Role of Ionizing Radiation in Shaping the Complex Multi-Layered Epigenome
Abstract
1. Chromatin Organization
2. Epigenetic Processes
2.1. Epigenetic Writers and Erasers in Chromatin Dynamics
2.2. DNA Methylation of CpG-Rich DNA Sequences
2.3. Post-Translational Modifications
2.4. Non-Coding RNA-Associated Gene Silencing
2.5. Replacement of Core Histones with Histone Variants
3. Epigenetic Processes Following Exposure to Ionizing Radiation
3.1. Ionizing Radiation and DNA Damage Induction
3.2. Chromatin Remodelers During Acute DNA Damage Response
3.3. Ubiquitylation, SUMOylation, and PARylation During DNA Damage Response
3.3.1. Ubiquitylation
3.3.2. SUMOylation
3.3.3. PARylation
3.4. Radiation-Induced Changes in DNA Methylation
3.4.1. Global DNA Methylation
3.4.2. Gene-Specific DNA Methylation
3.4.3. DNA Methylation of Repetitive Elements
3.5. Radiation-Induced Histone Modifications
3.5.1. Histone Phosphorylation
3.5.2. Histone Acetylation
3.5.3. Histone Methylation
3.6. Radiation-Induced Modulation of Non-Coding RNA Expression
3.7. Radiation-Induced Incorporation of Histone Variants
4. Radiation-Induced Epigenetic Changes and Premature Senescence
5. Radiation-Induced Epigenetic Changes and Individual Radiosensitivity
6. Susceptibility to Radiation-Induced Epigenetic Changes
7. Potential Challenges and Future Research Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATM | Ataxia Telangiectasia-Mutated |
| ATR | Ataxia Telangiectasia- and Rad3-related |
| BCL2 | B-Cell CLL/Lymphoma 2 |
| BRCA1 | Breast Cancer Type 1 Susceptibility Protein |
| CENPS | Centromere Protein S |
| CHD3 | Chromodomain Helicase DNA-Binding Protein 3 |
| DDR | DNA damage response |
| DNA | DeoxyRibonucleic Acid |
| DNMTs | DNA MethylTransferases |
| DSB | Double-Strand Break |
| EXO1 | Exonuclease 1 |
| GATA5 | GATA-Binding Protein 5 |
| Gy | Gray |
| HATs | Histone AcetylTransferases |
| HMTs | Histone MethylTransferases |
| INO80 | INO80 Complex ATPase Subunit |
| IR | Ionizing Radiation |
| HR | Homologous Recombination |
| KAP1 | KRAB domain-associated protein 1 |
| LINE-1 | Long Interspersed Nucleotide Element 1 |
| LET | linear energy transfer |
| MDC1 | Mediator Of DNA Damage Checkpoint 1 |
| MRN | MRE11-RAD50-NBS1 complex |
| NHEJ | Non-Homologous End-Joining |
| PCNA | Proliferating Cell Nuclear Antigen |
| PTMs | Post-Translational Modifications |
| PI3K | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase |
| AKT | AKT Serine/Threonine Kinase |
| RAD51 | DNA Repair Protein RAD51 Homolog |
| RAD52 | DNA Repair Protein RAD52 Homolog |
| RIF1 | Replication Timing Regulatory Factor 1 |
| RNF2/8/168 | Ring Finger Protein 2/8/168 |
| ROS | Reactive Oxygen Species |
| RPA1 | Replication Protein A1 |
| SAHF | Senescence-Associated Heterochromatin Foci |
| SASP | Senescence-Associated Secretory Phenotype |
| SSB | Single-Strand Break |
| STAT3 | Signal Transducer And Activator Of Transcription 3 |
| Sv | Sievert |
| IRF1 | Interferon Regulatory Factor 1 |
| SENP7 | SUMO Specific Peptidase 7 |
| SWI/SNF | Switch/Sucrose Non-Fermentable |
| TP53BP1 | Tumor Protein P53-Binding Protein 1 |
| WNT16 | Wnt Family Member 16 |
References
- Li, G.; Zhu, P. Structure and organization of chromatin fiber in the nucleus. FEBS Lett. 2015, 589 Pt A, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Cheema, M.S.; Ausio, J. The Structural Determinants behind the Epigenetic Role of Histone Variants. Genes 2015, 6, 685–713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, G.; Reinberg, D. Chromatin higher-order structures and gene regulation. Curr. Opin. Genet. Dev. 2011, 21, 175–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm 2023, 4, e292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.W.; Huang, K.; Yang, C.; Kang, C.S. Non-coding RNAs as regulators in epigenetics (Review). Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Oberdoerffer, P.; Miller, K.M. Histone H2A variants: Diversifying chromatin to ensure genome integrity. Semin. Cell Dev. Biol. 2023, 135, 59–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vergara, X.; Manjón, A.G.; de Haas, M.; Morris, B.; Schep, R.; Leemans, C.; Friskes, A.; Beijersbergen, R.L.; Sanders, M.A.; Medema, R.H.; et al. Widespread chromatin context-dependencies of DNA double-strand break repair proteins. Nat. Commun. 2024, 15, 5334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fabbrizi, M.R.; Warshowsky, K.E.; Zobel, C.L.; Hallahan, D.E.; Sharma, G.G. Molecular and epigenetic regulatory mechanisms of normal stem cell radiosensitivity. Cell Death Discov. 2018, 4, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Price, B.D.; D’Andrea, A.D. Chromatin remodeling at DNA double-strand breaks. Cell 2013, 152, 1344–1354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrod, A.; Lane, K.A.; Downs, J.A. The role of the SWI/SNF chromatin remodelling complex in the response to DNA double strand breaks. DNA Repair 2020, 93, 102919. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, X.; Chen, S.; Fernandes, N.; Price, B.D. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc. Natl. Acad. Sci. USA 2005, 102, 13182–13187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikura, M.; Furuya, K.; Matsuda, S.; Matsuda, R.; Shima, H.; Adachi, J.; Matsuda, T.; Shiraki, T.; Ikura, T. Acetylation of Histone H2AX at Lys 5 by the TIP60 Histone Acetyltransferase Complex Is Essential for the Dynamic Binding of NBS1 to Damaged Chromatin. Mol. Cell. Biol. 2015, 35, 4147–4157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Attikum, H.; Fritsch, O.; Hohn, B.; Gasser, S.M. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell 2004, 119, 777–788. [Google Scholar] [CrossRef] [PubMed]
- van Attikum, H.; Fritsch, O.; Gasser, S.M. Distinct roles for SWR1 and INO80 chromatin remodeling complexes at chromosomal double-strand breaks. EMBO J. 2007, 26, 4113–4125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karl, L.A.; Peritore, M.; Galanti, L.; Pfander, B. DNA Double Strand Break Repair and Its Control by Nucleosome Remodeling. Front Genet. 2021, 12, 821543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, S.P.; Durocher, D. Regulation of DNA damage responses by ubiquitin and SUMO. Mol. Cell 2013, 49, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Goldberg, A.L.; Mitch, W.E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 2006, 17, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Doil, C.; Mailand, N.; Bekker-Jensen, S.; Menard, P.; Larsen, D.H.; Pepperkok, R.; Ellenberg, J.; Panier, S.; Durocher, D.; Bartek, J.; et al. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell 2009, 136, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Messick, T.E.; Greenberg, R.A. The ubiquitin landscape at DNA double-strand breaks. J. Cell Biol. 2009, 187, 319–326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panier, S.; Durocher, D. Regulatory ubiquitylation in response to DNA double-strand breaks. DNA Repair 2009, 8, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Mattiroli, F.; Vissers, J.H.; van Dijk, W.J.; Ikpa, P.; Citterio, E.; Vermeulen, W.; Marteijn, J.A.; Sixma, T.K. RNF168 ubiquitinates K13-15 on H2A/H2AX to drive DNA damage signaling. Cell 2012, 150, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Fradet-Turcotte, A.; Canny, M.D.; Escribano-Díaz, C.; Orthwein, A.; Leung, C.C.Y.; Huang, H.; Landry, M.-C.; Kitevski-LeBlanc, J.; Noordermeer, S.M.; Sicheri, F.; et al. 53BP1 is a reader of the DNA-damage-induced H2A Lys 15 ubiquitin mark. Nature 2013, 499, 50–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinder, J.B.; Attwood, K.M.; Dellaire, G. Reading, writing, and repair: The role of ubiquitin and the ubiquitin-like proteins in DNA damage signaling and repair. Front. Genet. 2013, 4, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Callen, E.; Di Virgilio, M.; Kruhlak, M.J.; Nieto-Soler, M.; Wong, N.; Chen, H.-T.; Faryabi, R.B.; Polato, F.; Santos, M.; Starnes, L.M.; et al. 53BP1 mediates productive and mutagenic DNA repair through distinct phosphoprotein interactions. Cell 2013, 153, 1266–1280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, T.; Cronshaw, J.; Kanu, N.; Snijders, A.P.; Behrens, A. UBR5-mediated ubiquitination of ATMIN is required for ionizing radiation-induced ATM signaling and function. Proc. Natl. Acad. Sci. USA 2014, 111, 12091–12096. [Google Scholar] [CrossRef]
- Thorslund, T.; Ripplinger, A.; Hoffmann, S.; Wild, T.; Uckelmann, M.; Villumsen, B.; Narita, T.; Sixma, T.K.; Choudhary, C.; Bekker-Jensen, S.; et al. Histone H1 couples initiation and amplification of ubiquitin signalling after DNA damage. Nature 2015, 527, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Fouad, S.; Wells, O.S.; Hill, M.A.; D’aNgiolella, V. Cullin Ring Ubiquitin Ligases (CRLs) in Cancer: Responses to Ionizing Radiation (IR) Treatment. Front. Physiol. 2019, 10, 1144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoege, C.; Pfander, B.; Moldovan, G.L.; Pyrowolakis, G.; Jentsch, S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 2002, 419, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.R.; Boutell, C.; Keppler, M.; Densham, R.; Weekes, D.; Alamshah, A.; Butler, L.; Galanty, Y.; Pangon, L.; Kiuchi, T.; et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature 2009, 462, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Bekker-Jensen, S.; Mailand, N. The ubiquitin- and SUMO-dependent signaling response to DNA double-strand breaks. FEBS Lett. 2011, 585, 2914–2919. [Google Scholar] [CrossRef] [PubMed]
- Galanty, Y.; Belotserkovskaya, R.; Coates, J.; Jackson, S.P. RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Genes Dev. 2012, 26, 1179–1195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garvin, A.J.; Densham, R.M.; A Blair-Reid, S.; Pratt, K.M.; Stone, H.R.; Weekes, D.; Lawrence, K.J.; Morris, J.R. The deSUMOylase SENP7 promotes chromatin relaxation for homologous recombination DNA repair. EMBO Rep. 2013, 14, 975–983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chung, I.; Zhao, X. DNA break-induced sumoylation is enabled by collaboration between a SUMO ligase and the ssDNA-binding complex RPA. Genes Dev. 2015, 29, 1593–1598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eifler, K.; Vertegaal, A.C.O. SUMOylation-Mediated Regulation of Cell Cycle Progression and Cancer. Trends Biochem. Sci. 2015, 40, 779–793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bologna, S.; Altmannova, V.; Valtorta, E.; Koenig, C.; Liberali, P.; Gentili, C.; Anrather, D.; Ammerer, G.; Pelkmans, L.; Krejci, L.; et al. Sumoylation regulates EXO1 stability and processing of DNA damage. Cell Cycle 2015, 14, 2439–2450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahel, I.; Ahel, D.; Matsusaka, T.; Clark, A.J.; Pines, J.; Boulton, S.J.; West, S.C. Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature 2008, 451, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Haince, J.-F.; McDonald, D.; Rodrigue, A.; Déry, U.; Masson, J.-Y.; Hendzel, M.J.; Poirier, G.G. PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J. Biol. Chem. 2008, 283, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, A.J.; Timinszky, G.; Kong, S.E.; Jin, J.; Cai, Y.; Swanson, S.K.; Washburn, M.P.; Florens, L.; Ladurner, A.G.; Conaway, J.W.; et al. Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proc. Natl. Acad. Sci. USA 2009, 106, 13770–13774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krietsch, J.; Rouleau, M.; Pic, É.; Ethier, C.; Dawson, T.M.; Dawson, V.L.; Masson, J.-Y.; Poirier, G.G.; Gagné, J.-P. Reprogramming cellular events by poly(ADP-ribose)-binding proteins. Mol. Asp. Med. 2013, 34, 1066–1087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beck, C.; Robert, I.; Reina-San-Martin, B.; Schreiber, V.; Dantzer, F. Poly(ADP-ribose) polymerases in double-strand break repair: Focus on PARP1, PARP2 and PARP3. Exp. Cell Res. 2014, 329, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Baer, R.; Gautier, J. DNA double-strand break repair pathway choice and cancer. DNA Repair 2014, 19, 169–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chapman, J.R.; Taylor, M.R.; Boulton, S.J. Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell 2012, 47, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Panier, S.; Boulton, S.J. Double-strand break repair: 53BP1 comes into focus. Nat. Rev. Mol. Cell Biol. 2014, 15, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, M.; Callen, E.; Yamane, A.; Zhang, W.; Jankovic, M.; Gitlin, A.D.; Feldhahn, N.; Resch, W.; Oliveira, T.Y.; Chait, B.T.; et al. Rif1 prevents resection of DNA breaks and promotes immunoglobulin class switching. Science 2013, 339, 711–715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chapman, J.R.; Barral, P.; Vannier, J.-B.; Borel, V.; Steger, M.; Tomas-Loba, A.; Sartori, A.A.; Adams, I.R.; Batista, F.D.; Boulton, S.J. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol. Cell 2013, 49, 858–871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escribano-Díaz, C.; Orthwein, A.; Fradet-Turcotte, A.; Xing, M.; Young, J.T.; Tkáč, J.; Cook, M.A.; Rosebrock, A.P.; Munro, M.; Canny, M.D.; et al. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol. Cell 2013, 49, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Lottersberger, F.; Buonomo, S.B.; Sfeir, A.; de Lange, T. 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. Science 2013, 339, 700–704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Aroumougame, A.; Lobrich, M.; Li, Y.; Chen, D.; Chen, J.; Gong, Z. PTIP associates with Artemis to dictate DNA repair pathway choice. Genes Dev. 2014, 28, 2693–2698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boersma, V.; Moatti, N.; Segura-Bayona, S.; Peuscher, M.H.; van der Torre, J.; Wevers, B.A.; Orthwein, A.; Durocher, D.; Jacobs, J.J.L. MAD2L2 controls DNA repair at telomeres and DNA breaks by inhibiting 5′ end resection. Nature 2015, 521, 537–540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, G.; Chapman, J.R.; Brandsma, I.; Yuan, J.; Mistrik, M.; Bouwman, P.; Bartkova, J.; Gogola, E.; Warmerdam, D.; Barazas, M.; et al. REV7 counteracts DNA double-strand break resection and affects PARP inhibition. Nature 2015, 521, 541–544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ziv, Y.; Bielopolski, D.; Galanty, Y.; Lukas, C.; Taya, Y.; Schultz, D.C.; Lukas, J.; Bekker-Jensen, S.; Bartek, J.; Shiloh, Y. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat. Cell Biol. 2006, 8, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Teloni, F.; Altmeyer, M. Readers of poly(ADP-ribose): Designed to be fit for purpose. Nucleic Acids Res. 2016, 44, 993–1006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Golia, B.; Singh, H.R.; Timinszky, G. Poly-ADP-ribosylation signaling during DNA damage repair. Front. Biosci. 2015, 20, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Izhar, L.; Adamson, B.; Ciccia, A.; Lewis, J.; Pontano-Vaites, L.; Leng, Y.; Liang, A.C.; Westbrook, T.F.; Harper, J.W.; Elledge, S.J. A Systematic Analysis of Factors Localized to Damaged Chromatin Reveals PARP-Dependent Recruitment of Transcription Factors. Cell Rep. 2015, 11, 1486–1500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalinich, J.F.; Catravas, G.N.; Snyder, S.L. The effect of gamma radiation on DNA methylation. Radiat Res. 1989, 117, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Antwih, D.A.; Gabbara, K.M.; Lancaster, W.D.; Ruden, D.M.; Zielske, S.P. Radiation-induced epigenetic DNA methylation modification of radiation-response pathways. Epigenetics 2013, 8, 839–848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tawa, R.; Kimura, Y.; Komura, J.-I.; Miyamura, Y.; Kurishita, A.; Sasaki, M.S.; Sakurai, H.; Ono, T. Effects of X-ray irradiation on genomic DNA methylation levels in mouse tissues. J. Radiat. Res. 1998, 39, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.; Raiche, J.; Slovack, M.; Kovalchuk, O. Dose-dependence, sex- and tissue-specificity, and persistence of radiation-induced genomic DNA methylation changes. Biochem. Biophys. Res. Commun. 2004, 320, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.; Koturbash, I.; Tryndyak, V.; Hudson, D.; Stevenson, S.M.; Sedelnikova, O.; Bonner, W.; Kovalchuk, O. Fractionated low-dose radiation exposure leads to accumulation of DNA damage and profound alterations in DNA and histone methylation in the murine thymus. Mol. Cancer Res. 2005, 3, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, O.; Burke, P.; Besplug, J.; Slovack, M.; Filkowski, J.; Pogribny, I. Methylation changes in muscle and liver tissues of male and female mice exposed to acute and chronic low-dose X-ray-irradiation. Mutat. Res. 2004, 548, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Koturbash, I.; Pogribny, I.; Kovalchuk, O. Stable loss of global DNA methylation in the radiation-target tissue--a possible mechanism contributing to radiation carcinogenesis? Biochem. Biophys Res. Commun. 2005, 337, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Giotopoulos, G.; McCormick, C.; Cole, C.; Zanker, A.; Jawad, M.; Brown, R.; Plumb, M. DNA methylation during mouse hemopoietic differentiation and radiation-induced leukemia. Exp. Hematol. 2006, 34, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Loree, J.; Koturbash, I.; Kutanzi, K.; Baker, M.; Pogribny, I.; Kovalchuk, O. Radiation-induced molecular changes in rat mammary tissue: Possible implications for radiation-induced carcinogenesis. Int. J. Radiat. Biol. 2006, 82, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Miousse, I.R.; Kutanzi, K.R.; Koturbash, I. Effects of ionizing radiation on DNA methylation: From experimental biology to clinical applications. Int. J. Radiat. Biol. 2017, 93, 457–469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sallam, M.; Mysara, M.; Benotmane, M.A.; Tamarat, R.; Santos, S.C.R.; Crijns, A.P.G.; Spoor, D.; Van Nieuwerburgh, F.; Deforce, D.; Baatout, S.; et al. DNA Methylation Alterations in Fractionally Irradiated Rats and Breast Cancer Patients Receiving Radiotherapy. Int. J. Mol. Sci. 2022, 23, 16214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakata, A.; Sato, K.; Fujishima, Y.; Ting, V.G.S.; Nakayama, K.; Ariyoshi, K.; Tsuruoka, C.; Shang, Y.; Iizuka, D.; Kakinuma, S.; et al. Evaluation of Global DNA Methylation and Gene Expression of Izumo1 and Izumo1r in Gonads after High- and Low-Dose Radiation in Neonatal Mice. Biology 2021, 10, 1270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maierhofer, A.; Flunkert, J.; Dittrich, M.; Müller, T.; Schindler, D.; Nanda, I.; Haaf, T.; Amendola, R. Analysis of global DNA methylation changes in primary human fibroblasts in the early phase following X-ray irradiation. PLoS ONE 2017, 12, e0177442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koturbash, I.; Jadavji, N.M.; Kutanzi, K.; Rodriguez-Juarez, R.; Kogosov, D.; Metz, G.A.; Kovalchuk, O. Fractionated low-dose exposure to ionizing radiation leads to DNA damage, epigenetic dysregulation, and behavioral impairment. Environ. Epigenet. 2016, 2, dvw025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Zhang, Y.; Xu, K.; Mao, X.; Xue, L.; Liu, X.; Yu, H.; Chen, L.; Chu, X.; Castresana, J.S. Genome-wide screen of DNA methylation changes induced by low dose X-ray radiation in mice. PLoS ONE 2014, 9, e90804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyon, C.M.; Klinge, D.M.; Liechty, K.C.; Gentry, F.D.; March, T.H.; Kang, T.; Gilliland, F.D.; Adamova, G.; Rusinova, G.; Telnov, V.; et al. Radiation-induced lung adenocarcinoma is associated with increased frequency of genes inactivated by promoter hypermethylation. Radiat. Res. 2007, 168, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Kontic, M.; Stojsic, J.; Jovanovic, D.; Bunjevacki, V.; Ognjanovic, S.; Kuriger, J.; Puumala, S.; Nelson, H.H. Aberrant promoter methylation of CDH13 and MGMT genes is associated with clinicopathologic characteristics of primary non-small-cell lung carcinoma. Clin. Lung Cancer 2012, 13, 297–303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaudhry, M.A.; Omaruddin, R.A. Differential DNA methylation alterations in radiation-sensitive and -resistant cells. DNA Cell Biol. 2012, 31, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Liu, Y.; Liu, Y.; Zhang, C.; Yuan, B.; Zhang, L.; Sun, S.; Deng, D. Increased p16 DNA methylation in mouse thymic lymphoma induced by irradiation. PLoS ONE 2014, 9, e93850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bae, J.H.; Kim, J.G.; Heo, K.; Yang, K.; Kim, T.O.; Yi, J.M. Identification of radiation-induced aberrant hypomethylation in colon cancer. BMC Genomics. 2015, 16, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koturbash, I.; Boyko, A.; Rodriguez-Juarez, R.; McDonald, R.J.; Tryndyak, V.P.; Kovalchuk, I.; Pogribny, I.P.; Kovalchuk, O. Role of epigenetic effectors in maintenance of the long-term persistent bystander effect in spleen in vivo. Carcinogenesis 2007, 28, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- de Koning, A.P.; Gu, W.; Castoe, T.A.; Batzer, M.A.; Pollock, D.D. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 2011, 7, e1002384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goetz, W.; Morgan, M.N.M.; Baulch, J.E. The effect of radiation quality on genomic DNA methylation profiles in irradiated human cell lines. Radiat. Res. 2011, 175, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Prior, S.; Miousse, I.R.; Nzabarushimana, E.; Pathak, R.; Skinner, C.; Kutanzi, K.R.; Allen, A.R.; Raber, J.; Tackett, A.J.; Hauer-Jensen, M.; et al. Densely ionizing radiation affects DNA methylation of selective LINE-1 elements. Environ. Res. 2016, 150, 470–481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, S.; Jin, Y.; Zhang, W.; Yang, L.; Shen, Y.; Cao, Y.; Tong, J. Aberrant promoter methylation of p16(INK4a) and O(6)-methylguanine-DNA methyltransferase genes in workers at a Chinese uranium mine. J. Occup. Health 2006, 48, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Belinsky, S.A.; Klinge, D.M.; Liechty, K.C.; March, T.H.; Kang, T.; Gilliland, F.D.; Telnov, V. Plutonium targets the p16 gene for inactivation by promoter hypermethylation in human lung adenocarcinoma. Carcinogenesis 2004, 25, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Aypar, U.; Morgan, W.F.; Baulch, J.E. Radiation-induced epigenetic alterations after low and high LET irradiations. Mutat. Res. 2011, 707, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Koturbash, I.; Miousse, I.R.; Sridharan, V.; Nzabarushimana, E.; Skinner, C.M.; Melnyk, S.B.; Pavliv, O.; Hauer-Jensen, M.; Nelson, G.A.; Boerma, M. Radiation-induced changes in DNA methylation of repetitive elements in the mouse heart. Mutat. Res. 2016, 787, 43–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rübe, C.E.; Lorat, Y.; Schuler, N.; Schanz, S.; Wennemuth, G.; Rübe, C. DNA repair in the context of chromatin: New molecular insights by the nanoscale detection of DNA repair complexes using transmission electron microscopy. DNA Repair 2011, 10, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Timm, S.; Lorat, Y.; Jakob, B.; Taucher-Scholz, G.; Rübe, C.E. Clustered DNA damage concentrated in particle trajectories causes persistent large-scale rearrangements in chromatin architecture. Radiother. Oncol. 2018, 129, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Lorat, Y.; Brunner, C.U.; Schanz, S.; Jakob, B.; Taucher-Scholz, G.; Rübe, C.E. Nanoscale analysis of clustered DNA damage after high-LET irradiation by quantitative electron microscopy--the heavy burden to repair. DNA Repair 2015, 28, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Lorat, Y.; Reindl, J.; Isermann, A.; Rübe, C.; Friedl, A.A.; Rübe, C.E. Focused Ion Microbeam Irradiation Induces Clustering of DNA Double-Strand Breaks in Heterochromatin Visualized by Nanoscale-Resolution Electron Microscopy. Int. J. Mol. Sci. 2021, 22, 7638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lorat, Y.; Schanz, S.; Rube, C.E. Ultrastructural Insights into the Biological Significance of Persisting DNA Damage Foci after Low Doses of Ionizing Radiation. Clin. Cancer Res. 2016, 22, 5300–5311. [Google Scholar] [CrossRef] [PubMed]
- Lorat, Y.; Schanz, S.; Schuler, N.; Wennemuth, G.; Rube, C.; Rube, C.E. Beyond repair foci: DNA double-strand break repair in euchromatic and heterochromatic compartments analyzed by transmission electron microscopy. PLoS ONE 2012, 7, e38165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lorat, Y.; Timm, S.; Jakob, B.; Taucher-Scholz, G.; Rübe, C.E. Clustered double-strand breaks in heterochromatin perturb DNA repair after high linear energy transfer irradiation. Radiother. Oncol. 2016, 121, 154–161. [Google Scholar] [CrossRef] [PubMed]
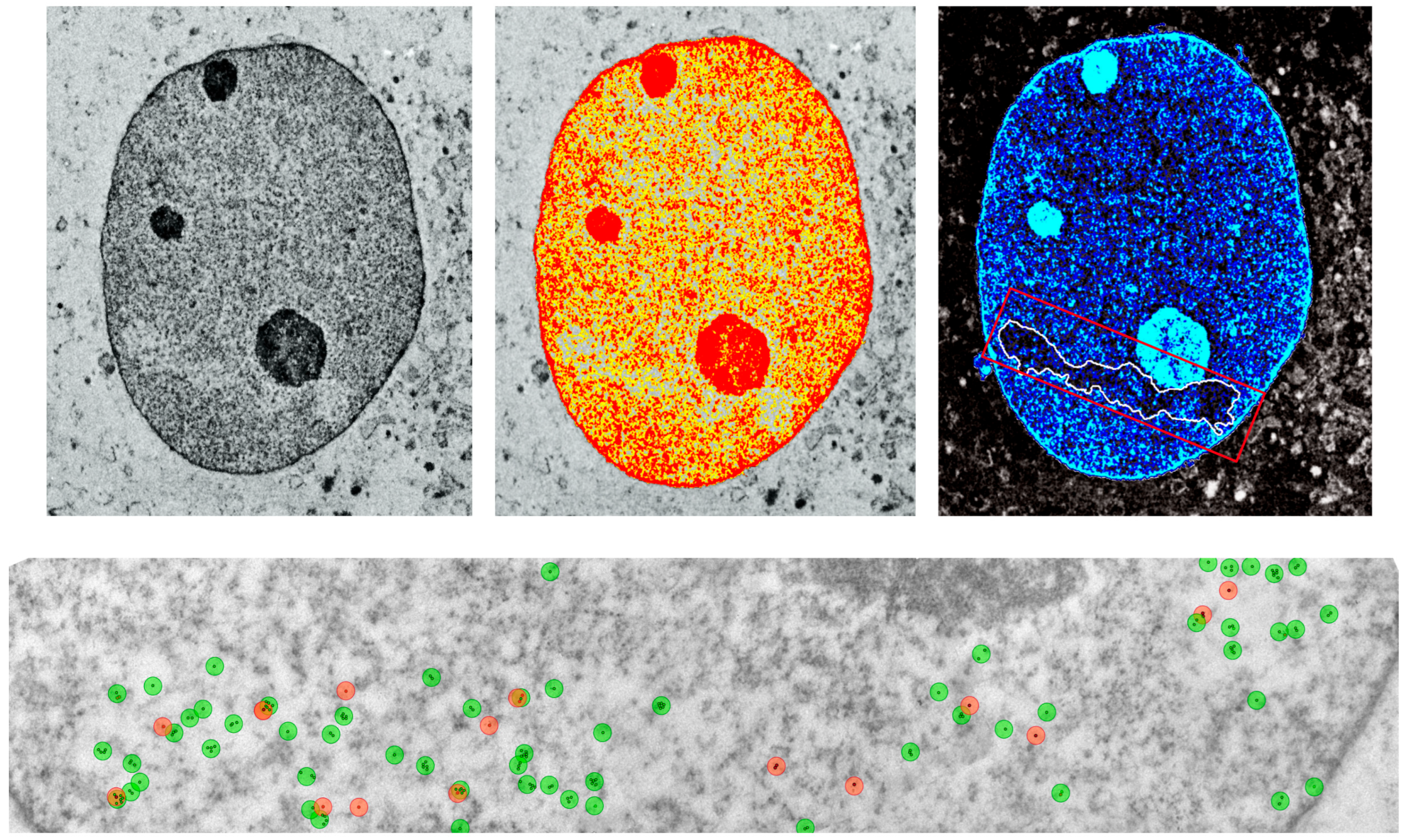
- Al-Razaq, M.A.A.; Isermann, A.; Hecht, M.; Rübe, C.E. Automated Image Analysis of Transmission Electron Micrographs: Nanoscale Evaluation of Radiation-Induced DNA Damage in the Context of Chromatin. Cells 2023, 12, 2427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pilch, D.R.; A Sedelnikova, O.; Redon, C.; Celeste, A.; Nussenzweig, A.; Bonner, W.M. Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem. Cell Biol. 2003, 81, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.A.; Pilch, D.R.; Redon, C.; Bonner, W.M. Histone H2AX in DNA damage and repair. Cancer Biol. Ther. 2003, 2, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Legube, G.; Trouche, D. Regulating histone acetyltransferases and deacetylases. EMBO Rep. 2003, 4, 944–947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shahbazian, M.D.; Grunstein, M. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 2007, 76, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.W.; Yu, D.Y.; Pray-Grant, M.G.; Qiu, Q.; Harmon, K.E.; Megee, P.C.; Grant, P.A.; Smith, M.M.; Christman, M.F. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 2002, 419, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, H.; Ui, A.; Otsuka, A.; Satoh, H.; Yokomi, I.; Nakajima, S.; Yasui, A.; Yokota, J.; Kohno, T. Histone acetylation by CBP and p300 at double-strand break sites facilitates SWI/SNF chromatin remodeling and the recruitment of non-homologous end joining factors. Oncogene 2011, 30, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Groselj, B.; Sharma, N.L.; Hamdy, F.C.; Kerr, M.; Kiltie, A.E. Histone deacetylase inhibitors as radiosensitisers: Effects on DNA damage signalling and repair. Br. J. Cancer 2013, 108, 748–754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Purrucker, J.C.; Fricke, A.; Ong, M.F.; Rube, C.; Rube, C.E.; Mahlknecht, U. HDAC inhibition radiosensitizes human normal tissue cells and reduces DNA Double-Strand Break repair capacity. Oncol. Rep. 2010, 23, 263–269. [Google Scholar] [PubMed]
- Zhang, F.; Zhang, T.; Teng, Z.-H.; Zhang, R.; Wang, J.-B.; Mei, Q.-B. Sensitization to gamma-irradiation-induced cell cycle arrest and apoptosis by the histone deacetylase inhibitor trichostatin A in non-small cell lung cancer (NSCLC) cells. Cancer Biol. Ther. 2009, 8, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Friedl, A.A.; Mazurek, B.; Seiler, D.M. Radiation-induced alterations in histone modification patterns and their potential impact on short-term radiation effects. Front. Oncol. 2012, 2, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gursoy-Yuzugullu, O.; Carman, C.; Serafim, R.B.; Myronakis, M.; Valente, V.; Price, B.D. Epigenetic therapy with inhibitors of histone methylation suppresses DNA damage signaling and increases glioma cell radiosensitivity. Oncotarget 2017, 8, 24518–24532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Shao, C. Histone methylation can either promote or reduce cellular radiosensitivity by regulating DNA repair pathways. Mutat Res Rev Mutat Res. 2021, 787, 108362. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Völker-Albert, M.; Bronkhorst, A.; Holdenrieder, S.; Imhof, A. Histone Modifications in Stem Cell Development and Their Clinical Implications. Stem Cell Rep. 2020, 15, 1196–1205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Metheetrairut, C.; Slack, F.J. MicroRNAs in the ionizing radiation response and in radiotherapy. Curr. Opin. Genet Dev. 2013, 23, 12–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wagner-Ecker, M.; Schwager, C.; Wirkner, U.; Abdollahi, A.; Huber, P.E. MicroRNA expression after ionizing radiation in human endothelial cells. Radiat Oncol. 2010, 5, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedroza-Torres, A.; Romero-Córdoba, S.L.; Montaño, S.; Peralta-Zaragoza, O.; Vélez-Uriza, D.E.; Arriaga-Canon, C.; Guajardo-Barreto, X.; Bautista-Sánchez, D.; Sosa-León, R.; Hernández-González, O.; et al. Radio-miRs: A comprehensive view of radioresistance-related microRNAs. Genetics 2024, 227, iyae097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lacombe, J.; Zenhausern, F. Emergence of miR-34a in radiation therapy. Crit. Rev. Oncol. Hematol. 2017, 109, 69–78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halimi, M.; Shahabi, A.; Moslemi, D.; Parsian, H.; Asghari, S.M.; Sariri, R.; Yeganeh, F.; Zabihi, E. Human serum miR-34a as an indicator of exposure to ionizing radiation. Radiat. Environ. Biophys. 2016, 55, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, X.; Tang, X.; Wang, P.; Wang, H.; Wang, Y. MiR-21 is continually elevated long-term in the brain after exposure to ionizing radiation. Radiat. Res. 2012, 177, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, R.; Saidijam, M.; Nikzad, S.; Tapak, L.; Alvandi, M.; Afshar, S. Human exposure to low dose ionizing radiation affects miR-21 and miR-625 expression levels. Mol. Biol. Rep. 2022, 49, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-P.; He, C.-Y.; Zhu, Z.-T. Role of microRNA-21 in radiosensitivity in non-small cell lung cancer cells by targeting PDCD4 gene. Oncotarget 2017, 8, 23675–23689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.; Liang, X.; Li, X.; Liu, X.; Zhu, M.; Gu, Y.; Zhou, P. MiRNA-21 functions in ionizing radiation-induced epithelium-to-mesenchymal transition (EMT) by downregulating PTEN. Toxicol. Res. 2019, 8, 328–340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gwak, H.-S.; Kim, T.H.; Jo, G.H.; Kim, Y.-J.; Kwak, H.-J.; Kim, J.H.; Yin, J.; Yoo, H.; Lee, S.H.; Park, J.B.; et al. Silencing of microRNA-21 confers radio-sensitivity through inhibition of the PI3K/AKT pathway and enhancing autophagy in malignant glioma cell lines. PLoS ONE 2012, 7, e47449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghafouri-Fard, S.; Abak, A.; Shoorei, H.; Mohaqiq, M.; Majidpoor, J.; Sayad, A.; Taheri, M. Regulatory role of microRNAs on PTEN signaling. Biomed. Pharmacother. 2021, 133, 110986. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, F.A.; Rodrigues, A.R.; Neto, F.S.L.; Peria, F.M.; Cirino, M.L.d.A.; Tirapelli, D.P.d.C.; Júnior, C.G.C. Apoptosis related microRNAs and MGMT in glioblastoma cell lines submitted to treatments with ionizing radiation and temozolomide. Rep. Pract. Oncol. Radiother. 2020, 25, 714–719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gasparini, P.; Lovat, F.; Fassan, M.; Casadei, L.; Cascione, L.; Jacob, N.K.; Carasi, S.; Palmieri, D.; Costinean, S.; Shapiro, C.L.; et al. Protective role of miR-155 in breast cancer through RAD51 targeting impairs homologous recombination after irradiation. Proc. Natl. Acad. Sci. USA 2014, 111, 4536–4541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Scheiber, M.N.; Neumann, C.; Calin, G.A.; Zhou, D. MicroRNA regulation of ionizing radiation-induced premature senescence. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 839–848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, X.; Yang, A.; McDonald, D.G.; Riemer, E.C.; Vanek, K.N.; Schulte, B.A.; Wang, G.Y. MiR-34a modulates ionizing radiation-induced senescence in lung cancer cells. Oncotarget 2017, 8, 69797–69807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.; Zhou, C.; Gao, F.; Cai, S.; Zhang, C.; Zhao, L.; Zhao, F.; Cao, F.; Lin, J.; Yang, Y.; et al. MiR-34a in age and tissue related radio-sensitivity and serum miR-34a as a novel indicator of radiation injury. Int. J. Biol. Sci. 2011, 7, 221–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bönisch, C.; Hake, S.B. Histone H2A variants in nucleosomes and chromatin: More or less stable? Nucleic Acids Res. 2012, 40, 10719–10741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Talbert, P.B.; Henikoff, S. Histone variants at a glance. J. Cell Sci. 2021, 134, jcs244749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paull, T.T.; Rogakou, E.P.; Yamazaki, V.; Kirchgessner, C.U.; Gellert, M.; Bonner, W.M. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000, 10, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Celeste, A.; Petersen, S.; Romanienko, P.J.; Fernandez-Capetillo, O.; Chen, H.T.; Sedelnikova, O.A.; Reina-San-Martin, B.; Coppola, V.; Meffre, E.; Difilippantonio, M.J.; et al. Genomic instability in mice lacking histone H2AX. Science 2002, 296, 922–927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bassing, C.H.; Chua, K.F.; Sekiguchi, J.; Suh, H.; Whitlow, S.R.; Fleming, J.C.; Monroe, B.C.; Ciccone, D.N.; Yan, C.; Vlasakova, K.; et al. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc. Natl. Acad. Sci. USA 2002, 99, 8173–8178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mangelinck, A.; Coudereau, C.; Courbeyrette, R.; Ourarhni, K.; Hamiche, A.; Redon, C.; Mann, C. The H2A.J histone variant contributes to Interferon-Stimulated Gene expression in senescence by its weak interaction with H1 and the derepression of repeated DNA sequences. bioRxiv 2020. bioRxiv: 2020.10.29.361204. [Google Scholar] [CrossRef]
- Isermann, A.; Mann, C.; Rube, C.E. Histone Variant H2A.J Marks Persistent DNA Damage and Triggers the Secretory Phenotype in Radiation-Induced Senescence. Int. J. Mol. Sci. 2020, 21, 9130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Contrepois, K.; Coudereau, C.; Benayoun, B.A.; Schuler, N.; Roux, P.-F.; Bischof, O.; Courbeyrette, R.; Carvalho, C.; Thuret, J.-Y.; Ma, Z.; et al. Histone variant H2A.J accumulates in senescent cells and promotes inflammatory gene expression. Nat. Commun. 2017, 8, 14995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Razaq, M.A.A.; Freyter, B.M.; Isermann, A.; Tewary, G.; Mangelinck, A.; Mann, C.; Rübe, C.E. Role of Histone Variant H2A.J in Fine-Tuning Chromatin Organization for the Establishment of Ionizing Radiation-Induced Senescence. Cells 2023, 12, 916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tewary, G.; Freyter, B.; Al-Razaq, M.A.; Auerbach, H.; Laschke, M.W.; Kübelbeck, T.; Kolb, A.; Mangelinck, A.; Mann, C.; Kramer, D.; et al. Immunomodulatory Effects of Histone Variant H2A.J in Ionizing Radiation Dermatitis. Int. J. Radiat. Oncol. Biol. Phys. 2023, 118, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Freyter, B.M.; Al-Razaq, M.A.A.; Hecht, M.; Rübe, C.; Rübe, C.E. Studies on Human Cultured Fibroblasts and Cutaneous Squamous Cell Carcinomas Suggest That Overexpression of Histone Variant H2A.J Promotes Radioresistance and Oncogenic Transformation. Genes 2024, 15, 851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Y.; Ayrapetov, M.K.; Xu, C.; Gursoy-Yuzugullu, O.; Hu, Y.; Price, B.D. Histone H2A.Z controls a critical chromatin remodeling step required for DNA double-strand break repair. Mol. Cell 2012, 48, 723–733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Z.; Bernstein, E. Histone variant macroH2A: From chromatin deposition to molecular function. Essays Biochem. 2019, 63, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.D.; Hamilton, G.A.; Park, J.W.; Gamble, M.J. MacroH2A1 Regulation of Poly(ADP-Ribose) Synthesis and Stability Prevents Necrosis and Promotes DNA Repair. Mol. Cell. Biol. 2019, 40, e00230-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, A.K.; Bhattacharya, S.; Khan, S.A.; Khade, B.; Gupta, S. Dynamic alteration in H3 serine 10 phosphorylation is G1-phase specific during ionization radiation induced DNA damage response in human cells. Mutat. Res. 2015, 773, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Deaton, A.M.; GómEz-RodrígUez, M.; Mieczkowski, J.; Tolstorukov, M.Y.; Kundu, S.; I Sadreyev, R.; Jansen, L.E.; E Kingston, R. Enhancer regions show high histone H3.3 turnover that changes during differentiation. Elife 2016, 5, e15316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haase, S.; Banerjee, K.; Mujeeb, A.A.; Hartlage, C.S.; Núñez, F.M.; Núñez, F.J.; Alghamri, M.S.; Kadiyala, P.; Carney, S.; Barissi, M.N.; et al. H3.3-G34 mutations impair DNA repair and promote cGAS/STING-mediated immune responses in pediatric high-grade glioma models. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seeber, A.; Gasser, S.M. Chromatin organization and dynamics in double-strand break repair. Curr. Opin Genet Dev. 2017, 43, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chandra, T.; Ewels, P.A.; Schoenfelder, S.; Furlan-Magaril, M.; Wingett, S.W.; Kirschner, K.; Thuret, J.-Y.; Andrews, S.; Fraser, P.; Reik, W. Global reorganization of the nuclear landscape in senescent cells. Cell Rep. 2015, 10, 471–483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Criscione, S.W.; Teo, Y.V.; Neretti, N. The Chromatin Landscape of Cellular Senescence. Trends Genet. 2016, 32, 751–761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duarte, L.F.; Young, A.R.J.; Wang, Z.; Wu, H.-A.; Panda, T.; Kou, Y.; Kapoor, A.; Hasson, D.; Mills, N.R.; Ma’aYan, A.; et al. Histone H3.3 and its proteolytically processed form drive a cellular senescence programme. Nat. Commun. 2014, 5, 5210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sen, P.; Shah, P.P.; Nativio, R.; Berger, S.L. Epigenetic Mechanisms of Longevity and Aging. Cell 2016, 166, 822–839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corpet, A.; Stucki, M. Chromatin maintenance and dynamics in senescence: A spotlight on SAHF formation and the epigenome of senescent cells. Chromosoma 2014, 123, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.; Sadaie, M.; Hoare, M.; Narita, M. Cellular senescence and its effector programs. Genes Dev. 2014, 28, 99–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freyter, B.M.; Al-Razaq, M.A.A.; Isermann, A.; Dietz, A.; Azimzadeh, O.; Hekking, L.; Gomolka, M.; Rübe, C.E. Nuclear Fragility in Radiation-Induced Senescence: Blebs and Tubes Visualized by 3D Electron Microscopy. Cells 2022, 11, 273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dasgupta, N.; Arnold, R.; Equey, A.; Gandhi, A.; Adams, P.D. The role of the dynamic epigenetic landscape in senescence: Orchestrating SASP expression. npj Aging 2024, 10, 48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vogin, G.; Foray, N. The law of Bergonie and Tribondeau: A nice formula for a first approximation. Int. J. Radiat Biol. 2013, 89, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ermolaeva, M.; Neri, F.; Ori, A.; Rudolph, K.L. Cellular and epigenetic drivers of stem cell ageing. Nat. Rev. Mol. Cell Biol. 2018, 19, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Alegria-Torres, J.A.; Baccarelli, A.; Bollati, V. Epigenetics and lifestyle. Epigenomics 2011, 3, 267–277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paksa, A.; Rajagopal, J. The epigenetic basis of cellular plasticity. Curr. Opin. Cell Biol. 2017, 49, 116–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schlesinger, S.; Meshorer, E. Open Chromatin, Epigenetic Plasticity, and Nuclear Organization in Pluripotency. Dev. Cell 2019, 48, 135–150. [Google Scholar] [CrossRef] [PubMed]
| Modification Type | Protein Complex | Key Mediators | Regulatory Function | References |
|---|---|---|---|---|
| Ubiquitylation | RNF8: Ring Finger Protein 8 | H2A, H2A.X: Lys-63 linked polyubiquitination, Lys-48-linked ubiquitination | DDR signaling, recruitment of DNA repair proteins, chromatin remodeling, checkpoint activation, regulator of DDR | Lecker et al., 2006 [21] Doil et al., 2009 [22] Messick & Greenberg 2009 [23] Panier & Durocher 2009 [24] Mattiroli et al., 2012 [25] Jackson & Durocher 2013 [20] Fradet-Turcotte et al., 2013 [26] Pinder et al., 2013 [27] Callen et al., 2013 [28] Zhang et al., 2014 [29] Thorslund et al., 2015 [30] Fouad et al., 2019 [31] |
| RNF168: Ring Finger Protein 168 | H1.2: Ubiquitination (type not specified) | DDR signaling, recruitment of DNA repair proteins (TP53BP1, BRCA1), formation of DNA damage foci, DNA repair | ||
| UBR5 Ubiquitin Protein Ligase E3 Component N-Recognin 5 | ATMIN (ATM Interactor): Ubiquitination at lysine 238 | ATM-MRN signaling, checkpoint activation, regulator of DDR | ||
| RNF2 Ring Finger Protein 2 | H2A.X: Monoubiquitination | Phosphorylated H2AX formation (γH2AX), MDC1/ATM recruitment, specific tag for epigenetic transcriptional repression | ||
| TIP60 Histone acetyltransferase KAT5 | H2A und H4: Acetylation-dependent ubiquitination | Modulation of nucleosome-DNA interactions, histone release, chromatin remodeling, transcriptional activation | ||
| Cullin-RING ligase E3 ubiquitin-protein ligase complex | EXO1, PCNA, CENPs: Polyubiquitination (lysine 6/33) | DDR signaling, recruitment of DNA repair proteins, cell cycle progression, signal transduction and transcription | ||
| SUMOylation | SWI/SNF SWItch/Sucrose Non-Fermentable | ATP-dependent chromatin remodeling by sliding and ejecting nucleosomes | Chromatin relaxation, phosphorylated H2AX (γH2AX), DSB repair, checkpoint maintenance | Hoege et al., 2002 [32] Morris et al., 2009 [33] Bekker-Jensen & Mailand 2011 [34] Galanty et al., 2012 [35] Garvin et al., 2013 [36] Chung & Zhao 2015 [37] Eifler & Vertegaal 2015 [38] Bologna et al., 2015 [39] |
| Brd4 Bromodomain-containing protein 4 | Chromatin reader protein that recognizes/binds acetylated histones | DDR signaling, phosphorylated H2AX (γH2AX), insulating regions from DDR by limiting spreading of H2A.X phosphorylation | ||
| NuRD Nucleosome Remodeling and Deacetylase | Multi-protein complex that combines HDAC with nucleosome remodeling activity, typically containing subunits HDAC1/2, CHD3/4 | Relaxation of heterochromatin via AP-1, chromatin remodeling | ||
| INO80 INO80 Complex ATPase Subunit | Incorporation and removal of alternate histones, e.g. histone variant H2A.Z | Chromatin remodeling, phosphorylated H2AX (γ-H2AX) interactions | ||
| PARylation | PARP1 Poly[ADP-ribose] polymerase 1 | By using NAD+ to synthesize poly ADPribose (PAR) and transferring PAR moieties to proteins, including repair factors, chromatin remodelers | Detection of DNA damage, decompaction of chromatin, interaction with multiple DNA repair factors to regulate DNA repair | Ahel et al., 2008 [40] Haince et al., 2008 [41] Gottschalk et al., 2009 [42] Krietsch et al., 2013 [43] Beck et al., 2014 [44] |
| DNA Methylation Pattern | Target Proteins | Regulatory Function | Biological Relevance | References |
|---|---|---|---|---|
| Global DNA methylation | Genome-wide DNA, germline and somatic cells, various organ tissues | Acute exposure (single or short-term) | Minimal or transient effects (within hours to days) | Kalinich et al., 1989 [59] Tawa et al., 1998 [61] Kovalchuk et al., 2004 [64] Pogribny et al., 2004 [62] Koturbash et al., 2005 [65] Giotopoulos et al., 2006 [66] Loree et al., 2006 [67] Antwih et al., 2013 [60] Wang et al., 2014 [52] Koturbash et al., 2016 [72] Maierhofer et al., 2017 [71] Miousse et al., 2017 [68] Sallam et al., 2022 [69] Nakata et al., 2021 [70] |
| Chronic and fractionated exposure (repeated or prolonged over time) | Mixed or time-dependent methylation effects: early hypomethylation followed by normalization or hypermethylation; differences based on dose-rate or fractionation | |||
| Gene-specific DNA methylation | Promoter regions of specific genes | Modifies transcriptional activity, leading to gene activation or silencing | Impact on cellular responses to IR and carcinogenesis | Lyon et al., 2007 [74] Kontic et al., 2012 [75] Chaudhry& Omaruddin 2012 [76] Antwih et al., 2013 [60] Song et al., 2014 [77] Bae et al., 2015 [78] |
| DNA repair (Rad23b, Ddit3) & cell cycle (p16INK4a, MGMT, GATA5) | DNA damage response | Genomic instability, Cancer risk | ||
| Endothelial function (PGRMC1, UNC119B, RERE, FNDC3B) | Cardiovascular regulation | Cardiovascular disease | ||
| Immune response (IL5RA, H2AFY, CTSA, LTC4S, RB1) | Immune signaling | Predictive of radiotherapy response | ||
| Tumor suppressors/oncogenes (RB1, JAK2, BCAM) | Genomic stability | Cancer progression, radioresistance | ||
| DNA methylation of repetitive elements | Repetitive elements (LINE-1, Alu elements) | Changes can lead to reactivation and retrotransposition | Genomic instability, cancer | Koturbash et al., 2007 [79] de Koning et al., 2011 [80] Goetz et al., 2011 [81] Prior et al., 2016 [82] Miousse et al., 2017 [68] |
| Modification Type | Target Histones | Regulatory Function | Biological Relevance | References |
|---|---|---|---|---|
| Histone Phosphorylation | Histone variant H2A.X (serine 139, γ-H2A.X) | Facilitates DNA damage sensing, recruits repair proteins, alters chromatin structure | Crucial for DSB repair, genome stability, formation of repair foci | Rogakou et al., 1998 [6] Pilch et al., 2003 [95] Sedelnikova et al., 2003 [96] |
| Histone Acetylation | Histones (lysine residues on N-terminal tails) | Neutralizes positive charge, chromatin condensation, influences repair pathway choice | Facilitates chromatin opening during DNA repair, impacts radiosensitivity | Bird et al., 2002 [99] Legube & Trouche 2003 [97] Zhang et al., 2009 [103] Purrucker et al., 2010 [102] Groselj et al., 2013 [101] |
| Histone Methylation | Histone H3 (K4, K9, K27, K36, K79) Histone H4 (K20) | Modulates chromatin accessibility, gene activation/repression, influences DNA repair & cell cycle | Affects DDR, chromatin structure, radiosensitivity, stem cell differentiation | Kouzarides, 2007 [104] Friedl et al., 2012 [105] Gursoy-Yuzugullu et al., 2017 [106] Zhou & Shao 2021 [107] |
| Modification | Target Proteins | Regulatory Function | Biological Relevance | References |
|---|---|---|---|---|
| miR-34a | c-Myc | Rapidly upregulated post-IR; modulates genes involved in DDR, including c-Myc | Predicts normal tissue toxicity and tumor radioresistance | He et al., 2017 [111] Lacombe & Zenhausern 2017 [113] Halimi et al., 2016 [114] |
| miR-21 | Tumor suppressor PDCD4, PTEN, RECK | Acts as oncomiR; negatively regulates tumor suppressor pathways | Contributes to increased cell survival, radioresistance, affects apoptosis and DNA repair | Shi et al., 2012 [115] Mahmoudi et al., 2022 [116] Jiang et al., 2017 [117] Liu et al., 2019 [118] Gwak et al., 2012 [119] Ghafouri-Fard et al., 2021 [120] |
| miR-16 | BCL2 | Targets anti-apoptotic BCL2; enhances radiotherapy effectiveness by sensitizing cells to apoptosis | Improves radiosensitivity by promoting apoptosis | Trevisan et al., 2020 [121] |
| miR-155 | RAD51 | Modulates DDR; influences HR pathway depending on context | Fine-tunes DNA repair, impacts radiation sensitivity or resistance | Gasparini et al., 2014 [122] Wang et al., 2011 [123] |
| Histone Variant | Modification by IR | Regulatory Function | Biological Relevance | References |
|---|---|---|---|---|
| H2A.X | Phosphorylation at S139 (γ-H2A.X); ubiquitination, acetylation | Phosphorylation destabilizes nucleosomes, recruits repair proteins, activates DDR | Early DSB marker; essential for efficient DSB repair; loss increases radiosensitivity and impairs genome stability | Rogakou et al., 1998 [6] Celeste et al., 2002 [130] Ikura et al., 2015 [16] Bassing et al., 2002 [131] |
| H2A.J | Phosphorylation at SQ motif; incorporation increases following IR exposure | Modulates chromatin accessibility, promotes inflammatory gene expression | radiation-induced senescence, inflammation, immune responses; overexpression can promote radioresistance and oncogenic transformation | Mangelinck et al., 2020 [132] Contrepois et al., 2017 [134] Isermann et al., 2020 [133] Freyter et al., 2024 [137] |
| H2A.Z | Rapid exchange at DSB sites | Promotes chromatin destabilization, increases accessibility for repair factors | Facilitates HR and NHEJ; crucial for efficient DNA repair | Xu et al., 2012 [138] |
| MacroH2A | Involved in chromatin compaction; regulation of PARP1 | Reduces chromatin accessibility and promotes gene repression | Modulates chromatin dynamics; involved in DNA repair and cell survival | Sun & Bernstein, 2019 [139] Ruiz et al., 2019 [140] |
| H3.3 | Incorporation during chromatin remodeling; increased at active regions | Associated with active transcription; facilitates chromatin accessibility | Impacts DNA repair efficiency; mutations impair repair and activate immune responses | Deaton et al., 2016 [142] Haase et al., 2022 [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rübe, C.E.; Al-razaq, M.A.A.; Meier, C.; Hecht, M.; Rübe, C. Role of Ionizing Radiation in Shaping the Complex Multi-Layered Epigenome. Epigenomes 2025, 9, 29. https://doi.org/10.3390/epigenomes9030029
Rübe CE, Al-razaq MAA, Meier C, Hecht M, Rübe C. Role of Ionizing Radiation in Shaping the Complex Multi-Layered Epigenome. Epigenomes. 2025; 9(3):29. https://doi.org/10.3390/epigenomes9030029
Chicago/Turabian StyleRübe, Claudia E., Mutaz A. Abd Al-razaq, Carola Meier, Markus Hecht, and Christian Rübe. 2025. "Role of Ionizing Radiation in Shaping the Complex Multi-Layered Epigenome" Epigenomes 9, no. 3: 29. https://doi.org/10.3390/epigenomes9030029
APA StyleRübe, C. E., Al-razaq, M. A. A., Meier, C., Hecht, M., & Rübe, C. (2025). Role of Ionizing Radiation in Shaping the Complex Multi-Layered Epigenome. Epigenomes, 9(3), 29. https://doi.org/10.3390/epigenomes9030029







