Acute Responses to Different Velocity Loss Thresholds during Squat Exercise with Blood-Flow Restriction in Strength-Trained Men
Abstract
1. Introduction
2. Materials and Methods
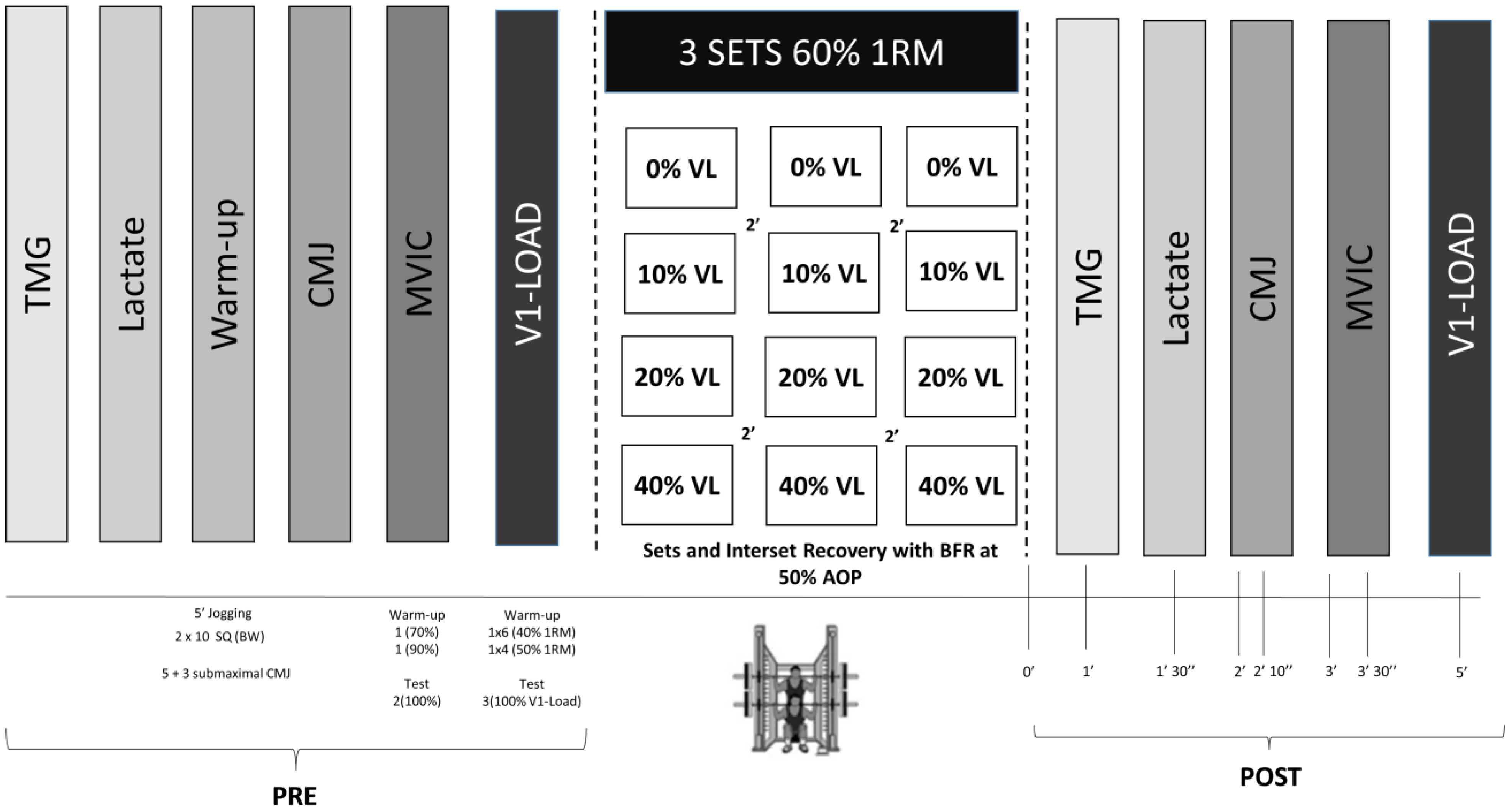
2.1. Study Design
2.2. Subjects
2.3. Testing Procedures
2.3.1. Arterial Occlusion Pressure
2.3.2. Progressive Loading Test
2.3.3. Resistance Exercise Protocol
2.3.4. Tensiomyography
2.3.5. Blood Lactate
2.3.6. Countermovement Jump
2.3.7. Maximal Voluntary Isometric Contraction
2.3.8. V1-Load Test
2.3.9. Electromyography Signal Acquisition
2.4. Statistical Analyses
3. Results
3.1. Descriptive Characteristics of the Resistance Exercise Protocols
3.2. Tensiomyography
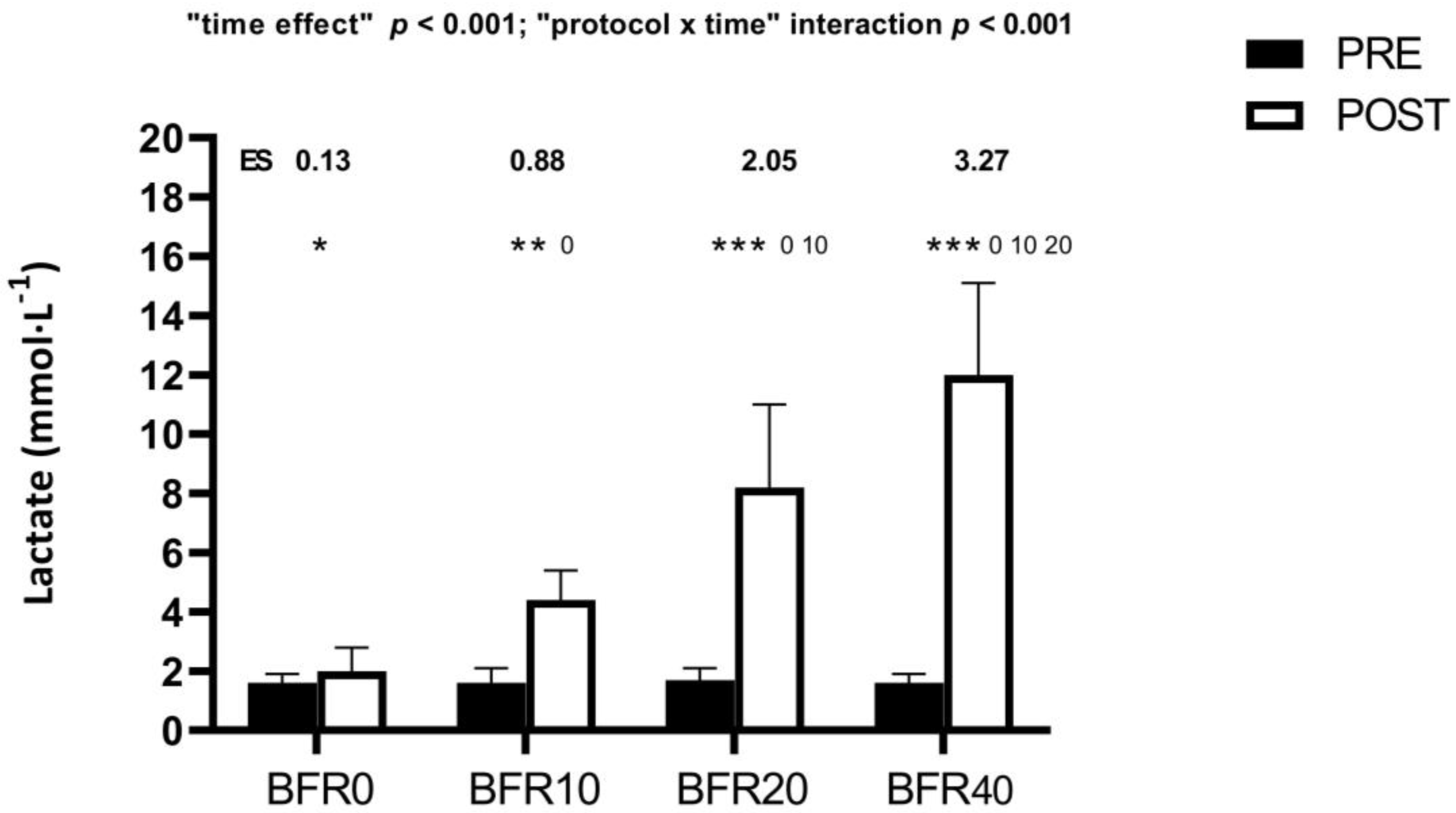
3.3. Blood Lactate
3.4. Mechanical Responses
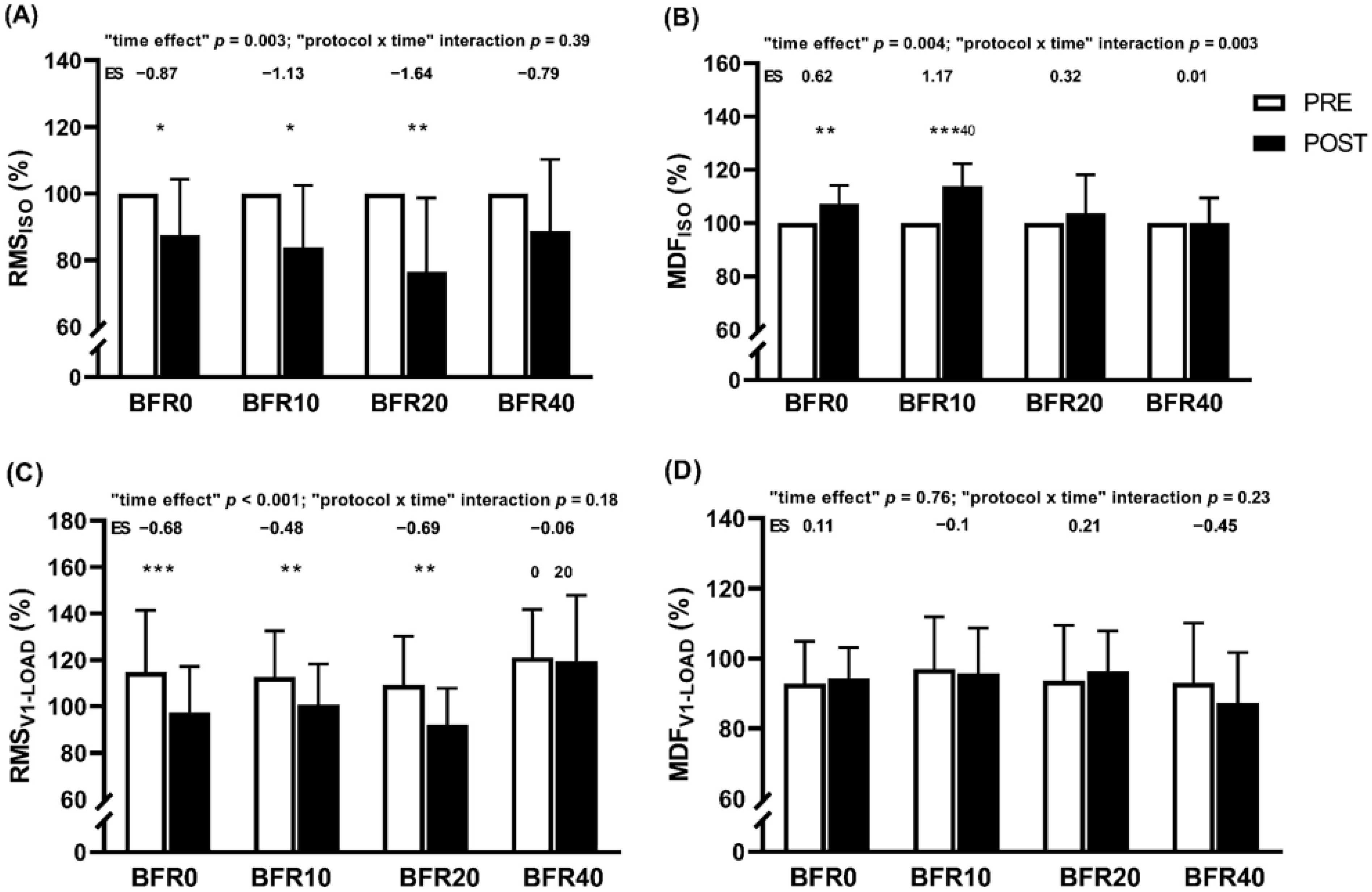
3.5. Neuromuscular Responses
4. Discussion
5. Conclusions
6. Practical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, B.R.; Loenneke, J.P.; Slattery, K.M.; Dascombe, B.J. Exercise with blood flow restriction: An updated evidence-based approach for enhanced muscular development. Sports Med. 2015, 45, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Larkin, K.A.; Macneil, R.G.; Dirain, M.; Sandesara, B.; Manini, T.M.; Buford, T.W. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med. Sci. Sports Exerc. 2012, 44, 2077–2083. [Google Scholar] [CrossRef] [PubMed]
- Takarada, Y.; Sato, Y.; Ishii, N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur. J. Appl. Physiol. 2002, 86, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Beekley, M.D.; Hinata, S.; Koizumi, K.; Sato, Y. Day-to-day change in muscle strength and MRI-measured skeletal muscle size during 7 days KAATSU resistance training: A case study. Int. J. KAATSU Train. Res. 2005, 1, 71–76. [Google Scholar] [CrossRef]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef]
- Lixandrao, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceicao, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of Muscle Strength and Mass Adaptations Between High-Load Resistance Training Versus Low-Load Resistance Training Associated with Blood-Flow Restriction: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Thiebaud, R.S.; Fahs, C.A.; Rossow, L.M.; Abe, T.; Bemben, M.G. Effect of cuff type on arterial occlusion. Clin. Physiol. Funct. Imaging 2013, 33, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Kim, D.; Mouser, J.G.; Allen, K.M.; Thiebaud, R.S.; Abe, T.; Bemben, M.G. Are there perceptual differences to varying levels of blood flow restriction? Physiol. Behav. 2016, 157, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front. Physiol. 2019, 10, 533. [Google Scholar] [CrossRef]
- Nielsen, J.L.; Frandsen, U.; Prokhorova, T.; Bech, R.D.; Nygaard, T.; Suetta, C.; Aagaard, P. Delayed Effect of Blood Flow-restricted Resistance Training on Rapid Force Capacity. Med. Sci. Sports Exerc. 2017, 49, 1157–1167. [Google Scholar] [CrossRef]
- Takada, S.; Okita, K.; Suga, T.; Omokawa, M.; Kadoguchi, T.; Sato, T.; Takahashi, M.; Yokota, T.; Hirabayashi, K.; Morita, N.; et al. Low-intensity exercise can increase muscle mass and strength proportionally to enhanced metabolic stress under ischemic conditions. J. Appl. Physiol. 2012, 113, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Paton, B.; Haddad, F.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Comparison of the acute perceptual and blood pressure response to heavy load and light load blood flow restriction resistance exercise in anterior cruciate ligament reconstruction patients and non-injured populations. Phys. Ther. Sport 2018, 33, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Okita, K.; Morita, N.; Yokota, T.; Hirabayashi, K.; Horiuchi, M.; Takada, S.; Omokawa, M.; Kinugawa, S.; Tsutsui, H. Dose effect on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. J. Appl. Physiol. 2010, 108, 1563–1567. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Fahs, C.A.; Rossow, L.M.; Abe, T.; Bemben, M.G. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med. Hypotheses 2012, 78, 151–154. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Abe, T.; Wilson, J.M.; Ugrinowitsch, C.; Bemben, M.G. Blood flow restriction: How does it work? Front. Physiol. 2012, 3, 392. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.L.; Aagaard, P.; Bech, R.D.; Nygaard, T.; Hvid, L.G.; Wernbom, M.; Suetta, C.; Frandsen, U. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J. Physiol. 2012, 590, 4351–4361. [Google Scholar] [CrossRef] [PubMed]
- Burgomaster, K.A.; Moore, D.R.; Schofield, L.M.; Phillips, S.M.; Sale, D.G.; Gibala, M.J. Resistance training with vascular occlusion: Metabolic adaptations in human muscle. Med. Sci. Sports Exerc. 2003, 35, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Bjørnsen, T.; Wernbom, M.; Kirketeig, A.; Paulsen, G.; Samnøy, L.; Bækken, L.; Cameron-Smith, D.; Berntsen, S.; Raastad, T. Type 1 Muscle Fiber Hypertrophy after Blood Flow-restricted Training in Powerlifters. Med. Sci. Sports Exerc. 2019, 51, 288–298. [Google Scholar] [CrossRef]
- Ortega-Becerra, M.; Sanchez-Moreno, M.; Pareja-Blanco, F. Effects of Cluster Set Configuration on Mechanical Performance and Neuromuscular Activity. J. Strength Cond. Res. 2021, 35, 310–317. [Google Scholar] [CrossRef]
- Sanchez-Medina, L.; Gonzalez-Badillo, J.J. Velocity loss as an indicator of neuromuscular fatigue during resistance training. Med. Sci. Sports Exerc. 2011, 43, 1725–1734. [Google Scholar] [CrossRef]
- Pareja-Blanco, F.; Rodriguez-Rosell, D.; Sanchez-Medina, L.; Ribas-Serna, J.; Lopez-Lopez, C.; Mora-Custodio, R.; Yanez-Garcia, J.M.; Gonzalez-Badillo, J.J. Acute and delayed response to resistance exercise leading or not leading to muscle failure. Clin. Physiol. Funct. Imaging 2017, 37, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rosell, D.; Yanez-Garcia, J.M.; Sanchez-Medina, L.; Mora-Custodio, R.; Gonzalez-Badillo, J.J. Relationship Between Velocity Loss and Repetitions in Reserve in the Bench Press and Back Squat Exercises. J. Strength Cond. Res. 2020, 34, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Weakley, J.; McLaren, S.; Ramirez-Lopez, C.; Garcia-Ramos, A.; Dalton-Barron, N.; Banyard, H.; Mann, B.; Weaving, D.; Jones, B. Application of velocity loss thresholds during free-weight resistance training: Responses and reproducibility of perceptual, metabolic, and neuromuscular outcomes. J. Sports Sci. 2020, 38, 477–485. [Google Scholar] [CrossRef]
- Pareja-Blanco, F.; Alcazar, J.; Cornejo-Daza, P.J.; Sanchez-Valdepenas, J.; Rodriguez-Lopez, C.; Hidalgo-de Mora, J.; Sanchez-Moreno, M.; Bachero-Mena, B.; Alegre, L.M.; Ortega-Becerra, M. Effects of velocity loss in the bench press exercise on strength gains, neuromuscular adaptations and muscle hypertrophy. Scand J. Med. Sci. Sports 2020, 30, 2154–2166. [Google Scholar] [CrossRef] [PubMed]
- Pareja-Blanco, F.; Alcazar, J.; Sánchez-Valdepeñas, J.; Cornejo-Daza, P.J.; Piqueras-Sanchiz, F.; Mora-Vela, R.; Sánchez-Moreno, M.; Bachero-Mena, B.; Ortega-Becerra, M.; Alegre, L.M. Velocity Loss as a Critical Variable Determining the Adaptations to Strength Training. Med. Sci. Sports Exerc. 2020, 52, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Pareja-Blanco, F.; Rodriguez-Rosell, D.; Sanchez-Medina, L.; Sanchis-Moysi, J.; Dorado, C.; Mora-Custodio, R.; Yanez-Garcia, J.M.; Morales-Alamo, D.; Perez-Suarez, I.; Calbet, J.A.L.; et al. Effects of velocity loss during resistance training on athletic performance, strength gains and muscle adaptations. Scand J. Med. Sci. Sports 2017, 27, 724–735. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Sieljacks, P.; Knudsen, L.; Wernbom, M.; Vissing, K. Body position influences arterial occlusion pressure: Implications for the standardization of pressure during blood flow restricted exercise. Eur. J. Appl. Physiol. 2018, 118, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Piqueras-Sanchiz, F.; Martin-Rodriguez, S.; Pareja-Blanco, F.; Baraja-Vegas, L.; Blazquez-Fernandez, J.; Bautista, I.J.; Garcia-Garcia, O. Mechanomyographic Measures of Muscle Contractile Properties are Influenced by Electrode Size and Stimulation Pulse Duration. Sci. Rep. 2020, 10, 8192. [Google Scholar] [CrossRef]
- Valencic, V.; Knez, N. Measuring of skeletal muscles’ dynamic properties. Artif. Organs 1997, 21, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Loturco, I.; Pereira, L.A.; Kobal, R.; Kitamura, K.; Ramírez-Campillo, R.; Zanetti, V.; Abad, C.C.; Nakamura, F.Y. Muscle Contraction Velocity: A Suitable Approach to Analyze the Functional Adaptations in Elite Soccer Players. J. Sports Sci. Med. 2016, 15, 483–491. [Google Scholar] [PubMed]
- Pyne, D.B.; Boston, T.; Martin, D.T.; Logan, A. Evaluation of the Lactate Pro blood lactate analyser. Eur. J. Appl. Physiol. 2000, 82, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: San Diego, CA, USA, 1985. [Google Scholar]
- Enoka, R.M.; Duchateau, J. Translating Fatigue to Human Performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef] [PubMed]
- Ducrocq, G.P.; Blain, G.M. Relationship between neuromuscular fatigue, muscle activation and the work done above the critical power during severe-intensity exercise. Exp. Physiol. 2022, 107, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Piqueras-Sanchiz, F.; Cornejo-Daza, P.J.; Sanchez-Valdepenas, J.; Bachero-Mena, B.; Sanchez-Moreno, M.; Martin-Rodriguez, S.; Garcia-Garcia, O.; Pareja-Blanco, F. Acute Mechanical, Neuromuscular, and Metabolic Responses to Different Set Configurations in Resistance Training. J. Strength Cond. Res. 2022, 36, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG: An update. J. Appl. Physiol. 2014, 117, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Bigland-Ritchie, B.R.; Dawson, N.J.; Johansson, R.S.; Lippold, O.C. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J. Physiol. 1986, 379, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Brody, L.R.; Pollock, M.T.; Roy, S.H.; De Luca, C.J.; Celli, B. pH-induced effects on median frequency and conduction velocity of the myoelectric signal. J. Appl. Physiol. 1991, 71, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Bruggen, T.A.; Baten, C.T.; Rutten, W.L.; Boom, H.B. The median frequency of the surface EMG power spectrum in relation to motor unit firing and action potential properties. J. Electromyogr. Kinesiol. 1992, 2, 15–25. [Google Scholar] [CrossRef]
- Hunter, A.M.; Galloway, S.D.; Smith, I.J.; Tallent, J.; Ditroilo, M.; Fairweather, M.M.; Howatson, G. Assessment of eccentric exercise-induced muscle damage of the elbow flexors by tensiomyography. J. Electromyogr. Kinesiol. 2012, 22, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, J.F.; Franz, A.; Mayer, C.; Zilkens, C.; Buhren, B.A.; Schrumpf, H.; Krauspe, R.; Behringer, M. Tensiomyography parameters and serum biomarkers after eccentric exercise of the elbow flexors. Eur. J. Appl. Physiol. 2019, 119, 455–464. [Google Scholar] [CrossRef]
- de Paula Simola, R.A.; Harms, N.; Raeder, C.; Kellmann, M.; Meyer, T.; Pfeiffer, M.; Ferrauti, A. Assessment of neuromuscular function after different strength training protocols using tensiomyography. J. Strength Cond. Res. 2015, 29, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Wernbom, M.; Aagaard, P. Muscle fibre activation and fatigue with low-load blood flow restricted resistance exercise-An integrative physiology review. Acta Physiol. 2020, 228, e13302. [Google Scholar] [CrossRef]
- Simunic, B.; Degens, H.; Rittweger, J.; Narici, M.; Mekjavic, I.B.; Pisot, R. Noninvasive estimation of myosin heavy chain composition in human skeletal muscle. Med. Sci. Sports Exerc. 2011, 43, 1619–1625. [Google Scholar] [CrossRef]
- Gorostiaga, E.M.; Navarro-Amezqueta, I.; Calbet, J.A.; Hellsten, Y.; Cusso, R.; Guerrero, M.; Granados, C.; Gonzalez-Izal, M.; Ibanez, J.; Izquierdo, M. Energy metabolism during repeated sets of leg press exercise leading to failure or not. PLoS ONE 2012, 7, e40621. [Google Scholar] [CrossRef]
- Pareja-Blanco, F.; Villalba-Fernandez, A.; Cornejo-Daza, P.J.; Sanchez-Valdepenas, J.; Gonzalez-Badillo, J.J. Time Course of Recovery Following Resistance Exercise with Different Loading Magnitudes and Velocity Loss in the Set. Sports 2019, 7, 59. [Google Scholar] [CrossRef]
- Zwarts, M.J.; Arendt-Nielsen, L. The influence of force and circulation on average muscle fibre conduction velocity during local muscle fatigue. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 58, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Solomonow, M.; Baten, C.; Smit, J.; Baratta, R.; Hermens, H.; D’Ambrosia, R.; Shoji, H. Electromyogram power spectra frequencies associated with motor unit recruitment strategies. J. Appl. Physiol. 1990, 68, 1177–1185. [Google Scholar] [CrossRef]
- Van der Hoeven, J.H.; Lange, F. Supernormal muscle fiber conduction velocity during intermittent isometric exercise in human muscle. J. Appl. Physiol. 1994, 77, 802–806. [Google Scholar] [CrossRef]
| Protocol | VL (%) | MPF (N) | MPP (W) | MPV (m·s−1) | Total Rep (n) | Rep Per Set (n) | RMS (%) | MDF (%) |
|---|---|---|---|---|---|---|---|---|
| BFR0 | 0.0 ± 0.0 | 838.7 ± 123.7 | 702.2 ± 112.2 | 0.91 ± 0.09 | 3.0 ± 0.0 | 1.0 ± 0.0 | 105.7 ± 30.6 | 96.0 ± 11.8 |
| BFR10 | 10.2 ± 1.6 0 | 813.5 ± 128.6 | 655.6 ± 95.1 0 | 0.88 ± 0.08 0 | 13.3 ± 3.7 0 | 4.4 ± 1.2 0 | 102.9 ± 17.1 | 95.7 ± 11.9 |
| BFR20 | 20.9 ± 2.1 0 10 | 776 ± 116.1 0 10 | 585.3 ± 86.9 0 10 | 0.81 ± 0.08 0 10 | 22 ± 5.3 0 10 | 7.3 ± 1.8 0 10 | 101.4 ± 19.1 | 93.5 ± 11.4 |
| BFR40 | 41.4 ± 4.0 0 10 20 | 727.3 ± 109.10 10 20 | 501.8 ± 71.3 0 10 20 | 0.73 ± 0.09 0 10 20 | 34.5 ± 7.3 0 10 20 | 11.5 ± 2.4 0 10 20 | 120.3 ± 23.7 0 10 20 | 88.5 ± 10.9 0 10 20 |
| BFR0 | BFR10 | BFR20 | BFR40 | Time Effect | Protocol × Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | ES | Pre | Post | ES | Pre | Post | ES | Pre | Post | ES | p-Value | p-Value | |
| Tc (ms) | 24.9 ± 4.2 | 24.0 ± 5.0 | −0.19 | 25.3 ± 3.5 | 21.6 ± 3.7 *** | −1.01 | 24.0 ± 3.3 | 23.3 ± 4.0 | −0.19 | 24.7 ± 4.8 | 25.5 ± 6.5 | 0.31 | 0.10 | 0.02 |
| Td (ms) | 23.8 ± 2.7 | 21.3 ± 2.8 *** | −0.89 | 23.7 ± 2.0 | 20.2 ± 1.9 *** | −1.76 | 22.8 ± 2.3 | 20.5 ± 2.0 *** | −1.05 | 23.7 ± 3.1 | 21.2 ± 2.6 ** | −0.86 | < 0.001 | 0.08 |
| Dm (mm) | 5.02 ± 1.71 | 4.27 ± 1.84 ** | −0.41 | 5.44 ± 1.61 | 4.27 ± 1.67 ** | −0.70 | 5.16 ± 1.89 | 3.73 ± 1.16 *** | −0.89 | 5.54 ± 1.85 | 3.07 ± 0.91 *** 0 10 | −1.66 | < 0.001 | 0.03 |
| Vd (mm·ms−1) | 0.104 ± 0.030 | 0.092 ± 0.033 * | −0.37 | 0.110 ± 0.028 | 0.101 ± 0.033 | −0.29 | 0.107 ± 0.036 | 0.084 ± 0.025 ** | −0.73 | 0.114 ± 0.034 | 0.067 ± 0.022 *** 0 10 20 | −1.61 | < 0.001 | 0.01 |
| BFR0 | BFR10 | BFR20 | BFR40 | Time Effect | Protocol × Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | ES | Pre | Post | ES | Pre | Post | ES | Pre | Post | ES | p-Value | p-Value | |
| CMJ (cm) | 39.3 ± 6.8 | 35.6 ± 7.0 *** | −0.56 | 39.2 ± 6.7 | 35.0 ± 5.9 *** | −0.63 | 39.3 ± 6.4 | 33.5 ± 5.4 *** 10 | −0.87 | 39.0 ± 6.7 | 28.7 ± 5.6 *** 0 10 20 | −1.55 | <0.001 | <0.001 |
| MIF (N) | 1157.2 ± 159.9 | 1032.3 ± 153.5 *** | −0.73 | 1149.5 ± 200.2 | 1028.5 ± 151.3 *** | −0.71 | 1146.5 ± 195.3 | 985.7 ± 152.3 *** | −0.94 | 1077.7 ± 155.3 | 912.2 ± 138.9 *** 0 10 | −0.97 | 0.12 | <0.001 |
| RFDmax (N·s−1) | 4478.8 ± 1189.9 | 3946.9 ± 1103.7 ** | −0.43 | 4112.5 ± 1197.7 | 3840.5 ± 1230.5 | −0.22 | 4015.4 ± 1463.2 | 3775.7 ± 1407.9 | −0.19 | 4078.4 ± 1068.7 | 3076.6 ± 960.2 *** 0 10 20 | −0.80 | <0.001 | <0.001 |
| MPF-V1 (N) | 867.7 ± 133.8 | 821.6 ± 132.9 *** | −0.35 | 860.4 ± 132. 9 | 834.1 ± 135.7 *** | −0.20 | 855.5 ± 129.9 | 826.2 ± 124.9 *** | −0.22 | 852.4 ± 132.4 | 789.7 ± 128.3 *** 0 20 | −0.48 | 0.05 | <0.001 |
| MPP-V1 (W) | 761.5 ± 129.4 | 665.0 ± 117.9 *** | −0.84 | 752.2 ± 119.8 | 690.1 ± 106.7 *** | −0.54 | 754.3 ± 117.4 | 673.2 ± 96.2 *** | −0.70 | 753.8 ± 121 | 590.1 ± 113.3 *** 0 10 20 | −1.42 | 0.006 | <0.001 |
| MPV-V1 (m·s−1) | 0.98 ± 0.09 | 0.88 ± 0.09 *** | −1.18 | 0.96 ± 0.09 | 0.89 ± 0.07 *** | −0.71 | 0.97 ± 0.06 | 0.88 ± 0.07 *** | −1.18 | 0.98 ± 0.07 | 0.80 ± 0.09 *** 0 10 20 | −2.13 | 0.004 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Valdepeñas, J.; Cornejo-Daza, P.J.; Rodiles-Guerrero, L.; Páez-Maldonado, J.A.; Sánchez-Moreno, M.; Bachero-Mena, B.; Saez de Villarreal, E.; Pareja-Blanco, F. Acute Responses to Different Velocity Loss Thresholds during Squat Exercise with Blood-Flow Restriction in Strength-Trained Men. Sports 2024, 12, 171. https://doi.org/10.3390/sports12060171
Sánchez-Valdepeñas J, Cornejo-Daza PJ, Rodiles-Guerrero L, Páez-Maldonado JA, Sánchez-Moreno M, Bachero-Mena B, Saez de Villarreal E, Pareja-Blanco F. Acute Responses to Different Velocity Loss Thresholds during Squat Exercise with Blood-Flow Restriction in Strength-Trained Men. Sports. 2024; 12(6):171. https://doi.org/10.3390/sports12060171
Chicago/Turabian StyleSánchez-Valdepeñas, Juan, Pedro J. Cornejo-Daza, Luis Rodiles-Guerrero, Jose A. Páez-Maldonado, Miguel Sánchez-Moreno, Beatriz Bachero-Mena, Eduardo Saez de Villarreal, and Fernando Pareja-Blanco. 2024. "Acute Responses to Different Velocity Loss Thresholds during Squat Exercise with Blood-Flow Restriction in Strength-Trained Men" Sports 12, no. 6: 171. https://doi.org/10.3390/sports12060171
APA StyleSánchez-Valdepeñas, J., Cornejo-Daza, P. J., Rodiles-Guerrero, L., Páez-Maldonado, J. A., Sánchez-Moreno, M., Bachero-Mena, B., Saez de Villarreal, E., & Pareja-Blanco, F. (2024). Acute Responses to Different Velocity Loss Thresholds during Squat Exercise with Blood-Flow Restriction in Strength-Trained Men. Sports, 12(6), 171. https://doi.org/10.3390/sports12060171





