Fecal Microbiota and Associated Metabolites Are Minimally Affected by Ten Weeks of Resistance Training in Younger and Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Participant Eligibility
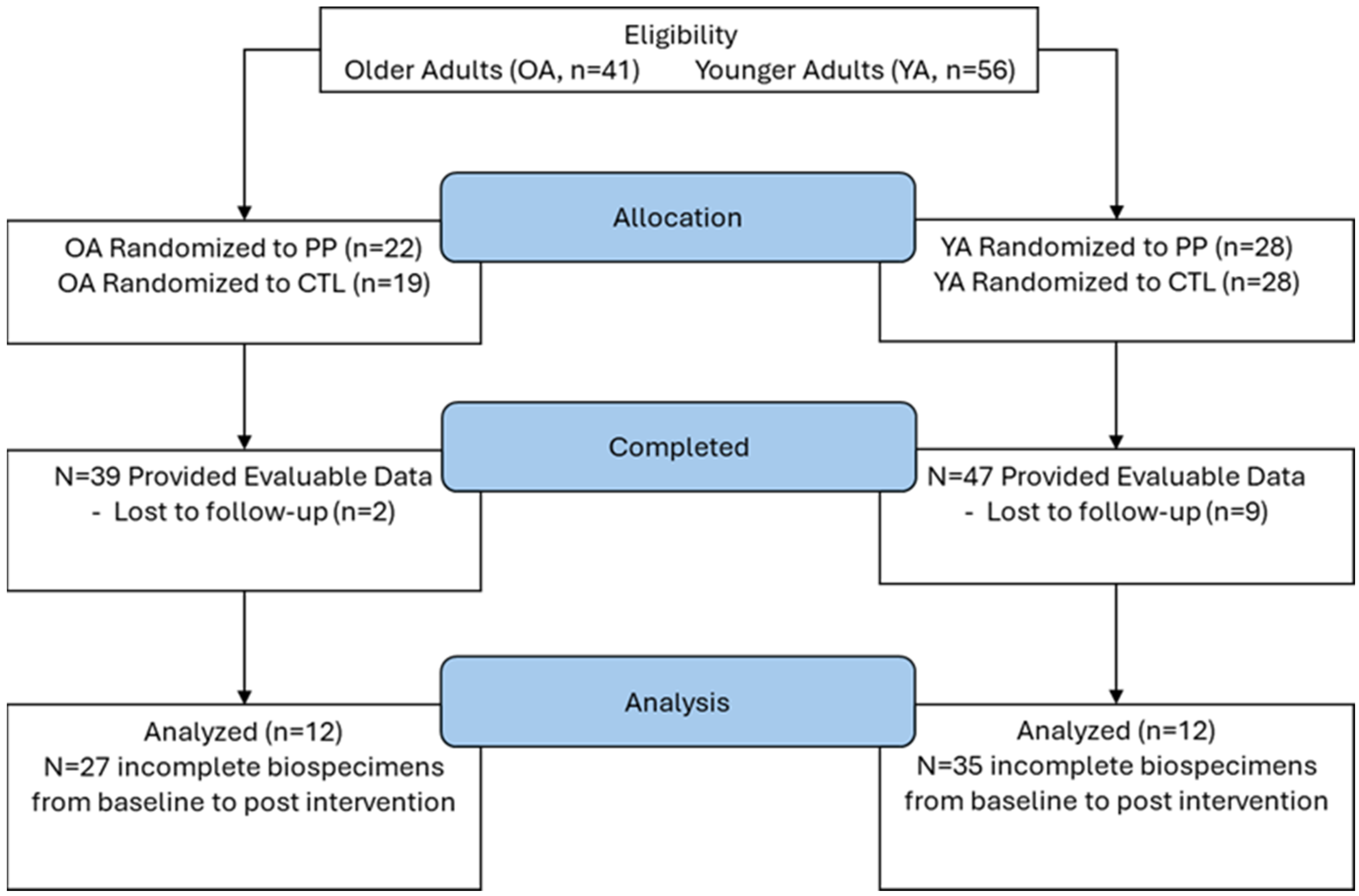
2.2. Study Design
2.3. Body Composition
2.4. Ultrasound for Vastus Lateralis Thickness
2.5. Supervised RT Program
2.6. Dietary Analysis
2.7. Fecal Microbiome Analysis
2.8. Fecal and Serum SCFA Analysis
2.9. Serum Biomarkers
2.10. Statistics
3. Results
3.1. Participant Characteristics and RT Outcomes
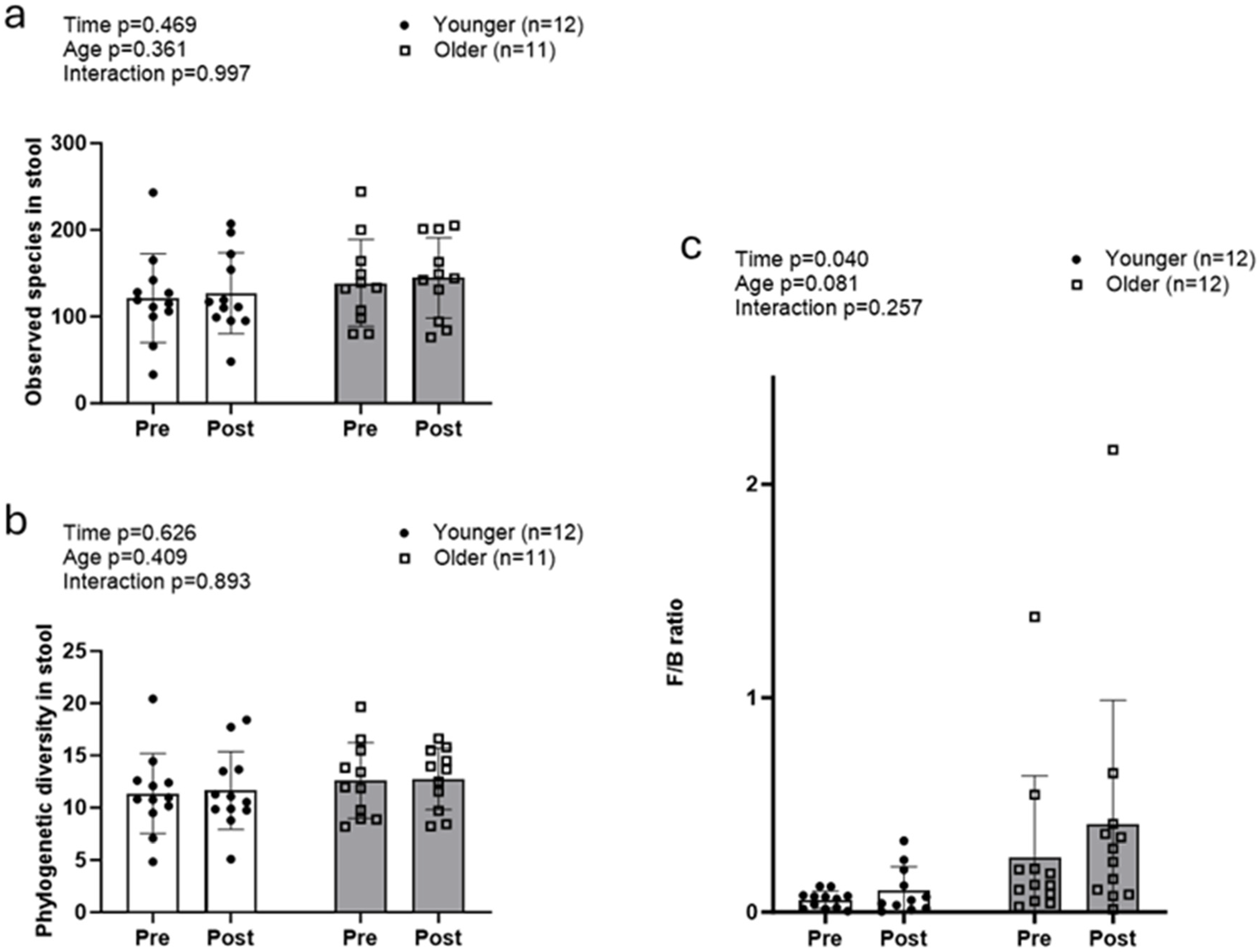
3.2. Fecal Microbiota Between Age Groups with Resistance Training
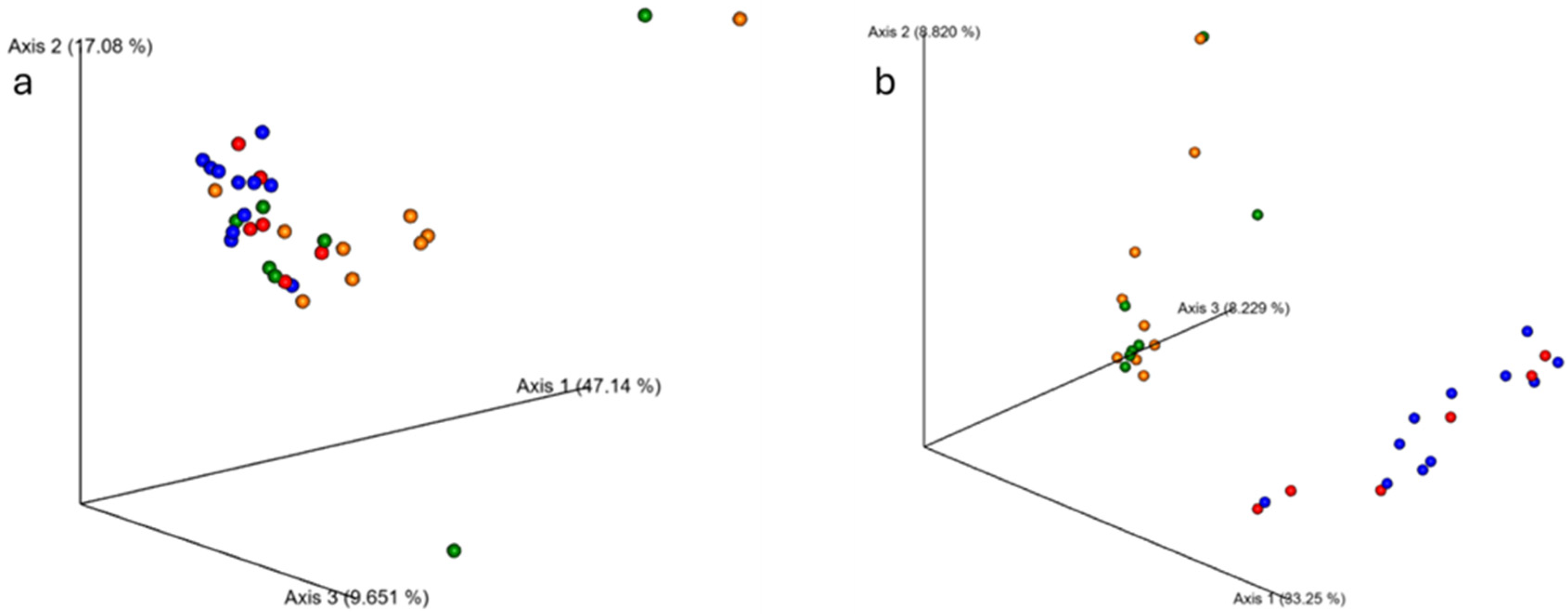
3.3. Beta Diversity Between Age Groups with Resistance Training
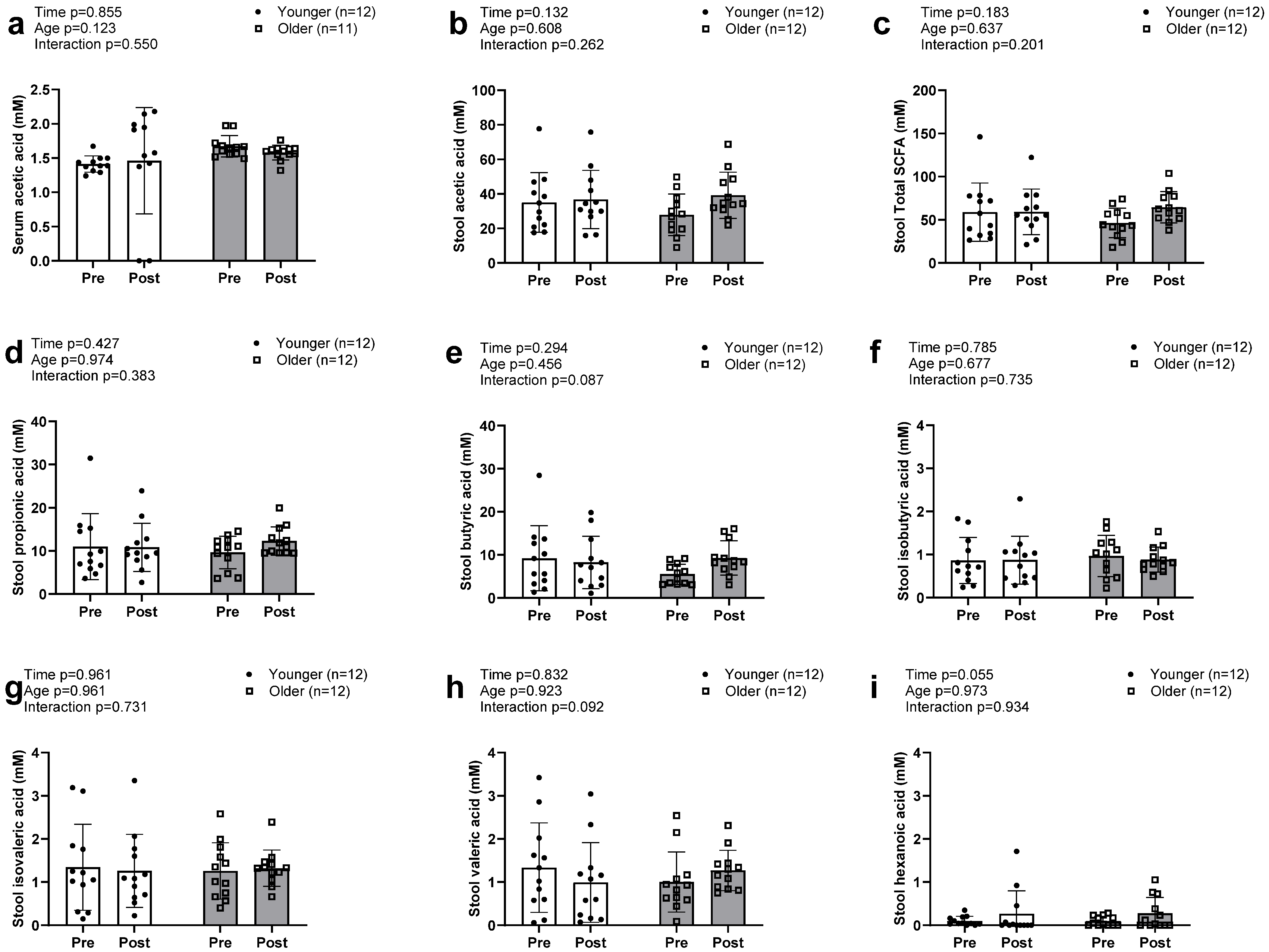
3.4. Serum and Fecal SCFAs Between Age Groups with Resistance Training
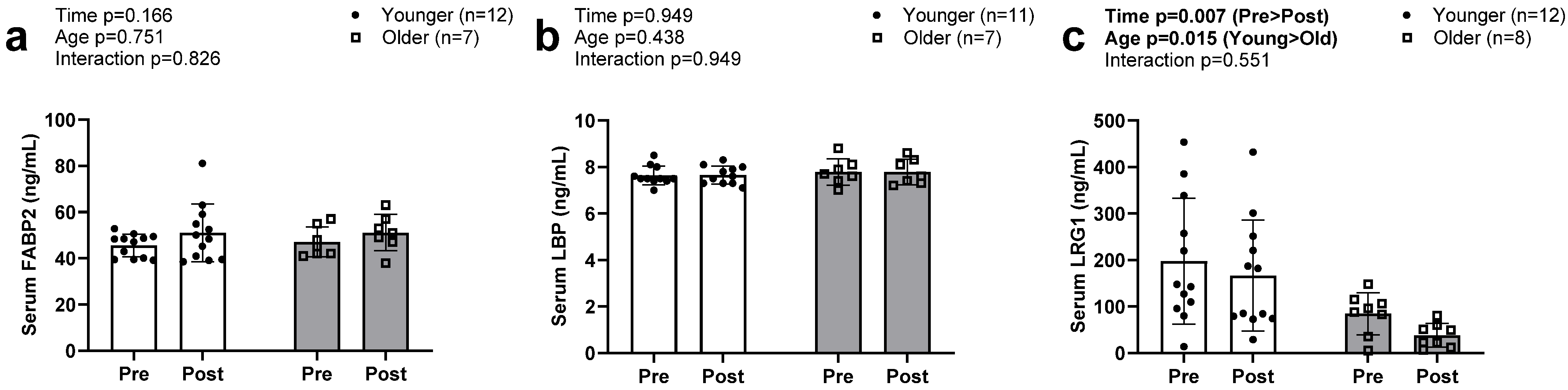
3.5. Serum Biomarkers of Intestinal Barrier Function
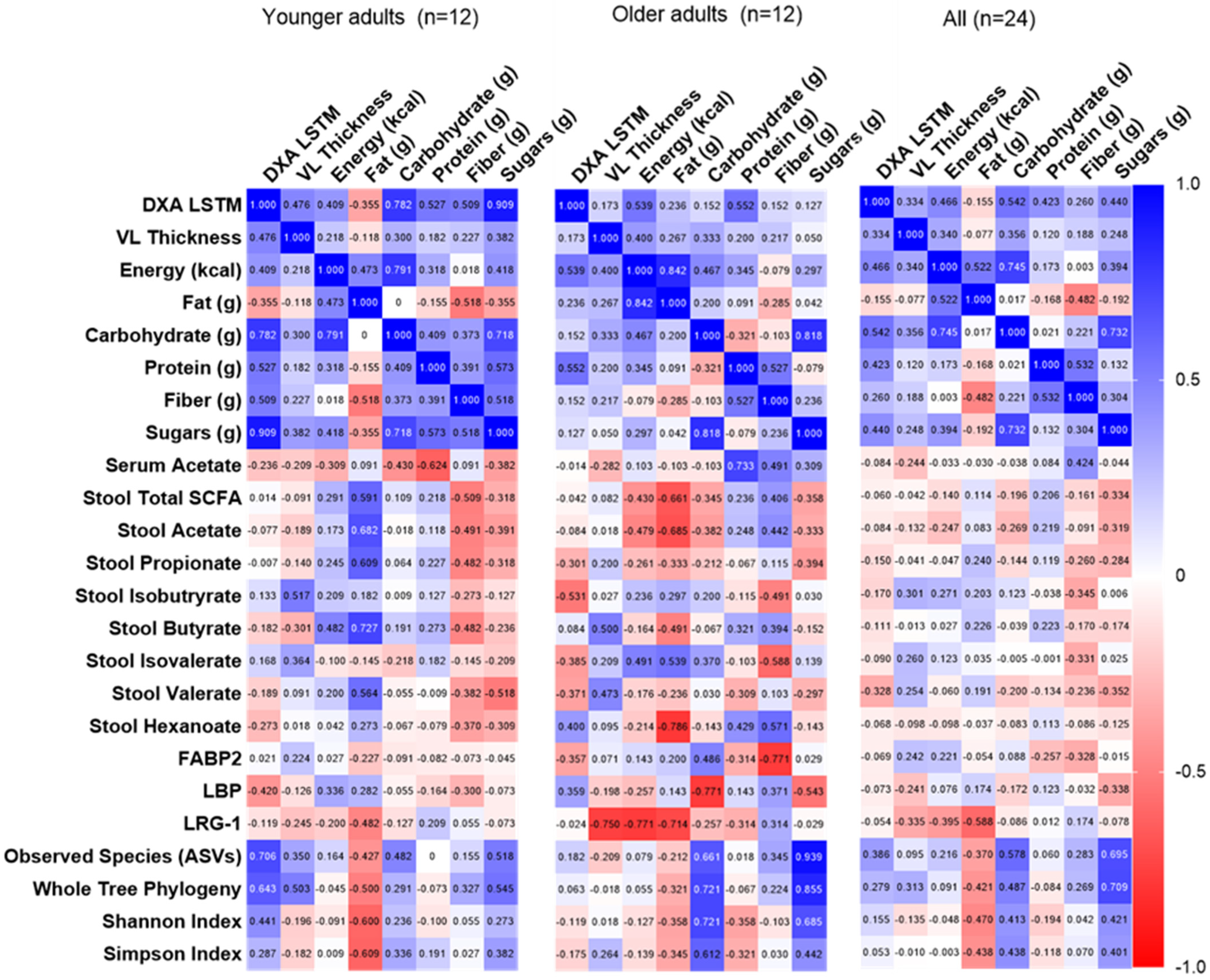
3.6. Correlations Among Dietary Intake, Fecal/Serum Measures, and Resistance Training Adaptations
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASV | Amplicon Sequence Variant |
| BMI | Body Mass Index |
| DXA | Dual-Energy X-ray Absorptiometry |
| ET | Endurance Training |
| FABP | Fatty Acid Binding Protein |
| F/B | Firmicutes to Bacteroidetes |
| FDR | False Discovery Rate |
| GC | Gas Chromatography |
| G × T | Group × Time |
| HMC | High Microbiome-Accessible Carbohydrate |
| IRB | Institutional Review Board |
| LBP | Lipopolysaccharide Binding Protein |
| LBM | Lean Body Mass |
| LMC | Low Microbiome-accessible Carbohydrate |
| LRG1 | Leucine-Rich Alpha-2 Glycoprotein 1 |
| LSTM | Lean Soft Tissue Mass |
| NDSR | Nutrition Data System for Research |
| OTU | Operational Taxonomic Unit |
| PCR | Polymerase Chain Reaction |
| PRE | Pre-intervention |
| POST | Post-intervention |
| RPE | Rating of Perceived Exertion |
| RT | Resistance Training |
| SCFA | Short-Chain Fatty Acid |
| USG | Urine Specific Gravity |
| VL | Vastus Lateralis |
References
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Correction: Yoo, J.Y., et al. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 2046. [Google Scholar] [CrossRef]
- Carmody, R.N.; Gerber, G.K.; Luevano, J.M., Jr.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Maurice, C.F.; Haiser, H.J.; Turnbaugh, P.J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.M.; Kornblith, L.Z.; Christie, S.A.; Conroy, A.S.; Nelson, M.F.; Campion, E.M.; Callcut, R.A.; Calfee, C.S.; Lamere, B.J.; Fadrosh, D.W.; et al. Characterizing the gut microbiome in trauma: Significant changes in microbial diversity occur early after severe injury. Trauma Surg. Acute Care Open 2017, 2, e000108. [Google Scholar] [CrossRef]
- Sittipo, P.; Shim, J.W.; Lee, Y.K. Microbial Metabolites Determine Host Health and the Status of Some Diseases. Int. J. Mol. Sci. 2019, 20, 5296. [Google Scholar] [CrossRef]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Yan, H.; Diao, H.; Xiao, Y.; Li, W.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Mao, X.; Luo, Y.; et al. Gut microbiota can transfer fiber characteristics and lipid metabolic profiles of skeletal muscle from pigs to germ-free mice. Sci. Rep. 2016, 6, 31786. [Google Scholar] [CrossRef]
- Fernández, J.; Fernández-Sanjurjo, M.; Iglesias-Gutiérrez, E.; Martínez-Camblor, P.; Villar, C.J.; Tomás-Zapico, C.; Fernández-García, B.; Lombó, F. Resistance and Endurance Exercise Training Induce Differential Changes in Gut Microbiota Composition in Murine Models. Front. Physiol. 2021, 12, 748854. [Google Scholar] [CrossRef]
- Cullen, J.M.A.; Shahzad, S.; Kanaley, J.A.; Ericsson, A.C.; Dhillon, J. The effects of 6 wk of resistance training on the gut microbiome and cardiometabolic health in young adults with overweight and obesity. J. Appl. Physiol. 2024, 136, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Remely, M.; Hippe, B.; Geretschlaeger, I.; Stegmayer, S.; Hoefinger, I.; Haslberger, A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: A pilot study. Wien. Klin. Wochenschr. 2015, 127, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Tang, G.; Du, Y.; Guan, H.; Jia, J.; Zhu, N.; Shi, Y.; Rong, S.; Yuan, W. Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2-mediated PI3K/Akt/mTOR signals. Br. J. Pharmacol. 2022, 179, 159–178. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Yu, C.; Liu, S.; Chen, L.; Shen, J.; Niu, Y.; Wang, T.; Zhang, W.; Fu, L. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J. Endocrinol. 2019, 243, 125–135. [Google Scholar] [CrossRef]
- Yang, L.; Lin, H.; Lin, W.; Xu, X. Exercise Ameliorates Insulin Resistance of Type 2 Diabetes through Motivating Short-Chain Fatty Acid-Mediated Skeletal Muscle Cell Autophagy. Biology 2020, 9, 203. [Google Scholar] [CrossRef]
- Erlandson, K.M.; Liu, J.; Johnson, R.; Dillon, S.; Jankowski, C.M.; Kroehl, M.; Robertson, C.E.; Frank, D.N.; Tuncil, Y.; Higgins, J.; et al. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther. Adv. Infect. Dis. 2021, 8, 20499361211027067. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Moore, J.H.; Smith, K.S.; Chen, D.; Lamb, D.A.; Smith, M.A.; Osburn, S.C.; Ruple, B.A.; Morrow, C.D.; Huggins, K.W.; McDonald, J.R.; et al. Exploring the Effects of Six Weeks of Resistance Training on the Fecal Microbiome of Older Adult Males: Secondary Analysis of a Peanut Protein Supplemented Randomized Controlled Trial. Sports 2022, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Cronin, O.; Barton, W.; Skuse, P.; Penney, N.C.; Garcia-Perez, I.; Murphy, E.F.; Woods, T.; Nugent, H.; Fanning, A.; Melgar, S.; et al. A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. mSystems 2018, 3, e00044-18. [Google Scholar] [CrossRef]
- Bycura, D.; Santos, A.C.; Shiffer, A.; Kyman, S.; Winfree, K.; Sutliffe, J.; Pearson, T.; Sonderegger, D.; Cope, E.; Caporaso, J.G. Impact of Different Exercise Modalities on the Human Gut Microbiome. Sports 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Aya, V.; Jimenez, P.; Muñoz, E.; Ramírez, J.D. Effects of exercise and physical activity on gut microbiota composition and function in older adults: A systematic review. BMC Geriatr. 2023, 23, 364. [Google Scholar] [CrossRef]
- Sexton, C.L.; Smith, M.A.; Smith, K.S.; Osburn, S.C.; Godwin, J.S.; Ruple, B.A.; Hendricks, A.M.; Mobley, C.B.; Goodlett, M.D.; Frugé, A.D.; et al. Effects of Peanut Protein Supplementation on Resistance Training Adaptations in Younger Adults. Nutrients 2021, 13, 3981. [Google Scholar] [CrossRef]
- Lamb, D.A.; Moore, J.H.; Smith, M.A.; Vann, C.G.; Osburn, S.C.; Ruple, B.A.; Fox, C.D.; Smith, K.S.; Altonji, O.M.; Power, Z.M.; et al. The effects of resistance training with or without peanut protein supplementation on skeletal muscle and strength adaptations in older individuals. J. Int. Soc. Sports Nutr. 2020, 17, 66. [Google Scholar] [CrossRef]
- Kumar, R.; Eipers, P.; Little, R.B.; Crowley, M.; Crossman, D.K.; Lefkowitz, E.J.; Morrow, C.D. Getting started with microbiome analysis: Sample acquisition to bioinformatics. Curr. Protoc. Hum. Genet. 2014, 82, 18.8.1–18.8.29. [Google Scholar] [CrossRef]
- Frugé, A.D.; Van der Pol, W.; Rogers, L.Q.; Morrow, C.D.; Tsuruta, Y.; Demark-Wahnefried, W. Fecal Akkermansia muciniphila Is Associated with Body Composition and Microbiota Diversity in Overweight and Obese Women with Breast Cancer Participating in a Presurgical Weight Loss Trial. J. Acad. Nutr. Diet. 2020, 120, 650–659. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Van Der Pol, W.J.; Kumar, R.; Morrow, C.D.; Blanchard, E.E.; Taylor, C.M.; Martin, D.H.; Lefkowitz, E.J.; Muzny, C.A. In Silico and Experimental Evaluation of Primer Sets for Species-Level Resolution of the Vaginal Microbiota Using 16S Ribosomal RNA Gene Sequencing. J. Infect. Dis. 2019, 219, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Estaki, M.; Jiang, L.; Bokulich, N.A.; McDonald, D.; González, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vázquez-Baeza, Y.; et al. QIIME 2 Enables Comprehensive End-to-End Analysis of Diverse Microbiome Data and Comparative Studies with Publicly Available Data. Curr. Protoc. Bioinform. 2020, 70, e100. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Zhao, G.; Nyman, M.; Jönsson, J.A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. BMC 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, J.F.; Nyman, M.; Jönsson, J.A. Determination of short-chain fatty acids in serum by hollow fiber supported liquid membrane extraction coupled with gas chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 846, 202–208. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Hoshino, Y.; Hosokawa, M.; Takeyama, H.; Higuchi, M. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol. Rep. 2018, 6, e13935. [Google Scholar] [CrossRef]
- Huang, L.; Li, T.; Zhou, M.; Deng, M.; Zhang, L.; Yi, L.; Zhu, J.; Zhu, X.; Mi, M. Hypoxia Improves Endurance Performance by Enhancing Short Chain Fatty Acids Production via Gut Microbiota Remodeling. Front. Microbiol. 2022, 12, 820691. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef]
- Eveleens Maarse, B.C.; Eggink, H.M.; Warnke, I.; Bijlsma, S.; van den Broek, T.J.; Oosterman, J.E.; Caspers, M.P.M.; Sybesma, W.; Gal, P.; van Kraaij, S.J.W.; et al. Impact of fibre supplementation on microbiome and resilience in healthy participants: A randomized, placebo-controlled clinical trial. Nutr. Metab. Cardiovasc. Dis. NMCD 2024, 34, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Numao, S.; Uchida, R.; Kurosaki, T.; Nakagaichi, M. Correction: Circulating Fatty Acid-binding Protein 4 Response to Acute Aerobic Exercise in Healthy Men. Int. J. Sports Med. 2023, 44, e1. [Google Scholar] [CrossRef] [PubMed]
- Shinzaki, S.; Matsuoka, K.; Iijima, H.; Mizuno, S.; Serada, S.; Fujimoto, M.; Arai, N.; Koyama, N.; Morii, E.; Watanabe, M.; et al. Leucine-rich Alpha-2 Glycoprotein is a Serum Biomarker of Mucosal Healing in Ulcerative Colitis. J. Crohn’s Colitis 2017, 11, 84–91. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, Q.; Hou, G.; Zhang, J.; Bai, J.; Lv, X.; Xie, Y.; Zhu, H.; Su, S.; Li, Y.; et al. Discovery of potential colorectal cancer serum biomarkers through quantitative proteomics on the colonic tissue interstitial fluids from the AOM-DSS mouse model. J. Proteom. 2016, 132, 31–40. [Google Scholar] [CrossRef]
- Yasutomi, E.; Inokuchi, T.; Hiraoka, S.; Takei, K.; Igawa, S.; Yamamoto, S.; Ohmori, M.; Oka, S.; Yamasaki, Y.; Kinugasa, H.; et al. Leucine-rich alpha-2 glycoprotein as a marker of mucosal healing in inflammatory bowel disease. Sci. Rep. 2021, 11, 11086. [Google Scholar] [CrossRef]
- Shen, J.; Obin, M.S.; Zhao, L. The gut microbiota, obesity and insulin resistance. Mol. Asp. Med. 2013, 34, 39–58. [Google Scholar] [CrossRef]
- Bell, D.S.H. Changes seen in gut bacteria content and distribution with obesity: Causation or association? Postgrad. Med. 2015, 127, 863–868. [Google Scholar] [CrossRef]
- De Keyzer, W.; Huybrechts, I.; De Vriendt, V.; Vandevijvere, S.; Slimani, N.; Van Oyen, H.; De Henauw, S. Repeated 24-hour recalls versus dietary records for estimating nutrient intakes in a national food consumption survey. Food Nutr. Res. 2011, 55, 7307. [Google Scholar] [CrossRef]
| Variable | Younger (n = 12) | Older (n = 12) | p-Values |
|---|---|---|---|
| Age (years) | 22 ± 2 | 59 ± 5 | p < 0.001 |
| Sex | 2 males/10 females | 4 males/8 females | |
| Body mass (kg) | Group p < 0.001 | ||
| PRE | 68.0 ± 10.3 | 96.1 ± 13.6 | Time p = 0.028 |
| POST | 69.1 ± 11.2 | 97.4 ± 15.1 | G × T p = 0.807 |
| DXA LSTM (kg) | Group p = 0.009 | ||
| PRE | 45.0 ± 7.2 | 54.8 ± 8.9 | Time p < 0.001 |
| POST | 46.6 ± 7.6 | 56.0 ± 8.9 | G × T p = 0.454 |
| VL thickness (cm) | Group p = 0.007 | ||
| PRE | 2.32 ± 0.32 | 1.93 ± 0.50 | Time p = 0.013 |
| POST | 2.54 ± 0.33 | 2.04 ± 0.34 | G × T p = 0.364 |
| Energy intake (kcal/d) | Group p = 0.146 | ||
| PRE | 1409.8 ± 431.5 | 1763.7 ± 566.6 | Time p = 0.804 |
| POST | 1438.6 ± 369.2 | 1705.1 ± 597.5 | G × T p = 0.470 |
| Fat intake (g/d) | Group p = 0.337 | ||
| PRE | 64.9 ± 17.7 | 73.1 ± 24.2 | Time p = 0.144 |
| POST | 58.4 ± 21.6 | 67.4 ± 26.1 | G × T p = 0.921 |
| Protein intake (g/d) | Group p = 0.010 | ||
| PRE | 54.2 ± 18.6 | 87.8 ± 31.8 | Time p = 0.003 |
| POST | 75.7 ± 17.4 | 107.9 ± 46.3 | G × T p = 0.909 |
| Carbohydrate intake (g/d) | Group p = 0.355 | ||
| PRE | 151.9 ± 75.6 | 189.4 ± 80.1 | Time p = 0.331 |
| POST | 154.6 ± 43.9 | 168.6 ± 63.9 | G × T p = 0.213 |
| Fiber intake (g/d) | Group p = 0.621 | ||
| PRE | 13.9 ± 6.9 | 14.2 ± 5.9 | Time p < 0.001 |
| POST | 21.8 ± 5.5 | 18.5 ± 10.9 | G × T p = 0.208 |
| Sugar intake (g/d) | Group p = 0.098 | ||
| PRE | 54.4 ± 47.2 | 75.8 ± 44.4 | Time p = 0.352 |
| POST | 43.3 ± 17.9 | 71.9 ± 37.4 | G × T p = 0.647 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agyin-Birikorang, A.; Lennon, S.; Smith, K.S.; Van Der Pol, W.; Smith, M.A.; Sexton, C.L.; Lamb, D.A.; Young, K.C.; Mobley, C.B.; Huggins, K.W.; et al. Fecal Microbiota and Associated Metabolites Are Minimally Affected by Ten Weeks of Resistance Training in Younger and Older Adults. Sports 2025, 13, 98. https://doi.org/10.3390/sports13040098
Agyin-Birikorang A, Lennon S, Smith KS, Van Der Pol W, Smith MA, Sexton CL, Lamb DA, Young KC, Mobley CB, Huggins KW, et al. Fecal Microbiota and Associated Metabolites Are Minimally Affected by Ten Weeks of Resistance Training in Younger and Older Adults. Sports. 2025; 13(4):98. https://doi.org/10.3390/sports13040098
Chicago/Turabian StyleAgyin-Birikorang, Anthony, Sarah Lennon, Kristen S. Smith, William Van Der Pol, Morgan A. Smith, Casey L. Sexton, Donald A. Lamb, Kaelin C. Young, Christopher Brooks Mobley, Kevin W. Huggins, and et al. 2025. "Fecal Microbiota and Associated Metabolites Are Minimally Affected by Ten Weeks of Resistance Training in Younger and Older Adults" Sports 13, no. 4: 98. https://doi.org/10.3390/sports13040098
APA StyleAgyin-Birikorang, A., Lennon, S., Smith, K. S., Van Der Pol, W., Smith, M. A., Sexton, C. L., Lamb, D. A., Young, K. C., Mobley, C. B., Huggins, K. W., Roberts, M. D., & Frugé, A. D. (2025). Fecal Microbiota and Associated Metabolites Are Minimally Affected by Ten Weeks of Resistance Training in Younger and Older Adults. Sports, 13(4), 98. https://doi.org/10.3390/sports13040098









