The Effect of Heart Rate Variability Biofeedback Training on Vagal Tone in Athletically Talented Secondary School Students
Abstract
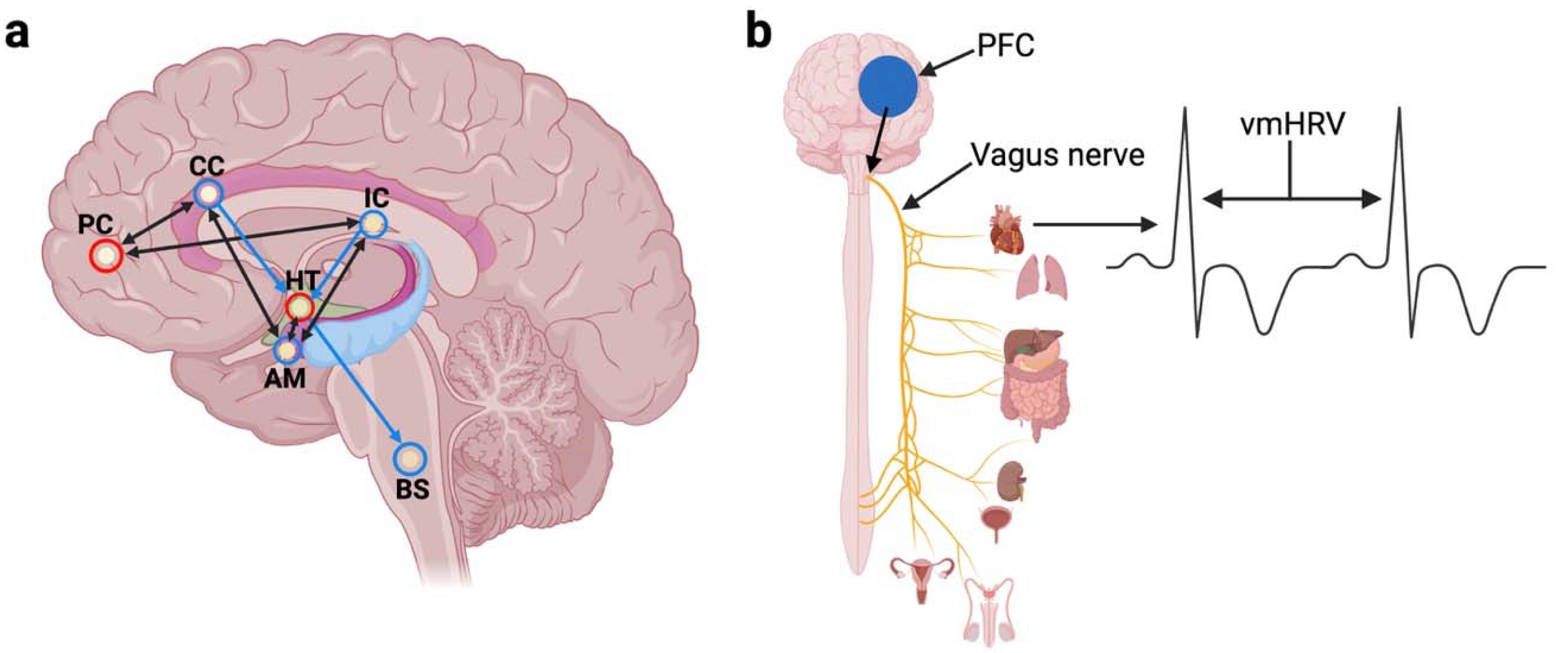
1. Introduction
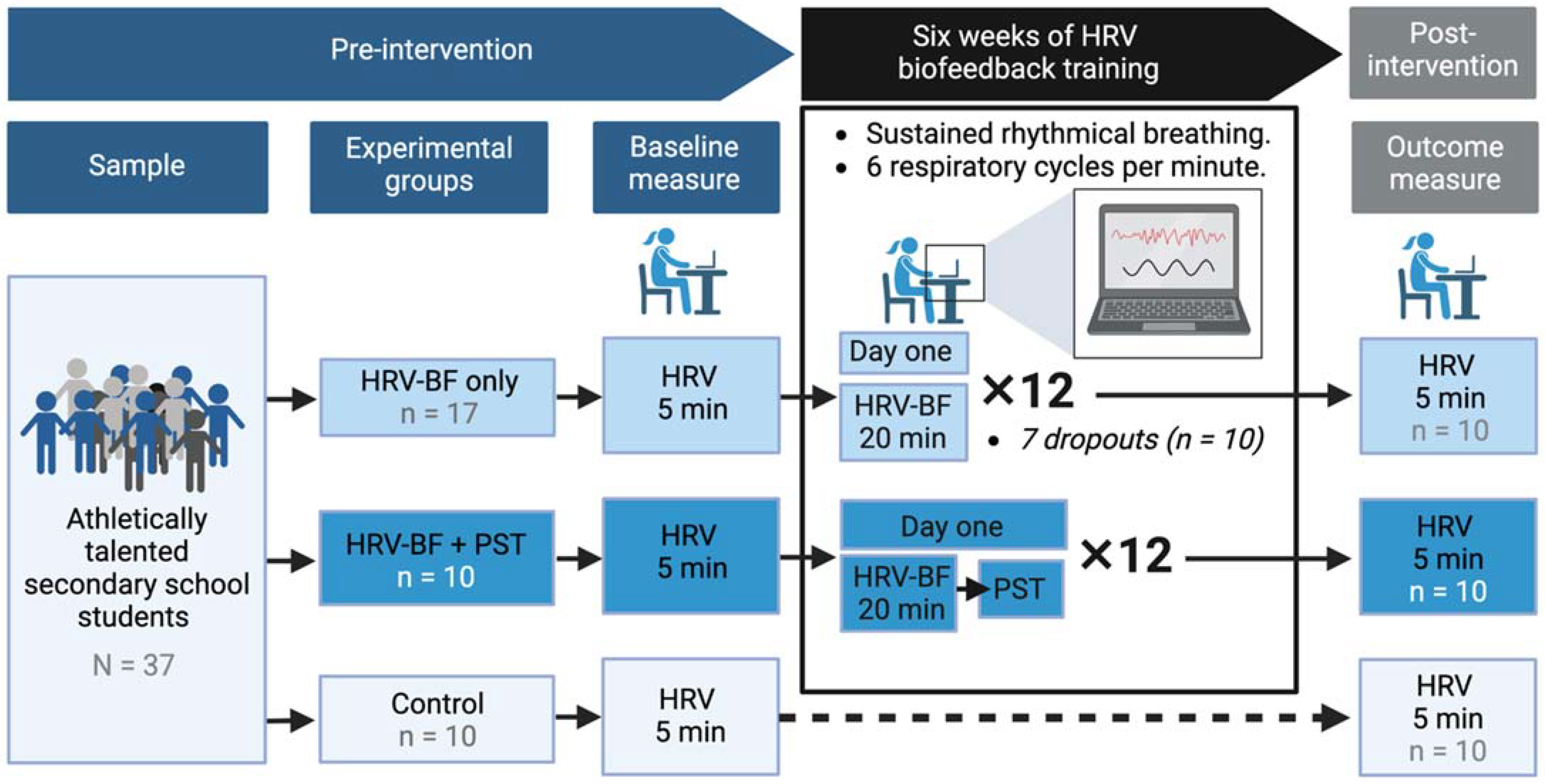
2. Materials and Methods
2.1. Participants and Background of the School
2.2. Ethics Statement
2.3. HRV Biofeedback Training Protocol
2.4. Heart Rate Variability Data Collection
2.5. Heart Rate Variability Data Reduction and Analysis
2.6. Heart Rate Variability Indices of Interest
2.7. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Appelhans, B.M.; Luecken, L.J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef]
- Thayer, J.F.; Ahs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007, 74, 224–242. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance HRV and CVD risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Kluttig, A.; Kuss, O.; Greiser, K.H. Ignoring lack of association of heart rate variability with cardiovascular disease and risk factors: Response to the manuscript “The relationship of autonomic imbalance, heart rate variability cardiovascular disease risk factors” by Julian F. Thayer, Shelby S. Yamamoto, Jos F. Brosschot. Int. J. Cardiol. 2010, 145, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Karavidas, M.K.; Lehrer, P.M.; Vaschillo, E.; Vaschillo, B.; Marin, H.; Buyske, S.; Malinovsky, I.; Radvanski, D.; Hassett, A. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl. Psychophysiol. Biofeedback 2007, 32, 19–30. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Thayer, J.F.; Brosschot, J.F. Psychosomatics and psychopathology: Looking up and down from the brain. Psychoneuroendocrinology 2005, 30, 1050–1058. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Benarroch, E.E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Benarroch, E.E. The central autonomic network. In Clinical Autonomic Disorders, 2nd ed.; Low, P.A., Ed.; Lippincott/Raven: Philadelphia, PA, USA, 1997; pp. 17–23. [Google Scholar]
- Thayer, J.F.; Siegle, G.J. Neurovisceral integration in cardiac and emotional regulation. IEEE Eng. Med. Biol. Mag. Q. Mag. Eng. Med. Biol. Soc. 2002, 21, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, P.; Kaur, K.; Sharma, A.; Shah, K.; Huseby, R.; Bhavsar, J.; Sgobba, P.; Zhang, Y. Heart rate variability biofeedback improves emotional and physical health and performance: A systematic review and meta analysis. Appl. Psychophysiol. Biofeedback 2020, 45, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Ask, T.F.; Lugo, R.G.; Sütterlin, S. The neuro-immuno-senescence integrative model (NISIM) on the negative association between parasympathetic activity and cellular senescence. Front. Neurosci. 2018, 12, 726. [Google Scholar] [CrossRef] [PubMed]
- De Couck, M.; Mravec, B.; Gidron, Y. You may need the vagus nerve to understand pathophysiology and to treat diseases. Clin. Sci. 2012, 122, 323–328. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Vaschillo, E.; Vaschillo, B. Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Appl. Psychophysiol. Biofeedback 2000, 25, 177–191. [Google Scholar] [CrossRef]
- Wheat, A.L.; Larkin, K.T. Biofeedback of heart rate variability and related physiology: A critical review. Appl. Psychophysiol. Biofeedback 2010, 35, 229–242. [Google Scholar] [CrossRef]
- Lehrer, P.M. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosom. Med. 2003, 65, 796–805. [Google Scholar] [CrossRef]
- Lehrer, P.; Carr, R.; Smetankine, A.; Vaschillo, E.; Peper, E.; Porges, S.; Edelberg, R.; Hamer, R.; Hochron, S. Respiratory sinus arrhythmia versus neck/trapezius EMG and incentive in spirometry biofeedback for asthma: A pilot study. Appl. Psychophysiol. Biofeedback 1997, 22, 95–109. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Vaschillo, E.; Vaschillo, B.; Lu, S.-E.; Scardella, A.; Siddique, M.; Habib, H. Biofeedback treatment for asthma. Chest 2004, 126, 352–361. [Google Scholar] [CrossRef]
- Lehrer, P.; Vaschillo, E.; Lu, S.; Eckberg, D.; Vaschillo, B.; Scardella, A.; Habib, R. Heart rate variability biofeedback: Effects of age on heart rate variability, baroreflex gain, and asthma. Chest 2006, 129, 278–284. [Google Scholar] [CrossRef]
- Cowan, M.; Pike, K.; Budzynski, H. Ovid: Psychosocial nursing therapy following sudden cardiac arrest: Impact on two-year survival. Nurs. Res. 2012, 50, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.M.; Gevirtz, R.N.; Scher, B.; Guarneri, E. Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. Am. Heart J. 2004, 147, E11. [Google Scholar] [CrossRef] [PubMed]
- Nolan, R.P.; Kamath, M.V.; Floras, J.S.; Stanley, J.; Pang, C.; Picton, P.; Young, Q.R. Heart rate variability biofeedback as a behavioural neurocardiac intervention to enhance vagal heart rate control. Am. Heart J. 2005, 149, 1137. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.; Lin, I.M.; Fan, S.Y.; Chien, C.L.; Lin, T.H. One-year cardiovascular prognosis of the randomized, controlled, short-term heart rate variability biofeedback among patients with coronary artery disease. Int. J. Behav. Med. 2018, 25, 271–282. [Google Scholar] [CrossRef]
- Giardino, N.D.; Chan, L.; Borson, S. Combined heart rate variability and pulse oximetry biofeedback for chronic obstructive pulmonary disease: Preliminary findings. Appl. Psychophysiol. Biofeedback 2004, 29, 121–133. [Google Scholar] [CrossRef]
- Hassett, A.L.; Radvanski, D.C.; Vaschillo, E.G.; Vaschillo, B.; Sigal, L.H.; Karavidas, M.K.; Buyske, S.; Lehrer, P.M. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Appl. Psychophysiol. Biofeedback 2007, 32, 1–10. [Google Scholar] [CrossRef]
- Windthorst, P.; Mazurak, N.; Kuske, M.; Hipp, A.; Giel, K.E.; Enck, P.; Nieß, A.; Zipfel, S.; Teufel, M. Heart rate variability biofeedback therapy and graded exercise training in management of chronic fatigue syndrome: An exploratory pilot study. J. Psychosom. Res. 2017, 93, 6–13. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gevirtz, R.N.; Brown, M.; Spira, J.; Guarneri, E.; Stoletniy, L. The effect of biofeedback on function in patients with heart failure. Appl. Psychophysiol. Biofeedback 2009, 34, 71–91. [Google Scholar] [CrossRef]
- Pizzoli, S.; Marzorati, C.; Gatti, D.; Monzani, D.; Mazzocco, K.; Pravettoni, G. A meta-analysis on heart rate variability biofeedback and depressive symptoms. Sci. Rep. 2021, 11, 6650. [Google Scholar] [CrossRef]
- Zucker, T.L.; Samuelson, K.W.; Muench, F.; Greenberg, M.A.; Gevirtz, R.N. The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: A pilot study. Appl. Psychophysiol. Biofeedback 2009, 34, 135–143. [Google Scholar] [CrossRef]
- Henriques, G.; Keffer, S.; Abrahamson, C.; Horst, S.J. Exploring the effectiveness of a computer-based heart rate variability biofeedback program in reducing anxiety in college students. Appl. Psychophysiol. Biofeedback 2011, 36, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Hallman, D.M.; Olsson, E.M.G.; von Schéele, B.; Melin, L.; Lyskov, E. Effects of heart rate variability biofeedback in subjects with stress-related chronic neck pain: A pilot study. Appl. Psychophysiol. Biofeedback 2011, 36, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Goessl, V.C.; Curtiss, J.E.; Hofmann, S.G. The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychol. Med. 2017, 47, 2578–2586. [Google Scholar] [CrossRef]
- Firth, A.; Sütterlin, S.; Lugo, R.G. Using Cognitive Behavioural Techniques to Improve Academic Achievement in Student-Athletes. Educ. Sci. 2019, 9, 89. [Google Scholar] [CrossRef]
- Gevirtz, R. The promise of heart rate variability biofeedback: Evidence-based applications. Biofeedback 2013, 41, 110–120. [Google Scholar] [CrossRef]
- Paul, M.; Garg, K. The effect of heart rate variability biofeedback on performance psychology of basketball players. Appl. Psychophysiol. Biofeedback 2012, 37, 131–144. [Google Scholar] [CrossRef]
- Lin, G.; Xiang, Q.; Fu, X.; Wang, S.; Wang, S.; Chen, S.; Shao, L.; Zhao, Y.; Wang, T. Heart rate variability biofeedback decreases blood pressure in pre-hypertensive subjects by improving autonomic function and baroreflex. J. Altern. Compelemt. Med. 2012, 18, 143–152. [Google Scholar] [CrossRef]
- Lehrer, P.M. How does heart rate variability biofeedback work? resonance, the baroreflex, and other mechanisms. Biofeedback 2013, 41, 26–31. [Google Scholar] [CrossRef]
- Vaschillo, E.; Lehrer, P.; Rishe, N.; Konstantinov, M. Heart rate variability biofeedback as a method for assessing baroreflex function: A preliminary study of resonance in the cardiovascular system. Appl. Psychophysiol. Biofeedback 2002, 27, 1–27. [Google Scholar] [CrossRef]
- Watso, J.C.; Babcock, M.C.; Migdal, K.U.; Robinson, A.T. The baroreflex effectiveness index as an early marker of autonomic dysfunction in heart failure. J. Physiol. 2017, 595, 5013–5014. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Parati, G.; Castiglioni, P.; Tordi, R.; Mancia, G.; Pedotti, A. Baroreflex effectiveness index: An additional measure of baroreflex control of heart rate in daily life. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 280, R744–R751. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Jeong, S.W. Arterial baroreflex impairment and functional plasticity of cardiac autonomic neurons in rat models of liver cirrhosis. Auton. Neurosci. Basic Clin. 2015, 192, 90. [Google Scholar] [CrossRef]
- Leyro, T.M.; Buckman, J.F.; Bates, M.E. Theoretical implications and clinical support for heart rate variability biofeedback for substance use disorders. Curr. Opin. Psychol. 2019, 30, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Schumann, A.; de la Cruz, F.; Köhler, S.; Brotte, L.; Bär, K.J. The Influence of Heart Rate Variability Biofeedback on Cardiac Regulation and Functional Brain Connectivity. Front. Neurosci. 2021, 15, 691988. [Google Scholar] [CrossRef]
- Siepmann, M.; Aykac, V.; Unterdörfer, J.; Petrowski, K.; Mueck-Weymann, M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl. Psychophysiol. Biofeedback 2008, 33, 195–201. [Google Scholar] [CrossRef]
- Cartwright, S. How can I enable the gifts and talents of my students to be in the driving seat of their learning? Gift. Educ. Int. 2012, 29, 262–273. [Google Scholar] [CrossRef]
- DCSF. The National Strategies: Handbook for Leading Teachers for Gifted and Talented Education; Department for Children, Schools and Families: London, UK, 2008.
- Bradshaw, J.; Ager, R.; Burge, B.; Wheater, R. PISA 2009: Achievement of 15-Year-Olds in England; National Foundation for Educational Research: Slough, UK, 2010; Volume 34. [Google Scholar]
- Wilshaw, M. Unseen Children-HMCI Speech. 2013. Available online: http://www.ofsted.gov.uk/resources/unseen-children-hmci-speech. (accessed on 20 July 2022).
- Cammann, H.; Michel, J. How to avoid misinterpretation of heart rate variability power spectra? Comput. Methods Programs Biomed. 2002, 68, 15–23. [Google Scholar] [CrossRef]
- Verlinde, D.; Beckers, F.; Ramaekers, D.; Aubert, A.E. Wavelet decomposition analysis of heart rate variability in aerobic athletes. Auton. Neurosci. Basic Clin. 2001, 90, 138–141. [Google Scholar] [CrossRef]
- Sandercock, G.R.; Bromley, P.D.; Brodie, D.A. The reliability of short-term measurements of heart rate variability. Int. J. Cardiol. 2005, 103, 238–247. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Takalo, R.; Hytti, H.; Ihalainen, H. Tutorial on univariate autoregressive spectral analysis. J. Clin. Monit. Comput. 2005, 19, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Kanazawa, H.; Aizawa, Y.; Ardell, J.L.; Shivkumar, K. Cardiac Innervation and Sudden Cardiac Death. Circ. Res. 2015, 116, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Berntson, G.G.; Lozano, D.L.; Chen, Y.J. Filter properties of root mean square successive difference (RMSSD) for heart rate. Psychophysiology 2005, 42, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Goedhart, A.D.; van der Sluis, S.; Houtveen, J.H.; Willemsen, G.; de Geus, E.J. Comparison of time and frequency domain measures of RSA in ambulatory recordings. Psychophysiology 2007, 44, 203–215. [Google Scholar] [CrossRef]
- JASP, Version 0.15. Computer software. JASP Team: Amsterdam, The Netherlands, 2021.
- Schumann, A.; Köhler, S.; Brotte, L.; Bär, K.-J. Effect of an 8-week smartphone-guided HRV-biofeedback intervention on autonomic function and impulsivity in healthy controls. Physiol. Meas. 2019, 40, 064001. [Google Scholar] [CrossRef]
- Khoshnoud, S.; Alvarez Igarzábal, F.; Wittmann, M. Peripheral-physiological and neural correlates of the flow experience while playing video games: A comprehensive review. PeerJ 2020, 8, e10520. [Google Scholar] [CrossRef]
- Michailidis, L.; Balaguer-Ballester, E.; He, X. Flow and immersion in video games: The aftermath of a conceptual challenge. Front. Psychol. 2018, 9, 1682. [Google Scholar] [CrossRef]
- Nasso, S.; Vanderhasselt, M.A.; Demeyer, I.; De Raedt, R. Autonomic regulation in response to stress: The influence of anticipatory emotion regulation strategies and trait rumination. Emotion 2019, 19, 443. [Google Scholar] [CrossRef]
- Brinkmann, A.E.; Press, S.A.; Helmert, E.; Hautzinger, M.; Khazan, I.; Vagedes, J. Comparing Effectiveness of HRV-Biofeedback and Mindfulness for Workplace Stress Reduction: A Randomized Controlled Trial. Appl. Psychophysiol. Biofeedback 2020, 45, 307–322. [Google Scholar] [CrossRef]
- van der Zwan, J.E.; de Vente, W.; Huizink, A.C.; Bögels, S.M.; de Bruin, E.I. Physical activity, mindfulness meditation, or heart rate variability biofeedback for stress reduction: A randomized controlled trial. Appl. Psychophysiol. Biofeedback 2015, 40, 257–268. [Google Scholar] [CrossRef]
- Zahn, D.; Adams, J.; Krohn, J.; Wenzel, M.; Mann, C.G.; Comille, L.K.; Jacobi-Scherberning, V.; Kubaik, T. Heart rate variability and self-control—A meta-analysis. Biol. Psychol. 2016, 115, 9–26. [Google Scholar] [CrossRef] [PubMed]
| SDNN | ||||
|---|---|---|---|---|
| Pre | Post | Fw | ω2 | |
| Control (n = 10) | 74.3 ± 23.7 | 77.5 ± 12.9 | 1.48 | 0.012 |
| HRV-BF (n = 9) | 72.3 ± 21 | 111.9 ± 76.8 | ||
| HRV-BF and PST (n = 10) | 66.5 ± 14.9 | 71.3 ± 34.3 | ||
| Sample Totals | 71 ± 19.7 | 86.1 ± 49.3 | ||
| Fb | 1.93 | |||
| w2 | 0.022 | |||
| RMSSD † | ||||
| Pre | Post | W | ES | |
| Control (n = 10) | 68.5 ± 23.3 | 65.5 ± 15.3 | 30 | 0.091 |
| HRV-BF (n = 9) | 72.3 ± 21 | 111.9 ± 76.8 | 10 | 0.164 |
| HRV-BF and PST (n = 10) | 61.4 ± 21.2 | 55.1 ± 25.7 | 33 | 0.200 |
| Sample Totals | 67.2 ± 21.6 | 76.3 ± 50.8 | ||
| Fb | ||||
| w2 | ||||
| HF HRV log | ||||
| Pre | Post | Fw | ω2 | |
| Control (n = 10) | 3 ± 55 | 3 ± 30 | 0.81 | 0.000 |
| HRV-BF (n = 9) | 2.9 ± 29 | 3.2 ± 50 | ||
| HRV-BF and PST (n = 10) | 2.9 ± 42 | 2.9 ± 45 | ||
| Sample Totals | 2.9 ± 43 | 3 ± 42 | ||
| Fb | 0.54 | |||
| w2 | 0.000 | |||
| pNN50 | ||||
| Pre | Post | Fw | ω2 | |
| Control (n = 10) | 39.5 ± 13.1 | 41.2 ± 11.6 | 1.29 | 0.035 |
| HRV-BF (n = 9) | 33 ± 12.9 | 39.8 ± 16.5 | ||
| HRV-BF and PST (n = 10) | 34.2 ± 13.7 | 28.6 ± 12.8 | ||
| Sample Totals | 82.3 ± 11.7 | 82 ± 13.5 | ||
| Fb | 1.81 | |||
| w2 | 0.020 | |||
| SDNN | ||||
|---|---|---|---|---|
| Pre | Post | Fw | ω2 | |
| Control (n = 10) | 74.3 ± 23.7 | 77.5 ± 12.9 | 0.778 | 0.000 |
| HRV-BF (n = 19) | 69.3 ± 17.8 | 90.7 ± 60.4 | ||
| Sample Totals | 71 ± 19.7 | 86.1 ± 49.3 | ||
| Fb | 0.145 | |||
| w2 | 0.000 | |||
| RMSSD | ||||
| Pre | Post | Fw | ω2 | |
| Control (n = 10) | 68.5 ± 23.3 | 65.5 ± 15.3 | 0.780 | 0.000 |
| HRV-BF (n = 19) | 66.6 ± 21 | 82.1 ± 61.7 | ||
| Sample Totals | 67.2 ± 21.6 | 76.3 ± 50.8 | ||
| Fb | 0.420 | |||
| w2 | 0.000 | |||
| HF HRV log | ||||
| Pre | Post | Fw | ω2 | |
| Control (n = 10) | 3 ± 55 | 3 ± 30 | 0.444 | 0.511 |
| HRV-BF (n = 19) | 2.9 ± 36 | 3 ± 48 | ||
| Sample Totals | 2.9 ± 43 | 3 ± 42 | ||
| Fb | 0.446 | |||
| w2 | 0.510 | |||
| pNN50 | ||||
| Pre | Post | Fw | ω2 | |
| Control (n = 10) | 39.5 ± 13.1 | 41.2 ± 11.6 | 0.045 | 0.834 |
| HRV-BF (n = 19) | 33 ± 13.0 | 33.9 ± 15.4 | ||
| Sample Totals | 82.3 ± 11.7 | 82 ± 13.5 | ||
| Fb | 2.49 | |||
| w2 | 0.122 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firth, A.M.; Ask, T.F.; Sütterlin, S.; Lugo, R.G. The Effect of Heart Rate Variability Biofeedback Training on Vagal Tone in Athletically Talented Secondary School Students. Sports 2022, 10, 146. https://doi.org/10.3390/sports10100146
Firth AM, Ask TF, Sütterlin S, Lugo RG. The Effect of Heart Rate Variability Biofeedback Training on Vagal Tone in Athletically Talented Secondary School Students. Sports. 2022; 10(10):146. https://doi.org/10.3390/sports10100146
Chicago/Turabian StyleFirth, Andrea M., Torvald F. Ask, Stefan Sütterlin, and Ricardo G. Lugo. 2022. "The Effect of Heart Rate Variability Biofeedback Training on Vagal Tone in Athletically Talented Secondary School Students" Sports 10, no. 10: 146. https://doi.org/10.3390/sports10100146
APA StyleFirth, A. M., Ask, T. F., Sütterlin, S., & Lugo, R. G. (2022). The Effect of Heart Rate Variability Biofeedback Training on Vagal Tone in Athletically Talented Secondary School Students. Sports, 10(10), 146. https://doi.org/10.3390/sports10100146






