Cystine/Glutamine Mixture Supplementation Attenuated Fatigue during Endurance Exercise in Healthy Young Men by Enhancing Fatty Acid Utilization
Abstract
:1. Introduction
2. Materials and Methods
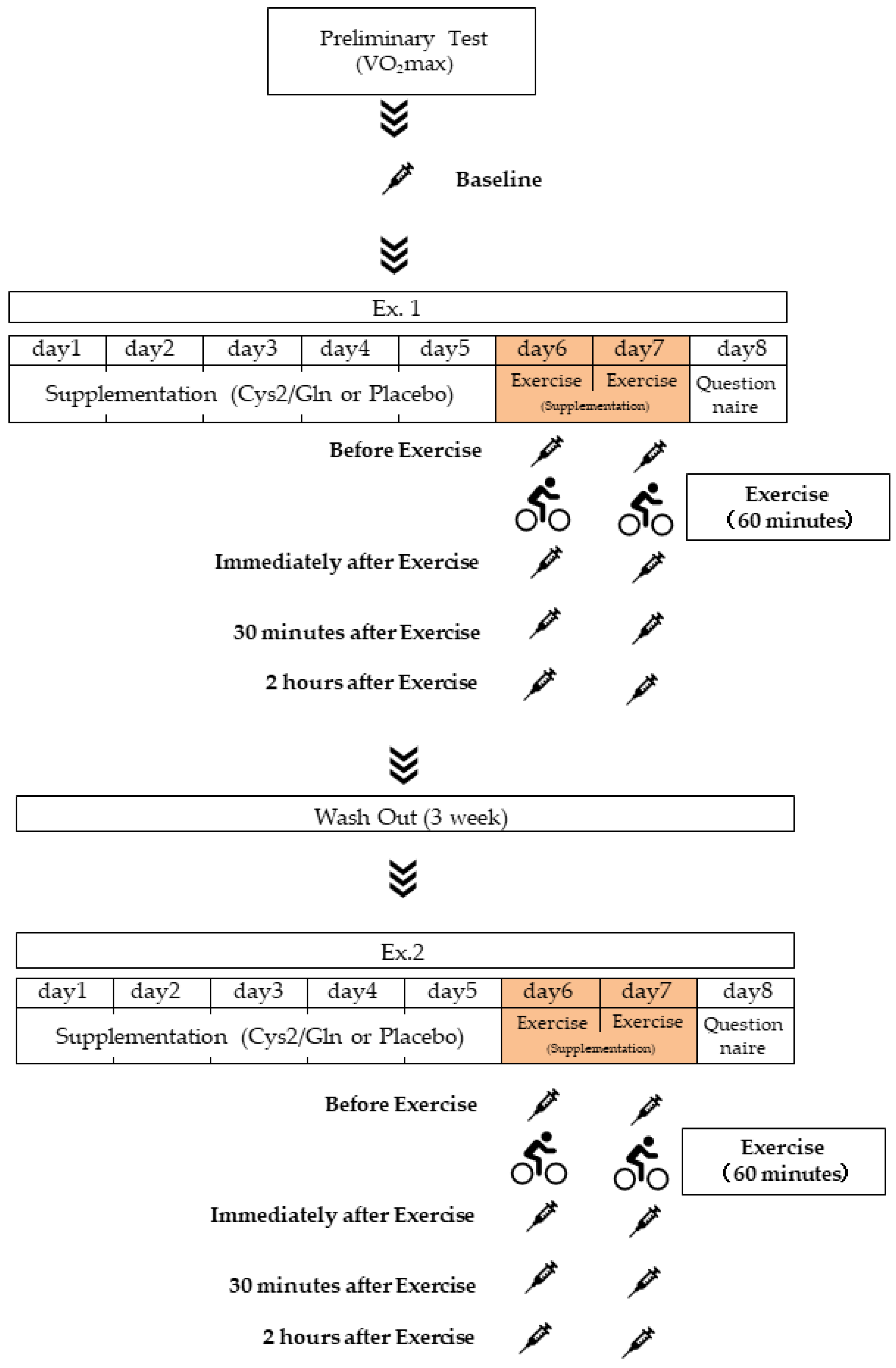
2.1. Experimental Design and Experimental Protocol
2.2. Participants and Amino Acid Mixture Supplementation
2.3. Blood Sampling and Biochemistry Analyses
2.4. Reagents for the Cell Culture and Cell Experiment
2.5. Cell Culture and Oxidative Stress with H2O2 Stimulation
2.6. Fatty Acid Oxidation Assay and Mitochondrial Oxygen Consumption Rate
2.7. Sample Size Calculations and Statistical Analyses
3. Results
3.1. Physiological Variables throughout the Experiment
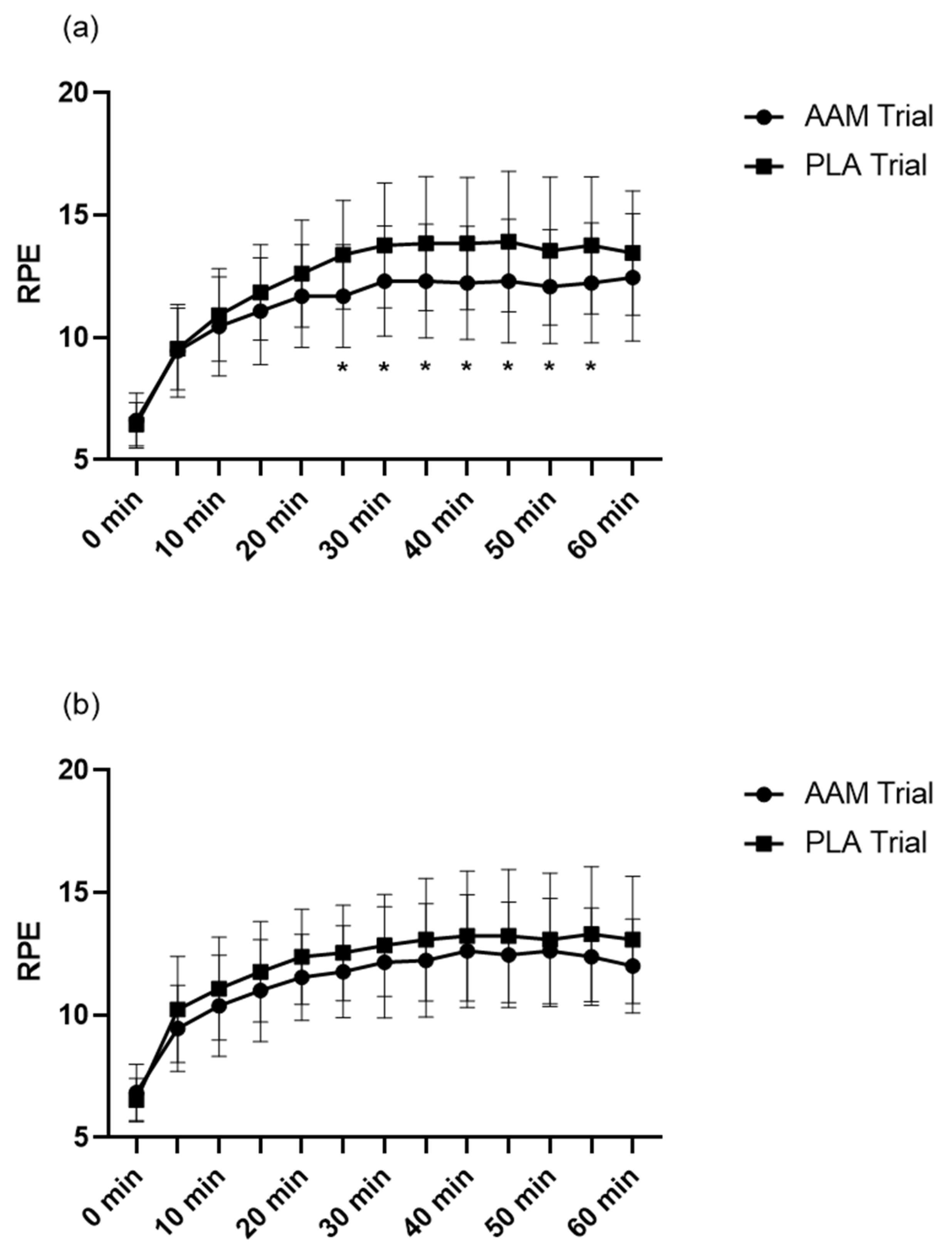
3.2. A 7-Day Amino Acid Supplementation Decreased Ratings of Perceived Exertion
3.3. A 7-Day Amino Acid Supplementation Enhanced Fatty Acid Utilization
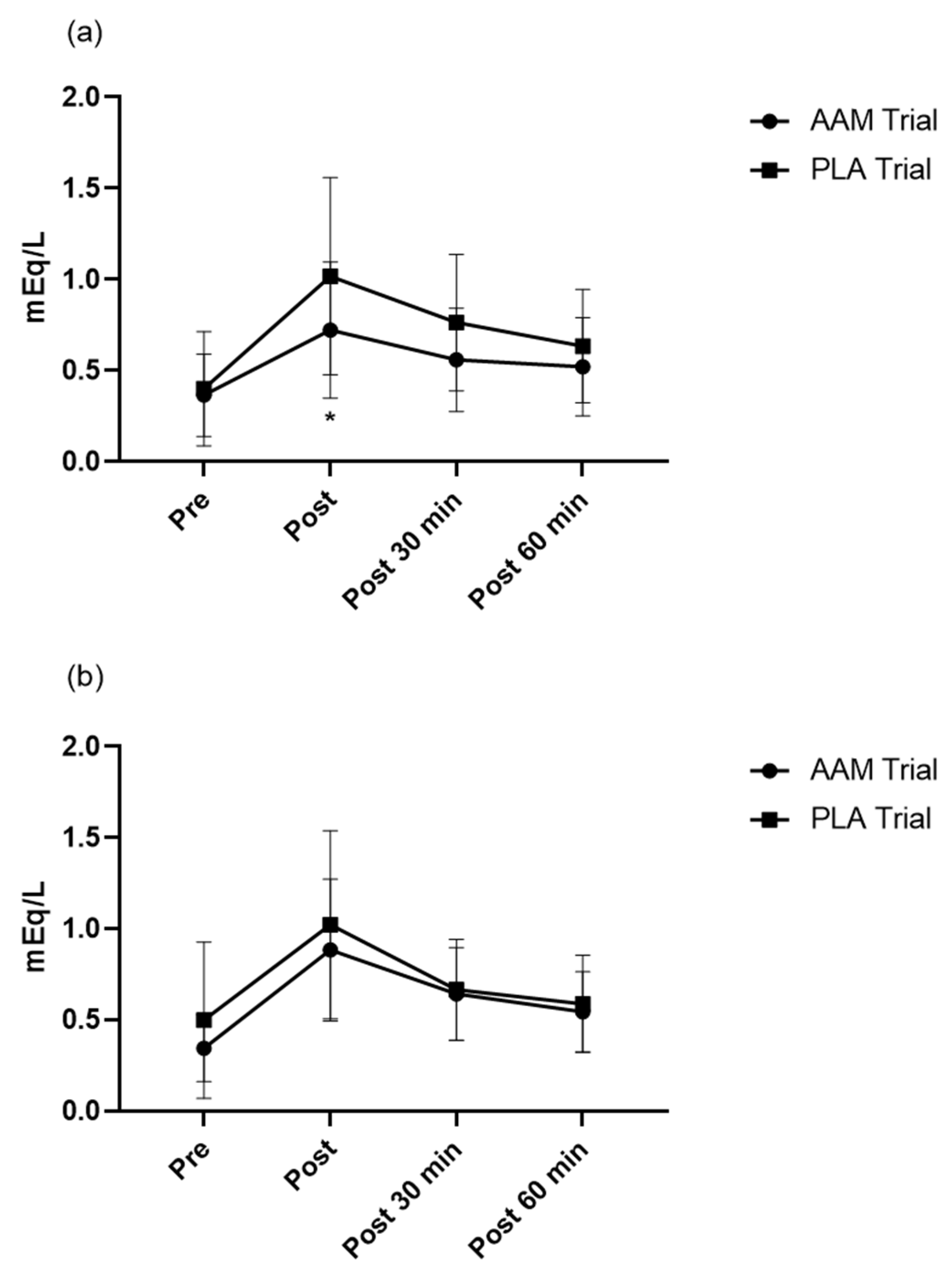
3.4. Effects of Exercise and Amino Acid Supplementation on Blood Biochemistry and the Subjective Physical Condition
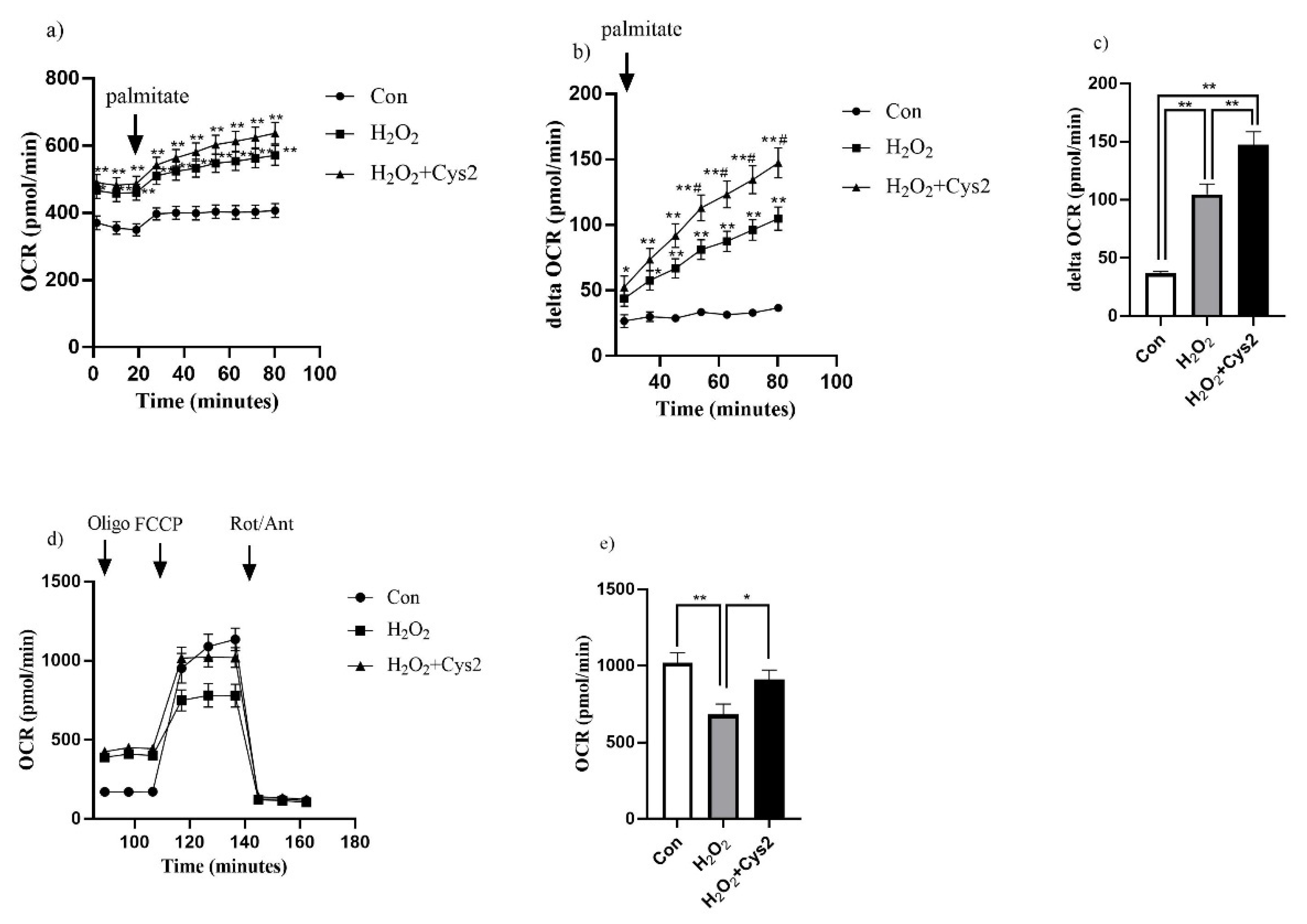
3.5. Effect of Cys2 on Mitochondrial Respiration and Fatty Acid Utilization in C2C12 Myotubes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xirouchaki, C.E.; Mangiafico, S.P.; Bate, K.; Ruan, Z.; Huang, A.M.; Tedjosiswoyo, B.W.; Lamont, B.; Pong, W.; Favaloro, J.; Blair, A.R.; et al. Impaired Glucose Metabolism and Exercise Capacity with Muscle-Specific Glycogen Synthase 1 (Gys1) Deletion in Adult Mice. Mol. Metab. 2016, 5, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Ma, S.; Tominaga, T.; Suzuki, K.; Liu, C. An 8-Week, Low Carbohydrate, High Fat, Ketogenic Diet Enhanced Exhaustive Exercise Capacity in Mice Part 2: Effect on Fatigue Recovery, Post-Exercise Biomarkers and Anti-Oxidation Capacity. Nutrients 2018, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Huang, Q.; Tominaga, T.; Liu, C.; Suzuki, K. An 8-Week Ketogenic Diet Alternated Interleukin-6, Ketolytic and Lipolytic Gene Expression, and Enhanced Exercise Capacity in Mice. Nutrients 2018, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Yada, K.; Suzuki, K.; Oginome, N.; Ma, S.; Fukuda, Y.; Iida, A.; Radak, Z. Single Dose Administration of Taheebo Polyphenol Enhances Endurance Capacity in Mice. Sci. Rep. 2018, 8, 14625. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A. Effect of increased fat availability on metabolism and exercise capacity. Med. Sci. Sports Exerc. 2002, 34, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Saltin, B.; Larsen, H.; Terrados, N.; Bangsbo, J.; Bak, T.; Kim, C.K.; Svedenhag, J.; Rolf, C.J. Aerobic exercise capacity at sea level and at altitude in Kenyan boys, junior and senior runners compared with Scandinavian runners. Scand. J. Med. Sci. Sports 2021, 31, 209–221. [Google Scholar] [CrossRef]
- Aoi, W.; Ogaya, Y.; Takami, M.; Konishi, T.; Sauchi, Y.; Park, E.Y.; Wada, S.; Sato, K.; Higashi, A. Glutathione Supplementation Suppresses Muscle Fatigue Induced by Prolonged Exercise via Improved Aerobic Metabolism. J. Int. Soc. Sports Nutr. 2015, 12, 7. [Google Scholar] [CrossRef]
- Kim, H.; Kojima, N.; Uchida, R.; Somekawa, S.; Inoue, N.; Kobayashi, H.; Osuka, Y. The Additive Effects of Exercise and Essential Amino Acid on Muscle Mass and Strength in Community-Dwelling Older Japanese Women with Muscle Mass Decline, but Not Weakness and Slowness: A Randomized Controlled and Placebo Trial. Aging Clin. Exp. Res. 2021, 33, 1841–1852. [Google Scholar] [CrossRef]
- Mizugaki, A.; Kato, H.; Takeda, T.; Inoue, Y.; Hasumura, M.; Hasegawa, T.; Murakami, H. Cystine reduces mitochondrial dysfunction in C2C12 myotubes under moderate oxidative stress induced by H2O2. Amino Acids 2022, 54, 1203–1213. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Sinha, P.; Clements, V.K.; Rodriguez, P.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells Inhibit T-Cell Activation by Depleting Cystine and Cysteine. Cancer Res. 2010, 70, 68–77. [Google Scholar] [CrossRef]
- Castell, L.M.; Poortmans, J.R.; Leclercq, R.; Brasseur, M.; Duchateau, J.; Newsholme, E.A. Some Aspects of the Acute Phase Response after a Marathon Race, and the Effects of Glutamine Supplementation. Eur. J. Appl. Physiol. 1996, 75, 47–53. [Google Scholar] [CrossRef]
- Dröge, W.; Eck, H.P.; Gmünder, H.; Mihm, S. Modulation of Lymphocyte Functions and Immune Responses by Cysteine and Cysteine Derivatives. Am. J. Med. 1991, 91, S140–S144. [Google Scholar] [CrossRef]
- de Siqueira, R.F.; Filho, H.C.M.; Fernandes, W.R. Glutamine Supplementation Affects Th1 and Th2 Cell Populations in Endurance Horses. Comp. Exer. Physiol. 2020, 16, 259–266. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Clerc, I.; Abba Moussa, D.; Vahlas, Z.; Tardito, S.; Oburoglu, L.; Hope, T.J.; Sitbon, M.; Dardalhon, V.; Mongellaz, C.; Taylor, N. Entry of Glucose- and Glutamine-Derived Carbons into the Citric Acid Cycle Supports Early Steps of HIV-1 Infection in CD4 T Cells. Nat. Metab. 2019, 1, 717–730. [Google Scholar] [CrossRef]
- Elias, R.J.; McClements, D.J.; Decker, E.A. Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase β-lactoglobulin in oil-in-water emulsions. J. Agric. Food Chem. 2005, 53, 10248–10253. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Campbell, K.S.; Reid, M.B. N-acetylcysteine in handgrip exercise: Plasma thiols and adverse reactions. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 146–154. [Google Scholar] [CrossRef]
- Yu, J.C.; Jiang, Z.M.; Li, D.M. Glutamine: A precursor of glutathione and its effect on liver. World J. Gastroenterol. 1999, 5, 143. [Google Scholar] [CrossRef]
- Ziegler, T.R.; Bazargan, N.; Leader, L.M.; Martindale, R.G. Glutamine and the Gastrointestinal Tract. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 355–362. [Google Scholar] [CrossRef]
- Iwashita, S.; Williams, P.; Jabbour, K.; Ueda, T.; Kobayashi, H.; Baier, S.; Flakoll, P.J. Impact of Glutamine Supplementation on Glucose Homeostasis during and after Exercise. J. Appl. Physiol. 2005, 99, 1858–1865. [Google Scholar] [CrossRef]
- Tanaka, K.A.K.; Kurihara, S.; Shibakusa, T.; Chiba, Y.; Mikami, T. Cystine Improves Survival Rates in a LPS-Induced Sepsis Mouse Model. Clin. Nutr. 2015, 34, 1159–1165. [Google Scholar] [CrossRef]
- Takagi, Y.; Kurihara, S.; Higashi, N.; Morikawa, S.; Kase, T.; Maeda, A.; Arisaka, H.; Shibahara, S.; Akiyama, Y. Combined Administration of L-Cystine and L-Theanine Enhances Immune Functions and Protects against Influenza Virus Infection in Aged Mice. J. Vet. Med. Sci. 2010, 72, 157. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Honda, H.; Oikawa, M.; Kakita, T.; Oyama, A.; Oishi, H.; Tochikubo, K.; Hashimoto, T.; Kurihara, S.; Shibakusa, T.; et al. Oral Administration of the Amino Acids Cystine and Theanine Attenuates the Adverse Events of S-1 Adjuvant Chemotherapy in Gastrointestinal Cancer Patients. Int. J. Clin. Oncol. 2016, 21, 1085–1090. [Google Scholar] [CrossRef]
- Williams, N. The Borg Rating of Perceived Exertion (RPE) Scale. Occu. Med. 2017, 67, 404–405. [Google Scholar] [CrossRef]
- Sasaki, E.; Umeda, T.; Takahashi, I.; Arata, K.; Yamamoto, Y.; Tanabe, M.; Oyamada, K.; Hashizume, E.; Nakaji, S. Effect of glutamine supplementation on neutrophil function in male judoists. Luminescence 2019, 28, 442–449. [Google Scholar] [CrossRef]
- Kurihara, S.; Yoshida, S.; Sukegawa, E.; Yoshimura, S.; Uchida, H.; Maeda, K.; Yamamoto, T. Evaluation of safety of long-term and excessive intake of l-cystine and l-theanine in healthy adult subjects. Seikatsu Eisei. 2008, 52, 229–236. (In Japanese) [Google Scholar] [CrossRef]
- Zuhl, M.; Schneider, S.; Lanphere, K.; Conn, C.; Dokladny, K.; Moseley, P. Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 2014, 48, 980–986. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Ainscow, E.K.; Brand, M.D. Top-down control analysis of ATP turnover, glycolysis and oxidative phosphorylation in rat hepatocytes. Eur. J. Biochem. 1999, 263, 671–685. [Google Scholar] [CrossRef]
- March, D.S.; Marchbank, T.; Playford, R.J.; Jones, A.W.; Thatcher, R.; Davison, G. Intestinal fatty acid-binding protein and gut permeability responses to exercise. Eur. J. Appl. Physiol. 2017, 117, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Corbi, G.; Conti, V.; Troisi, J.; Colucci, A.; Manzo, V.; Di Pietro, P.; Calabrese, M.C.; Carrizzo, A.; Vecchione, C.; Ferrara, N.; et al. Cardiac Rehabilitation Increases SIRT1 Activity and β-Hydroxybutyrate Levels and Decreases Oxidative Stress in Patients with HF with Preserved Ejection Fraction. Oxid. Med. Cell. Longev. 2019, 2019, 7049237. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, G.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 6116, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Caris, A.V.; Thomatieli-Santos, R.V. Carbohydrate and Glutamine Supplementation Attenuates the Increase in Rating of Perceived Exertion during Intense Exercise in Hypoxia Similar to 4200 m. Nutrients 2020, 12, 3797. [Google Scholar] [CrossRef] [PubMed]
- Clemente Plaza, N.; Reig García-Galbis, M.; Martínez-Espinosa, R.M. Effects of the Usage of L-Cysteine (l-Cys) on Human Health. Molecules 2018, 23, 575. [Google Scholar] [CrossRef] [PubMed]
- van Hall, G.; González-Alonso, J.; Sacchetti, M.; Saltin, B. Skeletal muscle substrate metabolism during exercise: Methodological considerations. Proc. Nutr. Soc. 1999, 58, 899–912. [Google Scholar] [CrossRef]
- Ma, S.; Yang, J.; Tominaga, T.; Liu, C.; Suzuki, K. A Low-Carbohydrate Ketogenic Diet and Treadmill Training Enhanced Fatty Acid Oxidation Capacity but Did Not Enhance Maximal Exercise Capacity in Mice. Nutrients 2021, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Hirano, R.; Haneda, Y.; Fukano, R.; Hara, M.; Furukawa, S. Amino Acids Exhibit Anti-Inflammatory Effects in Human Monocytic Leukemia Cell Line, THP-1 Cells. Inflamm. Res. 2011, 60, 1013. [Google Scholar] [CrossRef]
- Sakurada, S.; Kato, T.; Okamoto, T. Induction of Cytokines and ICAM-1 by Proinflammatory Cytokines in Primary Rheumatoid Synovial Fibroblasts and Inhibition by N-Acetyl-L-Cysteine and Aspirin. Int. Immunol. 1996, 8, 1483–1493. [Google Scholar] [CrossRef]
- Kawada, S.; Kobayashi, K.; Ohtani, M.; Fukusaki, C. Cystine and Theanine Supplementation Restores High-Intensity Resistance Exercise-Induced Attenuation of Natural Killer Cell Activity in Well-Trained Men. J. Strength Cond. Res. 2010, 24, 846–851. [Google Scholar] [CrossRef]
- Miyakuni, T.; Fukatsu, K.; Ri, M.; Murakoshi, S.; Inoue, Y.; Kurihara, S.; Takayama, T.; Yasuhara, H. Cystine and Theanine Improve Survival after Gut Ischemia-Reperfusion. Ann. Nutr. Metab. 2018, 73, 131–137. [Google Scholar] [CrossRef]
- Miyachi, T.; Tsuchiya, T.; Oyama, A.; Tsuchiya, T.; Abe, N.; Sato, A.; Chiba, Y.; Kurihara, S.; Shibakusa, T.; Mikami, T. Perioperative Oral Administration of Cystine and Theanine Enhances Recovery After Distal Gastrectomy. J. Parenter. Enteral. Nutr. 2013, 37, 384–391. [Google Scholar] [CrossRef]
- Kurihara, S.; Shibahara, S.; Arisaka, H.; Akiyama, Y. Enhancement of Antigen-Specific Immunoglobulin G Production in Mice by Co-Administration of L-Cystine and L-Theanine. J. Vet. Med. Sci. 2007, 69, 1263–1270. [Google Scholar] [CrossRef]
- Hamaguchi, R.; Tsuchiya, T.; Miyata, G.; Sato, T.; Takahashi, K.; Miura, K.; Oshio, H.; Ohori, H.; Ariyoshi, K.; Oyamada, S.; et al. Efficacy of Oral Administration of Cystine and Theanine in Colorectal Cancer Patients Undergoing Capecitabine-Based Adjuvant Chemotherapy after Surgery: A Multi-Institutional, Randomized, Double-Blinded, Placebo-Controlled, Phase II Trial (JORTC-CAM03). Support. Care Cancer 2020, 28, 3649–3657. [Google Scholar] [CrossRef]
- Kurihara, S.; Shibakusa, T.; Tanaka, K.A. Cystine and Theanine: Amino Acids as Oral Immunomodulative Nutrients. SpringerPlus 2013, 2, 635. [Google Scholar] [CrossRef]
- Laaksonen, D.E.; Atalay, M.; Niskanen, L.; Uusitupa, M.; Hänninen, O.; Sen, C.K. Blood Glutathione Homeostasis as a Determinant of Resting and Exercise-Induced Oxidative Stress in Young Men. Redox Rep. 1999, 4, 53–59. [Google Scholar] [CrossRef]
- Hasegawa, T.; Mizugaki, A.; Inoue, Y.; Kato, H.; Murakami, H. Cystine Reduces Tight Junction Permeability and Intestinal Inflammation Induced by Oxidative Stress in Caco-2 Cells. Amino Acids 2021, 53, 1021–1032. [Google Scholar] [CrossRef]
- Kerksick, C.; Willoughby, D. The Antioxidant Role of Glutathione and N-Acetyl-Cysteine Supplements and Exercise-Induced Oxidative Stress. J. Int. Soc. Sports Nutr. 2005, 2, 38. [Google Scholar] [CrossRef]
- Lemasters, J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005, 8, 3–5. [Google Scholar] [CrossRef]
- Loy, B.D.; Cameron, M.H.; O’Connor, P.J. Perceived fatigue and energy are independent unipolar states: Supporting evidence. Med. Hypotheses 2018, 113, 46–51. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Ono, M.; Mizugaki, A.; Kato, H.; Miyashita, M.; Suzuki, K. Cystine/Glutamine Mixture Supplementation Attenuated Fatigue during Endurance Exercise in Healthy Young Men by Enhancing Fatty Acid Utilization. Sports 2022, 10, 147. https://doi.org/10.3390/sports10100147
Ma S, Ono M, Mizugaki A, Kato H, Miyashita M, Suzuki K. Cystine/Glutamine Mixture Supplementation Attenuated Fatigue during Endurance Exercise in Healthy Young Men by Enhancing Fatty Acid Utilization. Sports. 2022; 10(10):147. https://doi.org/10.3390/sports10100147
Chicago/Turabian StyleMa, Sihui, Miho Ono, Ami Mizugaki, Hiroyuki Kato, Masashi Miyashita, and Katsuhiko Suzuki. 2022. "Cystine/Glutamine Mixture Supplementation Attenuated Fatigue during Endurance Exercise in Healthy Young Men by Enhancing Fatty Acid Utilization" Sports 10, no. 10: 147. https://doi.org/10.3390/sports10100147
APA StyleMa, S., Ono, M., Mizugaki, A., Kato, H., Miyashita, M., & Suzuki, K. (2022). Cystine/Glutamine Mixture Supplementation Attenuated Fatigue during Endurance Exercise in Healthy Young Men by Enhancing Fatty Acid Utilization. Sports, 10(10), 147. https://doi.org/10.3390/sports10100147






