Abstract
The purpose of this study was to examine changes in body fat, muscle mass, muscle thickness, and physical and cardiorespiratory fitness in overweight and obese individuals following progressive Tabata training. Thirty-six participants were randomly assigned to either the Tabata group (four progressive cycles of body-weight high-intensity intermittent training at 75–85% of maximum perceived exertion, 3 days/week for 12 weeks) or the control group. Body composition, muscle thickness, strength and endurance, and peak oxygen uptake (VO2peak) were measured at baseline and after the training period and compared between groups. No changes in body fat percentage and fat mass were found, but the waist-to-hip ratio was lower in the Tabata group (p = 0.043). The muscle mass percentages of the right (p = 0.026) and left legs (p = 0.043) increased, while the muscle thicknesses of the biceps, triceps, rectus femoris, and vastus intermedius were increased in the Tabata group (p < 0.05) to a greater extent than in the control group (p < 0.05). Muscle strength and endurance (p < 0.05), as well as VO2peak (p = 0.006), also increased in the Tabata group. Twelve weeks of modified Tabata training effectively increased muscle mass and thickness and physical and cardiorespiratory fitness, although it did not reduce fat mass in overweight and obese participants. The combination of this training with a dietary intervention may have a more obvious impact.
1. Introduction
Obesity is a major global health concern [1], with the prevalence of overweight and obesity individuals having doubled worldwide; in 2022, 16% of adults were overweight and 43% were obese [2]. These conditions present major risks for cardiovascular, neurodegenerative, respiratory, autoimmune, non-communicable, and musculoskeletal diseases, in addition to major cancers [3,4,5].
Typically, the management of overweight and obesity involves weight control, reducing abdominal fat, and improving cardiorespiratory fitness, with approaches to these goals including dietary therapy, exercise, lifestyle changes, medication, and bariatric surgery [6]. The American College of Sports Medicine [7] recommends moderate-to-vigorous-intensity aerobic exercise as a method of weight control.
High-intensity intermittent training (HIIT) is a form of interval training that alternates brief periods of intense exercise with complete rest [8]. The duration of the exercise and rest periods varies from 6 s to 4 min over a period of 2 to 15 weeks [9]. HIIT activities such as sprinting or cycling have been shown to reduce body fat, abdominal fat, and waist circumference with minimal weight loss, while improving maximal oxygen consumption (VO2max) in obese children, healthy adolescents, overweight women, and adults with type 2 diabetes [10,11]. Additionally, an 8-week Tabata HIIT program involving sedentary men increased their levels of irisin, a hormone that aids in fat utilization [12].
Tabata training, a form of HIIT, involves 20 s of intense exercise followed by 10 s of rest, repeated for 7–8 sets [13]. Originally developed for cycling [14], it has been adapted for other forms of exercise like running and body-weight workouts. The benefits of Tabata training include burning fat, heightened metabolism during and after workouts, and the enhancement of anaerobic and aerobic systems [13,14,15]. Previous studies have shown that 4 to 12 weeks of body-weight Tabata training can increase VO2max by 5–18% [16].
Most studies on the Tabata protocol have been conducted on healthy men and women or (primarily) athletes with low physical fitness levels, where improvements in muscle gene expression related to both sports performance and health promotion have been demonstrated [16,17]. Given the high intensity of Tabata training, adherence may be challenging; for example, a study of obese male adolescents reported a 6.3% withdrawal rate [18] and 90% compliance in sedentary participants [19]. These findings suggest that this intensity may reduce enjoyment for some participants [20], and a lower-intensity regimen could enhance motivation. The body-weight exercises commonly used in Tabata training, such as squats, jumps, and lunges, involve high-impact movements which may be unsuitable for overweight individuals, highlighting the need for modifications.
We conducted a literature review and identified the following research gaps: (1) HIIT with the Tabata protocol appears to enhance fat utilization by increasing serum irisin levels, which could benefit individuals seeking to reduce body fat; (2) the very high intensity of HIIT may reduce exercise compliance due to decreased enjoyment, particularly in adolescents and young adults; (3) high-impact exercises may not be suitable for overweight or obese individuals; and (4) the effectiveness of Tabata protocols in promoting weight loss has been examined in only a few previous studies [16].
As such, in this study, we aimed to examine whether modified Tabata HIIT changes body fat, fat mass, muscle mass, muscle thickness, and physical and cardiorespiratory fitness. The hypothesis was that the modified Tabata training would improve on these variables in overweight and obesity participants.
2. Materials and Methods
2.1. Study Design: Randomized and Non-Blinded
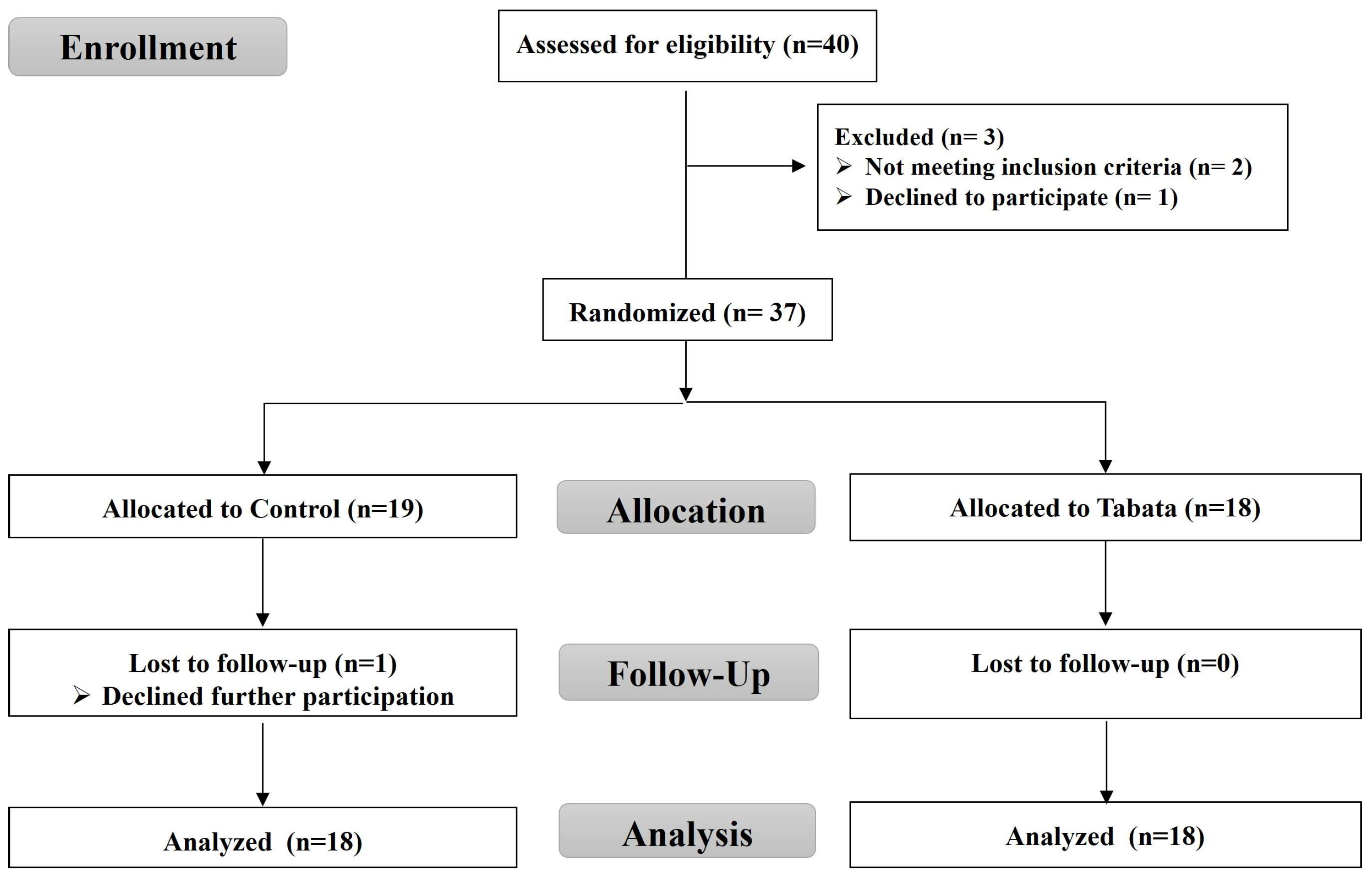
For this randomized controlled trial, as shown in the CONSORT flow diagram (Figure 1), we recruited overweight or obese individuals living in Chonburi Province starting in September 2022. This study used a stratified blocked randomization method to assign subjects to either the control or Tabata group using the RAND function in Microsoft Excel. This study was non-blinded, with the same researcher responsible for screening, randomization, allocation, data collection, and analysis.
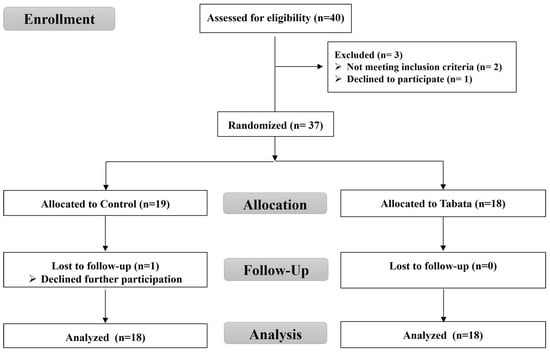
Figure 1.
CONSORT flow diagram.
This study was approved by the Burapha University Institutional Review Board on 24 May 2022 (ID: G-HS018/2565) and registered in the Thai Clinical Trials Registry (ID: TCTR20220518001). All participants gave written informed consent prior to screening.
2.2. Screening of Participants
Participants aged 18–30 years with a BMI > 22.9 kg/m2 [21] were included. The exclusion criteria were the consumption of certain drugs; cardiovascular, liver, renal, musculoskeletal, infectious, neurological, or psychiatric diseases or cancer; and regular smoking and alcohol consumption. A health history questionnaire was designed based on the inclusion and exclusion criteria and was used to screen for eligibility. It included questions about personal information such as gender, age, smoking history, use of supplements or tonics, history of lithium use, pregnancy or breastfeeding history, and various diseases specified in the inclusion and exclusion criteria. Additionally, the Baecke Habitual Physical Activity Questionnaire was used to determine activity levels (sedentary or active) [22,23]. A research assistant administered the health history and activity level questionnaires to the volunteers. These questionnaires were completed before the participants were divided into groups.
2.3. Sample Size
The sample size was calculated using the means and standard deviations of VO2max according to a previous study [24] that investigated the effects of a 4-week Tabata HIIT intervention in overweight individuals. With an α error of 0.05 and a test power of 0.98, the sample size was obtained using G*Power 3.1 [25]; accounting for a 10% dropout, the sample size was 18 per group (36 total).
2.4. Tabata Training
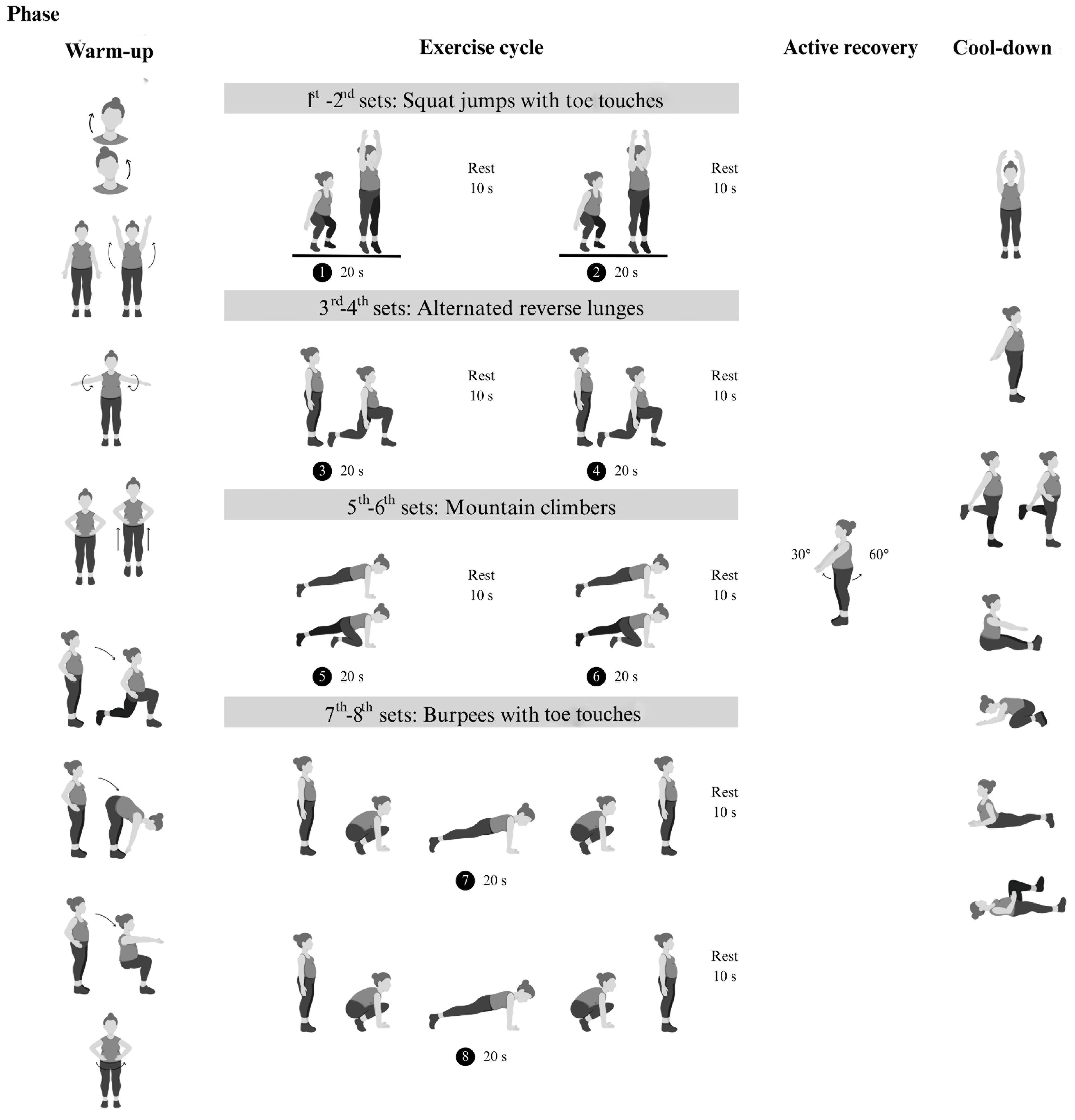
The modified Tabata training was a progressive home-based program consisting of body-weight exercises followed by active rest (Figure 2). This progressive body-weight HIIT program was designed based on biomechanical principles. Four body-weight exercises, including squat jumps with toe touches and alternating reverse lunge movements, primarily targeted the leg muscles (quadriceps, gastrocnemius) and core stabilizers (transversus abdominis, multifidus). Additionally, mountain climbers and burpees with toe touches movements engaged the upper limb muscles (biceps, triceps, pectoralis) in addition to the leg and core muscles. This training was designed to reduce exhaustion and prevent dropout [20]. To minimize the impact on the lower limb joints and reduce the risk of injury during unsupervised exercise, we modified the squat jumps and burpees by replacing jumps with standing on tiptoes. Each session included 4 min exercise cycles (20 s of exercise and 10 s of rest) followed by 4 min of active rest, totaling 8 min per cycle. Participants trained three days a week for 12 weeks, starting with two cycles for the first 4 weeks, then increasing to three cycles from weeks 5 to 8 and four cycles from weeks 9 to 12. The exercise intensity was set at 75–85% of maximum perceived exertion, with 4 min active rest periods where participants swung their arms at 40–50% exertion. Participants were provided with an exercise diagram and video clip to demonstrate the exercises. The exercise area was approximately 3 m in width and 3 m in length. The researcher monitored the correctness of exercise posture and compliance with the exercise plan. Appointments were scheduled for measurement and exercise sessions for each volunteer, and communication was conducted via online conference platforms such as Line, Google Meet, or Microsoft Teams, depending on convenience. During the session, the exercise video clip was played, and the researcher observed and provided real-time feedback to correct posture as needed while offering encouragement throughout the exercises.
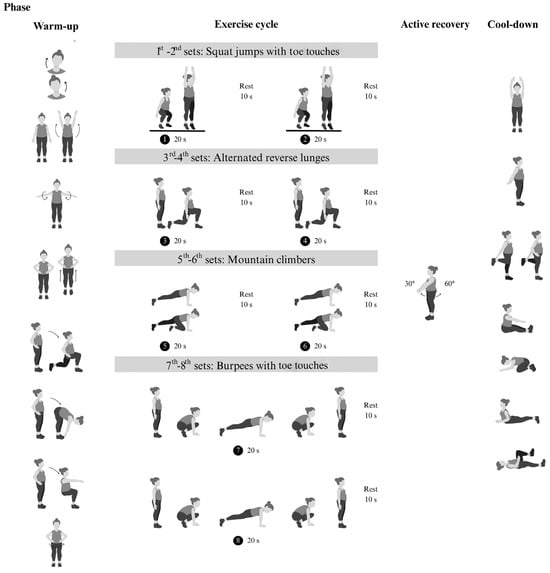
Figure 2.
Phases of exercise training: warm-up, 4 min exercise cycle (body-weight-bearing based on Tabata training method), 4 min active recovery, and cool-down.
Exercise intensity was assessed using the rating of perceived exertion (RPE) scale developed by Gunnar Borg [26]. This scale is a numerical rating system ranging from 6 (no exertion) to 20 (maximum effort). A rating of 7 to 11 represents extremely light to light intensity, 12 to 14 indicates moderate to somewhat hard intensity, and 15 to 17 represents hard to very hard intensity. Prior to data collection, the meaning of each rating was explained to the participants during the pre-test day. They were instructed to assess their exertion level during each set of exercises performed at home. Researchers recorded individual RPE scores to monitor exercise intensity and provided real-time encouragement to ensure participants reached the target intensity range specified in the exercise program.
The control group participants were asked to maintain their current physical activity and diet.
2.5. Study End Points
The primary outcomes were improvements in body composition, including body fat, fat mass, muscle mass, and muscle thickness. The secondary outcomes were improvements in fat distribution, including waist and hip circumference and ratio; physical fitness, including muscle strength and endurance; and cardiorespiratory fitness, including aerobic capacity. Both outcomes were measured before and after the 12-week interventions.
All outcome measurements were conducted by the same researcher, a certified physical therapist trained by an exercise biology expert before the study commenced. The measurements took place between 08:00 and 12:00 on the appointment date at the Exercise and Nutrition Innovation and Sciences Research Unit Room, Faculty of Allied Health Sciences, Burapha University. The order of measurements on each day is provided below.
2.5.1. Body Composition and Vital Signs
Body composition, including body weight, BMI, fat percentage, fat and muscle mass, water, protein, mineral, visceral fat, and basal metabolic rate, was measured using a bioelectrical impedance analyzer (InBody270, InBody Co., Ltd., Daejeon, Republic of Korea) [27].
Resting vital signs, including heart rate (HR), respiratory rate (RR), systolic and diastolic blood pressure (SBP and DBP), mean arterial pressure (MAP), and partial oxygen saturation (SpO2), were measured using a real-time vital sign bedside monitor (PVM-2701 Vismo, Nihon-Kohden, Tokyo, Japan) after the participant had been seated for 10 min. Each measurement was taken twice, with a 2 min interval between readings, and the average of the two values was reported.
2.5.2. Muscle Thickness
An ultrasound machine (M5 series, Shenzhen Mindray Bio-Medical, Shenzhen, China) with a 7.5 MHz probe was used to measure two upper limb muscles (biceps and triceps brachii) and two lower limb muscles (rectus femoris and vastus intermedius) in the dominant limb according to a published protocol [28,29]. Measurements were taken twice and analyzed using ImageJ software version 1.54g (Wayne Rasband, NIH, Bethesda, MD, USA), which showed a high intraclass correlation coefficient (0.999 for biceps and triceps, 0.996 for rectus femoris, and 0.989 for vastus intermedius). The average thickness was calculated from two images for analysis.
2.5.3. Physical Fitness
Lower-body muscle strength was assessed using the Leg Dynamometer Test [30] (Takei Back/Leg Dynamometer Digital, Tokyo, Japan), measuring quadriceps strength in kilograms. Upper-body muscle endurance was evaluated with the YMCA Bench Press Test [30], recording total successful repetitions (reliability r = 0.87).
2.5.4. Cardiorespiratory Fitness
An incremental treadmill test (Valiant 2 Sport, Lode, Groningen, The Netherlands) following the Bruce protocol [31] was used to determine the peak VO2 (VO2peak). Gas and volume calibrations were carried out before each test following the manufacturer’s guidelines. Breath-by-breath analysis was measured with a cardiopulmonary exercise testing system and software (MetaMax 3B, Cortex, Leipzig, Germany). HR, breathing frequency (BF), VO2, carbon dioxide production (VCO2), respiratory exchange ratio (RER), first ventilatory threshold (VT1), and second ventilatory threshold (VT2) were measured in real time and analyzed. The test was terminated when the participant reached 75–95% of maximum HR (HRmax), expressed volitional fatigue, or met the guideline termination criteria [7].
2.6. Statistical Analysis
Data were analyzed using SPSS version 26 (IBM Corp., Armonk, NY, USA) and expressed as mean ± standard deviation or median (range). The Kolmogorov–Smirnov test assessed normality, after which appropriate parametric or non-parametric tests were applied. Differences between groups were analyzed using the independent t-test. Since the body composition data were not normally distributed, non-parametric tests (e.g., the Mann–Whitney U test) were employed. Pre- and post-test differences within each group were analyzed utilizing the dependent t-test, except for the bench press test, which used the Wilcoxon signed-rank test. Statistical significance was set at p < 0.05. The effect size (ES) was calculated following Cohen’s guidelines, with values above 0.8 denoting large effects, 0.5–0.8 medium effects, and 0.2–0.5 small effects.
3. Results
3.1. Participant Characteristics and Feasibility
A total of 40 volunteers initially registered, with 37 meeting the criteria, 36 completing the study, and 1 control group participant withdrawing. The study enrollment process is illustrated in Figure 1. The baseline physical and physiological characteristics, as well as vital signs, are detailed in Table 1, with no significant differences between groups (all p > 0.05).

Table 1.
Physical and physiological characteristics and vital signs of participants.
None of the Tabata group dropped out, and no serious adverse events occurred. The RPE ranged from 7 to 11 during the warm-up period, 13 to 17 during exercise, and 9 to 11 during the cool-down period. Minor adverse events, including leg muscle cramps and dizziness in two participants, occurred during the fourth cycle of the final week, representing 11.11% of the training group. However, no participants discontinued the training. No participants experienced joint injuries, thus confirming our initial presuppositions.
3.2. Impact on Body Composition
The pretest body composition showed no significant differences between groups (Table 2, Table 3 and Table 4). After 12 weeks of training, changes in segmental muscle composition were observed. In the control group, the muscle mass and muscle percentage of the right leg, in addition to the muscle percentage of the left leg, decreased compared to the pre-test values (all p < 0.05). The Tabata group showed no significant change in muscle mass in both legs. Post-test comparisons between groups revealed that the Tabata group had a higher right leg muscle mass (0.18 ± 0.08 kg (p = 0.031, ES = 0.22)) and a higher muscle percentage in both legs (right: 2.08 ± 0.89%, p = 0.026, ES = 0.63; left: 1.82 ± 0.86%, p = 0.043, ES = 0.67). The control group showed increases in abdominal fat and fat percentage (p < 0.05), while the Tabata group showed no changes. Notably, the Tabata group had a significantly lower waist-to-hip ratio (WHR) (0.02 ± 0.01) relative to the control group (p = 0.043, ES = 0.83).

Table 2.
Whole-body composition of participants.

Table 3.
Segmental muscular composition of participants.

Table 4.
Segmental fat composition of participants.
3.3. Impact on Muscle Thickness
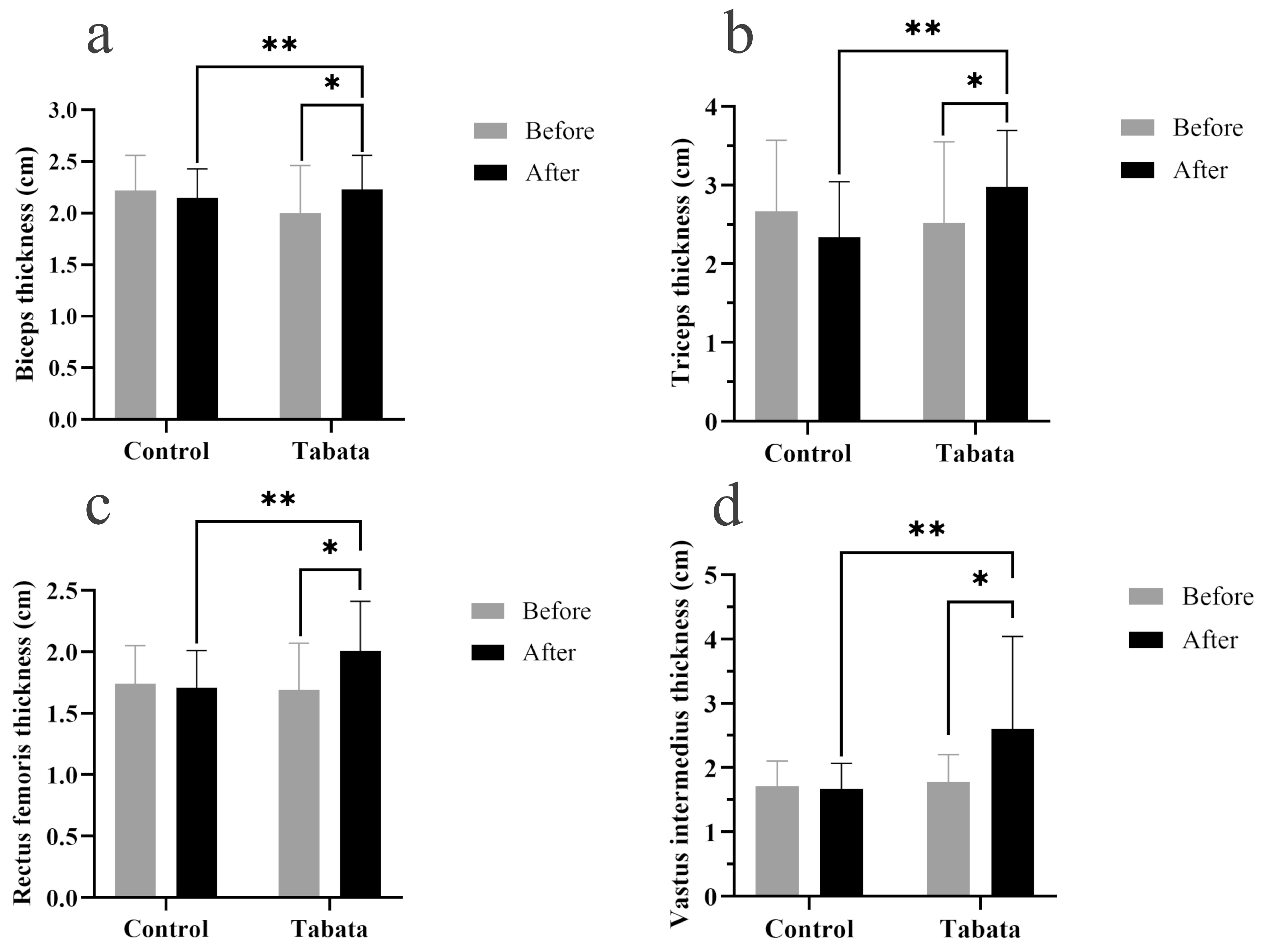
The Tabata group showed significant increases in muscle thicknesses of 0.30 ± 0.08 cm (biceps), 0.78 ± 0.25 cm (triceps), 0.35 ± 0.10 cm (rectus femoris), and 0.85 ± 0.34 cm (vastus intermedius) compared to both the pre-test period (all p < 0.05) and the control group (p = 0.001, ES = 0.26; p = 0.028, ES = 0.90; p = 0.003, ES = 0.84; p = 0.004, ES = 0.88, respectively), as shown in Figure 3 and Figure 4.
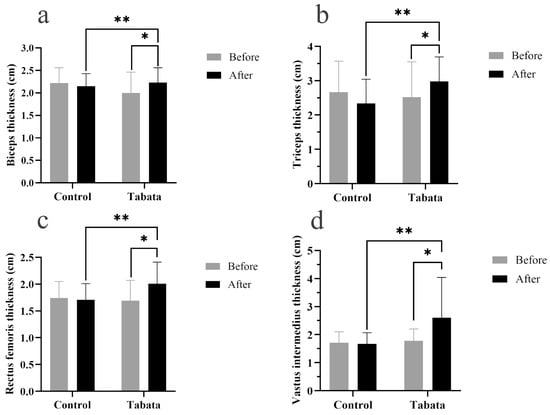
Figure 3.
Biceps muscle thickness (a), triceps muscle thickness (b), rectus femoris muscle thickness (c), and vastus intermedius muscle thickness (d) of participants in control and Tabata groups before and after 12-week intervention. *: p < 0.05 vs. before intervention, **: p < 0.05 vs. control group.
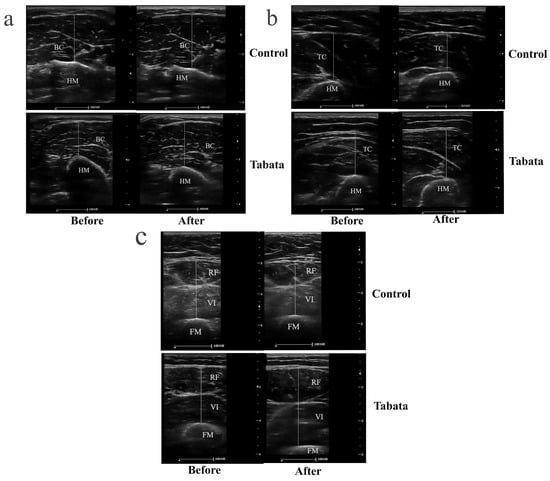
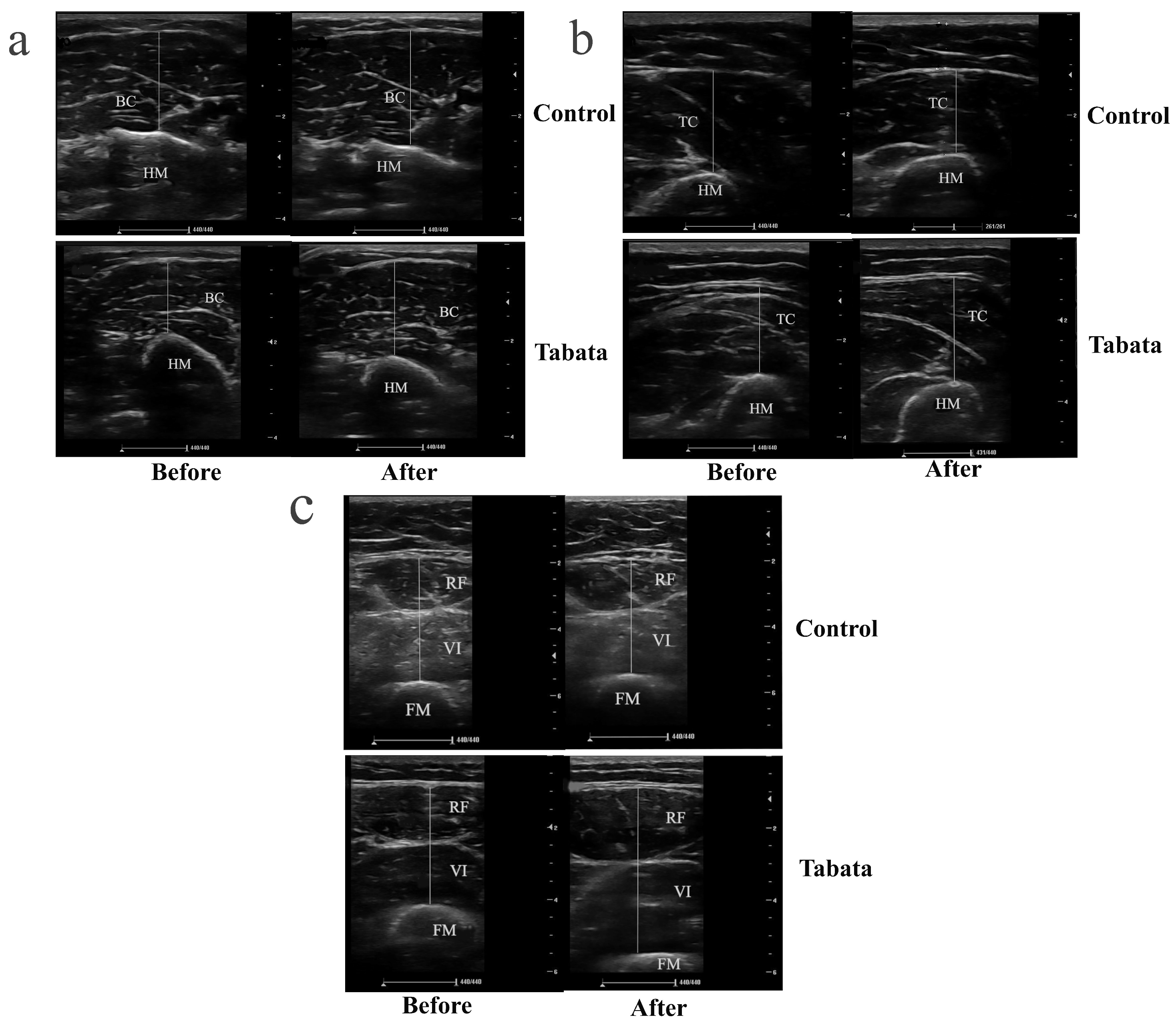
Figure 4.
Ultrasound images demonstrating muscle thickness of participants in control and Tabata groups before and after 12-week intervention. (a) Transverse scan of biceps muscle (BB), with the hyperechoic, curvilinear structure in the lower part of the image representing the humerus (HM). (b) Transverse scan of triceps muscle (TC), where the hyperechoic, curvilinear structure in the lower part of the image corresponds to the HM. (c) Transverse scan of quadriceps muscle, comprising rectus femoris (RF) and vastus intermedius (VI); the hyperechoic, curvilinear structure in the lower part of the image represents the femur (FM). Solid straight lines indicate the method used to measure the thickness of each muscle.
3.4. Impact on Physical Fitness
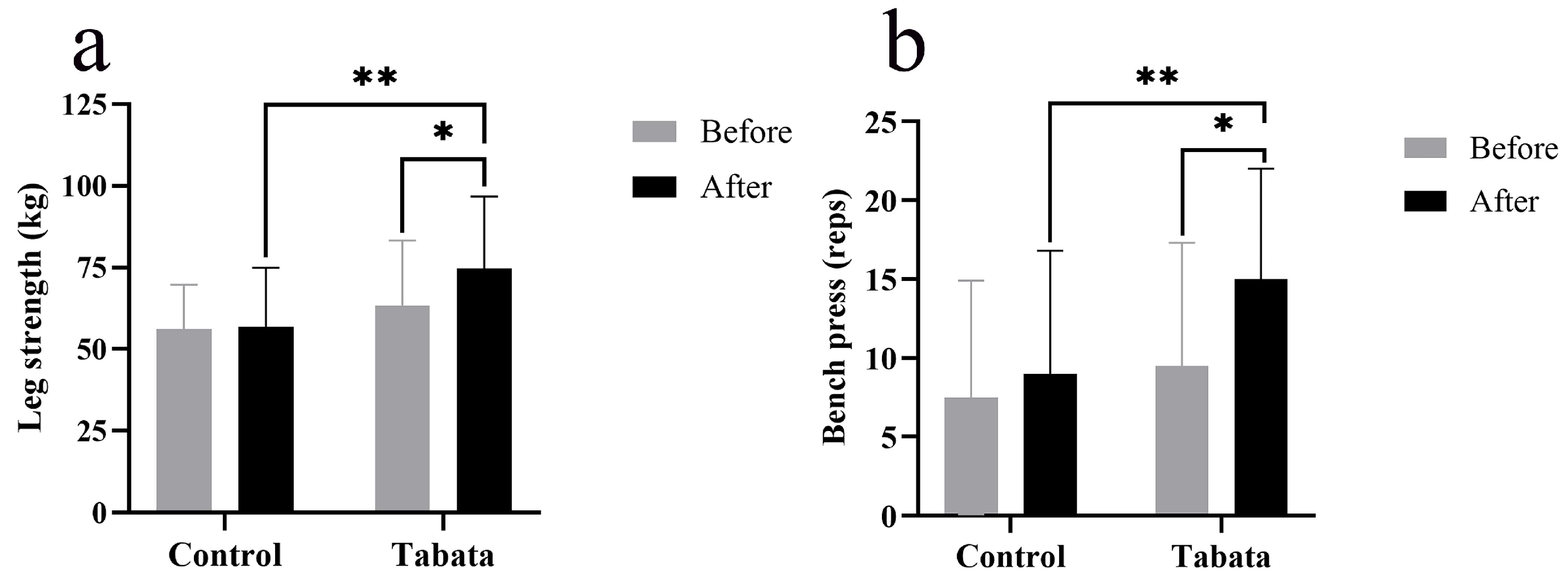
The Tabata group showed improvements in lower-body muscle strength, as evidenced by a 10.93 ± 4.27 kg increase in weight lifted compared to both the pre-test period (p = 0.004) and the control group (p = 0.015, ES = 0.89) (Figure 5a). Upper-body muscle endurance also improved, as indicated by a 5.00 ± 7.80 increase in bench press repetitions compared to both the pre-test period (p < 0.001) and the control group (p < 0.001, ES = 0.47) (Figure 5b).
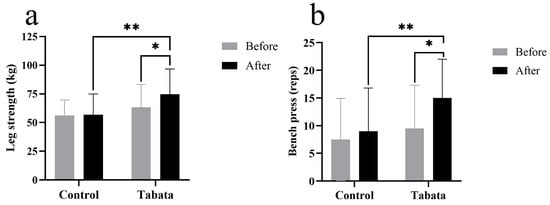
Figure 5.
Leg muscle strength (a) and arm muscle endurance (bench press) (b) of participants in control and Tabata groups before and after 12-week intervention. *: p < 0.05 vs. before intervention, **: p < 0.05 vs. control group.
3.5. Impact on Cardiorespiratory Fitness
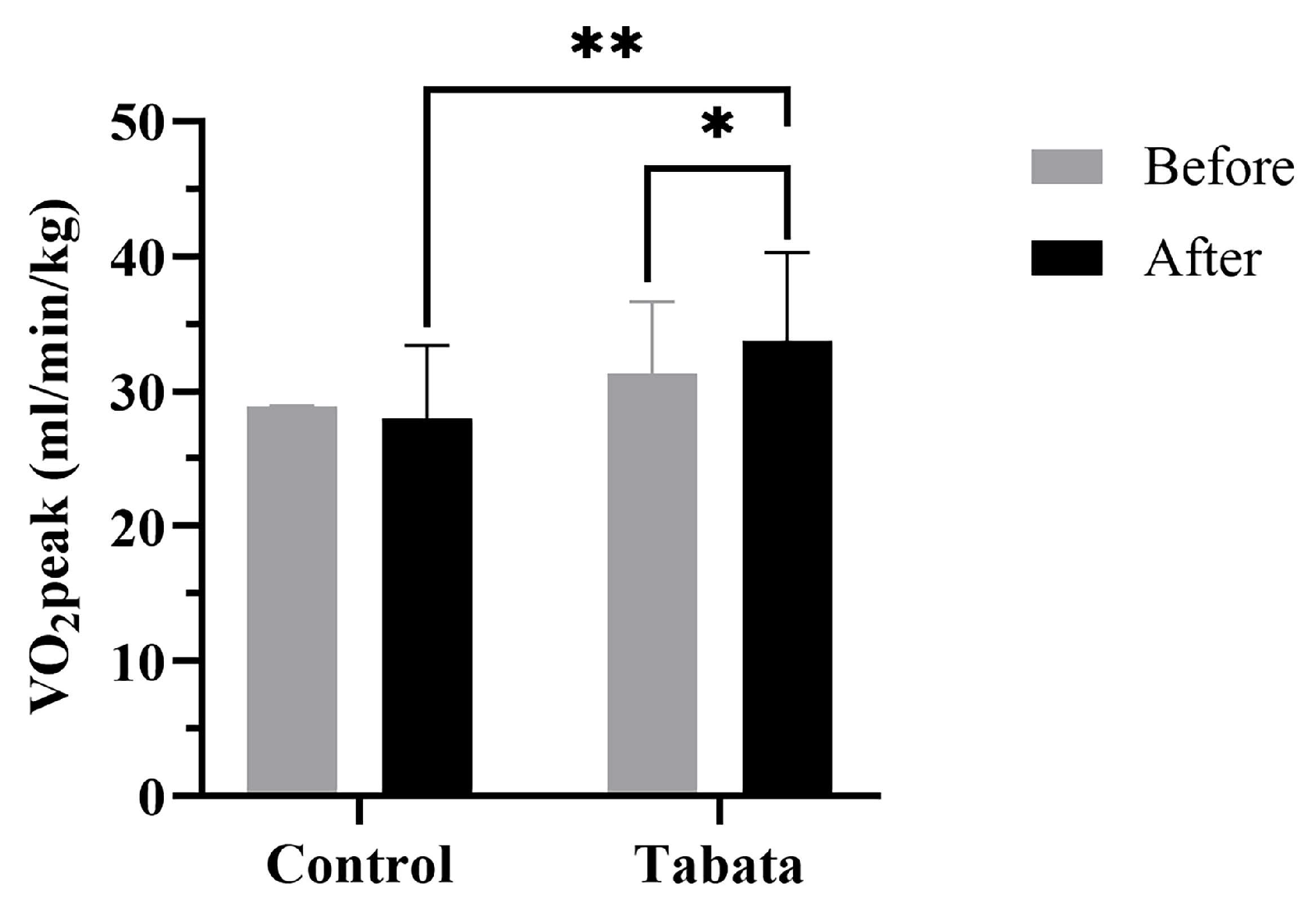
There were no significant differences in cardiorespiratory fitness shown between the two groups in the pre-test period (Table 5 and Figure 6). After 12 weeks, the control group showed a decrease in resting VO2 of 0.50 ± 0.70 mL/min/kg (p = 0.008). The Tabata group demonstrated greater improvements in anaerobic threshold and aerobic capacity, with increased VT2 (2.33 ± 1.53 mL/min/kg; p = 0.013) and VO2peak (3.33 ± 1.12 mL/min/kg; p = 0.006, ES = 0.56). Breathing frequency and RER increased in both groups, but no significant differences were seen between groups.

Table 5.
Cardiorespiratory fitness of participants.
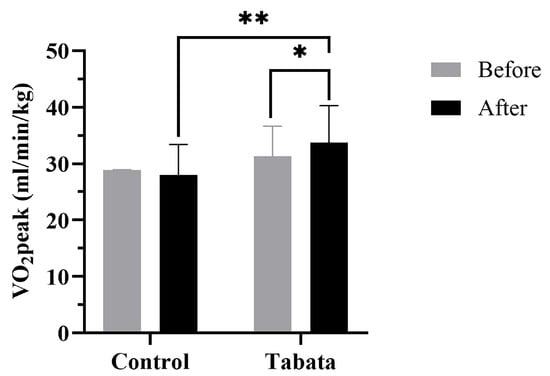
Figure 6.
Peak oxygen consumption (VO2peak) of participants in control and Tabata groups before and after 12-week intervention. *: p < 0.05 vs. before intervention, **: p < 0.05 vs. control group.
4. Discussion
This randomized control study provides evidence that the 12 weeks of modified Tabata training was safe and effective for overweight and obese participants. The program reduced WHR, enhanced leg muscle mass, and increased arm and leg muscle thickness, which collectively resulted in improvements in leg strength and arm endurance as well as aerobic capacity.
4.1. Modified Tabata Training Improves Body Proportions
A recent systematic review revealed that HIIT has the potential to facilitate weight loss in overweight and obese individuals [32]. Other investigations also reported positive effects, such as a 12-week HIIT program reducing body weight by 5.7 kg (8.3–3.1 kg) in obese adults [33,34]. However, the Tabata group did not demonstrate changes in either body weight or BMI; only minor changes were observed, similar to a previous study [35]. Furthermore, a systematic review also showed that HIIT can induce modest body composition improvements in overweight individuals without affecting body weight [36].
Following the 12-week HIIT intervention, the Tabata group demonstrated reduced WHR, leading to a more proportionate physique, similar to a previous study that showed reduced WHR and visceral fat in obese women [37]. However, only minimal reductions in other body composition indices were observed, which might be attributed to the lower intensity of exercise in this study (75–85% HRmax) relative to previous studies which reported more positive results (90–95% HRmax). This highlights the fact that heterogenous intensity and duration result in diverse outcomes. For example, the 12-week HIIT protocol reported by Astorino et al. (~75–95% HRmax) did not affect body weight or WHR [38]. Similarly, two recent studies revealed no changes in body weight or body composition in sedentary, overweight, or obese individuals following HIIT [39]. From a mechanistic point of view, two possible explanations for this lack of change are (1) behavioral adaptations that lead to an increase in food intake immediately following exercise, such as the consumption of snacks high in carbohydrates and fats, and (2) metabolic adaptations associated with the addition of a high-intensity exercise program. Specifically, a reduction in non-exercise activity thermogenesis (NEAT) may have occurred as a compensatory response to the increased exercise-induced energy expenditure in the experimental group [40,41].
4.2. Modified Tabata Training Improves Muscle Thickness
HIIT has been demonstrated to increase muscle thickness effectively in overweight and obese populations. Previous studies reported increases in vastus lateralis muscle thickness after 12 weeks of HIIT (Δ change = −3.17 ± 3.36 cm2) compared to traditional strength training, e.g., sprints (Δ change = −0.34 ± 2.36 cm2). The effects of HIIT on upper-body muscle thickness are less well documented; however, when HIIT protocols incorporate upper-body resistance exercises, such as push-ups, they also lead to increased muscle thickness [42]. Meanwhile, this study’s findings align with previous studies in which exercise types engaging both the upper and lower limb muscles, e.g., mountain climbers and burpees with toe touches, led to increased upper limb muscle thickness [43].
The hypertrophic effects of HIIT are attributed to increased muscle fiber recruitment, metabolic stress, and hormonal responses, including elevated levels of growth hormone, testosterone, and estrogen, all of which are linked to muscle growth [44,45,46]. The nature of HIIT induces metabolic stress, which is a key factor driving muscle hypertrophy [47].
4.3. Modified Tabata Training Improves Muscle Strength and Endurance
Studies have demonstrated that HIIT, particularly when involving sprinting or jumping, improves lower-body strength due to its high intensity [48]. Again, the effects on upper-body strength are generally less pronounced; however, HIIT protocols including upper-body resistance exercises such as push-ups can lead to improvements in this regard [49]. Our study is consistent with prior research, in that exercises involving both upper and lower limb muscles were shown to contribute to increased muscle strength [40]. The enhancement of strength caused by HIIT is thought to be a result of several factors, including the recruitment of fast-twitch muscle fibers and neuromuscular adaptations [50].
In addition, studies on HIIT’s effects on muscle endurance are limited, though HIIT has been demonstrated to augment muscle endurance by enhancing muscular enzyme content and activity [15]. A 4-week running and body-weight HIIT study reported enhanced muscle endurance, as evidenced by more burpees and toe-to-bar repetitions being performed [51]. These findings are consistent with the increase in bench press repetitions in our study. Moreover, sprint and cycling HIIT increased mitochondrial [52] and glycolytic enzyme activities [17]. These changes indicate that HIIT enhances protein expression, muscle adaptation, and energy expenditure in both anaerobic and aerobic systems.
4.4. Modified Tabata Training Improves Cardiorespiratory Fitness
The Tabata group demonstrated enhanced aerobic capacity. In line with this, a systematic review of 15 randomized controlled trials indicated that HIIT was more effective than traditional exercise in increasing VO2max [35]. Previous studies with obese and elderly participants also showed that 12–16 weeks of HIIT effectively improved VO2max and cardiovascular health [53,54]. A potential change after modified Tabata HIIT is enhanced muscle buffer capacity, which results in proportional glycolytic ATP production [55,56]. Furthermore, as reported in a review by Torma et al. [57], HIIT activates key pathways that enhance mitochondrial biogenesis and angiogenesis in skeletal muscle.
After identifying significant differences between the control and Tabata groups, Pearson correlation analysis revealed a positive correlation between VO2peak and VT2 (r = 0.844, p < 0.001), while Kendall correlation analysis identified a negative relationship between WHR and VT2 (r = −0.456, p = 0.008). The observed relationships suggest that modified Tabata training may enhance VO2peak, potentially due to the more efficient utilization of anaerobic energy pathways. Furthermore, as the proportion of visceral fat increases, aerobic energy pathway efficiency decreases.
Despite variations in exercise protocols, such as cycling, running in place, or calisthenics, most studies have found little to no difference in the effects of HIIT, provided that the exercise duration and rest periods range from 6 s to 4 min and the intensity reaches 75% of HR or VO2max [9]. A previous study comparing two HIIT protocols in 11 college students aged 19–27 years examined sprint interval cycling (four 30 s sets with a 4 min active recovery) versus high-intensity intermittent calisthenics (four sets of ten squats, ten push-ups, and five burpees, each performed for 30 s with a 4 min active recovery) over 9 days. The results showed no statistically significant differences between the two protocols in terms of %VO2peak (80.4 ± 5.3% vs. 77.6 ± 6.9%, respectively) [58]. Similarly, a 9-week study investigating the effects of four different high-intensity training modalities (HIIT, high-intensity functional training, high-intensity power training, and high-intensity endurance training) found that all four groups experienced an 8% improvement in VO2max, while the levels of brain-derived neurotrophic factor (BDNF), a key regulator of neurogenesis, remained unchanged in all groups [59]. These findings suggest that regardless of the specific exercise modality, when exercise and rest durations range from 6 s to 4 min over a period of 2 to 15 weeks, similar physiological benefits can be achieved [9].
4.5. Limitations of Study
This study has several limitations. Firstly, the exercise program for the Tabata group was home-based, with an adherence rate of 89.87% and a compliance rate of 100%. Exercise frequency and intensity were monitored online, and participants recorded their perceived exertion. To ensure accuracy and maintain intensity, participants were required to visit the research room every 4 weeks for guidance amid subsequent sessions. Although monitoring took place online, supervised programs may yield better adherence and control. Future studies should implement supervised exercise programs in a controlled setting and HR tracking should be used to enhance reliability. Secondly, eating behaviors were not strictly controlled due to being ecologically practicable and reliant on self-reported data. This limitation is particularly relevant to the control group, as changes in body composition could have been influenced by unreported dietary factors. In future research, implementing more stringent dietary control or more reliable monitoring methods is recommended. Thirdly, the impact of variables like increased protein intake and metabolic hormones (e.g., growth hormone, testosterone) was unaccounted for, and should thus be considered by future studies. Lastly, body composition data were obtained using an electrical impedance technique. While this method is highly reliable, more accurate techniques, such as Dual-Energy X-ray Absorptiometry (DEXA) or the BodPod, should be considered for directly measuring body composition, if feasible.
5. Conclusions
Modified Tabata training, a progressive body-weight HIIT program taking place over 12 weeks, proved safe and effective for overweight and obese participants. Improvements in WHR, leg muscle mass, and arm and leg muscle thickness were seen; furthermore, these improvements led to enhancements in physical fitness due to increased muscle strength and endurance, in addition to enhancements in cardiorespiratory fitness through increased aerobic capacity. These results suggest that this training method has potential in terms of improving body proportion and overall health among overweight and obese populations.
Author Contributions
Conceptualization, P.P. and T.P.; methodology, P.P. and T.P.; software, T.P.; validation, T.P.; formal analysis, T.P.; investigation, T.P.; resources, P.P.; data curation, T.P.; writing—original draft preparation, T.P.; writing—review and editing, P.P.; visualization, T.P.; supervision, P.P.; project administration, P.P.; funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Council of Thailand, grant number N41A660293/2566; the Graduated School, grant number 0118/2565; and the Faculty of Allied Health Sciences, grant number AHS08/2565, Burapha University.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Burapha University (protocol ID: G-HS018/2565 and approval date 24 May 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available on request to the corresponding author.
Acknowledgments
The authors appreciate all volunteers who participated in this study. We would like to express our sincere gratitude to Pornpimol Muanjai, an expert in exercise biology, for providing training on muscle thickness measurement.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BB | Biceps muscle |
| BF | Body fat |
| BMI | Body mass index |
| BMR | Basal metabolic rate |
| BW | Body weight |
| DBP | Diastolic blood pressure |
| ES | Effect size |
| FFM | Fat-free mass |
| FMs | Fat mass |
| FM | Femur |
| HM | Humerus |
| HIIT | High-intensity intermittent training |
| HR | Heart rate |
| HRmax | Maximal heart rate |
| MAP | Mean arterial pressure |
| MM | Muscle mass |
| NEAT | Non-exercise activity thermogenesis |
| REP | Rating of perceived exertion |
| RER | Respiratory exchange ratio |
| RF | Rectus femoris muscle |
| RR | Respiratory rate |
| SBP | Systolic blood pressure |
| SpO2 | Partial oxygen saturation |
| TC | Triceps muscle |
| VCO2 | Carbon dioxide production |
| VI | Vastus intermedius muscle |
| VO2 | Oxygen uptake |
| VO2max | Maximal oxygen consumption |
| VT | Ventilatory threshold |
| WHO | World Health Organization |
| WHR | Waist–hip ratio |
References
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Powis, J.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for 161 countries. BMJ Glob. Health 2022, 7, e009773. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Gerdes, M.W.; Martinez, S.G. Identification of Risk Factors Associated with Obesity and Overweight—A Machine Learning Overview. Sensors 2020, 20, 2734. [Google Scholar] [CrossRef]
- Jensen, M.; Ryan, D.; Donato, K.; Apovian, C.; Ard, J.; Comuzzie, A.; Hu, F.; Hubbard, V.; Jakicic, J.; Kushner, R.; et al. Expert Panel Report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014, 22 (Suppl. S2), S41–S410. [Google Scholar] [CrossRef]
- American College of Sports Medicine; Liguori, G.; Feito, Y.; Fountaine, C.; Roy, B.A. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2022. [Google Scholar]
- Tschakert, G.; Hofmann, P. High-Intensity Intermittent Exercise: Methodological and Physiological Aspects. Int. J. Sports Physiol. Perform. 2013, 8, 600–610. [Google Scholar] [CrossRef]
- Boutcher, S.H. High-intensity intermittent exercise and fat loss. J. Obes. 2011, 2011, 868305. [Google Scholar] [CrossRef]
- Cooper, S.B.; Dring, K.J.; Nevill, M.E. High-Intensity Intermittent Exercise: Effect on Young People’s Cardiometabolic Health and Cognition. Curr. Sports. Med. Rep. 2016, 15, 245–251. [Google Scholar] [CrossRef]
- Cassidy, S.; Thoma, C.; Hallsworth, K.; Parikh, J.; Hollingsworth, K.G.; Taylor, R.; Jakovljevic, D.G.; Trenell, M.I. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: A randomised controlled trial. Diabetologia 2016, 59, 56–66. [Google Scholar] [CrossRef]
- Murawska-Cialowicz, E.; Wolanski, P.; Zuwala-Jagiello, J.; Feito, Y.; Petr, M.; Kokstejn, J.; Stastny, P.; Goliński, D. Effect of HIIT with Tabata Protocol on Serum Irisin, Physical Performance, and Body Composition in Men. Int. J. Environ. Res. Public Health 2020, 17, 3589. [Google Scholar] [CrossRef] [PubMed]
- Tabata, I. Tabata training: One of the most energetically effective high-intensity intermittent training methods. J. Physiol. Sci. 2019, 69, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Tabata, I.; Nishimura, K.; Kouzaki, M.; Hirai, Y.; Ogita, F.; Miyachi, M.; Yamamoto, K. Effects of moderate-intensity endurance and high-intensity intermittent training on anaerobic capacity and VO2max. Med. Sci. Sports Exerc. 1996, 28, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Tabata, I.; Irisawa, K.; Kouzaki, M.; Nishimura, K.; Ogita, F.; Miyachi, M. Metabolic profile of high intensity intermittent exercises. Med. Sci. Sports Exerc. 1997, 29, 390–395. [Google Scholar] [CrossRef]
- Viana, R.B.; de Lira, C.A.B.; Naves, J.P.A.; Coswig, V.S.; Del Vecchio, F.B.; Gentil, P. Tabata protocol: A review of its application, variations and outcomes. Clin. Physiol. Funct. Imaging 2019, 39, 1–8. [Google Scholar] [CrossRef]
- Miyamoto-Mikami, E.; Tsuji, K.; Horii, N.; Hasegawa, N.; Fujie, S.; Homma, T.; Uchida, M.; Hamaoka, T.; Kanehisa, H.; Tabata, I.; et al. Gene expression profile of muscle adaptation to high-intensity intermittent exercise training in young men. Sci. Rep. 2018, 8, 16811. [Google Scholar] [CrossRef]
- Chuensiri, N.; Suksom, D.; Tanaka, H. Effects of High-Intensity Intermittent Training on Vascular Function in Obese Preadolescent Boys. Child. Obes. 2018, 14, 41–49. [Google Scholar] [CrossRef]
- Logan, G.R.; Harris, N.; Duncan, S.; Plank, L.D.; Merien, F.; Schofield, G. Low-Active Male Adolescents: A Dose Response to High-Intensity Interval Training. Med. Sci. Sports Exerc. 2016, 48, 481–490. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Hall, E.E.; Petruzzello, S.J. The relationship between exercise intensity and affective responses demystified: To crack the 40-year-old nut, replace the 40-year-old nutcracker! Ann. Behav. Med. 2008, 35, 136–149. [Google Scholar] [CrossRef]
- World Health Organization; Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, NSW, Australia, 2000. [Google Scholar]
- Jalayondeja, C.; Jalayondeja, W.; Vachalathiti, R.; Bovonsunthonchai, S.; Sakulsriprasert, P.; Kaewkhuntee, W.; Bunprajun, T.; Upiriyasakul, R. Cross-Cultural Adaptation of the Compendium of Physical Activity: Thai Translation and Content Validity. J. Med. Assoc. Thail. 2015, 98 (Suppl. S5), S53–S59. [Google Scholar]
- Baecke, J.A.; Burema, J.; Frijters, J.E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 1982, 36, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Triyulianti, S.P.A.; Utami, R. The Effect of High Intensity Interval Training on Maximal Oxygen Uptake (VO2max) in Overweight Adolescents. Int. J. Aging Health Mov. 2023, 5, 21–28. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Perceived exertion as an indicator of somatic stress. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef]
- Czartoryski, P.; Garcia, J.; Manimaleth, R.; Napolitano, P.; Weber, H.W.C.; Alvarez-Beaton, A.; Nieto, A.C.; Patel, A.; Peacock, C.; Banks, J.; et al. Body Composition Assessment: A Comparison of the DXA, InBody 270, and Omron. J. Exerc. Nutr. 2020, 3, 1–6. [Google Scholar]
- Hentzen, J.; van Wijk, L.; Buis, C.; Viddeleer, A.; Bock, G.; van der Schans, C.; Dam, G.; Kruijff, S.; Klaase, J. Impact and risk factors for clinically relevant surgery-related muscle loss in patients after major abdominal cancer surgery: Study protocol for a prospective observational cohort study (MUSCLE POWER). Int. J. Clin. Trials 2019, 6, 138. [Google Scholar] [CrossRef]
- Spineti, J.; Figueiredo, T.; Miranda, H.; de Salles, B.; Oliveira, L.; Simão, R. The effects of exercise order and periodized resistance training on maximum strength and muscle thickness. Int. Sport. J. 2014, 15, 374–390. [Google Scholar]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Bruce, R.A. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann. Clin. Res. 1971, 3, 323–332. [Google Scholar]
- Sanca-Valeriano, S.; Espinola-Sánchez, M.; Caballero-Alvarado, J.; Canelo-Aybar, C. Effect of high-intensity interval training compared to moderate-intensity continuous training on body composition and insulin sensitivity in overweight and obese adults: A systematic review and meta-analysis. Heliyon 2023, 9, e20402. [Google Scholar] [CrossRef]
- D’Amuri, A.; Sanz, J.M.; Capatti, E.; Di Vece, F.; Vaccari, F.; Lazzer, S.; Zuliani, G.; Dalla Nora, E.; Passaro, A. Effectiveness of high-intensity interval training for weight loss in adults with obesity: A randomised controlled non-inferiority trial. BMJ Open Sport Exerc. Med. 2021, 7, e001021. [Google Scholar] [CrossRef]
- Airin, S.; Linoby, A.; Mohamad Zaki, M.S.; Baki, H.; Sariman, H.; Esham, B.; Mohd Azam, M.Z.; Mohamed, M.N. The Effects of High-Intensity Interval Training and Continuous Training on Weight Loss and Body Composition in Overweight Females. In Proceedings of the International Colloquium on Sports Science, Exercise, Engineering and Technology 2014 (ICoSSEET 2014), Singapore, 7–9 April 2014; pp. 401–409. [Google Scholar]
- Türk, Y.; Theel, W.; Kasteleyn, M.J.; Franssen, F.M.E.; Hiemstra, P.S.; Rudolphus, A.; Taube, C.; Braunstahl, G.J. High intensity training in obesity: A Meta-analysis. Obes. Sci. Pract. 2017, 3, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.; van den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Ardavani, A.; Aziz, H.; Smith, K.; Atherton, P.J.; Phillips, B.E.; Idris, I. The Effects of Very Low Energy Diets and Low Energy Diets with Exercise Training on Skeletal Muscle Mass: A Narrative Review. Adv. Ther. 2021, 38, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; Schubert, M.M.; Palumbo, E.; Stirling, D.; McMillan, D.W. Effect of two doses of interval training on maximal fat oxidation in sedentary women. Med. Sci. Sports Exerc. 2013, 45, 1878–1886. [Google Scholar] [CrossRef]
- Tjønna, A.E.; Stølen, T.O.; Bye, A.; Volden, M.; Slørdahl, S.A.; Ødegård, R.; Skogvoll, E.; Wisløff, U. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin. Sci. 2009, 116, 317–326. [Google Scholar] [CrossRef]
- King, N.A.; Caudwell, P.; Hopkins, M.; Byrne, N.M.; Colley, R.; Hills, A.P.; Stubbs, J.R.; Blundell, J.E. Metabolic and behavioral compensatory responses to exercise interventions: Barriers to weight loss. Obesity 2007, 15, 1373–1383. [Google Scholar] [CrossRef]
- Melanson, E.L.; Keadle, S.K.; Donnelly, J.E.; Braun, B.; King, N.A. Resistance to exercise-induced weight loss: Compensatory behavioral adaptations. Med. Sci. Sports Exerc. 2013, 45, 1600. [Google Scholar] [CrossRef]
- Blue, M.N.; Smith-Ryan, A.E.; Trexler, E.T.; Hirsch, K.R. The effects of high intensity interval training on muscle size and quality in overweight and obese adults. J. Sci. Med. Sport 2018, 21, 207–212. [Google Scholar] [CrossRef]
- Abe, T.; DeHoyos, D.V.; Pollock, M.L.; Garzarella, L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur. J. Appl. Physiol. 2000, 81, 174–180. [Google Scholar] [CrossRef]
- Wideman, L.; Weltman, J.; Hartman, M.; Johannes, D.; Weltman, A. Growth Hormone Release During Acute and Chronic Aerobic and Resistance Exercise. Sports Med. 2002, 32, 987–1004. [Google Scholar] [CrossRef]
- Francois, M.E.; Little, J.P. Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectr. 2015, 28, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, W.; Xirouchaki, C.E.; El-Gilany, A.-H. The Comparative Effects of High-Intensity Interval Training and Traditional Resistance Training on Hormonal Responses in Young Women: A 10-Week Intervention Study. Sports 2025, 13, 67. [Google Scholar] [CrossRef]
- Zaroni, R.S.; Brigatto, F.A.; Schoenfeld, B.J.; Braz, T.V.; Benvenutti, J.C.; Germano, M.D.; Marchetti, P.H.; Aoki, M.S.; Lopes, C.R. High Resistance-Training Frequency Enhances Muscle Thickness in Resistance-Trained Men. J. Strength Cond. Res. 2019, 33 (Suppl. S1), S140–S151. [Google Scholar] [CrossRef] [PubMed]
- McRae, G.; Payne, A.; Zelt, J.G.; Scribbans, T.D.; Jung, M.E.; Little, J.P.; Gurd, B.J. Extremely low volume, whole-body aerobic-resistance training improves aerobic fitness and muscular endurance in females. Appl. Physiol. Nutr. Metab. 2012, 37, 1124–1131. [Google Scholar] [CrossRef]
- Caparrós-Manosalva, C.; Garrido-Muñoz, N.; Alvear-Constanzo, B.; Sanzana-Laurié, S.; Artigas-Arias, M.; Alegría-Molina, A.; Vidal-Seguel, N.; Espinoza-Araneda, J.; Huard, N.; Pagnussat, A.S.; et al. Effects of high-intensity interval training on lean mass, strength, and power of the lower limbs in healthy old and young people. Front. Physiol. 2023, 14, 1223069. [Google Scholar] [CrossRef]
- Laursen, P.B.; Jenkins, D.G. The scientific basis for high-intensity interval training: Optimising training programmes and maximising performance in highly trained endurance athletes. Sports Med. 2002, 32, 53–73. [Google Scholar] [CrossRef]
- Menz, V.; Marterer, N.; Amin, S.B.; Faulhaber, M.; Hansen, A.B.; Lawley, J.S. Functional vs. Running low-volume high-intensity interval training: Effects on vo2max and muscular endurance. J. Sports Sci. Med. 2019, 18, 497. [Google Scholar]
- Gibala, M.J.; McGee, S.L. Metabolic adaptations to short-term high-intensity interval training: A little pain for a lot of gain? Exerc. Sport Sci. Rev. 2008, 36, 58–63. [Google Scholar] [CrossRef]
- Rohmansyah, N.A.; Ka Praja, R.; Phanpheng, Y.; Hiruntrakul, A. High-Intensity Interval Training Versus Moderate-Intensity Continuous Training for Improving Physical Health in Elderly Women. Inquiry 2023, 60, 1–13. [Google Scholar] [CrossRef]
- Tjønna, A.E.; Lee, S.J.; Rognmo, Ø.; Stølen, T.O.; Bye, A.; Haram, P.M.; Loennechen, J.P.; Al-Share, Q.Y.; Skogvoll, E.; Slørdahl, S.A.; et al. Aerobic Interval Training Versus Continuous Moderate Exercise as a Treatment for the Metabolic Syndrome. Circulation 2008, 118, 346–354. [Google Scholar] [CrossRef]
- Bo, B.; Guo, A.; Kaila, S.J.; Hao, Z.; Zhang, H.; Wei, J.; Yao, Y. Elucidating the primary mechanisms of high-intensity interval training for improved cardiac fitness in obesity. Front. Physiol. 2023, 14, 1170324. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.L.; Costill, D.L.; Fink, W.J.; King, D.S. Effects of eight weeks of bicycle ergometer sprint training on human muscle buffer capacity. Int. J. Sports Med. 1986, 7, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Torma, F.; Gombos, Z.; Jokai, M.; Takeda, M.; Mimura, T.; Radak, Z. High intensity interval training and molecular adaptive response of skeletal muscle. Sports Med. Health Sci. 2019, 1, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Gist, N.H.; Freese, E.C.; Cureton, K.J. Comparison of responses to two high-intensity intermittent exercise protocols. J. Strength Cond. Res. 2014, 28, 3033–3040. [Google Scholar] [CrossRef]
- Murawska-Ciałowicz, E.; de Assis, G.G.; Clemente, F.M.; Feito, Y.; Stastny, P.; Zuwała-Jagiełło, J.; Bibrowicz, B.; Wolański, P. Effect of four different forms of high intensity training on BDNF response to Wingate and Graded Exercise Test. Sci. Rep. 2021, 11, 8599. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).