The Acute and Chronic Effects of Resistance and Aerobic Exercise in Hemostatic Balance: A Brief Review
Abstract
1. Introduction
2. Endurance Exercise and Hemostatic Balance
2.1. Acute Effects
2.1.1. Healthy Individuals and Athletes
Platelet Function
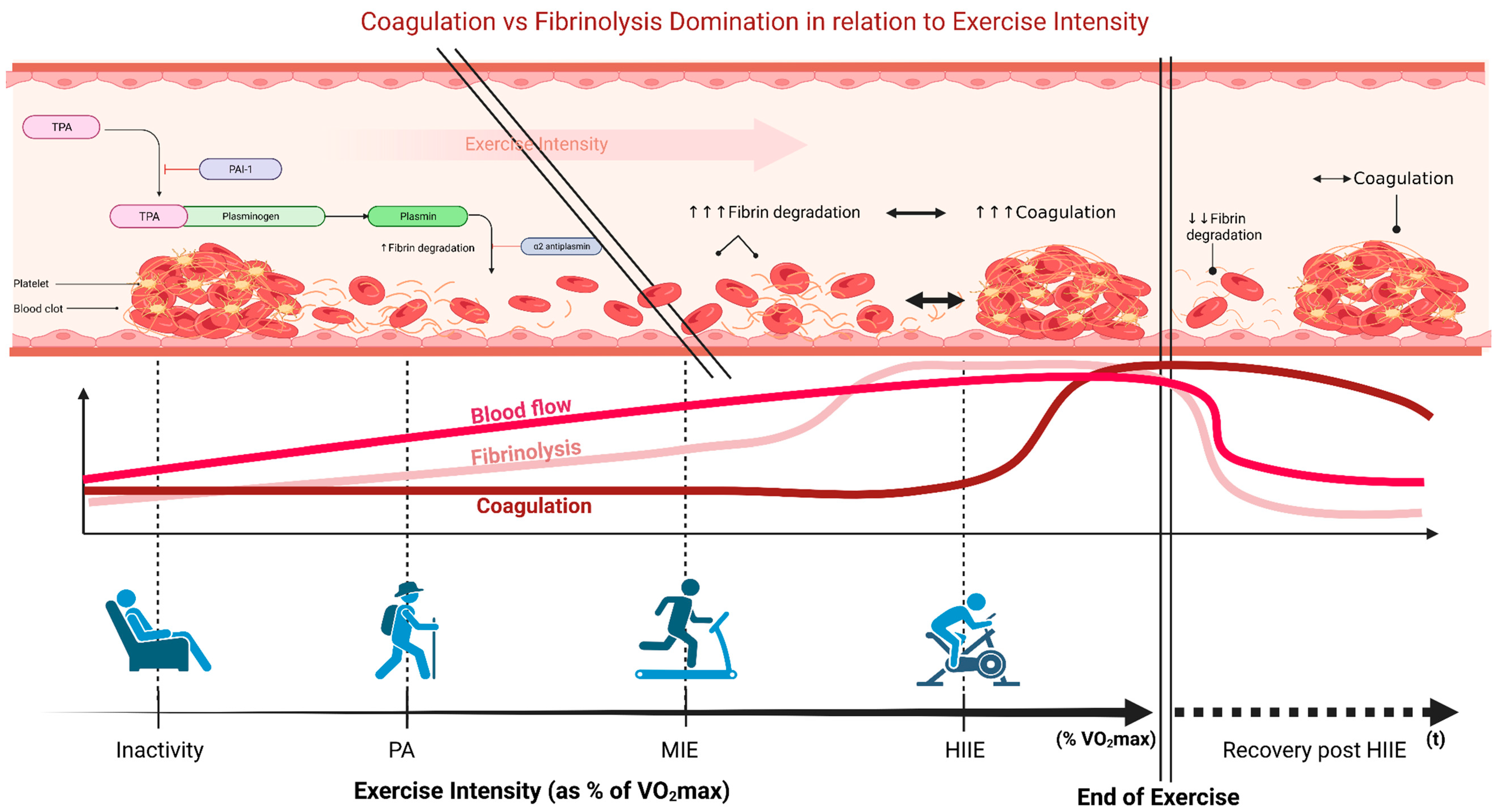
Coagulation
Fibrinolysis
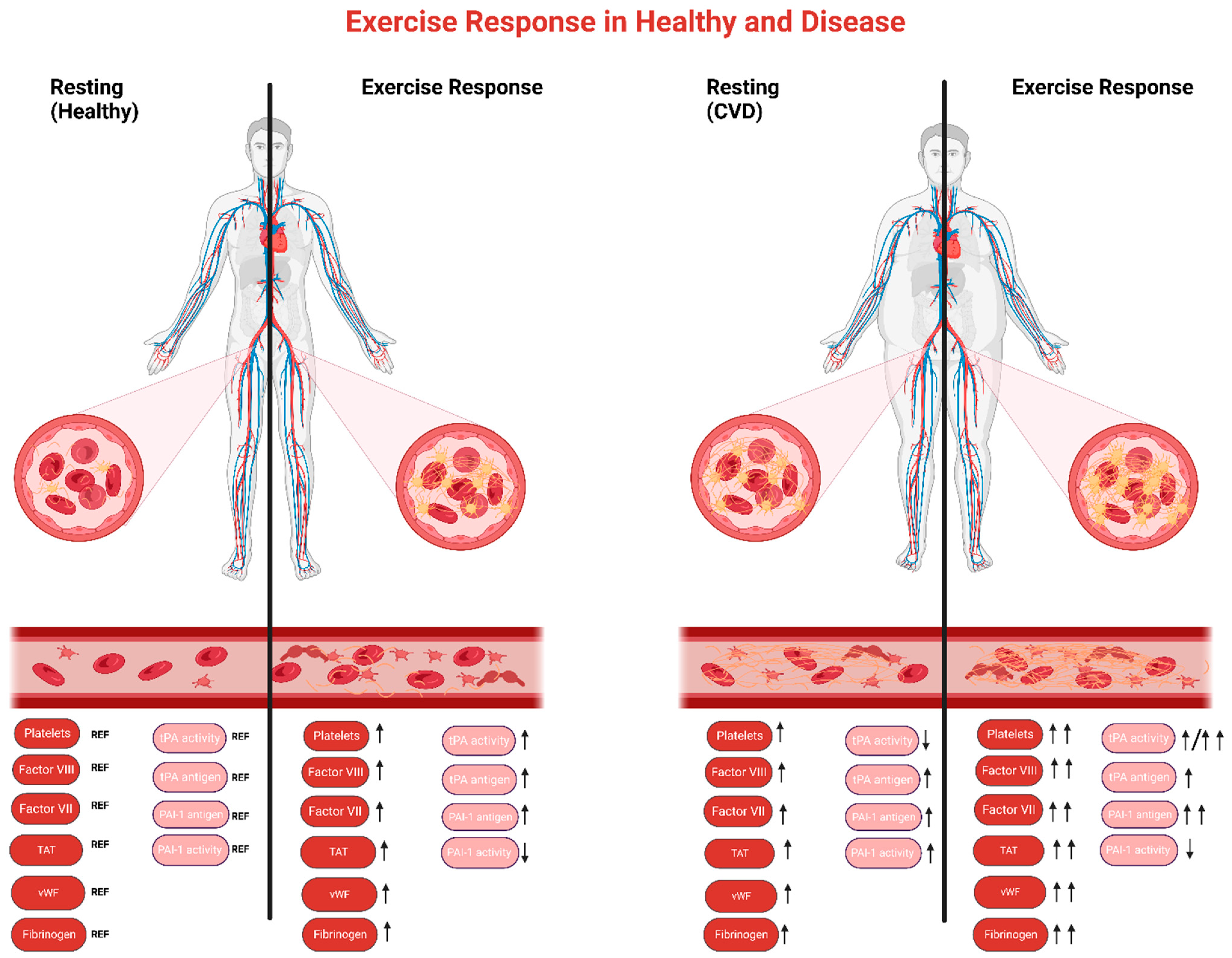
2.1.2. Patient Populations
Platelet Function
Coagulation and Fibrinolysis
2.2. Chronic Adaptations
2.2.1. Healthy Individuals and Athletes
Platelet Function
Coagulation
Fibrinolysis
2.2.2. Patient Populations
Platelet Function
Coagulation
Fibrinolysis
3. Resistance Exercise and Hemostatic Balance
3.1. Acute Effects
3.1.1. Healthy Individuals and Athletes
Platelet Function
Coagulation
Fibrinolysis
3.1.2. Patient Populations
Platelet Function
Fibrinolysis
3.2. Chronic Adaptations
3.2.1. Healthy Individuals and Athletes
Coagulation
Fibrinolysis
3.2.2. Patient Populations
Coagulation
4. High-Intensity Interval Exercise and Hemostatic Balance
4.1. Acute Effects
4.1.1. Healthy Individuals and Athletes
Platelet Function
Coagulation
Fibrinolysis
4.1.2. Patient Populations
Platelet Function
Coagulation
4.2. Chronic Adaptations
4.2.1. Healthy Individuals and Athletes
Platelet Function
Coagulation and Fibrinolysis
4.2.2. Patient Populations
Platelet Function
Coagulation
Fibrinolysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saint-Maurice, P.F.; Graubard, B.I.; Troiano, R.P.; Berrigan, D.; Galuska, D.A.; Fulton, J.E.; Matthews, C.E. Estimated number of deaths prevented through increased physical activity among US adults. JAMA Intern. Med. 2022, 182, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- Gorzelitz, J.; Trabert, B.; Katki, H.A.; Moore, S.C.; Watts, E.L.; Matthews, C.E. Independent and joint associations of weightlifting and aerobic activity with all-cause, cardiovascular disease and cancer mortality in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Br. J. Sports Med. 2022, 56, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, D.; Viken, H.; Steinshamn, S.L.; Dalen, H.; Støylen, A.; Loennechen, J.P.; Reitlo, L.S.; Zisko, N.; Bækkerud, F.H.; Tari, A.R.; et al. Effect of exercise training for five years on all cause mortality in older adults-the Generation 100 study: Randomised controlled trial. BMJ 2020, 371, m3485. [Google Scholar] [CrossRef]
- Pedisic, Z.; Shrestha, N.; Kovalchik, S.; Stamatakis, E.; Liangruenrom, N.; Grgic, J.; Titze, S.; Biddle, S.J.; Bauman, A.E.; Oja, P. Is running associated with a lower risk of all-cause, cardiovascular and cancer mortality, and is the more the better? A systematic review and meta-analysis. Br J. Sports Med. 2020, 54, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Momma, H.; Kawakami, R.; Honda, T.; Sawada, S.S. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: A systematic review and meta-analysis of cohort studies. Br. J. Sports Med. 2022, 56, 755–763. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; Lucia, A. Exercise is the real polypill. Physiology (Bethesda) 2013, 28, 330–358. [Google Scholar] [CrossRef]
- Gubert, C.; Hannan, A.J. Exercise mimetics: Harnessing the therapeutic effects of physical activity. Nat. Rev. Drug. Discov. 2021, 20, 862–879. [Google Scholar] [CrossRef]
- Athanasiou, N.; Bogdanis, G.C.; Mastorakos, G. Endocrine responses of the stress system to different types of exercise. Rev. Endocr. Metab. Disord. 2023, 24, 251–266. [Google Scholar] [CrossRef]
- Jeong, S.-W.; Kim, S.-H.; Kang, S.-H.; Kim, H.-J.; Yoon, C.-H.; Youn, T.-J.; Chae, I.-H. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur. Heart J. 2019, 40, 3547–3555. [Google Scholar] [CrossRef]
- Gong, Q.; Zhang, P.; Wang, J.; Ma, J.; An, Y.; Chen, Y.; Zhang, B.; Feng, X.; Li, H.; Chen, X.; et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019, 7, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Vermylen, J.G.; Chamone, D.A. The role of the fibrinolytic system in thromboembolism. Prog. Cardiovasc. Dis. 1979, 21, 255–266. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; de Boer, J.D.; Levi, M. The effect of inflammation on coagulation and vice versa. Curr. Opin. Infect. Dis. 2011, 24, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, P.K.; Adams, M.J. Thrombosis in systemic lupus erythematosus: Role of impaired fibrinolysis. Semin. Thromb. Hemost. 2013, 39, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A. Liver disease and hemostatic (dys)function. Semin. Thromb. Hemost. 2015, 41, 462–467. [Google Scholar] [CrossRef]
- Tran, H.D.N.; Moonshi, S.S.; Xu, Z.P.; Ta, H.T. Influence of nanoparticles on the haemostatic balance: Between thrombosis and haemorrhage. Biomater. Sci. 2021, 10, 10–50. [Google Scholar] [CrossRef]
- Thrall, G.; Lip, G.Y. Exercise and the prothrombotic state: A paradox of cardiovascular prevention or an enhanced prothrombotic state? Arterioscler. Thromb. Vasc. Biol. 2005, 25, 265–266. [Google Scholar] [CrossRef]
- Sharma, S.; Merghani, A.; Mont, L. Exercise and the heart: The good, the bad, and the ugly. Eur. Heart J. 2015, 36, 1445–1453. [Google Scholar] [CrossRef]
- Parry-Williams, G.; Sharma, S. The effects of endurance exercise on the heart: Panacea or poison? Nat. Rev. Cardiol. 2020, 17, 402–412. [Google Scholar] [CrossRef]
- Hilberg, T.; Ransmann, P.; Hagedorn, T. Sport and venous thromboembolism—Site, accompanying features, symptoms, and diagnosis. Dtsch. Arztebl. Int. 2021, 118, 181–187. [Google Scholar] [CrossRef]
- Smith, D.L.; Fernhall, B. Hemostasis: Coagulation and Fibrinolysis. In Advanced Cardiovascular Exercise Physiology; Smith, D.L., Fernhall, B., Eds.; Human Kinetics: Champaign, IL, USA, 2011; pp. 123–136. [Google Scholar]
- Neubauer, K.; Zieger, B. Endothelial cells and coagulation. Cell Tissue Res. 2022, 387, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Scridon, A. Platelets and their role in hemostasis and thrombosis-From physiology to pathophysiology and therapeutic implications. Int. J. Mol. Sci. 2022, 23, 12772. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2016, 2, 16045. [Google Scholar] [CrossRef]
- Frischmuth, T.; Hindberg, K.; Aukrust, P.; Ueland, T.; Braekkan, S.K.; Hansen, J.B.; Morelli, V.M. Elevated plasma levels of plasminogen activator inhibitor-1 are associated with risk of future incident venous thromboembolism. J. Thromb. Haemost. 2022, 20, 1618–1626. [Google Scholar] [CrossRef]
- Poli, K.A.; Tofler, G.H.; Larson, M.G.; Evans, J.C.; Sutherland, P.A.; Lipinska, I.; Mittleman, M.A.; Muller, J.E.; D’Agostino, R.B.; Wilson, P.W.F.; et al. Association of blood pressure with fibrinolytic potential in the Framingham Offspring population. Circulation 2000, 101, 264–269. [Google Scholar] [CrossRef]
- Hu, X.; Zan, X.; Xie, Z.; Li, Y.; Lin, S.; Li, H.; You, C. Association between plasminogen activator inhibitor-1 genetic polymorphisms and stroke susceptibility. Mol. Neurobiol. 2017, 54, 328–341. [Google Scholar] [CrossRef]
- Song, C.; Burgess, S.; Eicher, J.D.; O’Donnell, C.J.; Johnson, A.D. Causal effect of plasminogen activator inhibitor type 1 on coronary heart disease. J. Am. Heart Assoc. 2017, 6, e004918. [Google Scholar] [CrossRef]
- Oh, J.; Lee, H.J.; Song, J.H.; Park, S.I.; Kim, H. Plasminogen activator inhibitor-1 as an early potential diagnostic marker for Alzheimer’s disease. Exp. Gerontol. 2014, 60, 87–91. [Google Scholar] [CrossRef]
- Tsai, S.J. Role of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in psychological stress and depression. Oncotarget 2017, 8, 113258–113268. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Bordin Barbieri, N.; Weinmann, T.; Ziegelmann, P.K.; Duncan, B.B.; Inês Schmidt, M. Plasminogen activator inhibitor-1 and type 2 diabetes: A systematic review and meta-analysis of observational studies. Sci. Rep. 2016, 6, 17714. [Google Scholar] [CrossRef]
- Batiha, G.E.; Al-Kuraishy, H.M.; Al-Maiahy, T.J.; Al-Buhadily, A.K.; Saad, H.M.; Al-Gareeb, A.I.; Simal-Gandara, J. Plasminogen activator inhibitor 1 and gestational diabetes: The causal relationship. Diabetol. Metab. Syndr. 2022, 14, 127. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.A. Exercise and cardiovascular events: A double-edged sword? J. Sports Sci. 1999, 17, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Exercise is a double-edged sword for endothelial function. Hypertens. Res. 2016, 39, 61–63. [Google Scholar] [CrossRef]
- Du, F.; Wu, C. Review on the effect of exercise training on immune function. Biomed. Res. Int. 2022, 2022, 9933387. [Google Scholar] [CrossRef]
- Olsen, L.N.; Fischer, M.; Evans, P.A.; Gliemann, L.; Hellsten, Y. Does exercise influence the susceptibility to arterial thrombosis? An integrative perspective. Front. Physiol. 2021, 12, 636027. [Google Scholar] [CrossRef]
- Wang, J.S. Exercise prescription and thrombogenesis. J. Biomed. Sci. 2006, 13, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Haynes, A.; Linden, M.D.; Robey, E.; Naylor, L.H.; Ainslie, P.N.; Cox, K.L.; Lautenschlager, N.T.; Green, D.J. Beneficial impacts of regular exercise on platelet function in sedentary older adults: Evidence from a randomized 6-mo walking trial. J. Appl. Physiol. 2018, 125, 401–408. [Google Scholar] [CrossRef]
- Thompson, P.D.; Franklin, B.A.; Balady, G.J.; Blair, S.N.; Corrado, D.; Estes, N.A., 3rd; Fulton, J.E.; Gordon, N.F.; Haskell, W.L.; Link, M.S.; et al. Exercise and acute cardiovascular events placing the risks into perspective: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 2007, 115, 2358–2368. [Google Scholar] [CrossRef]
- Hvas, A.M.; Neergaard-Petersen, S. Influence of exercise on platelet function in patients with cardiovascular disease. Semin Thromb. Hemost. 2018, 44, 802–812. [Google Scholar] [CrossRef]
- Haynes, A.; Linden, M.D.; Robey, E.; Watts, G.F.; Barrett, P.H.R.; Naylor, L.H.; Green, D.J. Acute impact of different exercise modalities on arterial and platelet function. Med. Sci. Sports Exerc. 2018, 50, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Womack, C.J.; Nagelkirk, P.R.; Coughlin, A.M. Exercise-induced changes in coagulation and fibrinolysis in healthy populations and patients with cardiovascular disease. Sports Med. 2003, 33, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Moussalli, H.; Ledinski, G.; Leschnik, B.; Schlagenhauf, A.; Koestenberger, M.; Polt, G.; Cvirn, G. Effects of a single bout of walking exercise on blood coagulation parameters in obese women. J. Appl. Physiol. 2013, 115, 57–63. [Google Scholar] [CrossRef] [PubMed]
- van der Vorm, L.N.; Huskens, D.; Kicken, C.H.; Remijn, J.A.; Roest, M.; de Laat, B.; Miszta, A. Effects of repeated bouts of exercise on the hemostatic system. Semin. Thromb. Hemost. 2018, 44, 710–722. [Google Scholar] [CrossRef]
- Hilberg, T.; Menzel, K.; Wehmeier, U.F. Endurance training modifies exercise-induced activation of blood coagulation: RCT. Eur. J. Appl. Physiol. 2013, 113, 1423–1430. [Google Scholar] [CrossRef]
- Taniguchi, N.; Furui, H.; Yamauchi, K.; Sotobata, I. Effects of treadmill exercise on platelet functions and blood coagulating activities in healthy men. Jpn. Heart J. 1984, 25, 167–180. [Google Scholar] [CrossRef]
- Burghuber, O.; Sinzinger, H.; Silberbauer, K.; Wolf, C.; Haber, P. Decreased prostacyclin sensitivity of human platelets after jogging and squash. Prostaglandins Med. 1981, 6, 127–130. [Google Scholar] [CrossRef]
- Feng, D.L.; Murillo, J.; Jadhav, P.; McKenna, C.; Gebara, O.C.; Lipinska, I.; Muller, J.E.; Tofler, G.H. Upright posture and maximal exercise increase platelet aggregability and prostacyclin production in healthy male subjects. Br. J. Sports Med. 1999, 33, 401–404. [Google Scholar] [CrossRef]
- el-Sayed, M.S. Effects of exercise on blood coagulation, fibrinolysis and platelet aggregation. Sports Med. 1996, 22, 282–298. [Google Scholar] [CrossRef]
- Cadroy, Y.; Pillard, F.; Sakariassen, K.S.; Thalamas, C.; Boneu, B.; Riviere, D. Strenuous but not moderate exercise increases the thrombotic tendency in healthy sedentary male volunteers. J. Appl. Physiol. 2002, 93, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Cheng, L.J. Effect of strenuous, acute exercise on alpha2-adrenergic agonist-potentiated platelet activation. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.S.; Ali, N.; El-Sayed Ali, Z. Aggregation and activation of blood platelets in exercise and training. Sports Med. 2005, 35, 11–22. [Google Scholar] [CrossRef]
- Hawkey, C.M.; Britton, B.J.; Wood, W.G.; Peele, M.; Irving, M.H. Changes in blood catecholamine levels and blood coagulation and fibrinolytic activity in response to graded exercise in man. Br. J. Haematol. 1975, 29, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E. Effects of strenuous exercise on haemostasis. Br. J. Sports Med. 2003, 37, 433–435. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chen, J.K.; Wang, J.S. Strenuous exercise promotes shear-induced thrombin generation by increasing the shedding of procoagulant microparticles from platelets. Thromb. Haemost. 2010, 104, 293–301. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Nomura, S.; Miyake, T.; Kagawa, H.; Kitada, C.; Taniguchi, H.; Komiyama, Y.; Fujimura, Y.; Ikeda, Y.; Fukuhara, S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood 1996, 88, 3456–3464. [Google Scholar] [CrossRef]
- Austin, A.W.; Patterson, S.M.; von Känel, R. Hemoconcentration and hemostasis during acute stress: Interacting and independent effects. Ann. Behav. Med. 2011, 42, 153–173. [Google Scholar] [CrossRef]
- Brozovīc, M. Physiological mechanisms in coagulation and fibrinolysis. Br. Med. Bull. 1977, 33, 231–238. [Google Scholar] [CrossRef]
- Cohen, R.J.; Epstein, S.E.; Cohen, L.S.; Dennis, L.H. Alterations of fibrinolysis and blood coagulation induced by exercise, and the role of beta-adrenergic-receptor stimulation. Lancet 1968, 2, 1264–1266. [Google Scholar] [CrossRef]
- El-Sayed, M.S.; El-Sayed Ali, Z.; Ahmadizad, S. Exercise and training effects on blood haemostasis in health and disease: An update. Sports Med. 2004, 34, 181–200. [Google Scholar] [CrossRef]
- von Känel, R.; Dimsdale, J.E. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur. J. Haematol. 2000, 65, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Martignoni, E.; Sances, E.; Bono, G.; Nappi, G. Combined response of plasma and platelet catecholamines to different types of short-term stress. Life Sci. 1995, 56, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Golaszewska, A.; Misztal, T.; Marcinczyk, N.; Chabielska, E.; Rusak, T. Adrenaline may contribute to prothrombotic condition via augmentation of platelet procoagulant response, enhancement of fibrin formation, and attenuation of fibrinolysis. Front. Physiol. 2021, 12, 657881. [Google Scholar] [CrossRef]
- Ikarugi, H.; Taka, T.; Nakajima, S.; Noguchi, T.; Watanabe, S.; Sasaki, Y.; Haga, S.; Ueda, T.; Seki, J.; Yamamoto, J. Norepinephrine, but not epinephrine, enhances platelet reactivity and coagulation after exercise in humans. J. Appl. Physiol. 1999, 86, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Figures, W.R.; Scearce, L.M.; Wachtfogel, Y.; Chen, J.; Colman, R.F.; Colman, R.W. Platelet ADP receptor and alpha 2-adrenoreceptor interaction. Evidence for an ADP requirement for epinephrine-induced platelet activation and an influence of epinephrine on ADP binding. J. Biol. Chem. 1986, 261, 5981–5986. [Google Scholar] [CrossRef]
- Garcia-Alvarez, M.; Marik, P.; Bellomo, R. Stress hyperlactataemia: Present understanding and controversy. Lancet Diabetes Endocrinol. 2014, 2, 339–347. [Google Scholar] [CrossRef]
- Wang, J.S.; Jen, C.J.; Kung, H.C.; Lin, L.J.; Hsiue, T.R.; Chen, H.I. Different effects of strenuous exercise and moderate exercise on platelet function in men. Circulation 1994, 90, 2877–2885. [Google Scholar] [CrossRef]
- Drygas, W.K. Changes in blood platelet function, coagulation, and fibrinolytic activity in response to moderate, exhaustive, and prolonged exercise. Int. J. Sports Med. 1988, 9, 67–72. [Google Scholar] [CrossRef]
- Lockard, M.M.; Gopinathannair, R.; Paton, C.M.; Phares, D.A.; Hagberg, J.M. Exercise training-induced changes in coagulation factors in older adults. Med. Sci. Sports Exerc. 2007, 39, 587–592. [Google Scholar] [CrossRef]
- El-Sayed, M.S.; Sale, C.; Jones, P.G.; Chester, M. Blood hemostasis in exercise and training. Med. Sci. Sports Exerc. 2000, 32, 918–925. [Google Scholar] [CrossRef]
- Pinotti, M.; Bertolucci, C.; Portaluppi, F.; Colognesi, I.; Frigato, E.; Foà, A.; Bernardi, F. Daily and circadian rhythms of tissue factor pathway inhibitor and factor VII activity. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 646–649. [Google Scholar] [CrossRef]
- West, A.S.; Schønsted, M.I.; Iversen, H.K. Impact of the circadian clock on fibrinolysis and coagulation in healthy individuals and cardiovascular patients—A systematic review. Thromb. Res. 2021, 207, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Zadow, E.K.; Kitic, C.M.; Wu, S.S.X.; Fell, J.W.; Adams, M.J. Time of day and short-duration high-intensity exercise influences on coagulation and fibrinolysis. Eur. J. Sport Sci. 2018, 18, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Jilma, B.; Dirnberger, E.; Eichler, H.G.; Matulla, B.; Schmetterer, L.; Kapiotis, S.; Speiser, W.; Wagner, O.F. Partial blockade of nitric oxide synthase blunts the exercise-induced increase of von Willebrand factor antigen and of factor VIII in man. Thromb. Haemost. 1997, 78, 1268–1271. [Google Scholar] [CrossRef]
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.M.; Rakobowchuk, M.; Macdonald, M.J.; McGee, S.L.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef]
- Laursen, P.B.; Shing, C.M.; Peake, J.M.; Coombes, J.S.; Jenkins, D.G. Influence of high-intensity interval training on adaptations in well-trained cyclists. J. Strength Cond. Res. 2005, 19, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Ostrowski, S.R. Acute coagulopathy of trauma: Balancing progressive catecholamine induced endothelial activation and damage by fluid phase anticoagulation. Med. Hypotheses 2010, 75, 564–567. [Google Scholar] [CrossRef]
- Prentice, C.R.M.; Forbes, C.D.; Smith, S.M. Rise of factor VIII after exercise and adrenaline infusion, measured by immunological and biological techniques. Thromb. Res. 1972, 1, 493–505. [Google Scholar] [CrossRef]
- Weiss, C.; Seitel, G.; Bärtsch, P. Coagulation and fibrinolysis after moderate and very heavy exercise in healthy male subjects. Med. Sci. Sports Exerc. 1998, 30, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Bourey, R.E.; Santoro, S.A. Interactions of exercise, coagulation, platelets, and fibrinolysis—A brief review. Med. Sci. Sports Exerc. 1988, 20, 439–446. [Google Scholar] [CrossRef]
- Chandler, W.L.; Levy, W.C.; Veith, R.C.; Stratton, J.R. A kinetic model of the circulatory regulation of tissue plasminogen activator during exercise, epinephrine infusion, and endurance training. Blood 1993, 81, 3293–3302. [Google Scholar] [CrossRef]
- Kupchak, B.R.; Creighton, B.C.; Aristizabal, J.C.; Dunn-Lewis, C.; Volk, B.M.; Ballard, K.D.; Comstock, B.A.; Maresh, C.M.; Kraemer, W.J.; Volek, J.S. Beneficial effects of habitual resistance exercise training on coagulation and fibrinolytic responses. Thromb. Res. 2013, 131, e227–e234. [Google Scholar] [CrossRef]
- Yin, C.; Wang, Y.; Mo, C.; Yue, Z.; Sun, Y.; Hu, D. Influence of cardiopulmonary exercise test on platelet function in patients with coronary artery diseases on antiplatelet therapy. BMC Cardiovasc. Disord. 2022, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Wang, Y.; Yue, Z.; Hu, D.; Yin, C. Influence of exercise test on platelet function in patients with coronary arterial disease: A systematic review. Medicine 2021, 100, e24932. [Google Scholar] [CrossRef]
- Bøhn, S.K.; Thune, I.; Flote, V.G.; Frydenberg, H.; Bertheussen, G.F.; Husøy, A.; Fjeldheim, F.; Brunvoll, S.H.; Hjartåker, A.; Mowinckel, M.C.; et al. Effects of a 1-year physical activity intervention on markers of hemostasis among breast cancer survivors: A randomized controlled trial. TH Open 2021, 5, e14–e23. [Google Scholar] [CrossRef] [PubMed]
- Braschi, A. Acute exercise-induced changes in hemostatic and fibrinolytic properties: Analogies, similarities, and differences between normotensive subjects and patients with essential hypertension. Platelets 2019, 30, 675–689. [Google Scholar] [CrossRef]
- Gkaliagkousi, E.; Gavriilaki, E.; Douma, S. Effects of acute and chronic exercise in patients with essential hypertension: Benefits and risks. Am. J. Hypertens. 2015, 28, 429–439. [Google Scholar] [CrossRef]
- Kolasa-Trela, R.; Fil, K.; Wypasek, E.; Undas, A. Exercise stress testing enhances blood coagulation and impairs fibrinolysis in asymptomatic aortic valve stenosis. J. Cardiol. 2015, 65, 501–507. [Google Scholar] [CrossRef]
- Papathanasiou, G.; Tsamis, N.; Georgiadou, P.; Adamopoulos, S. Beneficial effects of physical training and methodology of exercise prescription in patients with heart failure. Hellenic. J. Cardiol. 2008, 49, 267–277. [Google Scholar] [PubMed]
- Papathanasiou, G.; Mitsiou, G.; Stamou, M.; Stasi, S.; Mamali, A.; Papageorgiou, E. Impact of physical activity on heart rate, blood pressure and rate-pressure product in healthy elderly. Health Sci. J. 2020, 14, 712. [Google Scholar]
- Kestin, A.S.; Ellis, P.A.; Barnard, M.R.; Errichetti, A.; Rosner, B.A.; Michelson, A.D. Effect of strenuous exercise on platelet activation state and reactivity. Circulation 1993, 88, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Jen, C.J.; Chen, H.I. Effects of exercise training and deconditioning on platelet function in men. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Turitto, V.T.; Weiss, H.J. Red blood cells: Their dual role in thrombus formation. Science 1980, 207, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Ruslan, N.H.; Ghosh, A.; Hassan, R. A comparative study on platelet activation markers between continuous and intermittent exercise training programs in healthy males. J. Hematol. 2014, 3, 72–75. [Google Scholar] [CrossRef]
- Lehmann, M.; Hasler, K.; Bergdolt, E.; Keul, J. Alpha-2-adrenoreceptor density on intact platelets and adrenaline-induced platelet aggregation in endurance- and nonendurance-trained subjects. Int. J. Sports Med. 1986, 7, 172–176. [Google Scholar] [CrossRef]
- El-Sayed, M.S.; Lin, X.; Rattu, A.J. Blood coagulation and fibrinolysis at rest and in response to maximal exercise before and after a physical conditioning programme. Blood Coagul. Fibrinolysis. 1995, 6, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Salvagno, G.L.; Montagana, M.; Guidi, G.C. Chronic influence of vigorous aerobic training on hemostasis. Blood Coagul. Fibrinolysis. 2005, 16, 533–534. [Google Scholar] [CrossRef]
- Dubach, P.; Myers, J.; Dziekan, G.; Goebbels, U.; Reinhart, W.; Muller, P.; Buser, P.; Stulz, P.; Vogt, P.; Ratti, R. Effect of high intensity exercise training on central hemodynamic responses to exercise in men with reduced left ventricular function. J. Am. Coll. Cardiol. 1997, 29, 1591–1598. [Google Scholar] [CrossRef]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef]
- Rauramaa, R.; Salonen, J.T.; Seppänen, K.; Salonen, R.; Venäläinen, J.M.; Ihanainen, M.; Rissanen, V. Inhibition of platelet aggregability by moderate-intensity physical exercise: A randomized clinical trial in overweight men. Circulation 1986, 74, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.W.; Mason Guest, M. Exercise, physical conditioning, blood coagulation and fibrinolysis. Thromb. Haemost. 1974, 31, 063–071. [Google Scholar] [CrossRef]
- De Paz, J.A.; Lasierra, J.; Villa, J.G.; Viladés, E.; Martín-Nuño, M.A.; González-Gallego, J. Changes in the fibrinolytic system associated with physical conditioning. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C.; Stavrinou, P.; Fatouros, I.G.; Philippou, A.; Chatzinikolaou, A.; Draganidis, D.; Ermidis, G.; Maridaki, M. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem. Toxicol. 2013, 61, 171–177. [Google Scholar] [CrossRef]
- Chen, Y.W.; Apostolakis, S.; Lip, G.Y. Exercise-induced changes in inflammatory processes: Implications for thrombogenesis in cardiovascular disease. Ann. Med. 2014, 46, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.L.; Fu, T.C.; Hsu, C.C.; Huang, S.C.; Lin, Y.T.; Wang, J.S. Cycling exercise training enhances platelet mitochondrial bioenergetics in patients with peripheral arterial disease: A randomized controlled trial. Thromb. Haemost. 2021, 121, 900–912. [Google Scholar] [CrossRef]
- Hsu, C.C.; Tsai, H.H.; Fu, T.C.; Wang, J.S. Exercise training enhances platelet mitochondrial bioenergetics in stroke patients: A randomized controlled trial. J. Clin. Med. 2019, 8, 2186. [Google Scholar] [CrossRef] [PubMed]
- Levi, M. Disseminated intravascular coagulation in cancer: An update. Semin. Thromb. Hemost. 2019, 45, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Hisada, Y.M.; Kasthuri, R.S.; Reeves, B.N.; Mackman, N. Cancer therapy-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1291–1305. [Google Scholar] [CrossRef]
- Abdol Razak, N.B.; Jones, G.; Bhandari, M.; Berndt, M.C.; Metharom, P. Cancer-associated thrombosis: An overview of mechanisms, risk factors, and treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef]
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L.J.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-associated thrombosis: The when, how and why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef]
- Muñoz Martín, A.J.; Ramírez, S.P.; Morán, L.O.; Zamorano, M.R.; Benéitez, M.C.V.; Salcedo, I.A.; Escobar, I.G.; Fernández, J.M.S. Pharmacological cancer treatment and venous thromboembolism risk. Eur. Heart J. Suppl. 2020, 22, C2–C14. [Google Scholar] [CrossRef]
- Peralta, R.; Thani, H.A.; Rizoli, S. Coagulopathy in the surgical patient: Trauma-induced and drug-induced coagulopathies. Curr. Opin. Crit. Care 2019, 25, 668–674. [Google Scholar] [CrossRef]
- Li, X.B.; Peng, K.W.; Ji, Z.H.; Yu, Y.; Liu, G.; Li, Y. Prevention of venous thromboembolism after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Development of a physiotherapy program. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619890415. [Google Scholar] [CrossRef]
- Lee, K.W.; Lip, G.Y. Acute versus habitual exercise, thrombogenesis and exercise intensity. Thromb. Haemost. 2004, 91, 416–419. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Trikoupis, I.G.; Papadopoulos, D.V.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Savvidou, O.D.; Kontogeorgakos, V.A.; Piovani, D.; Kriebardis, A.G.; et al. Higher coagulation activity in hip fracture patients: A case-control study using rotational thromboelastometry. Int. J. Lab. Hematol. 2021, 43, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Papadopoulos, D.V.; Trikoupis, I.G.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Piovani, D.; Kriebardis, A.G.; Gialeraki, A.; Nikolopoulos, G.K.; et al. Rotational thromboelastometry findings are associated with symptomatic venous thromboembolic complications after hip fracture surgery. Clin. Orthop. Relat. Res. 2021, 479, 2457–2467. [Google Scholar] [CrossRef]
- Pre- and Post-Operative Exercise in Patients with Hip Fracture. NCT05389800. Available online: ClinicalTrials.gov (accessed on 31 December 2022).
- Thrall, G.; Lane, D.; Carroll, D.; Lip, G.Y. A systematic review of the effects of acute psychological stress and physical activity on haemorheology, coagulation, fibrinolysis and platelet reactivity: Implications for the pathogenesis of acute coronary syndromes. Thromb. Res. 2007, 120, 819–847. [Google Scholar] [CrossRef]
- Zadow, E.K.; Wundersitz, D.W.T.; Hughes, D.L.; Adams, M.J.; Kingsley, M.I.C.; Blacklock, H.A.; Wu, S.S.X.; Benson, A.C.; Dutheil, F.; Gordon, B.A. Coronavirus (COVID-19), coagulation, and exercise: Interactions that may influence health outcomes. Semin Thromb. Hemost. 2020, 46, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Röcker, L.; Günay, S.; Gunga, H.C.; Hopfenmüller, W.; Ruf, A.; Patscheke, H.; Möckel, M. Activation of blood platelets in response to maximal isometric exercise of the dominant arm. Int. J. Sports. Med. 2000, 21, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kitamura, Y.; Ishizuka, S.; Yamada, S.; Aono, H.; Kawahara, T.; Sobue, T. Mortality of Japanese Olympic athletes in 1964 Tokyo Olympic Games. BMJ Open Sport Exerc. Med. 2021, 7, e000896. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, S.; Bediz, C.S.; Pişkin, O.; Aksu, I.; Topçu, A.; Yüksel, F.; Demirkan, F. The effect of the acute submaximal exercise on thrombin activatable fibrinolysis inhibitor levels in young sedentary males. Clin. Appl. Thromb. Hemost. 2011, 17, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Ogedegbe, H.O. An overview of hemostasis. Lab Med. 2002, 33, 948–953. [Google Scholar] [CrossRef][Green Version]
- Ferguson, E.W.; Bernier, L.L.; Banta, G.R.; Yu-Yahiro, J.; Schoomaker, E.B. Effects of exercise and conditioning on clotting and fibrinolytic activity in men. J. Appl. Physiol. 1987, 62, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Carter, C.; O’Brodovich, H.; Heigenhauser, G. Increases in factor VIII complex and fibrinolytic activity are dependent on exercise intensity. J. Appl. Physiol. 1986, 60, 1917–1922. [Google Scholar] [CrossRef]
- Szymanski, L.M.; Durstine, J.L.; Davis, P.G.; Dowda, M.; Pate, R.R. Factors affecting fibrinolytic potential: Cardiovascular fitness, body composition, and lipoprotein(a). Metabolism 1996, 45, 1427–1433. [Google Scholar] [CrossRef]
- Nascimento Alves, M.; Souza Soares, A.; Melo Marinho, P. Efficacy of resistance exercise during hemodialysis on improving lower limb muscle strength in patients with chronic kidney disease: A meta-analysis of randomized clinical trials. Physiother. Theory Pract. 2022, Online Ahead of Print, 1–11. [Google Scholar] [CrossRef]
- Heiwe, S.; Jacobson, S.H. Exercise training for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2011, Cd003236. [Google Scholar] [CrossRef]
- Rolnick, N.; de Sousa Neto, I.V.; da Fonseca, E.F.; Neves, R.V.P.; Rosa, T.D.S.; Nascimento, D.D.C. Potential implications of blood flow restriction exercise on patients with chronic kidney disease: A brief review. J. Exerc. Rehabil. 2022, 18, 81–95. [Google Scholar] [CrossRef]
- Stavres, J.; Singer, T.J.; Brochetti, A.; Kilbane, M.J.; Brose, S.W.; McDaniel, J. The feasibility of blood flow restriction exercise in patients with incomplete spinal cord injury. PM R 2018, 10, 1368–1379. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Timmons, M.K.; Dolbow, D.R.; Bengel, J.; Fugate-Laus, K.C.; Michener, L.A.; Gater, D.R. Electrical stimulation and blood flow restriction increase wrist extensor cross-sectional area and flow meditated dilatation following spinal cord injury. Eur. J. Appl. Physiol. 2016, 116, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.V.; Pereira, E.T.; Reguera-García, M.M.; Oliveira, C.E.P.; Moreira, O.C. Resistance training and muscle strength in people with spinal cord injury: A systematic review and meta-analysis. J. Bodyw. Mov. Ther. 2022, 29, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garzon, M.; Cantarero-Villanueva, I.; Legerén-Alvarez, M.; Gallart-Aragón, T.; Postigo-Martin, P.; González-Santos, Á.; Lozano-Lozano, M.; Martín-Martín, L.; Ortiz-Comino, L.; Castro-Martín, E.; et al. Prevention of chemotherapy-induced peripheral neuropathy with PRESIONA, a therapeutic exercise and blood flow restriction program: A randomized controlled study protocol. Phys. Ther. 2022, 102, pzab282. [Google Scholar] [CrossRef] [PubMed]
- deJong, A.T.; Womack, C.J.; Perrine, J.A.; Franklin, B.A. Hemostatic responses to resistance training in patients with coronary artery disease. J. Cardiopulm. Rehabil. 2006, 26, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Kambič, T.; Novaković, M.; Tomažin, K.; Strojnik, V.; Božič-Mijovski, M.; Jug, B. Hemodynamic and hemostatic response to blood flow restriction resistance exercise in coronary artery disease: A pilot randomized controlled trial. J. Cardiovasc. Nurs. 2021, 36, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.D.S.; Karabulut, M.; Bemben, M.G. The evolution of blood flow restricted exercise. Front. Physiol. 2021, 12, 747759. [Google Scholar] [CrossRef]
- Creighton, B.C.; Kupchak, B.R.; Aristizabal, J.C.; Flanagan, S.D.; Dunn-Lewis, C.; Volk, B.M.; Comstock, B.A.; Volek, J.S.; Hooper, D.R.; Szivak, T.K.; et al. Influence of training on markers of platelet activation in response to a bout of heavy resistance exercise. Eur. J. Appl. Physiol. 2013, 113, 2203–2209. [Google Scholar] [CrossRef]
- Ahmadizad, S.; El-Sayed, M.S.; Maclaren, D.P. Responses of platelet activation and function to a single bout of resistance exercise and recovery. Clin. Hemorheol. Microcirc. 2006, 35, 159–168. [Google Scholar]
- Ahmadizad, S.; El-Sayed, M.S. The effects of graded resistance exercise on platelet aggregation and activation. Med. Sci. Sports Exerc. 2003, 35, 1026–1032. [Google Scholar] [CrossRef][Green Version]
- Ahmadizad, S.; El-Sayed, M.S.; MacLaren, D.P. Effects of time of day and acute resistance exercise on platelet activation and function. Clin. Hemorheol. Microcirc. 2010, 45, 391–399. [Google Scholar] [CrossRef]
- Vind, J.; Gleerup, G.; Nielsen, P.T.; Winther, K. The impact of static work on fibrinolysis and platelet function. Thromb. Res. 1993, 72, 441–446. [Google Scholar] [CrossRef]
- Smith, D.L.; Fernhall, B. Advanced Cardiovascular Exercise Physiology; Human Kinetics: Champaign, IL, USA, 2011. [Google Scholar]
- Ahmadizad, S.; El-Sayed, M.S. The acute effects of resistance exercise on the main determinants of blood rheology. J. Sports Sci. 2005, 23, 243–249. [Google Scholar] [CrossRef]
- Craig, S.K.; Byrnes, W.C.; Fleck, S.J. Plasma volume during weight lifting. Int. J. Sports Med. 2008, 29, 89–95. [Google Scholar] [CrossRef]
- Chamberlain, K.G.; Tong, M.; Penington, D.G. Properties of the exchangeable splenic platelets released into the circulation during exercise-induced thrombocytosis. Am. J. Hematol. 1990, 34, 161–168. [Google Scholar] [CrossRef]
- Weyrich, A.S.; Zimmerman, G.A. Platelets in lung biology. Annu. Rev. Physiol. 2013, 75, 569–591. [Google Scholar] [CrossRef]
- Cherouveim, E.D.; Miliotis, P.G.; Dipla, K.; Koskolou, M.D.; Vrabas, I.S.; Geladas, N.D. The effect of muscle blood flow restriction on hemodynamics, cerebral oxygenation and activation at rest. Appl. Physiol. Nutr. Metab. 2021, 46, 1216–1224. [Google Scholar] [CrossRef]
- Cuffe, M.; Novak, J.; Saithna, A.; Strohmeyer, H.S.; Slaven, E. Current trends in blood flow restriction. Front. Physiol. 2022, 13, 882472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tian, G.; Wang, X. Effects of low-load blood flow restriction training on hemodynamic responses and vascular function in older adults: A meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 6750. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.D.C.; Petriz, B.; Oliveira, S.D.C.; Vieira, D.C.L.; Funghetto, S.S.; Silva, A.O.; Prestes, J. Effects of blood flow restriction exercise on hemostasis: A systematic review of randomized and non-randomized trials. Int. J. Gen. Med. 2019, 12, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.W.; Hackney, K.J.; Brown, S.L.; Noonan, B.C. Blood flow restriction resistance exercise as a rehabilitation modality following orthopaedic surgery: A review of venous thromboembolism risk. J. Orthop. Sports Phys. Ther. 2019, 49, 17–27. [Google Scholar] [CrossRef]
- Nagelkirk, P.R.; Scalzo, R.; Harber, M.; Kaminsky, L.A. The influence of acute resistance training and body composition on coagulation and fibrinolytic activity in low-risk women. Int. J. Sports Med. 2010, 31, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic syndrome pathophysiology and predisposing factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Fini, E.M.; Salimian, M.; Ahmadizad, S. Responses of platelet CD markers and indices to resistance exercise with and without blood flow restriction in patients with type 2 diabetes. Clin. Hemorheol. Microcirc. 2022, 80, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Naci, H.; Salcher-Konrad, M.; Dias, S.; Blum, M.R.; Sahoo, S.A.; Nunan, D.; Ioannidis, J.P.A. How does exercise treatment compare with antihypertensive medications? A network meta-analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br. J. Sports Med. 2019, 53, 859–869. [Google Scholar] [CrossRef]
- Silva, J.; Menêses, A.L.; Parmenter, B.J.; Ritti-Dias, R.M.; Farah, B.Q. Effects of resistance training on endothelial function: A systematic review and meta-analysis. Atherosclerosis 2021, 333, 91–99. [Google Scholar] [CrossRef]
- Nascimento Dda, C.; Neto, F.R.; de Santana, F.S.; da Silva, R.A.; Dos Santos-Neto, L.; Balsamo, S. The interactions between hemostasis and resistance training: A review. Int. J. Gen. Med. 2012, 5, 249–254. [Google Scholar] [CrossRef]
- Nagelkirk, P.R.; Soave, K.; Altherr, C.; Del Pozzi, A. Regular resistance training enhances fibrinolytic potential but does not affect coagulation. Med. Sci. Sports Exerc. 2021, 53, 2318–2323. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Baynard, T.; Jacobs, H.M.; Kessler, C.M.; Kanaley, J.A.; Fernhall, B. Fibrinolytic markers and vasodilatory capacity following acute exercise among men of differing training status. Eur. J. Appl. Physiol. 2007, 101, 595–602. [Google Scholar] [CrossRef]
- de Boer, A.; Kluft, C.; Kroon, J.M.; Kasper, F.J.; Schoemaker, H.C.; Pruis, J.; Breimer, D.D.; Soons, P.A.; Emeis, J.J.; Cohen, A.F. Liver blood flow as a major determinant of the clearance of recombinant human tissue-type plasminogen activator. Thromb. Haemost. 1992, 67, 83–87. [Google Scholar] [CrossRef]
- Speiser, W.; Langer, W.; Pschaick, A.; Selmayr, E.; Ibe, B.; Nowacki, P.E.; Müller-Berghaus, G. Increased blood fibrinolytic activity after physical exercise: Comparative study in individuals with different sporting activities and in patients after myocardial infarction taking part in a rehabilitation sports program. Thromb. Res. 1988, 51, 543–555. [Google Scholar] [CrossRef]
- Bogdanis, G.C.; Mastorakos, G.; Tsirigkakis, S.; Stavrinou, P.S.; Kabasakalis, A.; Mantzou, A.; Mougios, V. Bout duration in high-intensity interval exercise modifies hematologic, metabolic and antioxidant responses. J. Exerc. Sci. Fit. 2022, 20, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, E.; Johnson, N.A.; Powell, L.; Hamer, M.; Rangul, V.; Holtermann, A. Short and sporadic bouts in the 2018 US physical activity guidelines: Is high-intensity incidental physical activity the new HIIT? Br. J. Sports Med. 2019, 53, 1137–1139. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef]
- Gibala, M.J.; McGee, S.L. Metabolic adaptations to short-term high-intensity interval training: A little pain for a lot of gain? Exerc. Sport Sci. Rev. 2008, 36, 58–63. [Google Scholar] [CrossRef]
- Smith, D.L.; Horn, G.P.; Petruzzello, S.J.; Freund, G.G.; Bloom, S.I.; Fernhall, B. Hemostatic responses to multiple bouts of firefighting activity: Female vs. male differences in a high demand, high performance occupation. Int. J. Environ. Res. Public Health 2022, 19, 2124. [Google Scholar] [CrossRef] [PubMed]
- Karampour, S.; Gaeini, A.A. Response of coagulation and anti-coagulant factors of elite athletes following acute resistance and high-intensity interval training. J. Sports Med. Phys. Fit. 2018, 58, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ikarugi, H.; Shibata, M.; Shibata, S.; Ishii, H.; Taka, T.; Yamamoto, J. High intensity exercise enhances platelet reactivity to shear stress and coagulation during and after exercise. Pathophysiol. Haemost. Thromb. 2003, 33, 127–133. [Google Scholar] [CrossRef]
- Sackett, J.R.; Farrell, D.P.; Nagelkirk, P.R. Hemostatic adaptations to high intensity interval training in healthy adult men. Int. J. Sports Med. 2020, 41, 867–872. [Google Scholar] [CrossRef]
- Thomas, H.J.; Marsh, C.E.; Lester, L.; Maslen, B.A.; Naylor, L.H.; Green, D.J. Sex differences in cardiovascular risk factor responses to resistance and endurance training in younger subjects. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H67–H78. [Google Scholar] [CrossRef]
- Kargotich, S.; Goodman, C.; Keast, D.; Morton, A.R. The influence of exercise-induced plasma volume changes on the interpretation of biochemical parameters used for monitoring exercise, training and sport. Sports Med. 1998, 26, 101–117. [Google Scholar] [CrossRef]
- Austin, A.W.; Wirtz, P.H.; Patterson, S.M.; Stutz, M.; von Känel, R. Stress-induced alterations in coagulation: Assessment of a new hemoconcentration correction technique. Psychosom. Med. 2012, 74, 288–295. [Google Scholar] [CrossRef]
- Womack, C.J.; Rasmussen, J.M.; Vickers, D.G.; Paton, C.M.; Osmond, P.J.; Davis, G.L. Changes in fibrinolysis following exercise above and below lactate threshold. Thromb. Res. 2006, 118, 263–268. [Google Scholar] [CrossRef]
- Menzel, K.; Hilberg, T. Blood coagulation and fibrinolysis in healthy, untrained subjects: Effects of different exercise intensities controlled by individual anaerobic threshold. Eur. J. Appl. Physiol. 2011, 111, 253–260. [Google Scholar] [CrossRef]
- Weiss, C.; Welsch, B.; Albert, M.; Friedmann, B.; Strobel, G.; Jost, J.; Nawroth, P.; Bärtsch, P. Coagulation and thrombomodulin in response to exercise of different type and duration. Med. Sci. Sports Exerc. 1998, 30, 1205–1210. [Google Scholar] [CrossRef]
- Prisco, D.; Francalanci, I.; Filippini, M.; Hagi, M.I. Physical exercise and hemostasis. Int. J. Clin. Lab. Res. 1994, 24, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; El-Sayed, M.S.; Waterhouse, J.; Reilly, T. Activation and disturbance of blood haemostasis following strenuous physical exercise. Int. J. Sports Med. 1999, 20, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Paton, C.M.; Nagelkirk, P.R.; Coughlin, A.M.; Cooper, J.A.; Davis, G.A.; Hassouna, H.; Pivarnik, J.M.; Womack, C.J. Changes in von Willebrand factor and fibrinolysis following a post-exercise cool-down. Eur. J. Appl. Physiol. 2004, 92, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Jen, C.J.; Chen, H.I. Effects of chronic exercise and deconditioning on platelet function in women. J. Appl. Physiol. 1997, 83, 2080–2085. [Google Scholar] [CrossRef] [PubMed]
- Fellmann, N. Hormonal and plasma volume alterations following endurance exercise. A brief review. Sports Med. 1992, 13, 37–49. [Google Scholar] [CrossRef]
- Kjaer, M.; Secher, N.H.; Galbo, H. Physical stress and catecholamine release. Baillieres Clin. Endocrinol. Metab. 1987, 1, 279–298. [Google Scholar] [CrossRef]
- Aird, W.C. Spatial and temporal dynamics of the endothelium. J. Thromb. Haemost. 2005, 3, 1392–1406. [Google Scholar] [CrossRef] [PubMed]
- Mittleman, M.A.; Maclure, M.; Tofler, G.H.; Sherwood, J.B.; Goldberg, R.J.; Muller, J.E. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N. Engl. J. Med. 1993, 329, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Willich, S.N.; Lewis, M.; Löwel, H.; Arntz, H.R.; Schubert, F.; Schröder, R. Physical exertion as a trigger of acute myocardial infarction. Triggers and Mechanisms of Myocardial Infarction Study Group. N. Engl. J. Med. 1993, 329, 1684–1690. [Google Scholar] [CrossRef]
- Gawel, M.J.; Glover, V.; Burkitt, M.; Sandler, M.; Rose, F.C. The specific activity of platelet monoamine oxidase varies with platelet count during severe exercise and noradrenaline infusion. Psychopharmacology 1981, 72, 275–277. [Google Scholar] [CrossRef]
- Heber, S.; Fischer, B.; Sallaberger-Lehner, M.; Hausharter, M.; Ocenasek, H.; Gleiss, A.; Fischer, M.J.M.; Pokan, R.; Assinger, A.; Volf, I. Effects of high-intensity interval training on platelet function in cardiac rehabilitation: A randomised controlled trial. Heart 2020, 106, 69–79. [Google Scholar] [CrossRef]
- Kristiansen, J.; Grove, E.L.; Sjúrðarson, T.; Rasmussen, J.; Mohr, M.; Kristensen, S.D.; Hvas, A.M. Haemostasis and fibrinolysis after regular high-intensity interval training in patients with coronary artery disease: A randomised controlled trial. Open Heart 2022, 9, e002127. [Google Scholar] [CrossRef]
- Biskey, L.M. Effects of high intensity interval training on hemostasis and fibrinolysis in healthy males: Relationship to sympathetic nervous system activation. Med. Sci. Sports Exerc. 2015, 47, 299–300. [Google Scholar] [CrossRef]
- Heber, S.; Volf, I. Effects of physical (in)activity on platelet function. Biomed. Res. Int. 2015, 2015, 165078. [Google Scholar] [CrossRef]
- Heber, S.; Assinger, A.; Pokan, R.; Volf, I. Correlation between cardiorespiratory fitness and platelet function in healthy women. Med. Sci. Sports Exerc. 2016, 48, 1101–1110. [Google Scholar] [CrossRef][Green Version]
- Lundberg Slingsby, M.H.; Nyberg, M.; Egelund, J.; Mandrup, C.M.; Frikke-Schmidt, R.; Kirkby, N.S.; Hellsten, Y. Aerobic exercise training lowers platelet reactivity and improves platelet sensitivity to prostacyclin in pre- and postmenopausal women. J. Thromb. Haemost. 2017, 15, 2419–2431. [Google Scholar] [CrossRef]
- Lundberg Slingsby, M.H.; Gliemann, L.; Thrane, M.; Rytter, N.; Egelund, J.; Chan, M.V.; Armstrong, P.C.; Warner, T.D.; Hellsten, Y. Platelet responses to pharmacological and physiological interventions in middle-aged men with different habitual physical activity levels. Acta Physiol. (Oxf.) 2018, 223, e13028. [Google Scholar] [CrossRef]
- Krammer, U.D.B.; Sommer, A.; Tschida, S.; Mayer, A.; Lilja, S.V.; Switzeny, O.J.; Hippe, B.; Rust, P.; Haslberger, A.G. PGC-1α methylation, miR-23a, and miR-30e expression as biomarkers for exercise- and diet-induced mitochondrial biogenesis in capillary blood from healthy Individuals: A single-arm intervention. Sports 2022, 10, 73. [Google Scholar] [CrossRef]
- Spiliopoulou, P.; Gavriatopoulou, M.; Kastritis, E.; Dimopoulos, M.A.; Terzis, G. Exercise-induced changes in tumor growth via tumor immunity. Sports 2021, 9, 46. [Google Scholar] [CrossRef]
- Methenitis, S. A brief review on concurrent training: From laboratory to the field. Sports 2018, 6, 127. [Google Scholar] [CrossRef]
- Krekels, J.P.M.; Verhezen, P.W.M.; Henskens, Y.M.C. Platelet aggregation in healthy participants is not affected by smoking, drinking coffee, consuming a high-fat meal, or performing physical exercise. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029618782445. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M.; Gelati, M.; Lippi, G. The role of epigenetics in the regulation of hemostatic balance. Semin. Thromb. Hemost. 2021, 47, 53–62. [Google Scholar] [CrossRef]
- Patsouras, M.D.; Vlachoyiannopoulos, P.G. Evidence of epigenetic alterations in thrombosis and coagulation: A systematic review. J. Autoimmun. 2019, 104, 102347. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Ghosh, S. Environmental exposures, epigenetics and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Zdravkovic, S.; Wienke, A.; Pedersen, N.L.; Marenberg, M.E.; Yashin, A.I.; De Faire, U. Heritability of death from coronary heart disease: A 36-year follow-up of 20 966 Swedish twins. J. Intern. Med. 2002, 252, 247–254. [Google Scholar] [CrossRef]
- Drobni, Z.D.; Kolossvary, M.; Karady, J.; Jermendy, A.L.; Tarnoki, A.D.; Tarnoki, D.L.; Simon, J.; Szilveszter, B.; Littvay, L.; Voros, S.; et al. Heritability of coronary artery disease: Insights from a classical twin wtudy. Circ. Cardiovasc. Imaging 2022, 15, e013348. [Google Scholar] [CrossRef]
- McGee, S.L.; Hargreaves, M. Epigenetics and Exercise. Trends. Endocrinol. Metab. 2019, 30, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Seaborne, R.A.; Sharples, A.P. The interplay between exercise metabolism, epigenetics, and skeletal muscle remodeling. Exerc Sport Sci. Rev. 2020, 48, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Landry, P.; Plante, I.; Ouellet, D.L.; Perron, M.P.; Rousseau, G.; Provost, P. Existence of a microRNA pathway in anucleate platelets. Nat. Struct. Mol. Biol. 2009, 16, 961–966. [Google Scholar] [CrossRef]
- Radom-Aizik, S.; Zaldivar, F., Jr.; Oliver, S.; Galassetti, P.; Cooper, D.M. Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes. J. Appl. Physiol. 2010, 109, 252–261. [Google Scholar] [CrossRef]
- Radom-Aizik, S.; Zaldivar, F., Jr.; Leu, S.Y.; Adams, G.R.; Oliver, S.; Cooper, D.M. Effects of exercise on microRNA expression in young males peripheral blood mononuclear cells. Clin. Transl. Sci. 2012, 5, 32–38. [Google Scholar] [CrossRef]
- Nielsen, S.; Åkerström, T.; Rinnov, A.; Yfanti, C.; Scheele, C.; Pedersen, B.K.; Laye, M.J. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS ONE 2014, 9, e87308. [Google Scholar] [CrossRef]
- Baggish, A.L.; Hale, A.; Weiner, R.B.; Lewis, G.D.; Systrom, D.; Wang, F.; Wang, T.J.; Chan, S.Y. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J. Physiol. 2011, 589, 3983–3994. [Google Scholar] [CrossRef]
- Christensen, P.M.; Jacobs, R.A.; Bonne, T.; Flück, D.; Bangsbo, J.; Lundby, C. A short period of high-intensity interval training improves skeletal muscle mitochondrial function and pulmonary oxygen uptake kinetics. J. Appl. Physiol. 2016, 120, 1319–1327. [Google Scholar] [CrossRef]
- Poredoš, P.; Šabovič, M.; Božič Mijovski, M.; Nikolajević, J.; Antignani, P.L.; Paraskevas, K.I.; Mikhailidis, D.P.; Blinc, A. Inflammatory and prothrombotic biomarkers, DNA polymorphisms, microRNAs and personalized medicine for patients with peripheral arterial disease. Int. J. Mol. Sci. 2022, 23, 12054. [Google Scholar] [CrossRef]
- Yamamoto, H.; Morino, K.; Nishio, Y.; Ugi, S.; Yoshizaki, T.; Kashiwagi, A.; Maegawa, H. MicroRNA-494 regulates mitochondrial biogenesis in skeletal muscle through mitochondrial transcription factor A and Forkhead box j3. Am. J. Physiol. Endocrinol. Metab 2012, 303, E1419–E1427. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Mizushima, K.; Takanami, Y.; Kawai, Y.; Ichikawa, H.; Yoshikawa, T. The microRNA miR-696 regulates PGC-1{alpha} in mouse skeletal muscle in response to physical activity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E799–E806. [Google Scholar] [CrossRef] [PubMed]
- Laura, T.; Robert, G.W.; Marco, M.; Bernard, D.; Sinead, S.; Michael, H.; Niall, M.; Gerardene, M.-M.; Nastassia, N.; Marc-Antoine, C.; et al. Platelets: Functional biomarkers of epigenetic drift. In Homeostasis; Fernanda, L., Sergio Dos Anjos, G., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 93–120. [Google Scholar]
- Jansson, J.H.; Johansson, B.; Boman, K.; Nilsson, T.K. Hypo-fibrinolysis in patients with hypertension and elevated cholesterol. J. Intern. Med. 1991, 229, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Fleck, S.J. Cardiovascular adaptations to resistance training. Med. Sci. Sports Exerc. 1988, 20, S146–S151. [Google Scholar] [CrossRef] [PubMed]
- Abrahin, O.; Moraes-Ferreira, R.; Cortinhas-Alves, E.A.; Guerreiro, J.F. Is resistance training alone an antihypertensive therapy? A meta-analysis. J. Hum. Hypertens. 2021, 35, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Polito, M.D.; Dias, J.R., Jr.; Papst, R.R. Resistance training to reduce resting blood pressure and increase muscle strength in users and non-users of anti-hypertensive medication: A meta-analysis. Clin. Exp. Hypertens. 2021, 43, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Smart, N.A. Exercise training for blood pressure: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef]
- Pedralli, M.L.; Marschner, R.A.; Kollet, D.P.; Neto, S.G.; Eibel, B.; Tanaka, H.; Lehnen, A.M. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: A randomized clinical trial Exercise, endothelium and blood pressure. Sci. Rep. 2020, 10, 7628. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, H.L.; Gadelha, A.B.; Dutra, M.T.; Neves, R.V.P.; de Deus, L.A.; Moura, S.R.G.; Silva, V.L.; Reis, A.L.; Honorato, F.S.; de Araújo, T.B.; et al. Post-exercise hypotension following different resistance exercise protocols. Sport Sci. Health 2022, 18, 357–365. [Google Scholar] [CrossRef]
- Brito, A.d.F.; de Oliveira, C.V.C.; Brasileiro-Santos, M.d.S.; Santos, A.d.C. Resistance exercise with different volumes: Blood pressure response and forearm blood flow in the hypertensive elderly. Clin. Interv. Aging. 2014, 9, 2151–2158. [Google Scholar] [CrossRef][Green Version]
- Gargallo, P.; Casaña, J.; Suso-Martí, L.; Cuenca-Martínez, F.; López-Bueno, R.; Andersen, L.L.; López-Bueno, L.; Cuerda-Del Pino, A.; Calatayud, J. Minimal dose of resistance exercise required to induce immediate hypotension effect in older adults with hypertension: Randomized cross-over controlled trial. Int. J. Environ. Res. Public Health 2022, 19, 14218. [Google Scholar] [CrossRef]
- Shimokawa, H.; Satoh, K. Vascular function. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2359–2362. [Google Scholar] [CrossRef] [PubMed]
- Kyte, K.H.; Stensrud, T.; Berg, T.J.; Seljeflot, I.; Hisdal, J. Vascular function in Norwegian female elite runners: A cross-sectional, controlled study. Sports 2022, 10, 37. [Google Scholar] [CrossRef]
- Oikonomou, E.; Siasos, G.; Marinos, G.; Zaromitidou, M.; Athanasiou, D.; Fountoulakis, P.; Tsalamandris, S.; Charalambous, G.; Lazaros, G.; Vlachopoulos, C.; et al. High-intensity endurance and strength training in water polo Olympic team payers: Impact on arterial wall properties. Cardiology 2021, 146, 119–126. [Google Scholar] [CrossRef] [PubMed]
- de Mello, M.B.; Righi, N.C.; Schuch, F.B.; Signori, L.U.; da Silva, A.M.V. Effect of high-intensity interval training protocols on VO(2)max and HbA1c level in people with type 2 diabetes: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2022, 65, 101586. [Google Scholar] [CrossRef]
- Wen, D.; Utesch, T.; Wu, J.; Robertson, S.; Liu, J.; Hu, G.; Chen, H. Effects of different protocols of high intensity interval training for VO(2)max improvements in adults: A meta-analysis of randomised controlled trials. J. Sci. Med. Sport 2019, 22, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Tsirigkakis, S.; Mastorakos, G.; Koutedakis, Y.; Mougios, V.; Nevill, A.M.; Pafili, Z.; Bogdanis, G.C. Effects of two workload-matched high-intensity interval training protocols on regional body composition and fat oxidation in obese men. Nutrients 2021, 13, 1096. [Google Scholar] [CrossRef]
- Khalafi, M.; Sakhaei, M.H.; Kazeminasab, F.; Symonds, M.E.; Rosenkranz, S.K. The impact of high-intensity interval training on vascular function in adults: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 1046560. [Google Scholar] [CrossRef]
- Ramos, J.S.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.S.; Coombes, J.S. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sports Med. 2015, 45, 679–692. [Google Scholar] [CrossRef]
- O’Brien, M.W.; Johns, J.A.; Robinson, S.A.; Bungay, A.; Mekary, S.; Kimmerly, D.S. Impact of high-intensity interval training, moderate-intensity continuous training, and resistance training on endothelial function in older adults. Med. Sci. Sports Exerc. 2020, 52, 1057–1067. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Hernández-Quiñones, P.A.; Tordecilla-Sanders, A.; Álvarez, C.; Ramírez-Campillo, R.; Izquierdo, M.; Correa-Bautista, J.E.; Garcia-Hermoso, A.; Garcia, R.G. Effectiveness of HIIT compared to moderate continuous training in improving vascular parameters in inactive adults. Lipids Health Dis. 2019, 18, 42. [Google Scholar] [CrossRef]
- Way, K.L.; Sultana, R.N.; Sabag, A.; Baker, M.K.; Johnson, N.A. The effect of high Intensity interval training versus moderate intensity continuous training on arterial stiffness and 24h blood pressure responses: A systematic review and meta-analysis. J. Sci. Med. Sport 2019, 22, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Iellamo, F.; Caminiti, G.; Sposato, B.; Vitale, C.; Massaro, M.; Rosano, G.; Volterrani, M. Effect of high-intensity interval training versus moderate continuous training on 24-h blood pressure profile and insulin resistance in patients with chronic heart failure. Intern. Emerg. Med. 2014, 9, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.M.; Galliano, L.M.; Del Vecchio, F.B. Effectiveness of high-intensity interval training versus moderate-intensity continuous training in hypertensive patients: A systematic review and meta-analysis. Curr. Hypertens. Rep. 2020, 22, 26. [Google Scholar] [CrossRef]
- Hu, J.; Liu, M.; Yang, R.; Wang, L.; Liang, L.; Yang, Y.; Jia, S.; Chen, R.; Liu, Q.; Ren, Y.; et al. Effects of high-intensity interval training on improving arterial stiffness in Chinese female university students with normal weight obese: A pilot randomized controlled trial. J. Transl. Med. 2022, 20, 60. [Google Scholar] [CrossRef]
- Hasegawa, N.; Fujie, S.; Horii, N.; Miyamoto-Mikami, E.; Tsuji, K.; Uchida, M.; Hamaoka, T.; Tabata, I.; Iemitsu, M. Effects of different exercise modes on arterial stiffness and nitric oxide synthesis. Med. Sci. Sports Exerc. 2018, 50, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J.; Lippi, G. Coagulation update: What’s new in hemostasis testing? Thromb. Res. 2011, 127 (Suppl 2), S13–S16. [Google Scholar] [CrossRef]
- Kapsis, D.P.; Tsoukos, A.; Psarraki, M.P.; Douda, H.T.; Smilios, I.; Bogdanis, G.C. Changes in body composition and strength after 12 weeks of high-intensity functional training with two different loads in physically active men and women: A randomized controlled study. Sports 2022, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Posnakidis, G.; Aphamis, G.; Giannaki, C.D.; Mougios, V.; Bogdanis, G.C. The addition of high-load resistance exercises to a high-intensity functional training program elicits further improvements in body composition and strength: A randomized trial. Sports 2022, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C.; Stavrinou, P.S.; Tsirigkakis, S.; Mougios, V.; Astorino, T.A.; Mastorakos, G. Attenuated metabolic and cardiorespiratory responses to isoenergetic high-intensity interval exercise of short versus long bouts. Med. Sci. Sports Exerc. 2022, 54, 1199–1209. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Papadopoulos, D.V.; Trikoupis, I.G.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Vaiopoulos, A.G.; Piovani, D.; Nikolopoulos, G.K.; Kokoris, S.I.; et al. The prognostic performance of rotational thromboelastometry for excessive bleeding and increased transfusion requirements in hip fracture surgeries. Thromb. Haemost. 2022, 122, 895–904. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Papadopoulos, D.V.; Roustemis, A.G.; Trikoupis, I.G.; Piovani, D.; Tsante, K.A.; Mantzios, P.G.; Mavrogenis, A.F.; Sokou, R.; Kokoris, S.I.; et al. Rotational thromboelastometry predicts transfusion requirements in total joint arthroplasties. Semin. Thromb. Hemost. 2023, 49, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Loukopoulou, I.; Papadopoulos, D.V.; Trikoupis, I.G.; Roustemis, A.G.; Goumenos, S.; Sokou, R.; Tsante, K.A.; Kriebardis, A.G.; Koulouvaris, P.; et al. The hypercoagulable profile of patients with bone tumors: A pilot observational study using rotational thromboelastometry. Cancers 2022, 14, 3930. [Google Scholar] [CrossRef]
| Endurance Exercise | ||||||
|---|---|---|---|---|---|---|
| Acute Response | Chronic Adaptations | |||||
| Healthy/Patients (Post- versus Pre-Exercise Differences) | Patients vs. Healthy | Healthy/Patients (Post- versus Pre-Training Differences) | Patients vs. Healthy | |||
| Resting | After Exercise | Resting | After Exercise | |||
| Platelet Function | ||||||
| Platelet aggregation and activation | ↑↔ | ? | ↓ | ↓ | ||
| vWF binding | ↑ | |||||
| Platelet count | ↑ | |||||
| βTG | ↑↔ | ↓ | ||||
| Fibrinolysis | ||||||
| Clot lysis time | ↑ | |||||
| tPA activity | ↑ | ↑↔ | ↑ | ↑↔ | ↓ | ↑↔ |
| tPA antigen | ↑ | ↔ | ↑ | ↔ | ||
| PAI-1 activity | ↓ | ↓↔ | ↓ | ↓ | ↑ | ↔ |
| Coagulation | ||||||
| aPTT | ↓ | ↑↔ | ↓↔ | ↔ | ↔ | |
| PT | ↑ | ↑ | ↔ | ↔ | ↔ | ↔ |
| TT | ↑ | ↔ | ↔ | ↔ | ↔ | |
| FVIII antigen | ↑ | ↓↔ | ↔ | ↑ | ||
| vWF antigen/activity | ↑ | ↓↔ | ↔ | ↑ | ↑ | |
| ETP | ↑ | ↑ | ↓ | |||
| TAT | ↑ | ↑ | ↑ | ↑ | ||
| Fibrinogen | ↑ | ↑ | ↓ | ↑ | ||
| Resistance Exercise | ||||||
|---|---|---|---|---|---|---|
| Acute Response | Chronic Adaptations | |||||
| Healthy/Patients (Post- versus Pre-Exercise Differences) | Patients vs. Healthy | Healthy/Patients (Post- versus Pre-Training Differences) | Patients vs. Healthy | |||
| Resting | After Exercise | Resting | After Exercise | |||
| Platelet Function | ||||||
| Platelet aggregation and activation | ↑ | ↓ | ||||
| Platelet count | ↑ | |||||
| βTG | ↑ | ↓ | ||||
| Fibrinolysis | ||||||
| tPA activity | ↑ | ↔ | ↑↔ | ↑ | ||
| tPA antigen | ↔ | |||||
| PAI-1 antigen | ↓ | |||||
| PAI-1 activity | ↓↔ | ↔ | ↓↔ | |||
| Coagulation | ||||||
| aPTT | ↓ | ↓ | ||||
| PT | ↔ | |||||
| FVIII antigen | ↔ | |||||
| vWF antigen/activity | ↔ | |||||
| TAT | ↓ | |||||
| Fibrinogen | ↔ | ↔ | ↔ | |||
| High-Intensity Interval Exercise | ||||||
|---|---|---|---|---|---|---|
| Acute Response | Chronic Adaptations | |||||
| Healthy/Patients (Post- versus Pre-Exercise Differences) | Patients vs. Healthy | Healthy/Patients (Post- versus Pre-Training Differences) | Patients vs. Healthy | |||
| Resting | After Exercise | Resting | After Exercise | |||
| Platelet Function | ||||||
| Platelet aggregation and activation | ↑ | ↓↔ | ↓↔ | ↔ | ||
| vWF binding | ↓ | |||||
| Platelet count | ↓ | ↓ | ||||
| Fibrinolysis | ||||||
| tPA activity | ↑ | ↑ | ||||
| tPA antigen | ↑ | |||||
| PAI-1 activity | ↓ | ↓ | ↓ | |||
| Coagulation | ||||||
| aPTT | ↓ | ↔ | ||||
| PT | ↓ | ↔ | ||||
| FVIII antigen | ↑ | |||||
| TAT | ↔ | |||||
| Fibrinogen | ↓ | ↔ | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skouras, A.Z.; Antonakis-Karamintzas, D.; Tsantes, A.G.; Triantafyllou, A.; Papagiannis, G.; Tsolakis, C.; Koulouvaris, P. The Acute and Chronic Effects of Resistance and Aerobic Exercise in Hemostatic Balance: A Brief Review. Sports 2023, 11, 74. https://doi.org/10.3390/sports11040074
Skouras AZ, Antonakis-Karamintzas D, Tsantes AG, Triantafyllou A, Papagiannis G, Tsolakis C, Koulouvaris P. The Acute and Chronic Effects of Resistance and Aerobic Exercise in Hemostatic Balance: A Brief Review. Sports. 2023; 11(4):74. https://doi.org/10.3390/sports11040074
Chicago/Turabian StyleSkouras, Apostolos Z., Dimitrios Antonakis-Karamintzas, Andreas G. Tsantes, Athanasios Triantafyllou, Georgios Papagiannis, Charilaos Tsolakis, and Panagiotis Koulouvaris. 2023. "The Acute and Chronic Effects of Resistance and Aerobic Exercise in Hemostatic Balance: A Brief Review" Sports 11, no. 4: 74. https://doi.org/10.3390/sports11040074
APA StyleSkouras, A. Z., Antonakis-Karamintzas, D., Tsantes, A. G., Triantafyllou, A., Papagiannis, G., Tsolakis, C., & Koulouvaris, P. (2023). The Acute and Chronic Effects of Resistance and Aerobic Exercise in Hemostatic Balance: A Brief Review. Sports, 11(4), 74. https://doi.org/10.3390/sports11040074







