Epigenetic Mechanisms in Neurofibromatosis Types 1 and 2
Abstract
1. Introduction
2. The Epigenetic Landscape of NF1
2.1. An Overview of NF1
2.2. The Role of Epigenetic Mechanisms in NF1 Pathogenesis
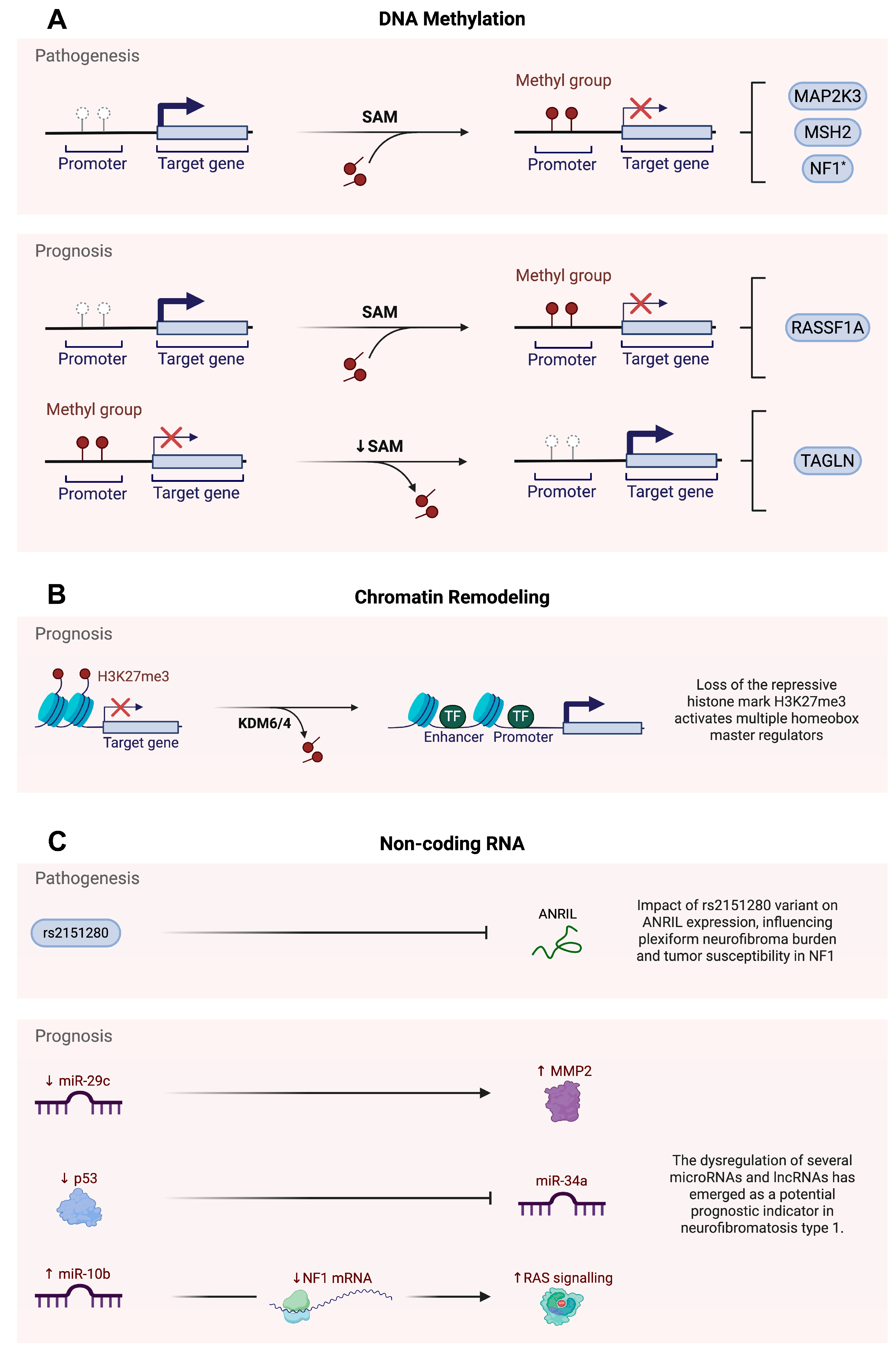
2.2.1. DNA Methylation
2.2.2. Non-Coding RNAs
2.3. The Prognostic Impact of Epigenetic Alterations in NF1
2.3.1. DNA Methylation
2.3.2. Histone Modifications
2.3.3. Non-Coding RNAs
3. The Epigenetic Architecture of NF2
3.1. An Overview of NF2
3.2. Epigenetic Drivers of NF2 Pathogenesis
3.2.1. DNA Methylation
3.2.2. Non-Coding RNAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANRIL | Antisense non-coding RNA in the INK4 locus |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A |
| CNF | Cutaneous neurofibroma |
| CpG | Cytosine–phosphate–guanine |
| CRE | cAMP response element |
| DNA | Deoxyribonucleic acid |
| EED | Embryonic ectoderm development |
| ERK | Extracellular signal-regulated kinase |
| H3K27me3 | Trimethylation of lysine 27 on histone H3 |
| HGAP | High-grade astrocytomas with piloid features |
| HOX | Homeobox |
| KDM4 | Lysine demethylase 4 |
| KDM6 | Lysine demethylase 6 |
| LncRNA | Long non-coding RNA |
| MAP2K3 | Mitogen-activated protein kinase 3 |
| MEK | Mitogen-activated protein kinase |
| MET | MET proto-oncogene, receptor tyrosine kinase |
| miRNA | MicroRNA |
| MKK3 | Mitogen-activated protein kinase 3 |
| MLH1 | MutL homolog 1 |
| MMP2 | Matrix metalloproteinase 2 |
| MPNST | Malignant peripheral nerve sheath tumour |
| mRNA | Messenger ribonucleic acid |
| MSH2 | MutS homolog 2 |
| MSH6 | MutS homolog 6 |
| MSP | Methylation-specific PCR |
| ncRNA | Non-coding RNA |
| NF-CAR | Neurofibromatosis consensus area of regulation |
| NF1 | Neurofibromatosis type 1 |
| NF1HCS | NF1 5′ highly conserved sequence |
| NF2 | Neurofibromatosis type 2 |
| NF2-SWN | NF2-related schwannomatosis |
| p53 | Tumour suppressor p53 |
| PCR | Polymerase chain reaction |
| PMEPA1 | Prostate transmembrane protein, androgen induced 1 |
| PMS2 | Postmeiotic segregation increased 2 |
| PNF | Plexiform neurofibroma |
| PRC2 | Polycomb repressive complex 2 |
| RAS | Rat sarcoma virus oncogene |
| RASSF1A | Ras association domain family member 1 isoform A |
| RNA | Ribonucleic acid |
| rs2151280 | Reference SNP ID 2151280 |
| SAM | S-adenosylmethionine |
| SK-ES-1 | Human Ewing sarcoma cell line |
| SNP | Single-nucleotide polymorphism |
| SP1 | Specificity protein 1 |
| SUZ12 | Suppressor of zeste 12 |
| TALGN | Transgelin |
| TF | Transcription factor |
| U-251 MG cells | Uppsala 251 Malignant Glioma cells |
| UTR | Untranslated region |
| VS | Vestibular schwannoma |
References
- Gürsoy, S.; Erçal, D. Genetic Evaluation of Common Neurocutaneous Syndromes. Pediatr. Neurol. 2018, 89, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Klar, N.; Cohen, B.; Lin, D.D.M. Neurocutaneous Syndromes. Handb. Clin. Neurol. 2016, 135, 565–589. [Google Scholar] [CrossRef]
- Hadjigavriel, G.; Stylianides, C.; Axarloglou, E.; Manthou, M.E.; Vakirlis, E.; Theotokis, P.; Meditskou, S.; Dermitzakis, I. Epigenetic Insights into Tuberous Sclerosis Complex, Von Hippel–Lindau Syndrome, and Ataxia–Telangiectasia. Epigenomes 2025, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Friedman, J.M. NF1 Gene and Neurofibromatosis 1. Am. J. Epidemiol. 2000, 151, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, M.; Polizzi, A.; Marceca, G.P.; Catanzaro, S.; Praticò, A.D.; Di Rocco, C. Introduction to Phacomatoses (Neurocutaneous Disorders) in Childhood. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2020, 36, 2229–2268. [Google Scholar] [CrossRef]
- Ruggieri, M.; Huson, S.M. The Clinical and Diagnostic Implications of Mosaicism in the Neurofibromatoses. Neurology 2001, 56, 1433–1443. [Google Scholar] [CrossRef]
- Kioutchoukova, I.; Foster, D.; Thakkar, R.; Ciesla, C.; Cabassa, J.S.; Strouse, J.; Kurz, H.; Lucke-Wold, B. Neurocutaneous Diseases: Diagnosis, Management, and Treatment. J. Clin. Med. 2024, 13, 1648. [Google Scholar] [CrossRef]
- De Ribaupierre, S.; Vernet, O.; Vinchon, M.; Rilliet, B. Phacomatosis and genetically determined tumors: The transition from childhood to adulthood. Neurochirurgie 2008, 54, 642–653. [Google Scholar] [CrossRef]
- Pérez-Grau, M.; Miró, N.; Prades, J.; Vergés, J.; Lareo, S.; Roca-Ribas, F. Neurofibromatosis type 2. Acta Otorrinolaringol. Esp. 2010, 61, 306–311. [Google Scholar] [CrossRef]
- Coy, S.; Rashid, R.; Stemmer-Rachamimov, A.; Santagata, S. An Update on the CNS Manifestations of Neurofibromatosis Type 2. Acta Neuropathol. 2020, 139, 643–665. [Google Scholar] [CrossRef]
- Moodley, M.; Lopez, K.R. Neurofibromatosis Type 1—An Update. Semin. Pediatr. Neurol. 2024, 52, 101172. [Google Scholar] [CrossRef]
- Anderson, J.L.; Gutmann, D.H. Chapter 4—Neurofibromatosis Type 1. In Handbook of Clinical Neurology; Islam, M.P., Roach, E.S., Eds.; Neurocutaneous Syndromes; Elsevier: Amsterdam, The Netherlands, 2015; Volume 132, pp. 75–86. [Google Scholar]
- Xiao, G.-H.; Chernoff, J.; Testa, J.R. NF2: The Wizardry of Merlin. Genes. Chromosomes Cancer 2003, 38, 389–399. [Google Scholar] [CrossRef]
- Jett, K.; Friedman, J.M. Clinical and Genetic Aspects of Neurofibromatosis 1. Genet. Med. 2010, 12, 1–11. [Google Scholar] [CrossRef]
- Sabbagh, A.; Pasmant, E.; Laurendeau, I.; Parfait, B.; Barbarot, S.; Guillot, B.; Combemale, P.; Ferkal, S.; Vidaud, M.; Aubourg, P.; et al. Unravelling the Genetic Basis of Variable Clinical Expression in Neurofibromatosis 1. Hum. Mol. Genet. 2009, 18, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, Y.; Miyawaki, S.; Nakatomi, H.; Ohara, K.; Hongo, H.; Dofuku, S.; Okano, A.; Takayanagi, S.; Ota, T.; Yoshimura, J.; et al. Early Prediction of Functional Prognosis in Neurofibromatosis Type 2 Patients Based on Genotype–Phenotype Correlation with Targeted Deep Sequencing. Sci. Rep. 2022, 12, 9543. [Google Scholar] [CrossRef]
- Peduto, C.; Zanobio, M.; Nigro, V.; Perrotta, S.; Piluso, G.; Santoro, C. Neurofibromatosis Type 1: Pediatric Aspects and Review of Genotype–Phenotype Correlations. Cancers 2023, 15, 1217. [Google Scholar] [CrossRef] [PubMed]
- Brown, R. Management of Central and Peripheral Nervous System Tumors in Patients with Neurofibromatosis. Curr. Oncol. Rep. 2023, 25, 1409–1417. [Google Scholar] [CrossRef]
- Lalvani, S.; Brown, R.M. Neurofibromatosis Type 1: Optimizing Management with a Multidisciplinary Approach. J. Multidiscip. Healthc. 2024, 17, 1803–1817. [Google Scholar] [CrossRef]
- Dermitzakis, I.; Theotokis, P.; Evangelidis, P.; Delilampou, E.; Evangelidis, N.; Chatzisavvidou, A.; Avramidou, E.; Manthou, M.E. CNS Border-Associated Macrophages: Ontogeny and Potential Implication in Disease. Curr. Issues Mol. Biol. 2023, 45, 4285–4300. [Google Scholar] [CrossRef]
- Dermitzakis, I.; Manthou, M.E.; Meditskou, S.; Miliaras, D.; Kesidou, E.; Boziki, M.; Petratos, S.; Grigoriadis, N.; Theotokis, P. Developmental Cues and Molecular Drivers in Myelinogenesis: Revisiting Early Life to Re-Evaluate the Integrity of CNS Myelin. Curr. Issues Mol. Biol. 2022, 44, 3208–3237. [Google Scholar] [CrossRef]
- Dermitzakis, I.; Manthou, M.E.; Meditskou, S.; Tremblay, M.-È.; Petratos, S.; Zoupi, L.; Boziki, M.; Kesidou, E.; Simeonidou, C.; Theotokis, P. Origin and Emergence of Microglia in the CNS-An Interesting (Hi)Story of an Eccentric Cell. Curr. Issues Mol. Biol. 2023, 45, 2609–2628. [Google Scholar] [CrossRef]
- Dermitzakis, I.; Kampitsi, D.D.; Manthou, M.E.; Evangelidis, P.; Vakirlis, E.; Meditskou, S.; Theotokis, P. Ontogeny of Skin Stem Cells and Molecular Underpinnings. Curr. Issues Mol. Biol. 2024, 46, 8118–8147. [Google Scholar] [CrossRef]
- Dermitzakis, I.; Chatzi, D.; Kyriakoudi, S.A.; Evangelidis, N.; Vakirlis, E.; Meditskou, S.; Theotokis, P.; Manthou, M.E. Skin Development and Disease: A Molecular Perspective. Curr. Issues Mol. Biol. 2024, 46, 8239–8267. [Google Scholar] [CrossRef] [PubMed]
- Dermitzakis, I.; Kyriakoudi, S.A.; Chatzianagnosti, S.; Chatzi, D.; Vakirlis, E.; Meditskou, S.; Manthou, M.E.; Theotokis, P. Epigenetics in Skin Homeostasis and Ageing. Epigenomes 2025, 9, 3. [Google Scholar] [CrossRef]
- Röhrich, M.; Koelsche, C.; Schrimpf, D.; Capper, D.; Sahm, F.; Kratz, A.; Reuss, J.; Hovestadt, V.; Jones, D.T.W.; Bewerunge-Hudler, M.; et al. Methylation-Based Classification of Benign and Malignant Peripheral Nerve Sheath Tumors. Acta Neuropathol. 2016, 131, 877–887. [Google Scholar] [CrossRef]
- Chen, W.-J.; Zhang, X.; Han, H.; Lv, J.-N.; Kang, E.-M.; Zhang, Y.-L.; Liu, W.-P.; He, X.-S.; Wang, J.; Wang, G.-H.; et al. The Different Role of YKL-40 in Glioblastoma Is a Function of MGMT Promoter Methylation Status. Cell Death Dis. 2020, 11, 668. [Google Scholar] [CrossRef]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.-Y.; Jones, D.T.W.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Quang, D.-A.K.; Tönjes, M.; et al. Driver Mutations in Histone H3.3 and Chromatin Remodelling Genes in Paediatric Glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef]
- Gorini, F.; Miceli, M.; de Antonellis, P.; Amente, S.; Zollo, M.; Ferrucci, V. Epigenetics and Immune Cells in Medulloblastoma. Front. Genet. 2023, 14, 1135404. [Google Scholar] [CrossRef] [PubMed]
- Cimino, P.J.; Ketchum, C.; Turakulov, R.; Singh, O.; Abdullaev, Z.; Giannini, C.; Pytel, P.; Lopez, G.Y.; Colman, H.; Nasrallah, M.P.; et al. Expanded Analysis of High-Grade Astrocytoma with Piloid Features Identifies an Epigenetically and Clinically Distinct Subtype Associated with Neurofibromatosis Type 1. Acta Neuropathol. 2023, 145, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, S.A.; Lind, G.E.; Kolberg, M.; Høland, M.; Bjerkehagen, B.; Sundby Hall, K.; van den Berg, E.; Mertens, F.; Smeland, S.; Picci, P.; et al. Methylated RASSF1A in Malignant Peripheral Nerve Sheath Tumors Identifies Neurofibromatosis Type 1 Patients with Inferior Prognosis. Neuro-Oncology 2015, 17, 63–69. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Ferner, R.E.; Listernick, R.H.; Korf, B.R.; Wolters, P.L.; Johnson, K.J. Neurofibromatosis Type 1. Nat. Rev. Dis. Primer 2017, 3, 17004. [Google Scholar] [CrossRef]
- Yap, Y.-S.; McPherson, J.R.; Ong, C.-K.; Rozen, S.G.; Teh, B.-T.; Lee, A.S.G.; Callen, D.F. The NF1 Gene Revisited—From Bench to Bedside. Oncotarget 2014, 5, 5873–5892. [Google Scholar] [CrossRef]
- Hernández-Martín, A.; Duat-Rodríguez, A. An Update on Neurofibromatosis Type 1: Not Just Café-Au-Lait Spots and Freckling. Part II. Other Skin Manifestations Characteristic of NF1. NF1 and Cancer. Actas Dermosifiliogr. 2016, 107, 465–473. [Google Scholar] [CrossRef]
- Miraglia, E.; Moliterni, E.; Iacovino, C.; Roberti, V.; Laghi, A.; Moramarco, A.; Giustini, S. Cutaneous Manifestations in Neurofibromatosis Type 1. Clin. Ter. 2020, 171, e371–e377. [Google Scholar] [CrossRef]
- Cassina, M.; Frizziero, L.; Opocher, E.; Parrozzani, R.; Sorrentino, U.; Viscardi, E.; Miglionico, G.; Midena, E.; Clementi, M.; Trevisson, E. Optic Pathway Glioma in Type 1 Neurofibromatosis: Review of Its Pathogenesis, Diagnostic Assessment, and Treatment Recommendations. Cancers 2019, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Summers, M.A.; Quinlan, K.G.; Payne, J.M.; Little, D.G.; North, K.N.; Schindeler, A. Skeletal Muscle and Motor Deficits in Neurofibromatosis Type 1. J. Musculoskelet. Neuronal Interact. 2015, 15, 161–170. [Google Scholar] [PubMed]
- Chinoy, A.; Vassallo, G.R.; Burkitt Wright, E.; Eelloo, J.; West, S.; Hupton, E.; Galloway, P.; Pilkington, A.; Padidela, R.; Mughal, M.Z. The Skeletal Muscle Phenotype of Children with Neurofibromatosis Type 1—A Clinical Perspective. J. Musculoskelet. Neuronal Interact. 2022, 22, 70–78. [Google Scholar] [PubMed]
- Sánchez Marco, S.B.; López Pisón, J.; Calvo Escribano, C.; González Viejo, I.; Miramar Gallart, M.D.; Samper Villagrasa, P. Neurological Manifestations of Neurofibromatosis Type 1: Our Experience. Neurologia 2022, 37, 325–333. [Google Scholar] [CrossRef]
- Swarup, M.S.; Gupta, S.; Singh, S.; Prakash, A.; Mehndiratta, A.; Garg, A. Phakomatoses: A Pictorial Review. Indian J. Radiol. Imaging 2020, 30, 195–205. [Google Scholar] [CrossRef]
- Belakhoua, S.M.; Rodriguez, F.J. Diagnostic Pathology of Tumors of Peripheral Nerve. Neurosurgery 2021, 88, 443–456. [Google Scholar] [CrossRef]
- Jouhilahti, E.-M.; Peltonen, S.; Heape, A.M.; Peltonen, J. The Pathoetiology of Neurofibromatosis 1. Am. J. Pathol. 2011, 178, 1932–1939. [Google Scholar] [CrossRef]
- Titze, S.; Peters, H.; Währisch, S.; Harder, T.; Guse, K.; Buske, A.; Tinschert, S.; Harder, A. Differential MSH2 Promoter Methylation in Blood Cells of Neurofibromatosis Type 1 (NF1) Patients. Eur. J. Hum. Genet. EJHG 2010, 18, 81–87. [Google Scholar] [CrossRef][Green Version]
- Grit, J.L.; Johnson, B.K.; Dischinger, P.S.; Essenburg, C.J.; Adams, M.; Campbell, S.; Pollard, K.; Pratilas, C.A.; Triche, T.J.; Graveel, C.R.; et al. Distinctive Epigenomic Alterations in NF1-Deficient Cutaneous and Plexiform Neurofibromas Drive Differential MKK/P38 Signaling. Epigenet. Chromatin 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Harder, A.; Titze, S.; Herbst, L.; Harder, T.; Guse, K.; Tinschert, S.; Kaufmann, D.; Rosenbaum, T.; Mautner, V.F.; Windt, E.; et al. Monozygotic Twins with Neurofibromatosis Type 1 (NF1) Display Differences in Methylation of NF1 Gene Promoter Elements, 5’ Untranslated Region, Exon and Intron 1. Twin Res. Hum. Genet. Off. J. Int. Soc. Twin Stud. 2010, 13, 582–594. [Google Scholar] [CrossRef]
- Harder, A.; Rosche, M.; Reuss, D.E.; Holtkamp, N.; Uhlmann, K.; Friedrich, R.; Mautner, V.F.; von Deimling, A. Methylation Analysis of the Neurofibromatosis Type 1 (NF1) Promoter in Peripheral Nerve Sheath Tumours. Eur. J. Cancer Oxf. Engl. 1990 2004, 40, 2820–2828. [Google Scholar] [CrossRef]
- Luijten, M.; Redeker, S.; van Noesel, M.M.; Troost, D.; Westerveld, A.; Hulsebos, T.J. Microsatellite Instability and Promoter Methylation as Possible Causes of NF1 Gene Inactivation in Neurofibromas. Eur. J. Hum. Genet. EJHG 2000, 8, 939–945. [Google Scholar] [CrossRef]
- Fishbein, L.; Eady, B.; Sanek, N.; Muir, D.; Wallace, M.R. Analysis of Somatic NF1 Promoter Methylation in Plexiform Neurofibromas and Schwann Cells. Cancer Genet. Cytogenet. 2005, 157, 181–186. [Google Scholar] [CrossRef]
- Horan, M.P.; Cooper, D.N.; Upadhyaya, M. Hypermethylation of the Neurofibromatosis Type 1 (NF1) Gene Promoter Is Not a Common Event in the Inactivation of the NF1 Gene in NF1-Specific Tumours. Hum. Genet. 2000, 107, 33–39. [Google Scholar] [CrossRef]
- Pasmant, E.; Sabbagh, A.; Masliah-Planchon, J.; Ortonne, N.; Laurendeau, I.; Melin, L.; Ferkal, S.; Hernandez, L.; Leroy, K.; Valeyrie-Allanore, L.; et al. Role of Noncoding RNA ANRIL in Genesis of Plexiform Neurofibromas in Neurofibromatosis Type 1. J. Natl. Cancer Inst. 2011, 103, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Mußotter, T.; Kluwe, L.; Högel, J.; Nguyen, R.; Cooper, D.N.; Mautner, V.-F.; Kehrer-Sawatzki, H. Non-Coding RNA ANRIL and the Number of Plexiform Neurofibromas in Patients with NF1 Microdeletions. BMC Med. Genet. 2012, 13, 98. [Google Scholar] [CrossRef]
- Tomczak, K.; Patel, M.S.; Bhalla, A.D.; Peterson, C.B.; Landers, S.M.; Callahan, S.C.; Zhang, D.; Wong, J.; Landry, J.P.; Lazar, A.J.; et al. Plasma DNA Methylation-Based Biomarkers for MPNST Detection in Patients with Neurofibromatosis Type 1. Mol. Carcinog. 2025, 64, 44–56. [Google Scholar] [CrossRef]
- Hirbe, A.C.; Dahiya, S.; Miller, C.A.; Li, T.; Fulton, R.S.; Zhang, X.; McDonald, S.; DeSchryver, K.; Duncavage, E.J.; Walrath, J.; et al. Whole Exome Sequencing Reveals the Order of Genetic Changes during Malignant Transformation and Metastasis in a Single Patient with NF1-Plexiform Neurofibroma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 4201–4211. [Google Scholar] [CrossRef]
- Henriques, C.M.; Ferreira, M.G. Telomere Length Is an Epigenetic Trait—Implications for the Use of Telomerase-Deficient Organisms to Model Human Disease. Dis. Model. Mech. 2024, 17, dmm050581. [Google Scholar] [CrossRef]
- Dogan, F.; Forsyth, N.R. Telomerase Regulation: A Role for Epigenetics. Cancers 2021, 13, 1213. [Google Scholar] [CrossRef] [PubMed]
- Park, G.-H.; Lee, S.-J.; Yim, H.; Han, J.-H.; Kim, H.J.; Sohn, Y.-B.; Ko, J.M.; Jeong, S.-Y. TAGLN Expression Is Upregulated in NF1-Associated Malignant Peripheral Nerve Sheath Tumors by Hypomethylation in Its Promoter and Subpromoter Regions. Oncol. Rep. 2014, 32, 1347–1354. [Google Scholar] [CrossRef][Green Version]
- Lee, W.; Teckie, S.; Wiesner, T.; Ran, L.; Prieto Granada, C.N.; Lin, M.; Zhu, S.; Cao, Z.; Liang, Y.; Sboner, A.; et al. PRC2 Is Recurrently Inactivated through EED or SUZ12 Loss in Malignant Peripheral Nerve Sheath Tumors. Nat. Genet. 2014, 46, 1227–1232. [Google Scholar] [CrossRef]
- Cleven, A.H.G.; Sannaa, G.A.A.; Briaire-de Bruijn, I.; Ingram, D.R.; van de Rijn, M.; Rubin, B.P.; de Vries, M.W.; Watson, K.L.; Torres, K.E.; Wang, W.-L.; et al. Loss of H3K27 Tri-Methylation Is a Diagnostic Marker for Malignant Peripheral Nerve Sheath Tumors and an Indicator for an Inferior Survival. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2016, 29, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, I.-M.; Fletcher, C.D.; Hornick, J.L. Loss of H3K27 Trimethylation Distinguishes Malignant Peripheral Nerve Sheath Tumors from Histologic Mimics. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2016, 29, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Torres, K.; Liu, J.; Hernandez, B.; Young, E.; Belushov, R.; Bolshakov, S.; Lazar, A.J.; Slopis, J.M.; McCutcheon, I.E.; et al. Autophagic Survival in Resistance to Histone Deacetylase Inhibitors: Novel Strategies to Treat Malignant Peripheral Nerve Sheath Tumors. Cancer Res. 2011, 71, 185–196. [Google Scholar] [CrossRef]
- De Raedt, T.; Beert, E.; Pasmant, E.; Luscan, A.; Brems, H.; Ortonne, N.; Helin, K.; Hornick, J.L.; Mautner, V.; Kehrer-Sawatzki, H.; et al. PRC2 Loss Amplifies Ras-Driven Transcription and Confers Sensitivity to BRD4-Based Therapies. Nature 2014, 514, 247–251. [Google Scholar] [CrossRef]
- Subramanian, S.; Thayanithy, V.; West, R.B.; Lee, C.-H.; Beck, A.H.; Zhu, S.; Downs-Kelly, E.; Montgomery, K.; Goldblum, J.R.; Hogendoorn, P.C.W.; et al. Genome-Wide Transcriptome Analyses Reveal P53 Inactivation Mediated Loss of miR-34a Expression in Malignant Peripheral Nerve Sheath Tumours. J. Pathol. 2010, 220, 58–70. [Google Scholar] [CrossRef]
- Chai, G.; Liu, N.; Ma, J.; Li, H.; Oblinger, J.L.; Prahalad, A.K.; Gong, M.; Chang, L.-S.; Wallace, M.; Muir, D.; et al. MicroRNA-10b Regulates Tumorigenesis in Neurofibromatosis Type 1. Cancer Sci. 2010, 101, 1997–2004. [Google Scholar] [CrossRef]
- Presneau, N.; Eskandarpour, M.; Shemais, T.; Henderson, S.; Halai, D.; Tirabosco, R.; Flanagan, A.M. MicroRNA Profiling of Peripheral Nerve Sheath Tumours Identifies miR-29c as a Tumour Suppressor Gene Involved in Tumour Progression. Br. J. Cancer 2013, 108, 964–972. [Google Scholar] [CrossRef]
- Brown, E.D.; Nassar, S.; Jagger, D.J. NF2-Related Schwannomatosis: A View from within the Inner Ear. Hear. Res. 2025, 460, 109226. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R. Current Understanding of Neurofibromatosis Type 1, 2, and Schwannomatosis. Int. J. Mol. Sci. 2021, 22, 5850. [Google Scholar] [CrossRef] [PubMed]
- Ghalavand, M.A.; Asghari, A.; Farhadi, M.; Taghizadeh-Hesary, F.; Garshasbi, M.; Falah, M. The Genetic Landscape and Possible Therapeutics of Neurofibromatosis Type 2. Cancer Cell Int. 2023, 23, 99. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Kwon, T.J.; Kim, U.-K.; Lee, W.-S. Genetic and Epigenetic Alterations of the NF2 Gene in Sporadic Vestibular Schwannomas. PLoS ONE 2012, 7, e30418. [Google Scholar] [CrossRef]
- Bachir, S.; Shah, S.; Shapiro, S.; Koehler, A.; Mahammedi, A.; Samy, R.N.; Zuccarello, M.; Schorry, E.; Sengupta, S. Neurofibromatosis Type 2 (NF2) and the Implications for Vestibular Schwannoma and Meningioma Pathogenesis. Int. J. Mol. Sci. 2021, 22, 690. [Google Scholar] [CrossRef]
- Ruggieri, M.; Praticò, A.D.; Evans, D.G. Diagnosis, Management, and New Therapeutic Options in Childhood Neurofibromatosis Type 2 and Related Forms. Semin. Pediatr. Neurol. 2015, 22, 240–258. [Google Scholar] [CrossRef]
- Ardern-Holmes, S.; Fisher, G.; North, K. Neurofibromatosis Type 2. J. Child Neurol. 2017, 32, 9–22. [Google Scholar] [CrossRef]
- Wiemels, J.; Wrensch, M.; Claus, E.B. Epidemiology and Etiology of Meningioma. J. Neurooncol. 2010, 99, 307–314. [Google Scholar] [CrossRef]
- Evans, D.G. NF2-Related Schwannomatosis. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Tamura, R.; Yo, M.; Toda, M. Historical Development of Diagnostic Criteria for NF2-Related Schwannomatosis. Neurol. Med. Chir. 2024, 64, 299–308. [Google Scholar] [CrossRef]
- Evans, D.G.R. Neurofibromatosis Type 2 (NF2): A Clinical and Molecular Review. Orphanet J. Rare Dis. 2009, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gomez, P.; Bello, M.J.; Alonso, M.E.; Lomas, J.; Arjona, D.; Campos, J.M.d.; Vaquero, J.; Isla, A.; Lassaletta, L.; Gutierrez, M.; et al. CpG Island Methylation in Sporadic and Neurofibromatis Type 2-Associated Schwannomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 5601–5606. [Google Scholar]
- Kino, T.; Takeshima, H.; Nakao, M.; Nishi, T.; Yamamoto, K.; Kimura, T.; Saito, Y.; Kochi, M.; Kuratsu, J.; Saya, H.; et al. Identification of the Cis-Acting Region in the NF2 Gene Promoter as a Potential Target for Mutation and Methylation-Dependent Silencing in Schwannoma. Genes Cells Devoted Mol. Cell. Mech. 2001, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martin, M.; Lassaletta, L.; de Campos, J.M.; Isla, A.; Gavilan, J.; Pinto, G.R.; Burbano, R.R.; Latif, F.; Melendez, B.; Castresana, J.S.; et al. Global Profiling in Vestibular Schwannomas Shows Critical Deregulation of microRNAs and Upregulation in Those Included in Chromosomal Region 14q32. PLoS ONE 2013, 8, e65868. [Google Scholar] [CrossRef]
- Torres-Martín, M.; Lassaletta, L.; de Campos, J.M.; Isla, A.; Pinto, G.R.; Burbano, R.R.; Melendez, B.; Castresana, J.S.; Rey, J.A. Genome-Wide Methylation Analysis in Vestibular Schwannomas Shows Putative Mechanisms of Gene Expression Modulation and Global Hypomethylation at the HOX Gene Cluster. Genes Chromosomes Cancer 2015, 54, 197–209. [Google Scholar] [CrossRef]
| Epigenetic Mechanism | Key Mediator | Impact | Effect of Modification | Ref. |
|---|---|---|---|---|
| DNA Methylation | NF2 promoter methylation | Yes | Leads to NF2 gene silencing, which may contribute to tumourigenesis | [76] |
| DNA Methylation | Methylation of three CpG sites within NF-CAR, a cis-acting regulatory region in the promoter of the NF2 gene | Yes | Inactivation of NF2, which leads to the development of schwannomas and other tumours in NF2 patients | [77] |
| DNA Methylation | Hypomethylation at HOX genes in several CpG sites; MiRNA-21, MET, and PMEPA1 showed promoter hypomethylation | Yes | Enhances oncogenic miRNA and gene expression; leads to pathogenesis of the vestibular schwannomas | [78] |
| DNA Methylation | No aberrant hypermethylation of the NF2 gene promoter region was found | No | Alternative mechanisms play a more critical role in the pathogenesis of vestibular schwannomas | [68] |
| MicroRNAs | MiR-10b, miR-206, miR-183, and miR-204 showed downregulation; miR-431, miR-221, miR-21, and miR-720 showed upregulation; non-coding RNAs in the 14q32 chromosomal region showed upregulation | Yes | Contributes to the development of schwannomas | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stylianides, C.; Hadjigavriel, G.; Theotokis, P.; Vakirlis, E.; Meditskou, S.; Manthou, M.E.; Dermitzakis, I. Epigenetic Mechanisms in Neurofibromatosis Types 1 and 2. Epigenomes 2025, 9, 30. https://doi.org/10.3390/epigenomes9030030
Stylianides C, Hadjigavriel G, Theotokis P, Vakirlis E, Meditskou S, Manthou ME, Dermitzakis I. Epigenetic Mechanisms in Neurofibromatosis Types 1 and 2. Epigenomes. 2025; 9(3):30. https://doi.org/10.3390/epigenomes9030030
Chicago/Turabian StyleStylianides, Christina, Gavriel Hadjigavriel, Paschalis Theotokis, Efstratios Vakirlis, Soultana Meditskou, Maria Eleni Manthou, and Iasonas Dermitzakis. 2025. "Epigenetic Mechanisms in Neurofibromatosis Types 1 and 2" Epigenomes 9, no. 3: 30. https://doi.org/10.3390/epigenomes9030030
APA StyleStylianides, C., Hadjigavriel, G., Theotokis, P., Vakirlis, E., Meditskou, S., Manthou, M. E., & Dermitzakis, I. (2025). Epigenetic Mechanisms in Neurofibromatosis Types 1 and 2. Epigenomes, 9(3), 30. https://doi.org/10.3390/epigenomes9030030









