Abstract
The aim of this systematic review (PROSPERO registration number CRD42024517069) was to investigate the effectiveness of exercise interventions in Post-Acute COVID-19 Syndrome (PACS). We searched on several databases and followed the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). We included randomized controlled trials that evaluate exercise interventions in adults (40–60 years old) diagnosed with PACS. The outcomes of interest were health-related quality of life (HRQoL) and functional fitness. Twenty studies were included after screening. Thirteen and fourteen studies were rated as “low” risk for HRQoL and functional fitness outcomes, respectively. Based on the evidence, an 8-week exercise protocol of aerobic training in combination with strength-based and breathing exercises was found to be safe and feasible while improving quality of life and functional fitness in people with PACS. Telerehabilitation can also be an option to avoid contagion and physical contact with the same beneficial effects. Future research should expand the knowledge about other types of exercise (i.e., water-based exercises) with high-quality trials and consider whether findings could be potentially transferable to recovery from a wider spectrum of viral infections.
1. Introduction
After the onset of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, long-term consequences emerged as latent symptoms for people recovered from the initial infection. Post-Acute COVID-19 Syndrome (PACS) is characterized by persistent, relapsing, or new symptoms that occur 30 or more days after the acute phase of SARS-CoV-2 infection, with a broad spectrum of physical and mental health manifestations that reduce quality of life [1]. PACS was first described in a Delphi study conducted by the World Health Organization (WHO) and then included in the International Classification of Diseases 10 and 11 (ICD-10 and ICD-11). People experiencing symptoms 4 weeks after the onset of the SARS-CoV-2 infection are thought to be affected by COVID-19 late sequelae or long COVID [2].
Long COVID is estimated to affect at least 10% of those recovering, corresponding to about 65 million people worldwide, with more than 200 reported symptoms including fatigue, cognitive deficits, sleep disorders, muscle weakness, and psychological problems [3,4,5]. A recent meta-analysis confirmed persistent symptoms for a minimum of 28 days post-infection, with COVID-19 survivors experiencing unresolved symptoms around four months after infection [6]. Possible mechanisms involve viral persistence, immune dysregulation, and blood cell changes, resembling other post-viral syndromes [7]. A total of 9.8% of patients with mild COVID-19 reported persistent symptoms one year post-infection, with an early phase (30–180 days) marked by hair loss, chest pain, cough, myalgia, and respiratory disorders, and a late phase (180–360 days), where most symptoms resolved [8]. Symptom severity defines three phenotypes (mild, moderate, severe), while clinical clusters (fatigue-like, respiratory, chronic pain, neurosensorial) describe different trajectories, with overlap indicating greater severity and reduced quality of life [9,10]. Stratifying patients by these profiles could help design targeted interventions and rehabilitation [9].
Several risk factors related to PACS were identified, including age (≥40 years old), female sex, frailty, emergency visits, and hospitalization due to COVID-19. Conversely, vaccination appears to reduce long COVID incidence, with a lower risk of dyspnoea [8,11], and increased protection with more doses. Identifying at-risk populations can contribute to the understanding and prevention of long COVID [11], addressing also the reduced quality of life [12].
The heterogeneity of symptoms that occurs in long COVID complicates the diagnosis and management of the condition. Exercise has emerged as a promising intervention [13]. However, evidence indicated some barriers to engaging in exercise and a lack of guidance regarding physical activity, pointing to the necessity for tailored exercise programs in individuals with long COVID [14]. Wright et al. [15] reported a marked decline in physical activity levels among individuals with long COVID and symptom exacerbation in response to physical exertion, highlighting the development of safe and effective exercise interventions [15]. Physically active individuals with a confirmed diagnosis of COVID-19 had a significantly lower risk of hospitalization, fewer hospital days, less respiratory distress, and a decreased need for oxygen support compared to sedentary people post-infection [16]. Similarly, physically active people with long COVID experienced lower hospitalization rates due to symptom exacerbation and a related better management, preventing disabilities and reducing the need for further interventions or medications [17].
Some trials have investigated the effects of physical exercise programs of variable duration and type in individuals with PACS, showing improvements in symptoms and quality of life. Some interventions, lasting from 1 to 2 weeks, assessed moderate-intensity aerobic exercise or a combination of endurance and balance training, yielding improvements in cardiorespiratory fitness and functional status [18], symptoms severity and immune function [19]. An 8-week protocol of aerobic training combined with strengthening exercises improved cardiorespiratory fitness, but neither quality of life nor persistent symptoms (dyspnoea, fatigue) improved [20]. Conversely, strengthening exercises of respiratory muscles improved quality of life but not exercise tolerance or lung function [21]. Finally, the current limitations in robust evidence make it difficult to publish effective interventions for long COVID [22]. Therefore, this systematic review investigated the impact of exercise in long COVID management, with a specific focus on the type of intervention and its effects on physical function and health-related quality of life.
2. Materials and Methods
The protocol of the systematic review was previously registered on PROSPERO (International Prospective Register of Systematic Reviews, CRD42024517069). The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [23]. The review question was “Is there scientific evidence concerning the effectiveness of exercise interventions in Post-Acute COVID-19 Syndrome”? The patients, interventions, control, outcome, and study design (PICOS) format [24,25] was based on the following:
- Population
This systematic review included studies focused on adults aged between 40 and 60 years old, with a confirmed diagnosis of PACS regardless of whether they required hospitalization for acute COVID-19 infection. Evidence showed that individuals aged 36–50 years had the highest proportion of long COVID diagnoses [7]. Similarly, a study characterizing PACS reported that adults aged 40–60 years were the most affected [10]. The studies including children, adolescents, and the elderly (over 60 years old), and people with ongoing physical or mental illness were excluded. Moreover, due to the heterogeneity in the definition of PACS, we did not apply restrictions related to diagnostic criteria or to the duration of symptom persistence, in order to include a larger number of studies potentially relevant to exercise interventions.
- Intervention(s)
This review considered studies without specific restrictions regarding exercise interventions to include all studies that addressed any type of physical exercise. Studies that were not focused on exercise intervention were excluded.
- Comparator(s)
This review considered studies that compared exercise interventions to any other standard management or care of PACS-diagnosed people.
- Outcomes
Health-related quality of life (HRQoL) and functional fitness were identified as outcomes. Therefore, the studies including exercise interventions to determine their impact on the quality of life and/or functional fitness of individuals with PACS were included in the systematic review.
- Studydesign
This systematic review included only randomized controlled trials (RCTs).
2.1. Search Strategy and Data Extraction
The literature search was performed through the indexed databases (BASE, EBSCO, EMBASE, PubMed, ScienceDirect, Scopus, and Web of Science) and clinical registers (Clinical Trials and Cochrane Library Register). The relevant articles published up to July 2025 were searched following a combination of keywords (e.g., Post-Acute COVID 19 Syndrome, long COVID, exercise, physical activity, training, health related quality life, physical fitness) and according to the specific search strategy of databases (e.g., possibility to use or not the Boolean operators and/or truncation of search terms). Once duplicates were removed from the total of RCTs identified, the screening process was conducted independently by two authors, and disagreements were verified by a third author. After the screening of title and abstract and availability check of the English full text, the remaining RCTs were considered eligible and further evaluated following the stated inclusion criteria. The references of the selected article were also considered. The relevant information from the selected articles was extracted and recorded in a spreadsheet (Excel® file, Microsoft® Excel® version 2502 Build 16.0.18526.20546, 64 bit), including study design and population demographics, details of the exercise interventions and comparators, outcome measures, and evaluation of the risk of bias.
2.2. Risk of Bias Assessment
A revised Cochrane risk of bias (RoB 2, version of 22 August 2019) tool for randomized trials was used to evaluate the quality of the included studies [26]. This tool considered the risk of bias arising from five domains: randomization, deviations from intended interventions, missing or incomplete outcome data, outcomes measurement, and selection of reported results. Each study was analysed for each outcome and domain with an algorithm-based approach, guided by responses to signalling questions. The quality assessment was performed by two authors independently, and disagreements were verified by a third author. An overall judgement was determined for each outcome and study as having “low”, “some concern”, or “high” risk of bias.
3. Results
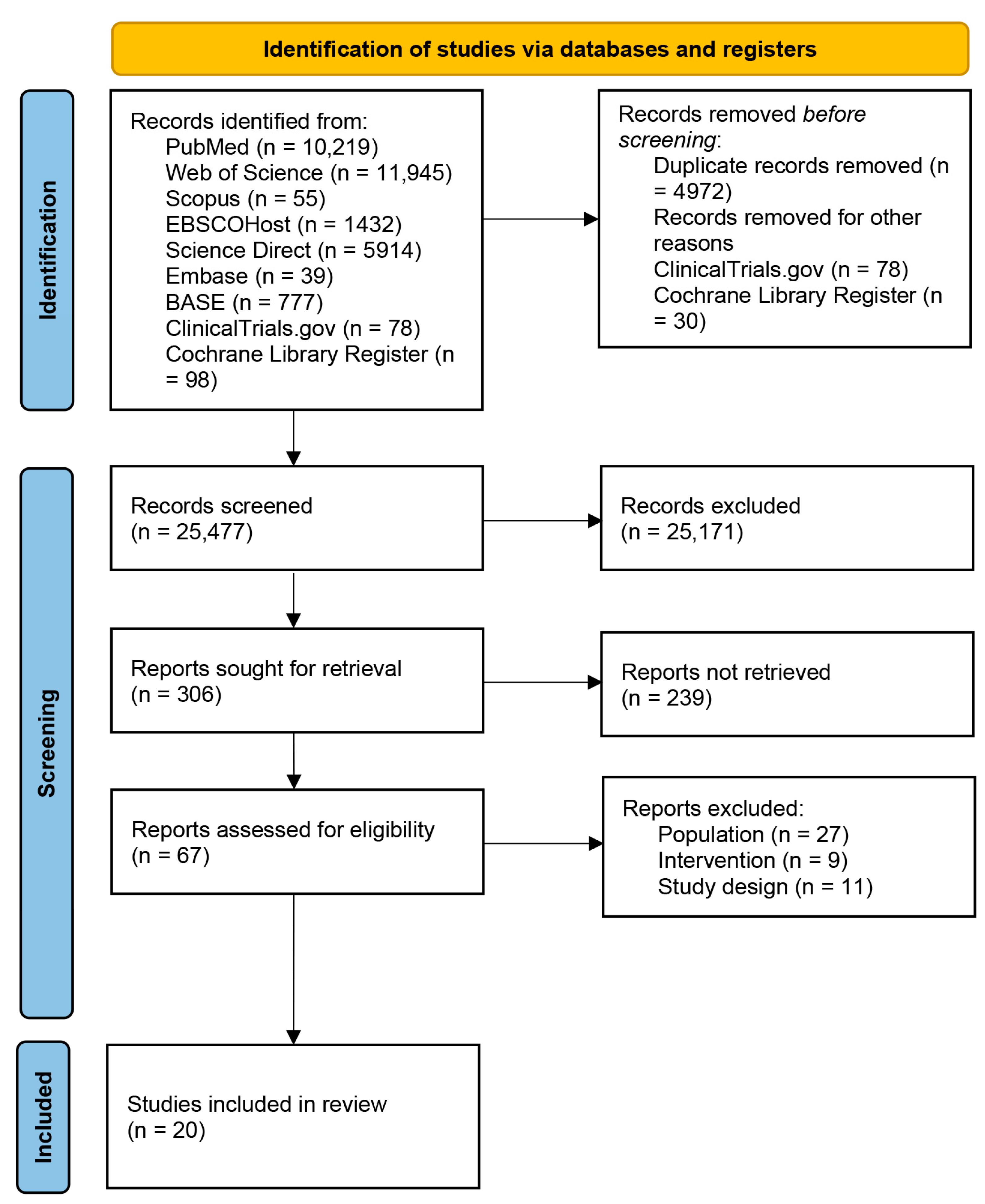
A total of 30,557 articles were identified from databases and registers (Figure 1). Before screening, 4972 duplicates were excluded. All the trials identified from ClinicalTrials.gov were excluded due to incomplete or unpublished results. Non-interventional studies, non-randomized trials, or out-of-scope studies were also excluded. Thirty studies were also excluded from the Cochrane Library Register due to unpublished results. Finally, a total of 25,171 articles were excluded for multiple reasons according to the PICOS framework (i.e., non-long-COVID population, non-interventional studies, non-controlled trials, studies that did not investigate the outcomes of interest, study designs other than RCTs). The remaining 306 articles were screened for title and abstract, and 67 were assessed for eligibility. Among these, 27 articles were excluded because the investigated population was identified as post-discharge patients, survivors, or patients recovered from COVID-19, thus without a diagnosis of PACS; 9 studies did not consider exercise intervention (e.g., electrical stimulation, vocal breath, etc.); and 11 studies were excluded due to the study designs (e.g., protocols, congress abstract, etc.). The remaining 20 articles were included in this systematic review.

Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram.
3.1. Studies Characteristics
One study was published in 2022 [27], seven studies were published in 2023 [28,29,30,31,32,33,34], nine studies were published in 2024 [35,36,37,38,39,40,41,42,43], and three studies were published in 2025 [44,45,46].
The sample size of the included studies ranged from 14 participants [42] to 585 participants [41]. Females were equally or mainly represented in all the studies except for [30,32,34,40] and Ref. [45], which included females at a lower percentage as compared to males.
The diagnosis of PACS was generally consistent across studies, relying on the persistence of symptoms lasting 3 months or more. Three studies recruited individuals with symptoms of PACS lasting from 4 to 6 weeks after SARS-CoV-2 infection [28,31,40]. One study did not report the duration of symptoms in the investigated population [34]. One study diagnosed PACS according to the dyspnea modified medical research council (mMRC) scale [43].
3.2. Exercise Interventions
The duration of exercise interventions ranged from a minimum of 2 weeks [31] to a maximum of 12 weeks [39,40,46], with a mode of 8 weeks in half of the studies. Where specified, the frequency of training sessions was three times per week with a duration of 60 min. In half of the studies, the execution of the exercise protocol was supervised via an app (Fisiotrack—https://fisiotrack.com/ -or other mobile phone application not specified) [28,40] or conducted in groups or in one-to-one online videoconferences. However, types of exercise differed across studies. Four studies [27,29,35,46] investigated the effects of concurrent training (i.e., resistance training in combination with aerobic exercise), setting the intensity of resistance training at 50% of one repetition maximum (1RM), at a moderate level for the aerobic training, and, once a week, light intensity was additionally planned. Similarly, a combination of aerobic exercise and resistance training was planned in seven studies [32,34,36,38,39,42,45]. Intensity of exercise was set at a moderate level, expressed by 60–70% of VO2max [32,36] or of the maximum heart rate [34,39], or by the Borg or OMNI Scale ranging from 4 to 6 and 8, respectively [35,42]. Breathing exercises were included in four studies in combination with aerobic training [37], strength-based exercises [31,45], or with pilates and yoga at variable intensities [41]. The other studies investigated the effects of (i) functional exercise (i.e., low-intensity strengthening exercise for large muscle groups) [28]; (ii) continuous aerobic training at 50% of workload [30]; and (iii) virtual reality-based program including high-intensity cycloergometer training [33].
The control groups varied across studies. Three studies compared the exercise group to general guidance on physical exercise and healthy habits [36] or WHO guidelines “Support for rehabilitation: Self-management after COVID-19 related illness” [27,29]. The other studies compared the experimental group to (i) no intervention, wait list, or usual care [31,35,37,38,39,40,41,44,45,46], (ii) exercise in a different setting (i.e., at hospital) [34], (iii) different exercise delivery (i.e., unsupervised, or traditional methods) [32,33,42,43], or (iv) different exercise protocols (i.e., aerobic or interval training) [28,30].
3.3. Outcomes Measured
The outcomes related to HRQoL and functional fitness were both investigated in 16 of 20 studies. Four studies investigated only the functional fitness as an outcome [31,33,38,43]. One study investigated the HRQoL as an outcome only [32]. The HRQoL was mainly evaluated with the European Quality of Life 5 Dimensions 5 Levels (EQ-5D-5L). The other studies used the 36-item short form health survey (SF-36) [30,39,44,46] or the shorter version (SF-12) [27,29,32,35]. Three studies used the Quality of Life Questionnaire (VQ11) [34], the St George’s Respiratory Questionnaire [37], and the World Health Organization Quality of Life Bref (WHO-QoL-BREF) [40], respectively. The functional fitness was evaluated with the Cardiopulmonary Exercise Testing (CPET) in eight studies [27,29,30,38,39,40,44,46], and in seven studies, the functional fitness was evaluated with the sit-to-stand test [28,34,42,43,44] or hand grip test [35,45]. Three studies used the 6 min walking test (6MWT) [31,33,37], and two studies used the International Physical Activity Questionnaire (IPAQ) [37,40].
All the included studies in this review were RCTs. Only one study was double-blind [31], and four were multicenter studies [32,35,37,41] (Table 1 and Table 2).

Table 1.
Study characteristics.

Table 2.
Comparative table summarizing modalities, dosage, and setting of exercise interventions.
3.4. Risk of Bias
The risk of bias of the included studies was assessed (Figure 2 and Figure 3). It is important to mention that 16 out of 20 studies evaluated both outcomes; thus, the assessment of the bias risk was performed separately. For studies evaluating only one between HRQoL and functional fitness, the assessment of bias risk was performed for the evaluated outcome only. Moreover, regardless of the investigated outcome, 3 out of 20 studies aimed at evaluating adherence to the intervention (“per-protocol effect”) [28,29,42], whilst the remaining studies aimed at evaluating the assignment to intervention (i.e., the “intention-to-treat” effect). Therefore, the risk of bias was independently assessed according to the nature of the effect of interest.

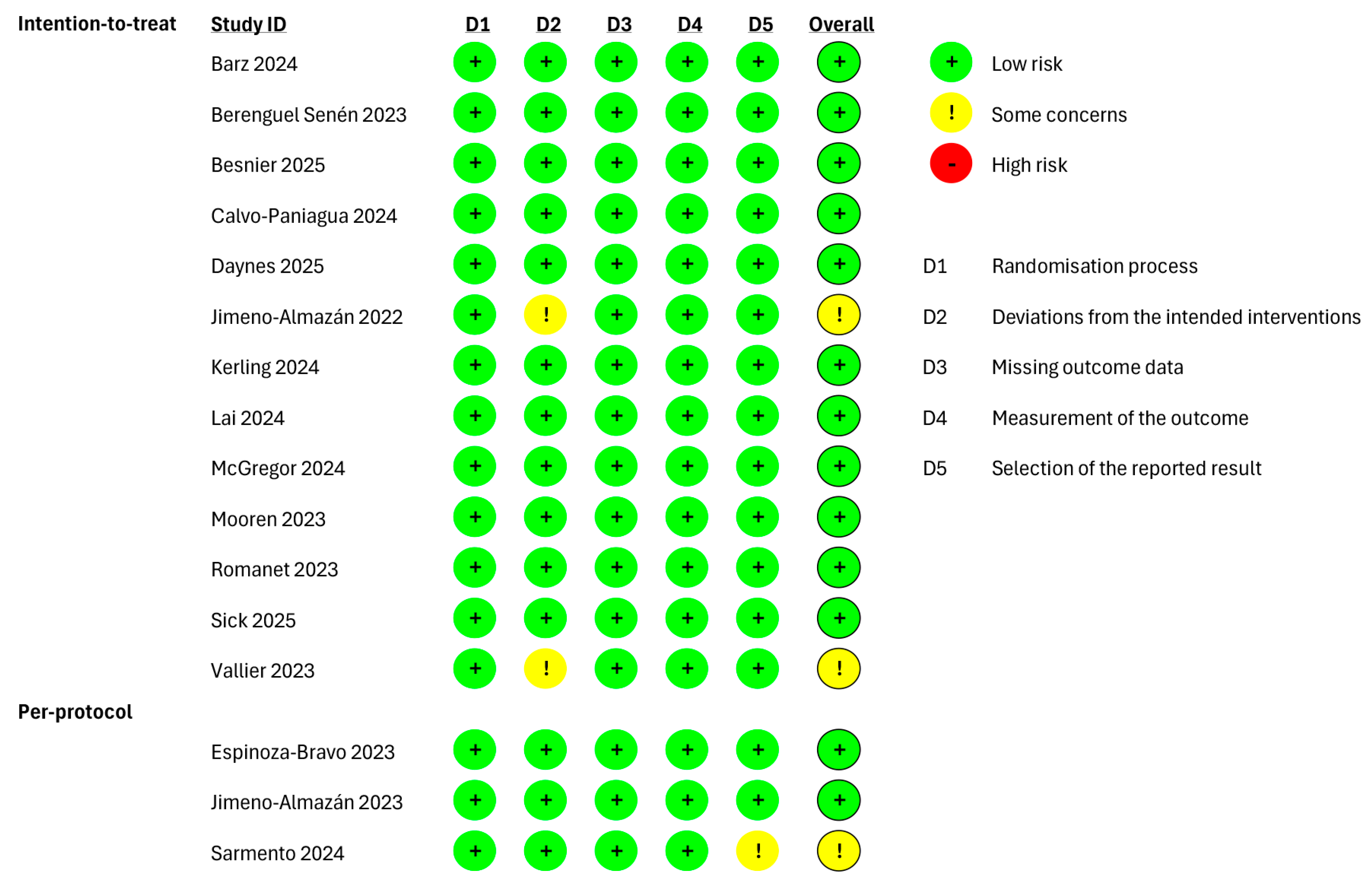
Figure 2.
Risk of bias of the included studies evaluating the health-related quality of life (HRQoL) as outcome and performing an “intention-to-treat” and “per-protocol” analyses. Barz 2024 [35], Berenguel Senén 2024 [36], Besnier 2025 [44], Calvo-Paniagua 2024 [37], Daynes 2025 [45], Jimeno-Almazán 2022 [27], Kerling 2024 [39], Lai 2024 [40], McGregor 2024 [41], Mooren 2023 [30], Romanet 2023 [32], Sick 2025 [46], Vallier 2023 [34], Espinoza-Bravo 2023 [28], Jimeno-Almazán 2023 [29], Sarmento 2024 [42].

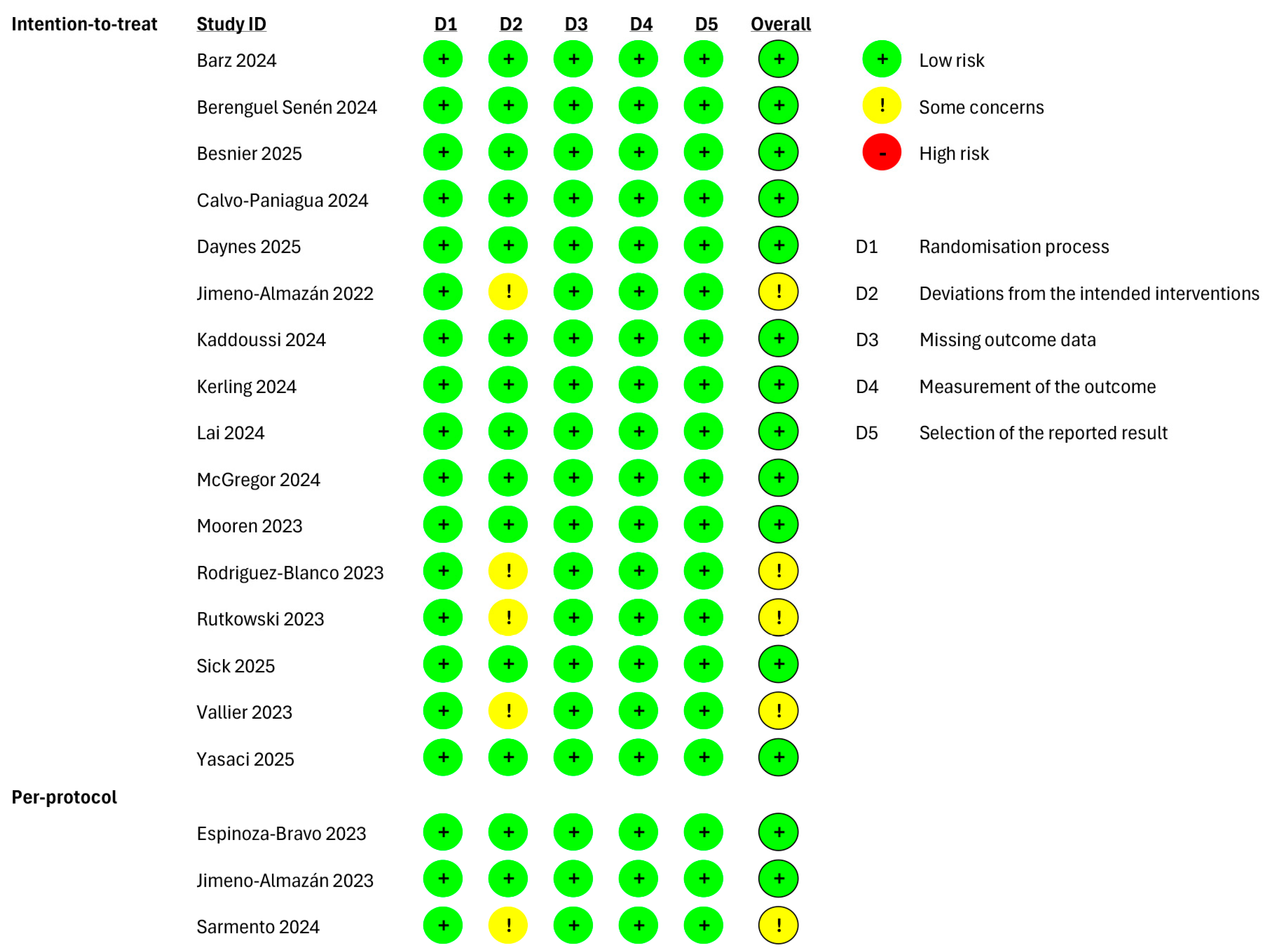
Figure 3.
Risk of bias of the included studies evaluating the functional fitness as outcome and performing an “intention-to-treat” and “per-protocol” analyses. Barz 2024 [35], Berenguel Senén 2024 [36], Besnier 2025 [44], Calvo-Paniagua 2024 [37], Daynes 2025 [45], Jimeno-Almazán 2022 [27], Kaddoussi 2024 [38], Kerling 2024 [39], Lai 2024 [40], McGregor 2024 [41], Mooren 2023 [30], Rodriguez-Blanco 2023 [31], Rutkowski 2023 [33], Sick 2025 [46], Vallier 2023 [34], Yasaci 2025 [43], Espinoza-Bravo 2023 [28], Jimeno-Almazán 2023 [29], Sarmento 2024 [42].
Regarding the studies investigating the HRQoL with an “intention-to-treat” analysis, the risk of bias was judged as “low”, except for two studies in which “some concerns” were identified due to deviations from the intended interventions [27,34]. Among the studies performing a “per-protocol” analysis, a bias in the selection of the reported result was observed in only one study [42].
The studies investigating the functional fitness, with an “intention-to-treat” analysis, were rated as “low”. Four studies were rated with “some concerns” [27,31,33,34], and Ref. [42] was rated with “some concerns” because of deviations from the intended interventions among the studies performing a “per-protocol” analysis.
The low-to-moderate risk of bias across studies supports the reliable effects of exercise interventions on HRQoL and functional fitness. However, the presence of some concerns may have influenced the observed effects. Consequently, while the results appear consistent, the confidence in the effect estimates remains moderate.
4. Discussion
The aim of this systematic review was to review the scientific literature to analyse whether any type of exercise intervention could improve health-related quality of life and physical function in people with a PACS diagnosis. A total of 20 RCTs was included in the systematic review, published from 2022 to 2025. The years of publication are consistent with the first characterization of PACS or “long COVID”. In the second part of 2020, a post-COVID syndrome was only suspected [47], and then it was preliminarily confirmed with a case series study [48] in which fatigue and dyspnoea were identified as persisting symptoms in the follow-up (i.e., after the discharge of acute COVID-19 illness). The RCTs included in this review recruited participants with a confirmed diagnosis of PACS according to [2]—3 months after SARS-CoV-19 infection. However, two studies referred to PACS involving individuals with persisting symptoms for at least 40 days [31] or 6 weeks [28], and in one study, the duration of symptom persistence was not specified [34]. The heterogeneity in the PACS definition can be explained by the fact that the authors have based their work on earlier studies, which described the condition in different ways [48,49], and a lack of worldwide consensus at the time of study publication. These initial differences in definitions have led to a variety of methods and understandings of how long COVID has been studied and managed. Nevertheless, as knowledge increased, the clinical definition of PACS evolved, and it is still ongoing [50].
The frequency, setting, and delivery of exercise protocols were not comparable to each other, even though the type of exercise protocol was similar among some studies. Notably, in half of the studies, the exercise delivery was totally remote or in combination with a face-to-face program (i.e., telerehabilitation) [28,31,34,37,39,40,41,42,43,45]. This aligns with the guidelines of the time, which recommended avoiding physical contact to prevent contagion. Studies have demonstrated that telerehabilitation can improve quality of life and functional capacity in patients with COVID-19 sequelae, with favourable outcomes in terms of costs and health benefits. These findings suggest that telerehabilitation could be a valuable tool for providing equitable rehabilitation options to a broader patient population [51]. In the remaining studies, where the protocol was administered in person (at the hospital), aerobic activity was the most frequently investigated, but in different forms (e.g., virtual reality, aerobic versus usual care group, interval versus continuous training) [30,32,33]. Ref. [36] evaluated a combination of inpatient and home-based protocol in which the aerobic training was performed at home. Separately, two studies evaluated concurrent training [27,29], but in one protocol, it was also combined with inspiratory muscle training [29]. Regardless of whether the exercise protocol was conducted in person or remotely, all the studies reported positive results or feasibility (i.e., higher percentage of adherence). Aerobic training could be performed as moderate interval or continuous training, showing similar positive results. However, functional training showed slightly better results when compared with aerobic training or as part of concurrent training, whilst virtual reality did not yield greater results than traditional methods. However, findings related to aerobic training, even in combination with other types of training, are consistent with what is reported in the literature [52,53]. Some studies were excluded from this review due to study design (i.e., non-RCTs, protocols, reviews). Moreover, this review did not include water-based exercise, for which some existing literature has reported benefits in managing PACS symptoms and reducing hospitalization over the long term [17,54]. Finally, two studies focused on breathing exercises and were aimed at improving the respiratory function in PACS individuals. Specifically, a 2-week protocol of breathing- and strength-based exercises was clinically effective [31] as well as an 8-week protocol consisting of breathing exercises, pilates, and yoga disciplines at variable intensities [41]. These findings were also previously reported by [55], although their results were not specific to the PACS condition, and the effectiveness of yoga alone remains to be demonstrated. Therefore, a multi-training approach could be effective in improving the recovery of people with PACS [56]. This could be explained by the release of exerkines—namely signalling moieties released during exercise—with a crosstalk effect between multiple body systems, and it serves as a strategy in the treatment of long COVID [57]. Furthermore, it has been demonstrated that physically active people with PACS recover quickly and fully as compared to sedentary individuals with PACS, confirming that exercise can mitigate the debilitating symptoms of PACS [58]. The diversity of control conditions across studies may have affected the observed results. Comparisons of no-intervention or waitlist controls with alternative exercise modalities or educational guidance may overestimate or underestimate the reported benefits. Variations in execution modalities within control groups could limit direct comparisons and the generalizability of results.
The quality of life was one of the outcomes investigated in this review because PACS-related symptomatology has been demonstrated to reduce the wellbeing and lifestyle in people experiencing this condition [9,10]. In fact, most of the included studies evaluated whether an exercise protocol could influence HRQoL, and almost all reported related improvements in people with PACS. Ref. [32] did not detect significant changes in quality of life when comparing the same aerobic training but supervised by a general or specialized physiotherapist. However, it should be noted that the assessment of HRQoL was performed with the SF-12, consisting of a mental and physical component, and significant changes were, nevertheless, reported for the physical part of the survey. In this regard, the other studies that used the SF-12 [27,29,35] reported positive results in both the physical and mental components of the survey. However, the exercise interventions were shorter, and the studies were not multicentric; thus, the results were not applicable to a larger and more diverse sample. Studies that used the longer version of the same questionnaire (SF-36) [30,39], with similar sample size and duration of the exercise protocol [39,46], reported significant improvements in HRQoL. The SF-36 appears more sensitive in capturing changes in both physical and mental health domains, while the shorter version may be less responsive to subtle improvements, especially in physical function or fatigue. Compared with the other tools used (EQ-5D-5L and PROMIS), current evidence is still limited on their responsiveness in PACS. Overall, the SF-36 may be preferable for detailed evaluation, whereas the SF-12 can be considered for practical assessment [59,60,61]. Therefore, given that the quality-of-life assessment methods varied across the included studies, the research methodology may have influenced the outcome of these studies. Similarly, the functional fitness assessment varied across studies. Notwithstanding, the outcome-related parameters were improved in all the included studies regardless of the exercise protocol characteristics (excluding virtual reality, which showed no superior change compared to traditional methods) [33]. The overall low risk of bias identified across studies indicates that the quality of the studies is mostly high. However, in the studies where “some concerns” were identified, the reason was primarily attributable to masking procedures, which were not always possible due to the nature of the interventions [27,31,33,34,42].
Among the limitations of this review, the heterogeneity of the intervention protocols should be acknowledged. Most studies were not comparable in terms of frequency, duration, and modality. Therefore, despite overall positive trends, the heterogeneity of protocols prevented quantification of the magnitude of improvements across studies, making it difficult to establish which type of exercise should be considered superior. Moreover, the studies were conducted based on the knowledge available at the time, leading to differences in investigation methodologies and target population (e.g., diagnosis of PACS differed in terms of symptoms duration and clinical definition). Another limitation concerns the variability of outcome assessment tools. Quality of life was measured using different questionnaires (e.g., SF-12 vs. SF-36). Similarly, functional fitness assessments varied across studies. Moreover, these outcomes were not systematically analysed as a primary outcome, which may have underestimated their role in relation to exercise interventions. Finally, although overall dropout rates were below 37%, lack of adherence in some studies may still have affected outcome reliability and should be considered in the design of future studies.
Future research should address methodological biases and include preventive measures as exercise protocols to monitor long-term impacts and to improve understanding and treatment of PACS condition [7]. It is crucial to expand ongoing intervention studies based on current knowledge and generate high-quality evidence for diverse long-COVID populations [4,10]. Moreover, a cohort study [62] compared long-term health outcomes following COVID-19 illness and seasonal influenza, suggesting that the management of long COVID with exercise protocol could be transferable to seasonal influenza, hypothesizing the same improvement in functional fitness and HRQoL parameters. Further research could, therefore, be conducted, focusing on the influence of exercise interventions while recovering from viral infections.
5. Conclusions
The management of PACS still requires further in-depth studies regarding the treatment of symptoms according to its clinical definition. This review shows that an intervention protocol based on physical exercise is safe and feasible, also via telerehabilitation. The duration of the intervention can range from 2 to 12 weeks, although a higher adherence has been observed with 8-week protocols, three to five sessions per week, 1 h/session. An aerobic activity may provide benefits, as moderate or continuous, with greater effects if combined with strength-based exercises and/or as concurrent training. Further benefits can be achieved with specific respiratory muscle training (i.e., breathing exercises). However, not all exercise types consistently improved HRQoL or symptoms, highlighting the need for individualized protocols. Recommendations should consider clinical phenotype, baseline functional capacity, and adherence potential. Therefore, while exercise interventions appear promising, their prescription should be tailored and carefully monitored rather than universally recommended.
Author Contributions
Conceptualization, V.P. and G.C.; methodology, V.P., A.G., F.L. and S.M.; software, V.P.; writing—original draft preparation, V.P.; writing—review and editing, V.P., O.d.M. and G.C.; supervision, M.V. and G.C.; funding acquisition, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by “Bando di Ateneo per la Ricerca 2022—Azione B” to G.C. (grant code MUR_DM737_2022_FIL_PROGETTI_B_CONDELLO_COFIN).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. WHO clinical case definition working group on post-COVID-19 condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, N.J.; Penfold, R.; Giunchiglia, V.; Bowyer, V.; Sudre, C.H.; Canas, L.S.; Deng, J.; Murray, B.; Kerfoot, E.; Antonelli, M.; et al. The effects of COVID-19 on cognitive performance in a community-based cohort: A COVID symptom study biobank prospective cohort study. eClinicalMedicine 2023, 62, 102086. [Google Scholar] [CrossRef] [PubMed]
- Hawke, L.D.; Nguyen, A.T.P.; Ski, C.F.; Thompson, D.R.; Ma, C.; Castle, D. Interventions for mental health, cognition, and psychological wellbeing in long COVID: A systematic review of registered trials. Psychol. Med. 2022, 52, 2426–2440. [Google Scholar] [CrossRef]
- Zhao, S.; Martin, E.M.; Reuken, P.A.; Scholcz, A.; Ganse-Dumrath, A.; Srowig, A.; Utech, I.; Kozik, V.; Radscheidt, M.; Brodoehl, S.; et al. Long COVID is associated with severe cognitive slowing: A multicentre cross-sectional study. eClinicalMedicine 2024, 68, 102434. [Google Scholar] [CrossRef] [PubMed]
- O’MAhoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. eClinicalMedicine 2022, 55, 101762. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; Ben David, S.S.; Lerner, U.; Bivas-Benita, M.; et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: Nationwide cohort study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef]
- Sivan, M.; Parkin, A.; Makower, S.; Greenwood, D.C. Post-COVID syndrome symptoms, functional disability, and clinical severity phenotypes in hospitalized and nonhospitalized individuals: A cross-sectional evaluation from a community COVID rehabilitation service. J. Med. Virol. 2022, 94, 1419–1427. [Google Scholar] [CrossRef]
- Gentilotti, E.; Górska, A.; Tami, A.; Gusinow, R.; Mirandola, M.; Baño, J.R.; Baena, Z.R.P.; Rossi, E.; Hasenauer, J.; Lopes-Rafegas, I.; et al. Clinical phenotypes and quality of life to define post-COVID-19 syndrome: A cluster analysis of the multinational, prospective ORCHESTRA cohort. eClinicalMedicine 2023, 62, 102107. [Google Scholar] [CrossRef]
- Jones, R.; Davis, A.; Stanley, B.; Julious, S.; Ryan, D.; Jackson, D.J.; Halpin, D.M.; Hickman, K.; Pinnock, H.; Quint, J.K.; et al. Risk predictors and symptom features of Long COVID within a broad primary care patient population including both tested and untested patients. Pragmat. Obs. Res. 2021, 12, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’HAra, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Scurati, R.; Papini, N.; Giussani, P.; Alberti, G.; Tringali, C. The challenge of Long COVID-19 management: From disease molecular hallmarks to the proposal of exercise as therapy. Int. J. Mol. Sci. 2022, 23, 12311. [Google Scholar] [CrossRef]
- Humphreys, H.; Kilby, L.; Kudiersky, N.; Copeland, R. Long COVID and the role of physical activity: A qualitative study. BMJ Open 2021, 11, e047632. [Google Scholar] [CrossRef]
- Wright, J.; Astill, S.L.; Sivan, M. The relationship between physical activity and Long COVID: A cross-sectional study. Int. J. Environ. Res. Public Health 2022, 19, 5093. [Google Scholar] [CrossRef]
- Gomide, E.B.G.; Mazzonetto, L.F.; Cordeiro, J.F.C.; Cordeiro, D.C.; Oliveira, A.d.S.; Fioco, E.M.; Venturini, A.C.R.; Abdalla, P.P.; Da Silva, L.S.L.; Júnior, M.F.T.; et al. Being physically active leads to better recovery prognosis for people diagnosed with COVID-19: A cross-sectional study. Int. J. Environ. Res. Public Health 2022, 19, 14908. [Google Scholar] [CrossRef]
- Grishechkina, I.A.; Lobanov, A.A.; Andronov, S.V.; Rachin, A.P.; Fesyun, A.D.; Ivanova, E.P.; Masiero, S.; Maccarone, M.C. Long-term outcomes of different rehabilitation programs in patients with long COVID syndrome: A cohort prospective study. Eur. J. Transl. Myol. 2023, 33, 11063. [Google Scholar] [CrossRef] [PubMed]
- Udina, C.; Ars, J.; Morandi, A.; Vilaró, J.; Cáceres, C.; Inzitari, M. Rehabilitation in adult post-COVID-19 patients in post-acute care with Therapeutic Exercise. J. Frailty Aging 2021, 10, 297–300. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Alawna, M. The effect of aerobic exercise on immune biomarkers and symptoms severity and progression in patients with COVID-19: A randomized control trial. J. Bodyw. Mov. Ther. 2021, 28, 425–432. [Google Scholar] [CrossRef]
- Mayer, K.P.; Steele, A.K.; Soper, M.K.; Branton, J.D.; Lusby, M.L.; Kalema, A.G.; E Dupont-Versteegden, E.; A Montgomery-Yates, A. Physical Therapy Management of an Individual with Post-COVID Syndrome: A Case Report. Phys. Ther. 2021, 101, pzab098. [Google Scholar] [CrossRef] [PubMed]
- Del Corral, T.; Fabero-Garrido, R.; Plaza-Manzano, G.; Fernández-de-Las-Peñas, C.; Navarro-Santana, M.; López-de-Uralde-Villanueva, I. Home-based respiratory muscle training on quality of life and exercise tolerance in long-term post-COVID-19: Randomized controlled trial. Ann. Phys. Rehabil. Med. 2023, 66, 101709. [Google Scholar] [CrossRef]
- Yelin, D.; Moschopoulos, C.D.; Margalit, I.; Gkrania-Klotsas, E.; Landi, F.; Stahl, J.-P.; Yahav, D. ESCMID rapid guidelines for assessment and management of long COVID. Clin. Microbiol. Infect. 2022, 28, 955–972. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brown, P.; Brunnhuber, K.; Chalkidou, K.; Chalmers, I.; Clarke, M.; Fenton, M.; Forbes, C.; Glanville, J.; Hicks, N.J.; Moody, J.; et al. How to formulate research recommendations. BMJ 2006, 333, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 4. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Jimeno-Almazán, A.; Franco-López, F.; Buendía-Romero, Á.; Martínez-Cava, A.; Sánchez-Agar, J.A.; Martínez, B.J.S.; Courel-Ibáñez, J.; Pallarés, J.G. Rehabilitation for post-COVID-19 condition through a supervised exercise intervention: A randomized controlled trial. Scand. J. Med. Sci. Sports 2022, 32, 1791–1801. [Google Scholar] [CrossRef]
- Espinoza-Bravo, C.; Arnal-Gómez, A.; Martínez-Arnau, F.M.; Núñez-Cortés, R.; Hernández-Guillén, D.; Flor-Rufino, C.; Cortés-Amador, S. Effectiveness of functional or aerobic exercise combined with breathing techniques in telerehabilitation for patients with Long COVID: A randomized controlled trial. Phys. Ther. 2023, 103, pzad118. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz, B.J.; Courel-Ibáñez, J.; Pallarés, J.G. Effects of a concurrent training, respiratory muscle exercise, and self-management recommendations on recovery from post-COVID-19 conditions: The RECOVE trial. J. Appl. Physiol. 1985 2023, 134, 95–104. [Google Scholar] [CrossRef]
- Mooren, J.M.; Garbsch, R.; Schäfer, H.; Kotewitsch, M.; Waranski, M.; Teschler, M.; Schmitz, B.; Mooren, F.C. Medical rehabilitation of patients with post-COVID-19 syndrome—A comparison of aerobic interval and continuous training. J. Clin. Med. 2023, 12, 6739. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, C.; Bernal-Utrera, C.; Anarte-Lazo, E.; Gonzalez-Gerez, J.J.; Saavedra-Hernandez, M. A 14-day therapeutic exercise telerehabilitation protocol of physiotherapy is effective in non-hospitalized post-COVID-19 conditions: A randomized controlled trial. J. Clin. Med. 2023, 12, 776. [Google Scholar] [CrossRef] [PubMed]
- Romanet, C.; Wormser, J.; Fels, A.; Lucas, P.; Prudat, C.; Sacco, E.; Bruel, C.; Plantefève, G.; Pene, F.; Chatellier, G.; et al. Effectiveness of exercise training on the dyspnoea of individuals with long COVID: A randomised controlled multicentre trial. Ann. Phys. Rehabil. Med. 2023, 66, 101765. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, S.; Bogacz, K.; Rutkowska, A.; Szczegielniak, J.; Casaburi, R. Inpatient post-COVID-19 rehabilitation program featuring virtual reality—Preliminary results of randomized controlled trial. Front. Public Health 2023, 11, 1121554. [Google Scholar] [CrossRef]
- Vallier, J.-M.; Simon, C.; Bronstein, A.; Dumont, M.; Jobic, A.; Paleiron, N.; Mely, L. Randomized controlled trial of home-based vs. hospital-based pulmonary rehabilitation in post COVID-19 patients. Eur. J. Phys. Rehabil. Med. 2023, 59, 103–110. [Google Scholar] [CrossRef]
- Barz, A.; Berger, J.; Speicher, M.; Morsch, A.; Wanjek, M.; Rissland, J.; Jäger, J. Effects of a symptom-titrated exercise program on fatigue and quality of life in people with post-COVID condition—A randomized controlled trial. Sci. Rep. 2024, 14, 30511. [Google Scholar] [CrossRef]
- Senén, A.B.; Fernández, A.G.; López, J.G.; Rodríguez, J.B.; Brejano, M.G.; Guillén, P.C.; Porras, C.d.C.; Perea, C.M.; Miravalles, E.G.; Blanco, Á.S.; et al. Functional rehabilitation based on therapeutic exercise training in patients with postacute COVID syndrome (RECOVER). Rev. Esp. Cardiol. 2024, 77, 167–175. [Google Scholar] [CrossRef]
- Calvo-Paniagua, J.; Díaz-Arribas, M.J.; Valera-Calero, J.A.; Ramos-Sánchez, M.O.; Fernández-De-Las-Peñas, C.; Navarro-Santana, M.J.; del Corral, T.; Plaza-Manzano, G. Educational, exercise, and occupational therapy-based telerehabilitation program versus “wait-and-see” for improving self-perceived exertion in patients with post-covid fatigue and dyspnea: A randomized clinical trial. Am. J. Phys. Med. Rehabil. 2024, 103, 797–804. [Google Scholar] [CrossRef]
- Kaddoussi, R.; Rejeb, H.; Kalai, A.; Zaara, E.; Rouetbi, N.; Frih, Z.B.S.; Zmijewski, P.; Ben Saad, H. Effects of a cardiopulmonary rehabilitation programme on submaximal exercise in Tunisian patients with long-COVID19: A randomized clinical trial. Biol. Sport 2024, 41, 197–217. [Google Scholar] [CrossRef]
- Kerling, A.; Beyer, S.; Dirks, M.; Scharbau, M.; Hennemann, A.-K.; Dopfer-Jablonka, A.; Lampe, V.; Salzmann, J.H.W.; Tegtbur, U.; Drick, N.; et al. Effects of a randomized-controlled and online-supported physical activity intervention on exercise capacity, fatigue and health related quality of life in patients with post-COVID-19 syndrome. BMC Sports Sci. Med. Rehabil. 2024, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Lin, C.H.; Chao, T.C.; Lin, C.-H.; Chang, C.-C.; Huang, C.-Y.; Chiang, S.-L. Effectiveness of a 12-week telerehabilitation training in people with long COVID: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2024, 67, 101853. [Google Scholar] [CrossRef]
- McGregor, G.; Sandhu, H.; Bruce, J.; Sheehan, B.; McWilliams, D.; Yeung, J.; Jones, C.; Lara, B.; Alleyne, S.; Smith, J.; et al. Clinical effectiveness of an online supervised group physical and mental health rehabilitation programme for adults with post-covid-19 condition (REGAIN study): Multicentre randomised controlled trial. BMJ 2024, 384, e076506. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, A.; Adodo, R.; Hodges, G.; Webber, S.C.; Sanchez-Ramirez, D.C. Virtual pulmonary rehabilitation approaches in patients with post COVID syndrome: A pilot study. BMC Pulm. Med. 2024, 24, 139. [Google Scholar] [CrossRef]
- Yasacı, Z.; Mustafaoglu, R.; Ozgur, O.; Kuveloglu, B.; Esen, Y.; Ozmen, O.; Yalcinkaya, E.Y. Virtual recovery: Efficacy of telerehabilitation on dyspnea, pain, and functional capacity in post-COVID-19 syndrome. Ir. J. Med. Sci. 2025, 194, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Besnier, F.; Malo, J.; Mohammadi, H.; Clavet, S.; Klai, C.; Martin, N.; Bérubé, B.; Lecchino, C.; Iglesies-Grau, J.; Vincent, T.; et al. Effects of Cardiopulmonary Rehabilitation on Cardiorespiratory Fitness and Clinical Symptom Burden in Long COVID: Results From the COVID-Rehab Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2025, 104, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Daynes, E.; Evans, R.A.; Greening, N.J.; Bishop, N.C.; Yates, T.; Lozano-Rojas, D.; Ntotsis, K.; Richardson, M.; Baldwin, M.M.; Hamrouni, M.; et al. Post-Hospitalisation COVID-19 Rehabilitation (PHOSP-R): A randomised controlled trial of exercise-based rehabilitation. Eur. Respir. J. 2025, 65, 2402152. [Google Scholar] [CrossRef] [PubMed]
- Sick, J.; Steinbacher, V.; Kotnik, D.; König, F.; Recking, T.; Bengsch, D.; König, D. Exercise rehabilitation in post COVID-19 patients: A randomized controlled trial of different training modalities. Eur. J. Phys. Rehabil. Med. 2025, 61, 130–140. [Google Scholar] [CrossRef]
- Lamprecht, B. Gibt es ein post-COVID-syndrom? [Is there a post-COVID syndrome?]. Pneumologe 2020, 17, 398–405. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Collins, E.; Shaver, N.; Little, J. The ongoing importance of patient-informed, collaborative research in advancing the definition and harmonization of post COVID-19 condition (PCC) subtypes across diverse populations. eBioMedicine 2025, 113, 105592. [Google Scholar] [CrossRef]
- Chandan, J.S.; Brown, K.R.; Simms-Williams, N.; Bashir, N.Z.; Camaradou, J.; Heining, D.; Turner, G.M.; Rivera, S.C.; Hotham, R.; Minhas, S.; et al. Non-pharmacological therapies for post-viral syndromes, including long COVID: A systematic review. Int. J. Environ. Res. Public Health 2023, 20, 3477. [Google Scholar] [CrossRef]
- Araújo, B.T.S.; Barros, A.E.V.R.; Nunes, D.T.X.; de Aguiar, M.I.R.; Mastroianni, V.W.; de Souza, J.A.F.; Fernades, J.; Campos, S.L.; Brandão, D.C.; de Andrade, A.D. Effects of continuous aerobic training associated with resistance training on maximal and submaximal exercise tolerance, fatigue, and quality of life of patients post-COVID-19. Physiother. Res. Int. 2023, 28, e1972. [Google Scholar] [CrossRef]
- Delevatti, R.S.; Danielevicz, A.; Sirydakis, M.E.; de Melo, P.U.G.; Freitas, C.d.l.R.; Rech, C.R.; Guglielmo, L.G.A.; Speretta, G.F.F.; Hansen, F.; Fonseca, F.R.; et al. Effects of physical training on functional, clinical, morphological, behavioural and psychosocial outcomes in post-COVID-19 infection: COVID-19 and REhabilitation study (CORE-study)—A study protocol for a randomised controlled clinical trial. Trials 2023, 24, 39. [Google Scholar] [CrossRef]
- Lobanov, A.A.; Grishechkina, I.A.; Andronov, S.V.; Barashkov, G.N.; Popov, A.I.; D.FEsyun, A.; Ivanova, E.P.; Maccarone, M.C.; Masiero, S. Can aquatic exercises contribute to the improvement of the gait stereotype function in patients with Long COVID outcomes? Eur. J. Transl. Myol. 2022, 32, 10698. [Google Scholar] [CrossRef]
- Capela Santos, D.; Jaconiano, S.; Macedo, S.; Ribeiro, F.; Ponte, S.; Soares, P.; Boaventura, P. Yoga for COVID-19: An ancient practice for a new condition—A literature review. Complement. Ther. Clin. Pract. 2023, 50, 101717. [Google Scholar] [CrossRef] [PubMed]
- Cattadori, G.; Di Marco, S.; Baravelli, M.; Picozzi, A.; Ambrosio, G. Exercise training in post-COVID-19 patients: The need for a multifactorial protocol for a multifactorial pathophysiology. J. Clin. Med. 2022, 11, 2228. [Google Scholar] [CrossRef]
- Torres, G.; Constantinou, D.; Gradidge, P.; Patel, D.; Patricios, J. Exercise is the most important medicine for COVID-19. Curr. Sports Med. Rep. 2023, 22, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Coscia, F.; Mancinelli, R.; Gigliotti, P.V.; Checcaglini, F.; Fanò-Illic, G. Physical activity effects on muscle fatigue in sport in active adults with long COVID-19: An observational study. Diagnostics 2023, 13, 1336. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of the EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: A cohort study. Lancet Infect. Dis. 2024, 24, 239–255. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).