Filho, Q.J.F. Cardiorespiratory and nutritional status through anthropometric patterns of health in 12–14-year-old schoolchildren in urban and rural areas of the araucanía region, chile. J. Phys. Educ. Sport 2017, 17, 348–354. [Google Scholar]
- Navarrete, F.C.; Floody, P.D.; Mayorga, D.J.; Poblete, A.O. Low levels of physical performance, VO2max and high prevalence of obesity among school children from 9 to 14 years of age. Nutr. Hosp. 2016, 33, 1045–1051. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaidis, P.T.; Kintziou, E.; Georgoudis, G.; Afonso, J.; Vancini, R.L.; Knechtle, B. The Effect of Body Mass Index on Acute Cardiometabolic Responses to Graded Exercise Testing in Children: A Narrative Review. Sports 2018, 6, 103. https://doi.org/10.3390/sports6040103
Nikolaidis PT, Kintziou E, Georgoudis G, Afonso J, Vancini RL, Knechtle B. The Effect of Body Mass Index on Acute Cardiometabolic Responses to Graded Exercise Testing in Children: A Narrative Review. Sports. 2018; 6(4):103. https://doi.org/10.3390/sports6040103
Chicago/Turabian StyleNikolaidis, Pantelis T., Eleni Kintziou, Georgios Georgoudis, José Afonso, Rodrigo L. Vancini, and Beat Knechtle. 2018. "The Effect of Body Mass Index on Acute Cardiometabolic Responses to Graded Exercise Testing in Children: A Narrative Review" Sports 6, no. 4: 103. https://doi.org/10.3390/sports6040103
APA StyleNikolaidis, P. T., Kintziou, E., Georgoudis, G., Afonso, J., Vancini, R. L., & Knechtle, B. (2018). The Effect of Body Mass Index on Acute Cardiometabolic Responses to Graded Exercise Testing in Children: A Narrative Review. Sports, 6(4), 103. https://doi.org/10.3390/sports6040103







