Effects of Different Types of Exercise on Kidney Diseases
Abstract
:1. Introduction
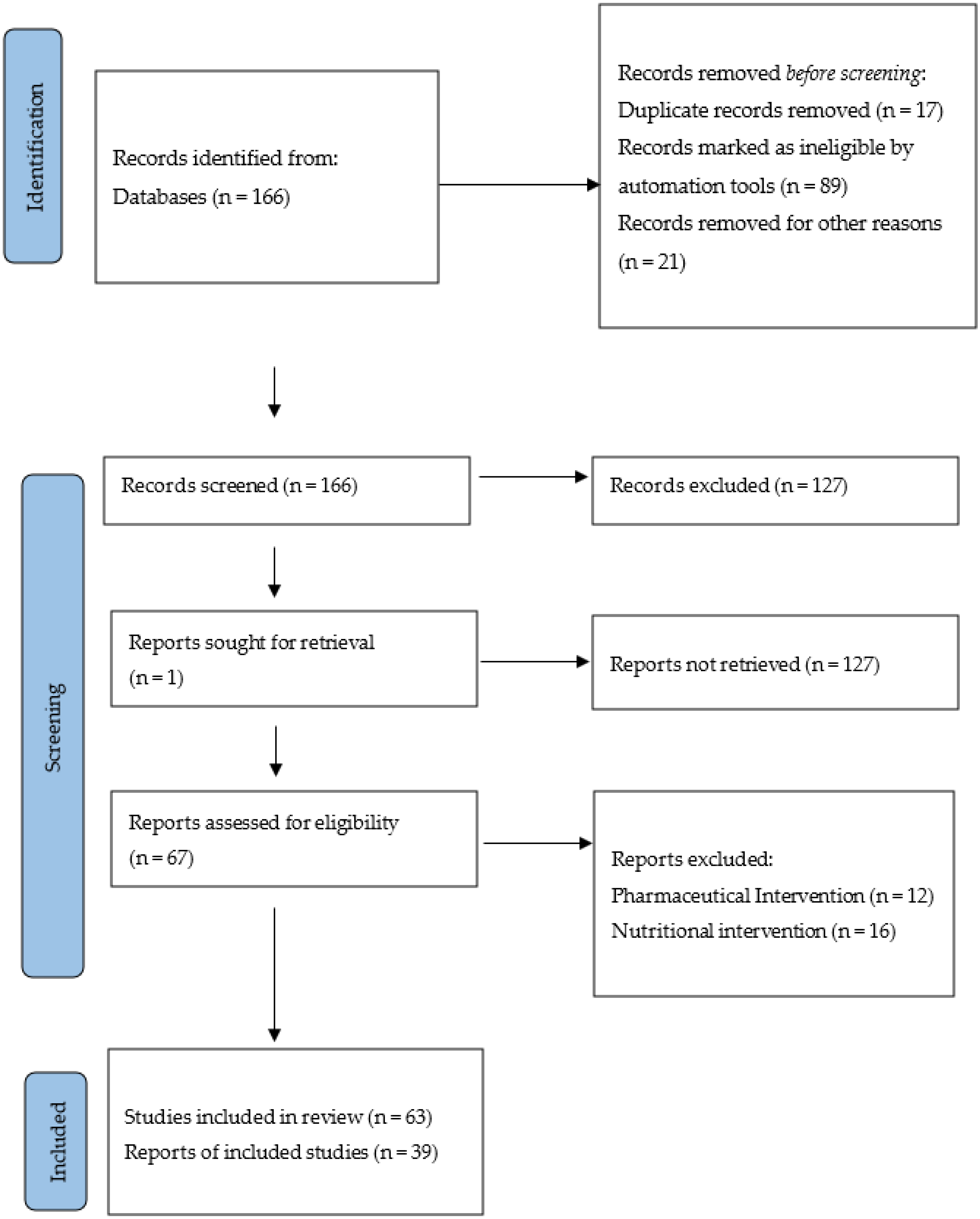
2. Materials and Methods
3. Results
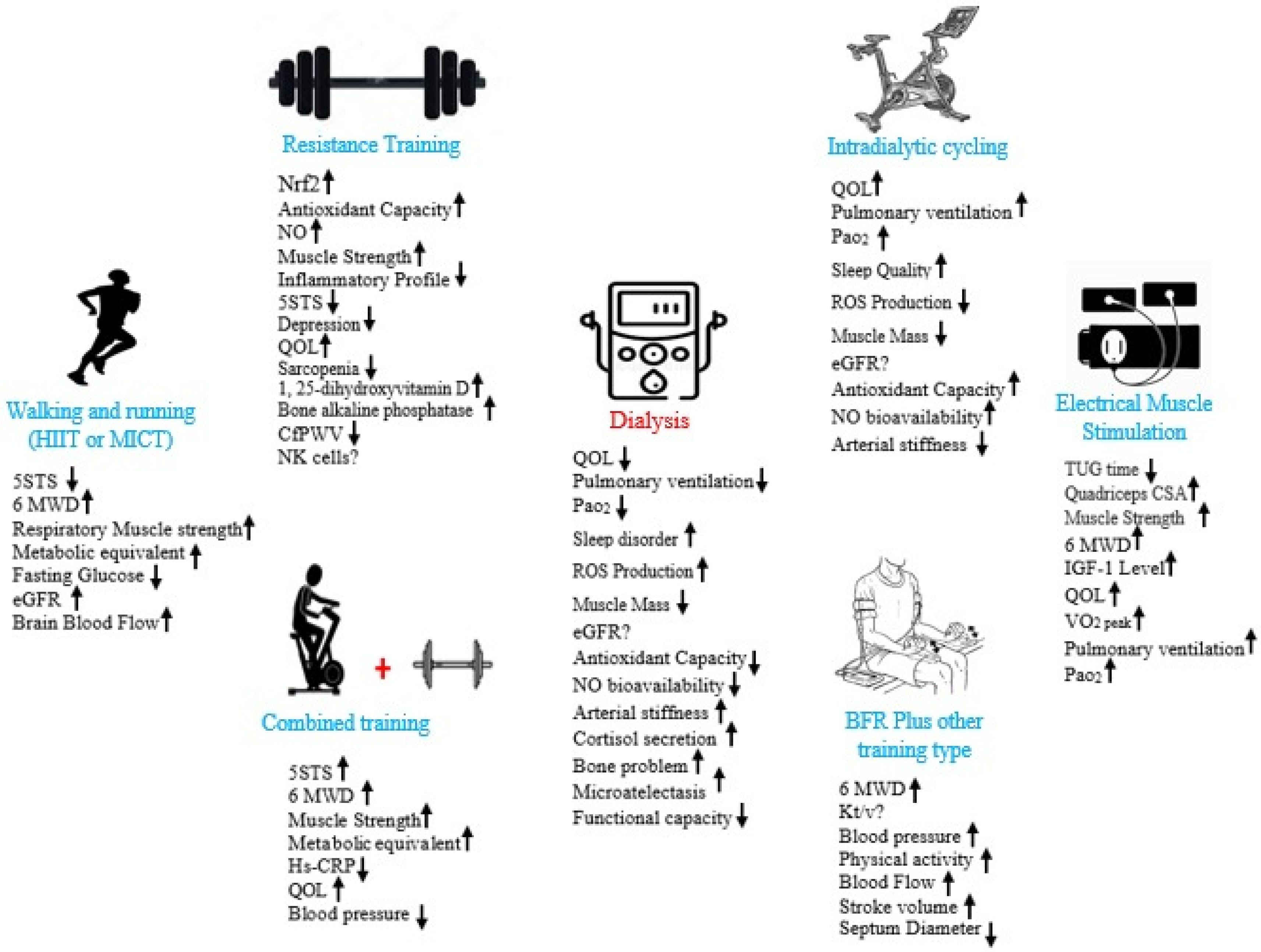
3.1. Aerobic and Functional Exercise in Kidney Diseases
3.2. Resistance Exercise and Kidney Diseases
3.3. Combined Exercise and Kidney Diseases
3.4. Blood Flow Restriction (BFR) Exercise and Kidney Diseases
3.5. High-Intensity Interval Training and Kidney Diseases
3.6. Electrical Muscle Stimulation and Kidney Diseases
3.7. Exercise and Rehabiliation in Patients with Neurogenic Bladder, Polycystic Kidney Disease, and Glomerulonephritis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Data from: Global prevalence of chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Barcellos, F.C.; Del Vecchio, F.B.; Reges, A.; Mielke, G.; Santos, I.S.; Umpierre, D.; Bohlke, M.; Hallal, P. Exercise in patients with hypertension and chronic kidney disease: A randomized controlled trial. J. Hum. Hypertens. 2018, 32, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Inker, L.A. Assessment of Glomerular Filtration Rate in Health and Disease: A State of the Art Review. Clin. Pharmacol. Ther. 2017, 102, 405–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, M.; Yasuda, Y.; Kato, S.; Arafuka, H.; Goto, M.; Hayashi, M.; Kajita, E.; Maruyama, S. The effectiveness and safety of modest exercise in Japanese patients with chronic kidney disease: A single-armed interventional study. Clin. Exp. Nephrol. 2016, 20, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Yang, B.; Fan, F.; Li, P.; Yang, L.; Guo, Y. Effects of individualized exercise program on physical function, psychological dimensions, and health-related quality of life in patients with chronic kidney disease: A randomized controlled trial in China. Int. J. Nurs. Pract. 2017, 23, e12519. [Google Scholar] [CrossRef]
- Watson, E.L.; Gould, D.W.; Wilkinson, T.J.; Xenophontos, S.; Clarke, A.L.; Vogt, B.P.; Viana, J.; Smith, A.C. Twelve-week combined resistance and aerobic training confers greater benefits than aerobic training alone in nondialysis CKD. Am. J. Physiol. Physiol. 2018, 314, F1188–F1196. [Google Scholar] [CrossRef] [Green Version]
- Böhm, J.; Monteiro, M.B.; Andrade, F.P.; Veronese, F.V.; Thomé, F.S. Acute effects of intradialytic aerobic exercise on solute removal, blood gases and oxidative stress in patients with chronic kidney disease. J. Bras. Nefrol. 2017, 39, 172–180. [Google Scholar] [CrossRef]
- Jeong, J.H.; Biruete, A.; Fernhall, B.; Wilund, K.R. Effects of acute intradialytic exercise on cardiovascular responses in hemodialysis patients. Hemodial. Int. 2018, 22, 524–533. [Google Scholar] [CrossRef]
- Gomes, T.S.; Aoike, D.T.; Baria, F.; Graciolli, F.G.; Moyses, R.M.; Cuppari, L. Effect of Aerobic Exercise on Markers of Bone Metabolism of Overweight and Obese Patients with Chronic Kidney Disease. J. Ren. Nutr. 2017, 27, 364–371. [Google Scholar] [CrossRef]
- Belik, F.S.; Silva, V.R.O.E.; Braga, G.P.; Bazan, R.; Vogt, B.P.; Caramori, J.C.T.; Barretti, P.; de Souza Gonçalves, R.; Bôas, P.J.F.V.; Hueb, J.C. Influence of intradialytic aerobic training in cerebral blood flow and cognitive function in patients with chronic kidney disease: A pilot randomized controlled trial. Nephron 2018, 140, 9–17. [Google Scholar]
- Fuhro, M.I.; Dorneles, G.P.; Andrade, F.P.; Romão, P.R.T.; Peres, A.; Monteiro, M.B. Acute exercise during hemodialysis prevents the decrease in natural killer cells in patients with chronic kidney disease: A pilot study. Int. Urol. Nephrol. 2018, 50, 527–534. [Google Scholar] [CrossRef]
- Kirkman, D.; Muth, B.; Stock, J.; Townsend, R.R.; Edwards, D.G. Cardiopulmonary exercise testing reveals subclinical abnormalities in chronic kidney disease. Eur. J. Prev. Cardiol. 2018, 25, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lv, A.; Wang, J.; Zhang, B.; Xu, N.; Zhai, Z.; Gao, J.; Wang, Y.; Li, T.; Ni, C. The effect of intradialytic combined exercise on hemodialysis efficiency in end-stage renal disease patients: A randomized-controlled trial. Int. Urol. Nephrol. 2020, 52, 969–976. [Google Scholar] [CrossRef]
- Toyama, K.; Sugiyama, S.; Oka, H.; Sumida, H.; Ogawa, H. Exercise therapy correlates with improving renal function through modifying lipid metabolism in patients with cardiovascular disease and chronic kidney disease. J. Cardiol. 2010, 56, 142–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wang, Y.; Xiong, L.; Luo, Y.; Huang, Z.; Yi, B. Exercise therapy improves eGFR, and reduces blood pressure and BMI in non-dialysis CKD patients: Evidence from a meta-analysis. BMC Nephrol. 2019, 20, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sasaki, T.; Yamamoto, S.; Hayashi, H.; Ako, S.; Tanaka, Y. Effects of exercise on kidney and physical function in patients with non-dialysis chronic kidney disease: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 18195. [Google Scholar] [CrossRef] [PubMed]
- Aoike, D.T.; Baria, F.; Kamimura, M.A.; Ammirati, A.; De Mello, M.T.; Cuppari, L. Impact of home-based aerobic exercise on the physical capacity of overweight patients with chronic kidney disease. Int. Urol. Nephrol. 2015, 47, 359–367. [Google Scholar] [CrossRef]
- Howden, E.J.; Coombes, J.; Strand, H.; Douglas, B.; Campbell, K.; Isbel, N.M. Exercise Training in CKD: Efficacy, Adherence, and Safety. Am. J. Kidney Dis. 2015, 65, 583–591. [Google Scholar] [CrossRef]
- Spada, T.C.; Silva, J.M.R.D.; Francisco, L.S.; Marçal, L.J.; Antonangelo, L.; Zanetta, D.M.T.; Yu, L.; Burdmann, E.A. High intensity resistance training causes muscle damage and increases biomarkers of acute kidney injury in healthy individuals. PLoS ONE 2018, 13, e0205791. [Google Scholar] [CrossRef]
- Honda, S.; Kawasaki, T.; Kamitani, T.; Kiyota, K. Rhabdomyolysis after High Intensity Resistance Training. Intern. Med. 2017, 56, 1175–1178. [Google Scholar] [CrossRef] [Green Version]
- Beetham, K.S.; Howden, E.J.; Fassett, R.G.; Petersen, A.; Trewin, A.J.; Isbel, N.M.; Coombes, J.S. High-intensity interval training in chronic kidney disease: A randomized pilot study. Scand. J. Med. Sci. Sports 2019, 29, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.A.B.; Ta, B.V.; Iqbal, S.; Gomez, M.Y.-H.; Mavrakanas, T.; Barré, P.; Vasilevsky, M.; Rahme, E.; Daskalopoulou, S.S. The Impact of Intradialytic Pedaling Exercise on Arterial Stiffness: A Pilot Randomized Controlled Trial in a Hemodialysis Population. Am. J. Hypertens. 2017, 31, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.O.; Belik, F.S.; Hueb, J.C.; Gonçalves, R.D.S.; Caramori, J.C.T.; Vogt, B.P.; Barretti, P.; Bazan, S.G.Z.; De Stefano, G.M.M.F.; Martin, L.C.; et al. Aerobic Exercise Training and Nontraditional Cardiovascular Risk Factors in Hemodialysis Patients: Results from a Prospective Randomized Trial. Cardiorenal Med. 2019, 9, 391–399. [Google Scholar] [CrossRef]
- Graham-Brown, M.P.M.; March, D.S.; Churchward, D.R.; Young, H.M.L.; Dungey, M.; Lloyd, S.; Brunskill, N.J.; Smith, A.C.; McCann, G.P.; Burton, J.O. Design and methods of CYCLE-HD: Improving cardiovascular health in patients with end stage renal disease using a structured programme of exercise: A randomised control trial. BMC Nephrol. 2016, 17, 69. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Lv, A.; Wang, J.; Xu, N.; Ma, G.; Zhai, Z.; Zhang, B.; Gao, J.; Ni, C. Exercise Training and Outcomes in Hemodialysis Patients: Systematic Review and Meta-Analysis. Am. J. Nephrol. 2019, 50, 240–254. [Google Scholar] [CrossRef]
- Dashtidehkordi, A.; Shahgholian, N.; Attari, F. Exercise during hemodialysis and health promoting behaviors: A clinical trial. BMC Nephrol. 2019, 20, 96. [Google Scholar] [CrossRef] [Green Version]
- Carney, R.M.; Templeton, B.; Hong, B.A.; Harter, H.R.; Hagberg, J.M.; Schechtman, K.B.; Goldberg, A.P. Exercise Training Reduces Depression and Increases the Performance of Pleasant Activities in Hemodialysis Patients. Nephron Exp. Nephrol. 1987, 47, 194–198. [Google Scholar] [CrossRef]
- Pomidori, L.; Lamberti, N.; Malagoni, A.M.; Manfredini, F.; Pozzato, E.; Felisatti, M.; Catizone, L.; Barillà, A.; Zuccalà, A.; Tripepi, G.; et al. Respiratory muscle impairment in dialysis patients: Can minimal dose of exercise limit the damage? A Preliminary study in a sample of patients enrolled in the EXCITE trial. J. Nephrol. 2016, 29, 863–869. [Google Scholar] [CrossRef]
- Kanbay, M.; Afsar, B.; Siriopol, D.; Unal, H.U.; Karaman, M.; Saglam, M.; Gezer, M.; Taş, A.; Eyileten, T.; Guler, A.K.; et al. Endostatin in chronic kidney disease: Associations with inflammation, vascular abnormalities, cardiovascular events and survival. Eur. J. Intern. Med. 2016, 33, 81–87. [Google Scholar] [CrossRef]
- Santamaría, R.; Díaz-Tocados, J.M.; De Mier, M.V.P.-R.; Robles, A.; Salmerón-Rodríguez, M.D.; Ruiz, E.; Vergara, N.; Aguilera-Tejero, E.; Raya, A.; Ortega, R.; et al. Increased Phosphaturia Accelerates The Decline in Renal Function: A Search for Mechanisms. Sci. Rep. 2018, 8, 13701. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Mallamaci, F.; D’Arrigo, G.; Baggetta, R.; Bolignano, D.; Torino, C.; Lamberti, N.; Bertoli, S.; Ciurlino, D.; Rocca-Rey, L.; et al. Exercise in Patients on Dialysis: A Multicenter, Randomized Clinical Trial. J. Am. Soc. Nephrol. 2017, 28, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.S.; Rowed, K.; Shearer, J.; Macrae, J.M.; Parker, K. Impact of intradialytic exercise intensity on urea clearance in hemodialysis patients. Appl. Physiol. Nutr. Metab. 2018, 43, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Hamza, E.; Metzinger, L.; Meuth, V.M.-L. Uremic Toxins Affect Erythropoiesis during the Course of Chronic Kidney Disease: A Review. Cells 2020, 9, 2039. [Google Scholar] [CrossRef]
- Corrêa, H.D.L.; Moura, S.R.G.; Neves, R.V.P.; Tzanno-Martins, C.; Souza, M.K.; Haro, A.S.; Costa, F.; Silva, J.A.B.; Stone, W.; Honorato, F.S.; et al. Resistance training improves sleep quality, redox balance and inflammatory profile in maintenance hemodialysis patients: A randomized controlled trial. Sci. Rep. 2020, 10, 11708. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, I.; Polkinghorne, K.; Atkins, R.C.; Kerr, P.G. Time and exercise improve phosphate removal in hemodialysis patients. Am. J. Kidney Dis. 2004, 43, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, K.; Nigam, S.; Snell, P.; Hsu, M.C.; Pak, C.Y.C. Assessment of the Pathogenetic Role of Physical Exercise in Renal Stone Formation. J. Clin. Endocrinol. Metab. 1987, 65, 974–979. [Google Scholar] [CrossRef]
- Baggetta, R.; on behalf of the EXCITE Working Group; D’Arrigo, G.; Torino, C.; ElHafeez, S.A.; Manfredini, F.; Mallamaci, F.; Zoccali, C.; Tripepi, G. Effect of a home based, low intensity, physical exercise program in older adults dialysis patients: A secondary analysis of the excite trial. BMC Geriatr. 2018, 18, 248. [Google Scholar] [CrossRef] [Green Version]
- Bogataj, Š.; Pajek, J.; Ponikvar, J.B.; Hadžić, V.; Pajek, M. Kinesiologist-guided functional exercise in addition to intradialytic cycling program in end-stage kidney disease patients: A randomised controlled trial. Sci. Rep. 2020, 10, 5717. [Google Scholar] [CrossRef]
- Parsons, T.L.; Toffelmire, E.B.; King-VanVlack, C.E. Exercise Training During Hemodialysis Improves Dialysis Efficacy and Physical Performance. Arch. Phys. Med. Rehabil. 2004, 87, 680–687. [Google Scholar] [CrossRef]
- Ulf, G.; Bronas, H.P.; Hannan, M. Cognitive Impairment in Chronic Kidney Disease: Vascular Milieu and the Potential Therapeutic Role of Exercise. BioMed Res. Int. 2017, 2017, 2726369. [Google Scholar] [CrossRef]
- Chu, N.; McAdams-DeMarco, M.A. Exercise and cognitive function in patients with end-stage kidney disease. Semin. Dial. 2019, 32, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.L.; Greening, N.J.; Viana, J.L.; Aulakh, J.; Bodicoat, D.H.; Barratt, J.; Feehally, J.; Smith, A.C. Progressive Resistance Exercise Training in CKD: A Feasibility Study. Am. J. Kidney Dis. 2014, 66, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.-H.; Lee, J.-Y.; Lee, S.; Park, H.; Choi, S.-W.; Kim, J.C. Effect of intradialytic exercise on daily physical activity and sleep quality in maintenance hemodialysis patients. Int. Urol. Nephrol. 2018, 50, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.L.; Viana, J.; Wimbury, D.; Martin, N.; Greening, N.; Barratt, J.; Smith, A.C. The Effect of Resistance Exercise on Inflammatory and Myogenic Markers in Patients with Chronic Kidney Disease. Front. Physiol. 2017, 8, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheema, B.S.; Chan, D.; Fahey, P.; Atlantis, E. Effect of Progressive Resistance Training on Measures of Skeletal Muscle Hypertrophy, Muscular Strength and Health-Related Quality of Life in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Sports Med. 2014, 44, 1125–1138. [Google Scholar] [CrossRef]
- Lopes, L.C.C.; Mota, J.F.; Prestes, J.; Schincaglia, R.M.; Silva, D.M.; Queiroz, N.P.; Freitas, A.T.V.D.S.; Lira, F.D.S.; Peixoto, M.D.R.G. Intradialytic Resistance Training Improves Functional Capacity and Lean Mass Gain in Individuals on Hemodialysis: A Randomized Pilot Trial. Arch. Phys. Med. Rehabil. 2019, 100, 2151–2158. [Google Scholar] [CrossRef]
- Chan, D.; Green, S.; Singh, M.F.; Barnard, R.; Cheema, B.S. Development, feasibility, and efficacy of a customized exercise device to deliver intradialytic resistance training in patients with end stage renal disease: Non-randomized controlled crossover trial. Hemodial. Int. 2016, 20, 650–660. [Google Scholar] [CrossRef]
- Abreu, C.; Cardozo, L.; Stockler-Pinto, M.; Esgalhado, M.; Barboza, J.; Frauches, R.; Mafra, D. Does resistance exercise performed during dialysis modulate Nrf2 and NF-κB in patients with chronic kidney disease? Life Sci. 2017, 188, 192–197. [Google Scholar] [CrossRef]
- M’Baya-Moutoula, E.; Louvet, L.; Molinié, R.; Guerrera, I.C.; Cerutti, C.; Fourdinier, O.; Nourry, V.; Gutierrez, L.; Morlière, P.; Mesnard, F.; et al. A multi-omics analysis of the regulatory changes induced by miR-223 in a monocyte/macrophage cell line. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2664–2678. [Google Scholar] [CrossRef]
- Baker, R.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kracht, M.; Müller-Ladner, U.; Schmitz, M.L. Mutual regulation of metabolic processes and proinflammatory NF-κB signaling. J. Allergy Clin. Immunol. 2020, 146, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.N.; Fraser, S.; Barnard, R.; Haines, T.; Ockerby, C.; Street, M.; Wang, W.C.; Daly, R. Effects of an intradialytic resistance training programme on physical function: A prospective stepped-wedge randomized controlled trial. Nephrol. Dial. Transplant. 2016, 31, 1302–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, K.; Speyer, E.; Stengel, B.; Frimat, L.; Sime, W.N.; Massy, Z.A.; Fouque, D.; Laville, M.; Combe, C.; Jacquelinet, C.; et al. Perceived Health and Quality of Life in Patients With CKD, Including Those with Kidney Failure: Findings from National Surveys in France. Am. J. Kidney Dis. 2020, 75, 868–878. [Google Scholar] [CrossRef]
- Roshanravan, B.; Gamboa, J.; Wilund, K. Exercise and CKD: Skeletal Muscle Dysfunction and Practical Application of Exercise to Prevent and Treat Physical Impairments in CKD. Am. J. Kidney Dis. 2017, 69, 837–852. [Google Scholar] [CrossRef]
- Zoccali, C.; Vanholder, R.; Massy, Z.A.; Ortiz, A.; Sarafidis, P.; Dekker, F.W.; Fliser, D.; Fouque, D.; Heine, G.H.; Jager, K.J.; et al. The systemic nature of CKD. Nat. Rev. Nephrol. 2017, 13, 344–358. [Google Scholar] [CrossRef]
- Hong, A.R.; Kim, S.W. Effects of Resistance Exercise on Bone Health. Endocrinol. Metab. 2018, 33, 435–444. [Google Scholar] [CrossRef]
- Russo, C.R. The effects of exercise on bone. Basic concepts and implications for the prevention of fractures. Clin. Cases Miner. Bone Metab. 2009, 6, 223–228. [Google Scholar]
- Cardoso, D.F.; Marques, E.A.; Leal, D.V.; Ferreira, A.; Baker, L.A.; Smith, A.C.; Viana, J.L. Impact of physical activity and exercise on bone health in patients with chronic kidney disease: A systematic review of observational and experimental studies. BMC Nephrol. 2020, 21, 334. [Google Scholar] [CrossRef]
- Marinho, S.M.S.D.A.; Mafra, D.; Pelletier, S.; Hage, V.; Teuma, C.; Laville, M.; Eduardo, J.C.C.; Fouque, D. In Hemodialysis Patients, Intradialytic Resistance Exercise Improves Osteoblast Function: A Pilot Study. J. Ren. Nutr. 2016, 26, 341–345. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Edwards, D.G.; Lennon-Edwards, S. Exercise as an Adjunct Therapy in Chronic Kidney Disease. Ren. Nutr. Forum 2014, 33, 1–8. [Google Scholar]
- Orcy, R.B.; Dias, P.S.; Seus, T.L.; Barcellos, F.C.; Bohlke, M. Combined Resistance and Aerobic Exercise is Better than Resistance Training Alone to Improve Functional Performance of Haemodialysis Patients—Results of a Randomized Controlled Trial. Physiother. Res. Int. 2012, 17, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, L.; Wang, Y.; Wang, C.; Hu, R.; Wu, Y. Effects of combined aerobic and resistance exercise on renal function in adult patients with chronic kidney disease: A systematic review and meta-analysis. Clin. Rehabil. 2020, 34, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Washida, N.; Morimoto, K.; Muraoka, K.; Kasai, T.; Yamaki, K.; Miyashita, K.; Wakino, S.; Itoh, H. Home-based Aerobic Exercise and Resistance Training in Peritoneal Dialysis Patients: A Randomized Controlled Trial. Sci. Rep. 2019, 9, 2632. [Google Scholar] [CrossRef] [Green Version]
- Hiraki, K.; Shibagaki, Y.; Izawa, K.P.; Hotta, C.; Wakamiya, A.; Sakurada, T.; Yasuda, T.; Kimura, K. Effects of home-based exercise on pre-dialysis chronic kidney disease patients: A randomized pilot and feasibility trial. BMC Nephrol. 2017, 18, 198. [Google Scholar] [CrossRef] [Green Version]
- Van Bergen, M.; Takken, T.; Engelbert, R.; Groothoff, J.; Nauta, J.; van Hoeck, K.; Helders, P.; Lilien, M. Exercise training in pediatric patients with end-stage renal disease. Pediatr. Nephrol. 2009, 24, 619–622. [Google Scholar] [CrossRef] [Green Version]
- Thompson, S.; Wiebe, N.; Gyenes, G.; Davies, R.; Radhakrishnan, J.; Graham, M. Physical Activity in Renal Disease (PAIRED) and the effect on hypertension: Study protocol for a randomized controlled trial. Trials 2019, 20, 109. [Google Scholar] [CrossRef]
- Hellberg, M.; Höglund, P.; Svensson, P.; Clyne, N. Comparing effects of 4 months of two self-administered exercise training programs on physical performance in patients with chronic kidney disease: RENEXC—A randomized controlled trial. PLoS ONE. 2018, 13, e0207349. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, M.J.; Brumby, C.; Fraser, S.F.; McMahon, L.P.; Bennett, P.N.; Warmington, S.A. Hemodynamic and perceptual responses to blood flow-restricted exercise among patients undergoing dialysis. Am. J. Physiol. Physiol. 2020, 318, F843–F850. [Google Scholar] [CrossRef]
- Cardoso, R.K.; Araujo, A.M.; Del Vechio, F.B.; Bohlke, M.; Barcellos, F.C.; Oses, J.P.; De Freitas, M.P.; Rombaldi, A.J. Intradialytic exercise with blood flow restriction is more effective than conventional exercise in improving walking endurance in hemodialysis patients: A randomized controlled trial. Clin. Rehabil. 2020, 34, 91–98. [Google Scholar] [CrossRef]
- Nilsson, B.B.; Bunæs-Næss, H.; Edvardsen, E.; Stenehjem, A.-E. High-intensity interval training in haemodialysis patients: A pilot randomised controlled trial. BMJ Open Sport Exerc. Med. 2019, 5, e000617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moholdt, T.; Aamot, I.L.; Granøien, I.; Gjerde, L.; Myklebust, G.; Walderhaug, L.; Brattbakk, L.; Hole, T.; Graven, T.; Stølen, T.O.; et al. Aerobic interval training increases peak oxygen uptake more than usual care exercise training in myocardial infarction patients: A randomized controlled study. Clin. Rehabil. 2012, 26, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Tjønna, A.E.; Leinan, I.M.; Bartnes, A.T.; Jenssen, B.M.; Gibala, M.J.; Winett, R.A.; Wisløff, U. Low- and high-volume of intensive endurance training significantly improves maximal oxygen uptake after 10-weeks of training in healthy men. PLoS ONE 2013, 8, e65382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falahati, A.; Arazi, H.; Suzuki, K. Acute Responses of Cardiac Biomarkers to Intermittent and Continuous Exercise Are Related to Age Difference but Not I/D Polymorphism in the ACE Gene. Front. Physiol. 2020, 11, 665. [Google Scholar] [CrossRef]
- Di Iorio, B.; Torraca, S.; Gustaferro, P.; Fazeli, G.; Heidland, A. High-frequency external muscle stimulation in acute kidney injury (AKI): Potential shortening of its clinical course. Clin. Nephrol. 2013, 79, S37–S45. [Google Scholar]
- Roxo, R.S.; Xavier, V.B.; Miorin, L.A.; Magalhães, A.O.; Sens, Y.A.; Alves, V.L. Impact of neuromuscular electrical stimulation on functional capacity of patients with chronic kidney disease on hemodialysis. J. Bras. Nefrol. 2016, 38, 344–350. [Google Scholar] [CrossRef]
- Suzuki, T.; Ikeda, M.; Minami, M.; Matayoshi, Y.; Nakao, M.; Nakamura, T.; Abo, M. Beneficial Effect of Intradialytic Electrical Muscle Stimulation in Hemodialysis Patients: A Randomized Controlled Trial. Artif. Organs. 2018, 42, 899–910. [Google Scholar] [CrossRef]
- McGregor, G.; Ennis, S.; Powell, R.; Hamborg, T.; Raymond, N.T.; Owen, W.; Aldridge, N.; Evans, G.; Goodby, J.; Hewins, S.; et al. Feasibility and effects of intra-dialytic low-frequency electrical muscle stimulation and cycle training: A pilot randomized controlled trial. PLoS ONE 2018, 13, e0200354. [Google Scholar] [CrossRef] [Green Version]
- Brüggemann, A.K.; Mello, C.; Dal Pont, T.; Kunzler, D.H.; Martins, D.F.; Bobinski, F.; Yamaguti, W.P.; Paulin, E. Effects of Neuromuscular Electrical Stimulation During Hemodialysis on eripheral Muscle Strength and Exercise Capacity: A Randomized Clinical Trial. Arch. Phys. Med. Rehabil. 2017, 98, 822–831.e1. [Google Scholar] [CrossRef]
- Yamagata, K.; Hoshino, J.; Sugiyama, H.; Hanafusa, N.; Shibagaki, Y.; Komatsu, Y.; Konta, T.; Fujii, N.; Kanda, E.; Sofue, T.; et al. Clinical practice guideline for renal rehabilitation: Systematic reviews and recommendations of exercise therapies in patients with kidney diseases. Ren. Replace. Ther. 2019, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Cornacoff, J.B.; Hebert, L.A.; Sharma, H.M.; Bay, W.H.; Young, D.C. Adverse Effect of Exercise on Immune Complex-Mediated Glomerulonephritis. Nephron Exp. Nephrol. 1985, 40, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Oh, D.-J.; Sung, B.M. FP291neurogenic bladder and chronic kidney disease in spinal cord injury patients. Nephrol. Dial. Transplant. 2015, 30, iii164. [Google Scholar] [CrossRef] [Green Version]
- Andrade, R.C.; Neto, J.A.; Andrade, L.; Oliveira, T.S.; Santos, D.N.; Oliveira, C.J.; Prado, M.J.; Carvalho, E.M. Effects of Physiotherapy in the Treatment of Neurogenic Bladder in Patients Infected with Human T-Lymphotropic Virus 1. Urology 2016, 89, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J.; Chapman, A.B.; Guay-Woodford, L.M.; Bae, K.T.; King, B.F., Jr.; Wetzel, L.H.; Baumgarten, D.A.; Kenney, P.J.; Harris, P.C.; Klahr, S.; et al. CRISP Investigators. Volume progression in polycystic kidney disease. N. Engl. J. Med. 2006, 354, 2122–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Vea, A.; Bardají, A.; Gutierrez, C.; Garca, C.; Peralta, C.; Marcas, L.; Oliver, J.A. Exercise blood pressure, cardiac structure, and diastolic function in young normotensive patients with polycystic kidney disease: A prehypertensive state. Am. J. Kidney Dis. 2004, 44, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, N.L.; Cunha, T.M.; Heilberg, I.P.; Higa, E.M.S.; Nishiura, J.L.; Neder, J.A.; Almeida, W.S.; Schor, N. Exercise Capacity in Polycystic Kidney Disease. Am. J. Kidney Dis. 2014, 64, 239–246. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Wisse, B.E. The Inflammatory Syndrome: The Role of Adipose Tissue Cytokines in Metabolic Disorders Linked to Obesity. J. Am. Soc. Nephrol. 2004, 15, 2792–2800. [Google Scholar] [CrossRef] [Green Version]
- Asad, A.; Burton, J.O.; March, D.S. Exercise as a therapeutic option for acute kidney injury: Mechanisms and considerations for the design of future clinical studies. BMC Nephrol. 2020, 21, 446. [Google Scholar] [CrossRef]
- Starkie, R.; Ostrowski, S.; Jauffred, S.; Febbraio, M.; Pedersen, B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003, 17, 884–886. [Google Scholar] [CrossRef]
- Stump, C.S. Physical Activity in the Prevention of Chronic Kidney Disease. Cardiorenal Med. 2011, 1, 164–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntyre, C.W.; Selby, N.; Sigrist, M.; Pearce, L.E.; Mercer, T.H.; Naish, P.F. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol. Dial. Transplant. 2006, 21, 2210–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, S.J.; Kim, T.J.; Yoon, S.Y.; Chung, J.H.; Hwang, H.J. Relationship between Stage of Chronic Kidney Disease and Sarcopenia in Korean Aged 40 Years and Older Using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008–2011. PLoS ONE 2015, 10, e0130740. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.L.; Suzuki, K. Systemic Inflammation Mediates the Effects of Endotoxemia in the Mechanisms of Heat Stroke. Biol. Med. 2017, 9, 1. [Google Scholar] [CrossRef]
- Radak, Z.; Torma, F.; Berkes, I.; Goto, S.; Mimura, T.; Posa, A.; Balogh, L.; Boldogh, I.; Suzuki, K.; Higuchi, M.; et al. Exercise effects on physiological function during aging. Free Radic. Biol. Med. 2019, 132, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Nemes, R.; Koltai, E.; Taylor, A.W.; Suzuki, K.; Gyori, F.; Radak, Z. Reactive Oxygen and Nitrogen Species Regulate Key Metabolic, Anabolic, and Catabolic Pathways in Skeletal Muscle. Antioxidants 2018, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef]
| Author, Year | Country | Sample Size | Age (Years) Mean ± SD | Duration of Dialysis (Months) Mean ± SD | Time | CKD Stage | Duration | Frequency | Intervention | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | ||||||||
| Dashtidehkordi et al. (2019) [27] | Iran | 24 | 22 | 51.22 | 55.64 | 5.48 | 4.48 | Intradialytic | HD | 8 weeks | 3 times/week | Two half hours with 5 min intervals using a stationary bicycle; the intensity was self-selected | Stationary bicycle during hemodialysis could enhance health-promoting behaviors |
| Böhm et al. (2017) [7] | Brazil | 11 | 12 | 52 ± 5 | 53 ± 3 | 20 (8–64) | 19 (10–45) | Intradialytic | HD | 1 session | -- | 30 min aerobic exercise with intensity between 60–70% of HRmax | Increased phosphorus serum concentration and decreased total antioxidant capacity, increased oxygen partial pressure and saturation, no change in acid base |
| e Silva et al. (2019) [24] | Brazil | 15 | 15 | 58 ± 15.0 | 50 ± 17.2 | 26.0 ± 14.58 | 21.0 ± 27.1 | Intradialytic | HD | 3 months | 3 times/week | 30 min without interruption, between 65% and 75% of the HRmax | Significant improvement in flow-mediated vasodilation, reduction in left-ventricular hypertrophy and serum aldosterone |
| Gomes et al. (2017) [9] | Brazil | 24 | 15 | 55.5 ± 8.3 | 55.5 ± 8.3 | - | - | Home-based | Obese P-HD | 24 weeks | 3 times/week | 30 min 3 times per week with increments of 10 min in duration every 4 weeks until week 8 | Aerobic training did not promote relevant changes in the bone metabolism markers |
| Belik et al. (2018) [10] | Brazil | 7 | 8 | 50.3 ± 17.24 | 57.8 ± 15.01 | 26.0 ± 14.58 | 21.1 ± 27.10 | Intradialytic | HD | 16 weeks | 3 times/week | 30 min with training range of 65–75% HRmax | Significant improvement of cognitive impairment and basilar maximum blood flow velocity in trained patients |
| Author, Year | Country | Sample Size | Age (Years) Mean ± SD | Duration of Dialysis (Months) Mean ± SD | Time | CKD Stage | Duration | Frequency | Intervention | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | ||||||||
| Corrêa et al. (2020) [35] | Brazil | 30 | 25 | 66.0 ± 4.0 | 65.7 ± 3.8 | 60.7 ± 8.0 | 59.8 ± 7.7 | Intradialytic | HD | 3 months | 3 times/week | Consisted of 11 exercises with 2 weeks of familiarization; 3 sets of 8–12 repetitions with 2 min of rest between sets | Decreased ferritin, sleep efficiency improvement |
| Lopes et al. (2019) [47] | Brazil | 30 | 20 | (MLG) 56.2 ± 12.5 and 48.1 ± 10.8 (HLG) | 56.9 ± 12.4 | (MLG) 72.1 ± 50.3 and 45.7 ± 39.3 (HLG) | 53.2 ± 44.1 | Intradialytic | HD | 12 weeks | 3 times/week | Each session involved 5 exercises; exercise was performed until volitional fatigue; the duration of the sessions varied between 20 and 40 min | Increased lean leg mass; improvements in pain and physical function; prevalence of sarcopenia was reduced by 14.3% and 25%; and no change in cytokines was observed |
| Abreu et al. (2017) [49] | Brazil | 25 | 19 | 45.07 ± 15.2 | 42.5 ± 13.5 | 71.2 ± 45.5 | 70.1 ± 49.9 | Intradialytic | HD | 12 weeks | 3 times/week | Elastic bands ranged from 1.6 to 10.0 kg, and the load used in exercises performed with ankle cuffs ranged from 1.0 to 12.0 kg; intensity set at 60% of 1RM, since CKD patients are mostly debilitated | Resistance exercises are able to induce Nrf2 activation in CKD patients on HD |
| Bennett et al. (2016) [53] | Australia | 171 | - | 68.1 (12.6) | - | 44 (26.0–85.5) | - | Intradialytic | HD | 12 to 36 weeks | 2 times/week | Two sets of 15–20 repetitions for each exercise; the resistance exercises were made progressively harder using different color-graded elastic bands; training consisted of six lower- and upper-body resistance exercises | Exercise training led to significant improvements in physical function as measured by STS and TUG |
| Chan et al. (2016) [48] | Australia | 22 | - | In total 71.3 6 11.0 | In total 24 (15–56) | Intradialytic | HD | 26 weeks | 2 times/week | 2 upper-body and 3 lower-body exercises, unilaterally and bilaterally, both before and during dialysis, with loads of 2.5 to 59 kg | Lower body strength and health-related quality of life (HRQoL) subscales significantly increased and a trend toward reduced depression was noted | ||
| Author, Year | Country | Sample Size | Age (Years) Mean ± SD | Durationof Dialysis (Months) Mean ± SD | Time | CKD Stage | Type | Duration | Frequency | Intervention | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | |||||||||
| Huang et al. (2020) [13] | China | n = 16 | n = 16 | 43.81 ± 10.25 | 37.63 ± 10.31 | 26 (29.75) | 43 (89) | Intradialytic | HD | CE | 24-week | 3 times/week | 5 min warm-up, cool-down, and 30 min cycling at a RPE of 12–14 | Systolic and diastolic blood pressure significantly decreased by 8.5 and 6.5 mmHg, respectively |
| Van Bergen et al., 2009 [66] | Netherlands | 20 | _ | Ranged between 8 and 18 yrs, mean 17.6 | A history of ESRD treated with either HD or PD for more than 3 months | HD | AE, RE, and game | 12 weeks | 2 times/week | 50 min sessions including 5 min warm-up period; each session involved aerobic training (with an intensity ranging from 55 to 90% HRmax), resistance exercises with no heavy load, and active games | Exercise capacity and muscle strength were higher after the exercise program in patients who completed training | |||
| Uchiyama et al. (2019) [64] | Japan | 24 | 23 | 64.9 ± 9.2 | 63.2 ± 9.5 | Unclear | Unclear | Home-based | PD | RE and AE | 24-week | 3 times/week (AE), 2 times/week (RE) | AE at 40–60% of the HRmax as determined in the baseline ISWT, with an RPE of 11–13; the program started at 20 min/session and progressed to 30 min/session; RT was prescribed at 70% (1RM) to train a variety of upper- and lower-body muscle groups | Physical functioning, emotional functioning, and role/social component summary significantly improved in the exercise group; serum albumin was maintained in the exercise group |
| Watson et al. (2018) [6] | UK | 21 AE | 20 RE | 63 (58–71) | 63 (51–69) | Unclear | Unclear | Unclear | CKD patients (stages 3b–5) | CE and AE | 12-week | 3 times/week | 30 min of moderate intensity exercise at 70–80% HRmax in CE, and only 20 min of AE were performed; resistance exercise consisted of leg extension; training load (in kg) was set at 70% 1RM, and patients performed 3 sets of 12–15 repetitions and 2–3 min rest interval | Combination of resistance and aerobic exercise confers more increases in muscle mass and strength than aerobic exercise alone |
| Barcellos et al. (2018) [2] | Brazil | 76 | 74 | 65.0 (1.2) | 65.1 (1.3) | Unclear | Unclear | Gym | CKD patients (stages 2–4) with hypertension | RE and AE | 16-week | 3 times/week (AE) | 10 min of initial warm-up, 60 min physical exercise sessions | Significant decreases in hs-CRP and fasting blood sugar, and increase in functional capacity in exercise group |
| Cho et al. (2018) [44] | South Korea | 33 | 13 | 51 ± 22 | 55 ± 64 | 54.8 ± 96.4 (AE), 47.6 ± 79.2 (RE) and 87.8 ± 70.5 (CE) | 61.4 ± 36.5 | Intradialytic | HD | AE, RE, and CE | 12-week | 3 times/week | AE at 60–70% of an individual’s maximal capacity, RE program consisted of seven exercises for 3 sets of 10–15 repetitions (RPE 13–15), CE group performed both the AE and RE | Improvement in daily physical activity and sleep quality, increase in metabolic equivalent but not to RE, and decrease in sedentary bouts |
| Thompson1 et al. (2019) [67] | Canada | 80 | 80 | Older than 18 yrs | More than 18 yrs | Unclear | Unclear | Home-based exercise | eGFR (15–44 mL/min per 1.73 m2) with hypertension (SBP > 130 mmHg) | AE and RE | 24-week | 3 times/week | Moderate-intensity aerobic exercise (50–60% heart rate reserve) supplemented with isometric resistance exercise in two phases: (1) supervised, facility-based, weekly, and home-based sessions (8 weeks); (2) home-based sessions (16 weeks) | A decrease in blood pressure in response to aerobic exercise and isometric contraction was observed and showed that aerobic exercise followed by isometric resistance training is feasible for patients with CKD |
| Author, Year | Country | Sample Size | Age (Years) Mean ± SD | Durationof Dialysis (Months) Mean ± SD | Time | CKD Stage | Type | Duration | Frequency | Intervention | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | |||||||||
| Clarkson et al. (2020) [69] | Australia | n = 10 | - | Unclear | Unclear | Unclear | Unclear | Intradialytic and off hemodialysis | ESKD (stage V); eGFR < 15 mL·min−1·1.73 m−2; HD > 12 wk | BFR + AE and AE | 2 sessions | - | 5-min cycling warmup and cool-down at a self-selected cadence; two 10 min bouts of cycling separated by a 20 min rest period; workload for each 10 min bout was between 10 W and 30 W | BFR did not affect ultrafiltration; BFR is comparable to standard aerobic exercise |
| Cardoso et al. (2020) [70] | Brazil | BFREG (n = 19), CEG (n = 20) | 20 | BFREG, 49.4 ± 15.9–59.8 ± 16.1 CEG, | 48.2 ± 13.6 | 54 (17.8–87) BFR-CEG 25 (12–69) | 33 (9–52.5) | Intradialytic | HD | BFR + AE and AE | 12 Weeks | 3 times/week | CEG 20 min training session, intensity increase from 60% to 76% HRmax BFR caused 50% reduction in arterial blood flow during 12 weeks of training | Increased walking distance in BFR+AE group in comparison with two other groups |
| Author, Year | Country | Sample Size | Age (Years) Mean ± SD | Durationof Dialysis (Months) Mean ± SD | Time | CKD Stage | Type | Duration | Frequency | Intervention | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | |||||||||
| Nilsson et al. (2019) [71] | Norway | 14 | 6 | 59.5 (55–67) | 67 (51–69) | Unclear | Unclear | Intradialytic | HD | HIIT and MICT | 22 weeks | 2 times/week | HIIT or MICT cycling two times per week for a total of 32 sessions over 16–22 weeks; the exercise intervention lasted 45 min | Increased VO2peak |
| Beetham et al., (2019) [21] | Australia | 14 | - | Ranged between 18 and 75 yrs | Unclear | Unclear | Unclear | Sage 3–4 CKD patients | HIIT and MICT | 12 weeks | 3 times/week | HIIT performed in 4 × 4 min intervals with 80–95% HRmax and MICT performed as 40 min run with 65% HRmax | There was no significant difference between HIIT and MICT in muscle protein synthesis and time efficieny of training | |
| Author, Year | Country | Sample Size | Age (Years) Mean ± SD | Durationof Dialysis (Months) Mean ± SD | Time | CKD Stage | Type | Duration | Frequency | Intervention | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | |||||||||
| Suzuki et al. (2018) [77] | Japan | 13 | 13 | 662.6 ± 12.8 | 651.6± 8.1 | 281.6 ± 24.2 | 304.6 ± 23.6 | Intradialytic | HD | EMS | 8 weeks | 3 times/week | EMS protocol was performed 3 times a week for 8 weeks in lower extremity; waveform simulation produced co-contractions in the lower extremity muscle groups at a frequency of 20 Hz with a pulse width of 250.l s; each duty cycle included a 5 s stimulation period with a 2 s pause for a period of 20 min | EMS could be an effective exercise training tool for HD patients with either muscle wasting, weakness, or sarcopenia |
| McGregor et al. (2018) [78] | UK | 33 | 18 | C- 52.1 [44.2; 59.9] LF-EMS 51.5 [42.3; 60.6] | 54.3 [46.0; 62.5] | Unclear | Unclear | Intradialytic | HD | EMS | 10 weeks | 3 times/week | Two weeks of familiarization allowed participants to become accustomed to the sensation of LF-EMS and progress to at least 30 min of stimulation; cycling was performed for up to one hour per session (minimum of 50 min), initially at a workload (Watts) equivalent to that achieved at 40–60% VO2 reserve during CPET; a five min warmup and cool-down | Cardio-respiratory reserve (VO2peak) and leg strength were improved; arterial structure and function were unaffected. |
| Brüggemann et al. (2017) [79] | Brazil | 51 in total | Unclear | Unclear | Unclear | Unclear | Intradialytic | HD | NMS | 12 sessions | 3 times/week | Neuromuscular electrical stimulation with 50 Hz and 24 medium intensity of 72.90 mA, and LG used 5 Hz and medium intensity of 13.85 mA | Increased 6 MWTD in both groups and improved physical capacity | |
| Author, Year | Country | Sample Size | Age (Years) Mean ± SD | CKD Stage | Type | Duration | Frequency | Intervention | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | ||||||||
| Andrade et al. (2016) [83] | Brazil | 21 | - | 54 ± 12 | - | HTLV-1-infected individuals with NB | EMS | 10–40 sessions | 2 times/week | Low-frequency biphasic current of 12 Hz, 0.2 milliseconds for 30 min; medium-frequency, (50 Hz, 250 μs), with an intermittent 3 s stimulus followed by 1 s of rest for 30 min | Reduction in the overactive bladder symptom score from 10 ± 4 to 6 ± 3; increase in the perineal muscle strength and improvement in symptoms of urinary urgency, frequency, incontinence, and nocturia |
| Reinecke et al. (2014) [86] | Brazil | 26 | 30 healthy subjects | Ranged between 19 and 39 yrs | ADPKD | AE | 1 session | - | 20 min single bout of cycle ergometer exercise with 60% of VO2peak was performed | Young, normotensive patients with polycystic KD and preserved kidney function had inadequate responses of nitric oxide and ADMA levels to acute exercise compared to healthy subjects; additionally, low aerobic capacity was observed in polycystic KD patients | |
| Yamagata et al. (2019) [80] | Japan | Unclear | Unclear | Ranged between 10 and 69 yrs | Glomerulonephritis, nephrotic syndrome, non-dialysis-dependent CKD | Most include AE | Different | Different | Various types of exercise included Bruce stress treadmill test and bicycle | Temporarily increased proteinuria; results showed a 7.1% increase in renal function after exercise. additionally, a higher VO2peak was observed in active patients | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arazi, H.; Mohabbat, M.; Saidie, P.; Falahati, A.; Suzuki, K. Effects of Different Types of Exercise on Kidney Diseases. Sports 2022, 10, 42. https://doi.org/10.3390/sports10030042
Arazi H, Mohabbat M, Saidie P, Falahati A, Suzuki K. Effects of Different Types of Exercise on Kidney Diseases. Sports. 2022; 10(3):42. https://doi.org/10.3390/sports10030042
Chicago/Turabian StyleArazi, Hamid, Majid Mohabbat, Payam Saidie, Akram Falahati, and Katsuhiko Suzuki. 2022. "Effects of Different Types of Exercise on Kidney Diseases" Sports 10, no. 3: 42. https://doi.org/10.3390/sports10030042
APA StyleArazi, H., Mohabbat, M., Saidie, P., Falahati, A., & Suzuki, K. (2022). Effects of Different Types of Exercise on Kidney Diseases. Sports, 10(3), 42. https://doi.org/10.3390/sports10030042







