The Past, Present, and Future of the Hemlock Woolly Adelgid (Adelges tsugae) and Its Ecological Interactions with Eastern Hemlock (Tsuga canadensis) Forests
Abstract
:1. Introduction
2. The Hemlock—Hemlock Woolly Adelgid System
2.1. The Hemlock Woolly Adelgid
2.2. Effects of the Adelgid on Vegetation Structure and Composition
2.3. Effects of the Adelgid and Hemlock Decline on Associated Fauna
2.4. Ecosystem-Level Changes
3. Adelgid Spread Models
3.1. Understanding and Predicting the Spread of the Adelgid
3.2. Forecasting the Ecosystem Consequences of Hemlock Loss
4. New Forecasts
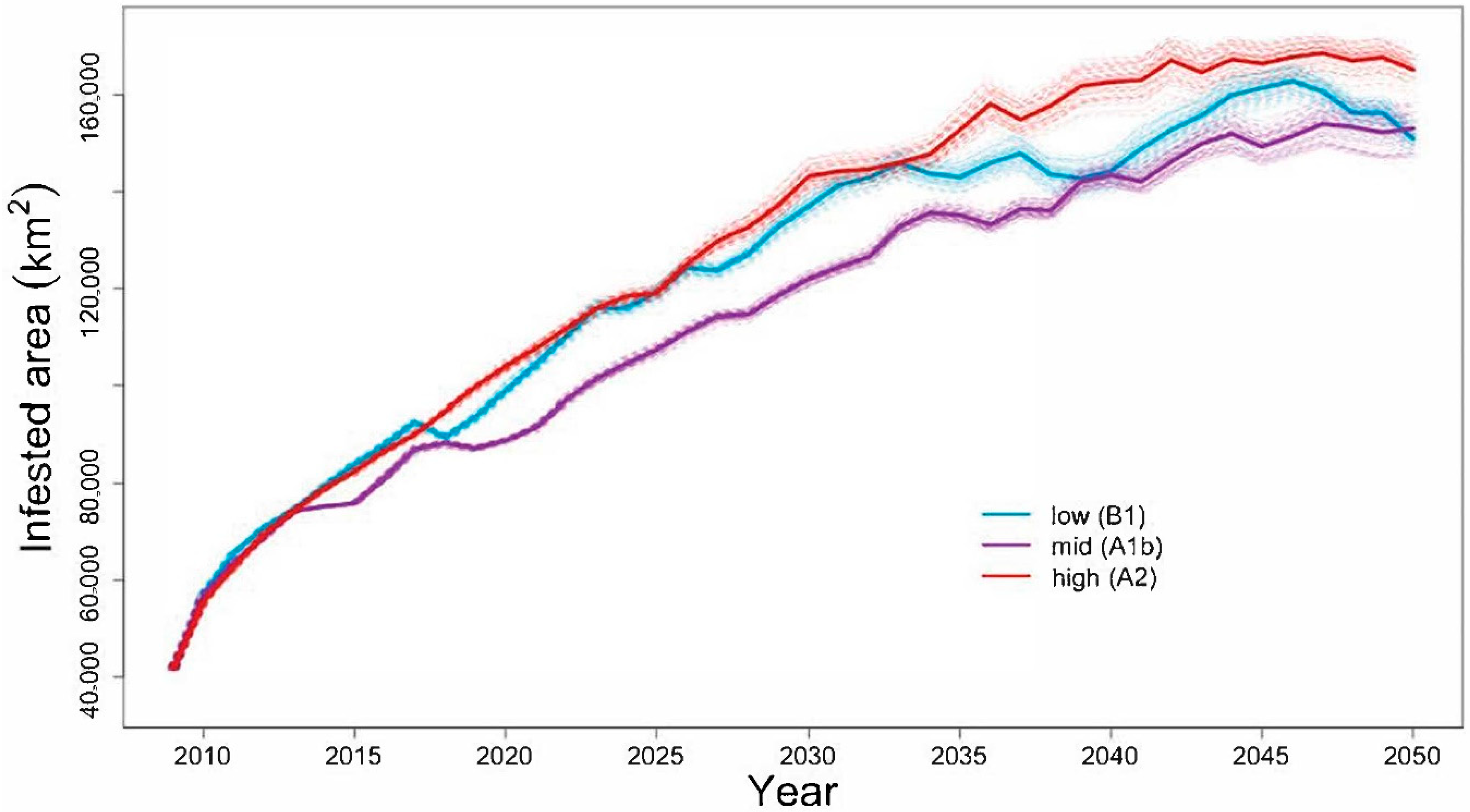
4.1. Will the Adelgid Continue to Spread?
Model Evaluation
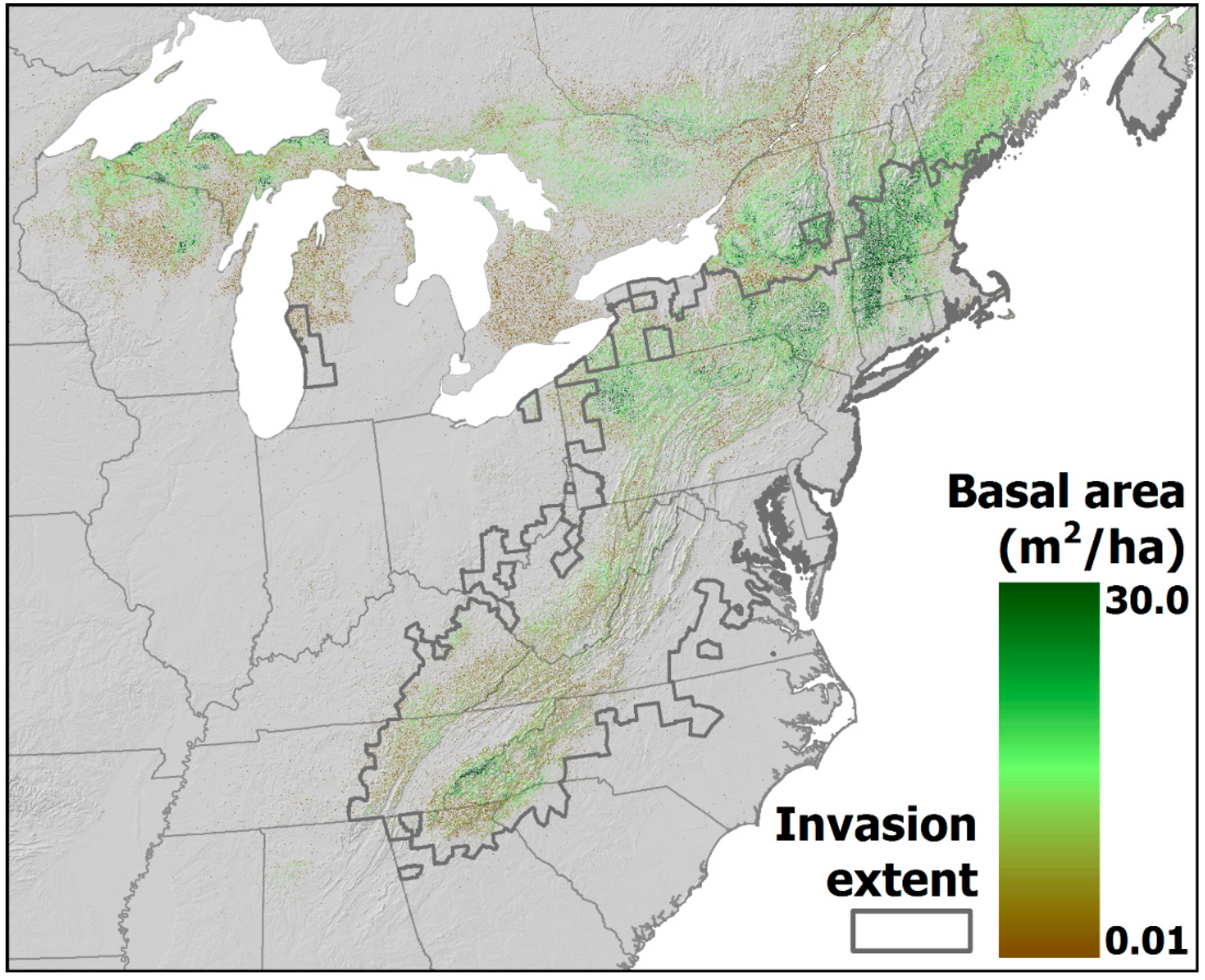
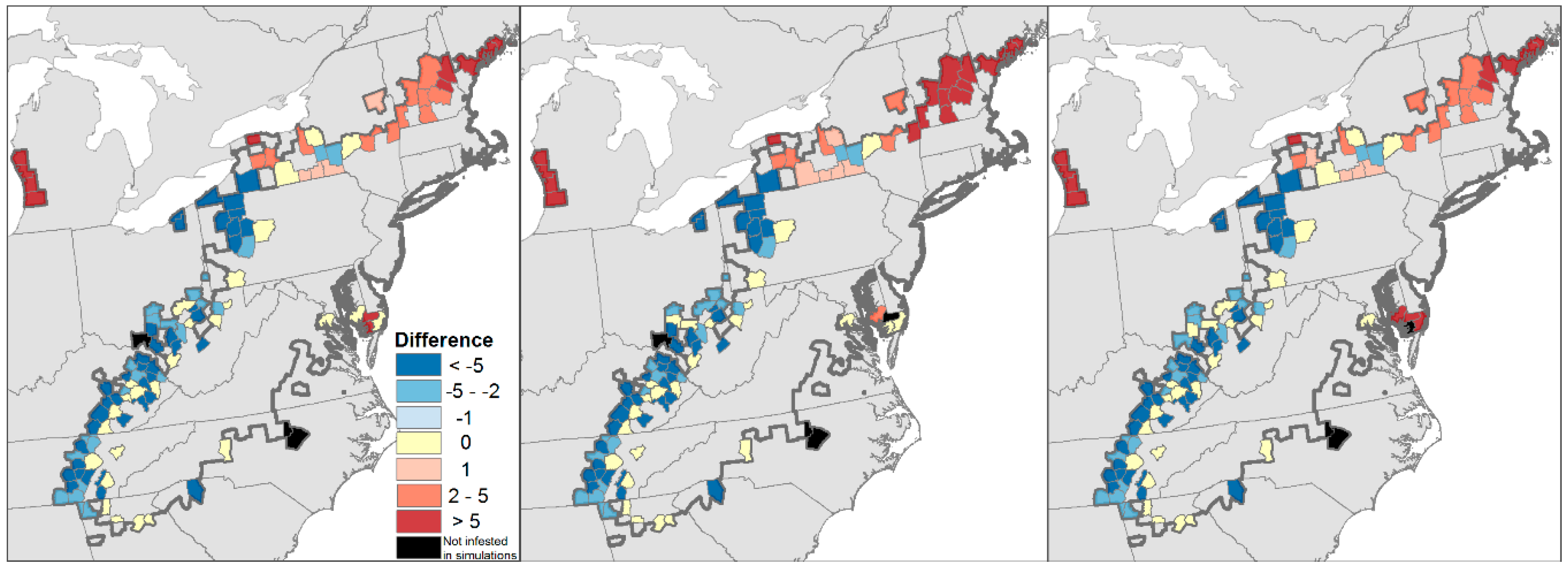
4.2. Projected Losses of Hemlock from Eastern Forests
4.3. Projected Changes in Ecosystem Dynamics in Eastern Forests
4.4. Interactions between the Adelgid and Other Species
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dayton, P.K. Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. In Proceedings of the Colloquium on Conservation Problems in Antarctica, Lawrence, KS, USA; 1972; pp. 81–96. [Google Scholar]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Tingley, M.W.; Orwig, D.A.; Field, R.; Motzkin, G. Avian response to removal of a forest dominant: Consequences of hemlock woolly adelgid infestations. J. Biogeogr. 2002, 29, 1505–1516. [Google Scholar] [CrossRef]
- Ellison, A.M.; Chen, J.; Díaz, D.; Kammerer-Burnham, C.; Lau, M. Changes in ant community structure and composition associated with hemlock decline in New England. In Proceedings of the 3rd Symposium on Hemlock Woolly Adelgid in the Eastern United States; USDA Forest Service: Morgantown, WV, USA, 2005. [Google Scholar]
- Dilling, C.; Lambdin, P.; Grant, J.; Buck, L. Insect guild structure associated with eastern hemlock in the southern Appalachians. Environ. Entomol. 2007, 36, 1408–1414. [Google Scholar] [CrossRef]
- Mathewson, B. The Relative Abundance of Eastern Red-Backed Salamanders in Eastern Hemlock-dominated and Mixed Deciduous Forests at Harvard Forest. Northeast. Nat. 2009, 16, 1–12. [Google Scholar] [CrossRef]
- Rohr, J.R.; Mahan, C.G.; Kim, K.C. Response of arthropod biodiversity to foundation species declines: The case of the eastern hemlock. For. Ecol. Manag. 2009, 258, 1503–1510. [Google Scholar] [CrossRef]
- Mallis, R.E.; Rieske, L.K. Arboreal spiders in eastern hemlock. Environ. Entomol. 2011, 40, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Sackett, T.E.; Record, S.; Bewick, S.; Baiser, B.; Sanders, N.J.; Ellison, A.M. Response of macroarthropod assemblages to the loss of hemlock (Tsuga canadensis), a foundation species. Ecosphere 2011, 2, art74. [Google Scholar] [CrossRef]
- Snyder, C.D.; Young, J.A.; Lemarié, D.P.; Smith, D.R. Influence of eastern hemlock (Tsuga canadensis) forests on aquatic invertebrate assemblages in headwater streams. Can. J. Fish. Aquatic Sci. 2002, 59, 262–275. [Google Scholar] [CrossRef]
- Siderhurst, L.A.; Griscom, H.P.; Hudy, M.; Bortolot, Z.J. Changes in light levels and stream temperatures with loss of eastern hemlock (Tsuga canadensis) at a southern Appalachian stream: Implications for brook trout. For. Ecol. Manag. 2010, 260, 1677–1688. [Google Scholar] [CrossRef]
- Yamasaki, M.; DeGraaf, R.M.; Lanier, J.W. Wildlife habitat associations in eastern hemlock-birds, smaller mammals, and forest carnivores. In Proceedings of the Symposium on Sustainable Management of Hemlock Ecosystems in Eastern North America; Gen. Tech. Rep. NE-267; McManus Katherine, A., Shields Kathleen, S., Souto Dennis, R., Eds.; US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Newtown Square, PA, USA, 2000; pp. 135–143. [Google Scholar]
- Lustenhouwer, M.N.; Nicoll, L.; Ellison, A.M. Microclimatic effects of the loss of a foundation species from New England forests. Ecosphere 2012, 3, art26. [Google Scholar] [CrossRef]
- Hadley, J.L.; Kuzeja, P.S.; Daley, M.J.; Phillips, N.G.; Mulcahy, T.; Singh, S. Water use and carbon exchange of red oak-and eastern hemlock-dominated forests in the northeastern USA: Implications for ecosystem-level effects of hemlock woolly adelgid. Tree Physiol. 2008, 28, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Cobb, R.C.; Orwig, D.A.; Currie, S. Decomposition of green foliage in eastern hemlock forests of southern New England impacted by hemlock woolly adelgid infestations. Can. J. For. Res. 2006, 36, 1331–1341. [Google Scholar] [CrossRef]
- Nuckolls, A.E.; Wurzburger, N.; Ford, C.R.; Hendrick, R.L.; Vose, J.M.; Kloeppel, B.D. Hemlock Declines Rapidly with Hemlock Woolly Adelgid Infestation: Impacts on the Carbon Cycle of Southern Appalachian Forests. Ecosystems 2009, 12, 179–190. [Google Scholar] [CrossRef]
- Ford, C.R.; Elliott, K.J.; Clinton, B.D.; Kloeppel, B.D.; Vose, J.M. Forest dynamics following eastern hemlock mortality in the southern Appalachians. Oikos 2012, 121, 523–536. [Google Scholar] [CrossRef]
- Jenkins, J.C.; Aber, J.D.; Canham, C.D. Hemlock woolly adelgid impacts on community structure and N cycling rates in eastern hemlock forests. Can. J. For. Res. 1999, 29, 630–645. [Google Scholar] [CrossRef]
- Stadler, B.; Müller, T.; Orwig, D.; Cobb, R. Hemlock Woolly Adelgid in New England Forests: Canopy Impacts Transforming Ecosystem Processes and Landscapes. Ecosystems 2005, 8, 233–247. [Google Scholar] [CrossRef]
- Orwig, D.A.; Cobb, R.C.; D’Amato, A.W.; Kizlinski, M.L.; Foster, D.R. Multi-year ecosystem response to hemlock woolly adelgid infestation in southern New England forests. Can. J. For. Res. 2008, 38, 834–843. [Google Scholar] [CrossRef]
- Knoepp, J.D.; Vose, J.M.; Clinton, B.D.; Hunter, M.D. Hemlock infestation and mortality: Impacts on nutrient pools and cycling in Appalachian forests. Soil Sci. Soc. Am. J. 2011, 75, 1935–1945. [Google Scholar] [CrossRef]
- Orwig, D.A.; Barker Plotkin, A.A.; Davidson, E.A.; Lux, H.; Savage, K.E.; Ellison, A.M. Foundation species loss affects vegetation structure more than ecosystem function in a northeastern USA forest. PeerJ 2013, 1, e41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendrick, J.A.; Ribbons, R.R.; Classen, A.T.; Ellison, A.M. Changes in canopy structure and ant assemblages affect soil ecosystem variables as a foundation species declines. Ecosphere 2015, 6, 1–20. [Google Scholar] [CrossRef]
- Foster, D.R.; Zebryk, T.M. Long-term vegetation dynamics and disturbance history of a Tsuga-dominated forest in New England. Ecology 1993, 74, 982–998. [Google Scholar] [CrossRef]
- Paciorek, C.J.; McLachlan, J.S. Mapping ancient forests: Bayesian inference for spatio-temporal trends in forest composition using the fossil pollen proxy record. J. Am. Stat. Assoc. 2009, 104, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.B. Outbreaks of Forest Pathogens in Quaternary History. In Proceedings of the Fourth International Conference on Palynology, Lucknow, India; 1981. [Google Scholar]
- Foster, D.R.; Oswald, W.W.; Faison, E.K.; Doughty, E.D.; Hansen, B.C. A climatic driver for abrupt mid-Holocene vegetation dynamics and the hemlock decline in New England. Ecology 2006, 87, 2959–2966. [Google Scholar] [CrossRef]
- Marsicek, J.P.; Shuman, B.; Brewer, S.; Foster, D.R.; Oswald, W.W. Moisture and temperature changes associated with the mid-Holocene Tsuga decline in the northeastern United States. Quat. Sci. Rev. 2013, 80, 129–142. [Google Scholar] [CrossRef]
- Oswald, W.W.; Doughty, E.D.; Foster, D.R.; Shuman, B.N.; Wagner, D.L. Evaluating the role of insects in the middle-Holocene Tsuga decline. J. Torrey Bot. Soc. 2017, 144, 35–39. [Google Scholar] [CrossRef]
- Smith, W.B.; Miles, P.D.; Perry, C.H.; Pugh, S.A. Forest resources of the United States, 2007: A technical document supporting the forest service 2010 RPA Assessment. Gen. Tech. Rep.-USDA For. Serv. 2009, WO-78. [Google Scholar] [CrossRef]
- Rogers, R.S. Forests dominated by hemlock (Tsuga canadensis): Distribution as related to site and postsettlement history. Can. J. Bot. 1978, 56, 843–854. [Google Scholar] [CrossRef]
- USNVC (United States National Vegetation Classification). United States National Vegetation Classificatoin Database, V2.01. Federal Geographic Data Committee, Vegetation Subcommittee, Washington, DC, USA [usnvc.org], 2017. Available online: http://usnvc.org/ (accessed on 23 November 2018).
- Fitzpatrick, M.C.; Preisser, E.L.; Porter, A.; Elkinton, J.; Ellison, A.M. Modeling range dynamics in heterogeneous landscapes: Invasion of the hemlock woolly adelgid in eastern North America. Ecol. Appl. 2012, 22, 472–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havill, N.P.; Montgomery, M.E.; Yu, G.; Shiyake, S.; Caccone, A. Mitochondrial DNA from hemlock woolly adelgid (Hemiptera: Adelgidae) suggests cryptic speciation and pinpoints the source of the introduction to eastern North America. Ann. Entomol. Soc. Am. 2006, 99, 195–203. [Google Scholar] [CrossRef]
- Havill, N.P.; Shiyake, S.; Lamb Galloway, A.; Foottit, R.G.; Yu, G.; Paradis, A.; Elkinton, J.; Montgomery, M.E.; Sano, M.; Caccone, A. Ancient and modern colonization of North America by hemlock woolly adelgid, Adelges tsugae (Hemiptera: Adelgidae), an invasive insect from East Asia. Mol. Ecol. 2016, 25, 2065–2080. [Google Scholar] [CrossRef] [PubMed]
- Canadian Food Inspection Agency. Hemlock Woolly Adelgid—Adelges tsugae (Annand). Available online: http://www.inspection.gc.ca/plants/plant-pests-invasive-species/insects/hemlock-woolly-adelgid/eng/1325610383502/1325610993895 (accessed on 21 October 2018).
- McClure, M.S. Evidence of a Polymorphic Life Cycle in the Hemlock Woolly Adelgid, Adelges tsugae (Homoptera: Adelgidae). Ann. Entomol. Soc. Am. 1989, 82, 50–54. [Google Scholar] [CrossRef]
- Preisser, E.L.; Lodge, A.G.; Orwig, D.A.; Elkinton, J.S. Range expansion and population dynamics of co-occurring invasive herbivores. Biol. Invasions 2008, 10, 201–213. [Google Scholar] [CrossRef]
- Orwig, D.A.; Foster, D.R.; Mausel, D.L. Landscape patterns of hemlock decline in New England due to the introduced hemlock woolly adelgid. J. Biogeogr. 2002, 29, 1475–1487. [Google Scholar] [CrossRef]
- McKenzie, E.A.; Elkinton, J.S.; Casagrande, R.A.; Preisser, E.L.; Mayer, M. Terpene chemistry of eastern hemlocks resistant to hemlock woolly adelgid. J. Chem. Ecol. 2014, 40, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Ingwell, L.L.; Preisser, E.L. Using citizen science programs to identify host resistance in pest-invaded forests. Conserv. Biol. 2011, 25, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.; Baiser, B.; D’Amato, A.; Ellison, A.M.; Plotkin, A.B.; Orwig, D.; Oswald, W.; Thompson, J. Hemlock: A Forest Giant on the Edge; Yale University Press: New Haven, CT, USA, 2014. [Google Scholar]
- Morin, R.S.; Liebhold, A.M. Invasions by two non-native insects alter regional forest species composition and successional trajectories. For. Ecol. Manag. 2015, 341, 67–74. [Google Scholar] [CrossRef]
- Orwig, D.A.; Foster, D.R. Forest response to the introduced hemlock woolly adelgid in southern New England, USA. J. Torrey Bot. Soc. 1998, 125, 60–73. [Google Scholar] [CrossRef]
- Abella, S.R. Forest decline after a 15-year “perfect storm” of invasion by hemlock woolly adelgid, drought, and hurricanes. Biol. Invasions 2018, 20, 695–707. [Google Scholar] [CrossRef]
- Brantley, S.T.; Miniat, C.F.; Elliott, K.J.; Laseter, S.H.; Vose, J.M. Changes to southern Appalachian water yield and stormflow after loss of a foundation species. Ecohydrology 2015, 8, 518–528. [Google Scholar] [CrossRef]
- Young, J.A.; Morton, D.D. Modeling landscape-level impacts of HWA in Shenandoah National Park. In Proceedings of the Hemlock Woolly Adelgid in the Eastern United States Symposium, East Brunswick, NJ, USA, 5 February 2002. [Google Scholar]
- Orwig, D.A.; Thompson, J.R.; Povak, N.A.; Manner, M.; Niebyl, D.; Foster, D.R. A foundation tree at the precipice: Tsuga canadensis health after the arrival of Adelges tsugae in central New England. Ecosphere 2012, 3, 1–16. [Google Scholar] [CrossRef]
- Small, M.J.; Small, C.J.; Dreyer, G.D. Changes in a hemlock-dominated forest following woolly adelgid infestation in southern New England. J. Torrey Bot. Soc. 2005, 132, 458–470. [Google Scholar] [CrossRef]
- Preisser, E.L.; Miller-Pierce, M.R.; Vansant, J.; Orwig, D.A. Eastern hemlock (Tsuga canadensis) regeneration in the presence of hemlock woolly adelgid (Adelges tsugae) and elongate hemlock scale (Fiorinia externa). Can. J. For. Res. 2011, 41, 2433–2439. [Google Scholar] [CrossRef]
- Eschtruth, A.K.; Cleavitt, N.L.; Battles, J.J.; Evans, R.A.; Fahey, T.J. Vegetation dynamics in declining eastern hemlock stands: 9 years of forest response to hemlock woolly adelgid infestation. Can. J. For. Res. 2006, 36, 1435–1450. [Google Scholar] [CrossRef]
- Cleavitt, N.L.; Eschtruth, A.K.; Battles, J.J.; Fahey, T.J. Bryophyte response to eastern hemlock decline caused by hemlock woolly adelgid infestation. J. Torrey Bot. Soc. 2008, 135, 12–25. [Google Scholar] [CrossRef]
- Jackson, M.R.; Bellemare, J. The potential for indirect negative effects of exotic insect species on a liverwort, Bazzania trilobata (Lepidoziaceae), mediated by the decline of a foundation tree species, Tsuga canadensis (Pinaceae). J. Torrey Bot. Soc. 2018, 145, 183–194. [Google Scholar] [CrossRef]
- Foster, D.R.; Orwig, D.A. Preemptive and salvage harvesting of New England forests: When doing nothing is a viable alternative. Conserv. Biol. 2006, 20, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Kizlinski, M.L.; Orwig, D.A.; Cobb, R.C.; Foster, D.R. Direct and indirect ecosystem consequences of an invasive pest on forests dominated by eastern hemlock. J. Biogeogr. 2002, 29, 1489–1503. [Google Scholar] [CrossRef]
- Sullivan, K.A.; Ellison, A.M. The Seed Bank of Hemlock Forests: Implications for Forest Regeneration Following Hemlock Decline. J. Torrey Bot. Soc. 2006, 133, 393–402. [Google Scholar] [CrossRef]
- Farnsworth, E.J.; Barker Plotkin, A.A.; Ellison, A.M. The relative contributions of seed bank, seed rain, and understory vegetation dynamics to the reorganization of Tsuga canadensis forests after loss due to logging or simulated attack by Adelges tsugae. Can. J. For. Res. 2012, 42, 2090–2105. [Google Scholar] [CrossRef] [Green Version]
- Zukswert, J.M.; Bellemare, J.; Rhodes, A.L.; Sweezy, T.; Gallogly, M.; Acevedo, S.; Taylor, R.S. Forest community structure differs, but not ecosystem processes, 25 years after eastern hemlock removal in an accidental experiment. Southeast. Nat. 2014, 13, 61–87. [Google Scholar]
- Benzinger, J. Hemlock decline and breeding birds. I. Hemlock ecology. Rec. N. J. Birds 1994, 20, 2–12. [Google Scholar]
- Toenies, M.J.; Miller, D.A.; Marshall, M.R.; Stauffer, G.E. Shifts in vegetation and avian community structure following the decline of a foundational forest species, the eastern hemlock. Condor 2018, 120, 489–506. [Google Scholar] [CrossRef]
- Becker, D.A.; Brittingham, M.C.; Goguen, C.B. Effects of hemlock woolly adelgid on breeding birds at Fort Indiantown Gap, Pennsylvania. Northeast. Nat. 2008, 227–240. [Google Scholar] [CrossRef]
- Lishawa, S.C.; Bergdahl, D.R.; Costa, S.D. Winter conditions in eastern hemlock and mixed-hardwood deer wintering areas of Vermont. Can. J. For. Res. 2007, 37, 697–703. [Google Scholar] [CrossRef]
- Faison, E.K.; DeStefano, S.; Foster, D.R.; Plotkin, A.B. Functional response of ungulate browsers in disturbed eastern hemlock forests. For. Ecol. Manag. 2016, 362, 177–183. [Google Scholar] [CrossRef]
- Eschtruth, A.K.; Battles, J.J. Deer herbivory alters forest response to canopy decline caused by an exotic insect pest. Ecol. Appl. 2008, 18, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Degrassi, A.L. Hemlock woolly adelgid invasion affects microhabitat characteristics and small mammal communities. Biol. Invasions 2018, 20, 2173–2186. [Google Scholar] [CrossRef]
- Siddig, A.A.H.; Ellison, A.M.; Mathewson, B.G. Assessing the impacts of the decline of Tsuga canadensis stands on two amphibian species in a New England forest. Ecosphere 2016, 7, e01574. [Google Scholar] [CrossRef]
- Ingwell, L.L.; Miller-Pierce, M.; Trotter, R.T., III; Preisser, E.L. Vegetation and invertebrate community response to Eastern Hemlock decline in southern New England. Northeast. Nat. 2012, 19, 541–558. [Google Scholar] [CrossRef]
- Snyder, C.D.; Young, J.A.; Ross, R.M.; Smith, D.R. Long-term effects of hemlock forest decline on headwater stream communities. In Proceedings of the Third Symposium on Hemlock Woolly Adelgid in the Eastern United States, Ashville, NC, USA, 1–3 February 2005; pp. 42–55. [Google Scholar]
- Ross, R.M.; Bennett, R.M.; Snyder, C.D.; Young, J.A.; Smith, D.R.; Lemarie, D.P. Influence of eastern hemlock (Tsuga canadensis L.) on fish community structure and function in headwater streams of the Delaware River basin. Ecol. Freshw. Fish 2003, 12, 60–65. [Google Scholar] [CrossRef]
- Webster, J.; Morkeski, K.; Wojculewski, C.; Niederlehner, B.; Benfield, E.; Elliott, K. Effects of hemlock mortality on streams in the southern Appalachian Mountains. Am. Midl. Nat. 2012, 112–131. [Google Scholar] [CrossRef]
- Rowell, T.J.; Sobczak, W.V. Will stream periphyton respond to increases in light following forecasted regional hemlock mortality? J. Freshw. Ecol. 2008, 23, 33–40. [Google Scholar] [CrossRef]
- Pitt, D.B.; Batzer, D.P. Potential impacts on stream macroinvertebrates of an influx of woody debris from eastern hemlock demise. For. Sci. 2015, 61, 737–746. [Google Scholar] [CrossRef]
- Evans, D.M.; Dolloff, C.A.; Aust, W.M.; Villamagna, A.M. Effects of Eastern Hemlock Decline on large wood loads in streams of the Appalachian Mountains. JAWRA J. Am. Water Resour. Assoc. 2012, 48, 266–276. [Google Scholar] [CrossRef]
- Adkins, J.K.; Rieske, L.K. Loss of a foundation forest species due to an exotic invader impacts terrestrial arthropod communities. For. Ecol. Manag. 2013, 295, 126–135. [Google Scholar] [CrossRef]
- Adkins, J.K.; Rieske, L.K. A terrestrial invader threatens a benthic community: Potential effects of hemlock woolly adelgid-induced loss of eastern hemlock on invertebrate shredders in headwater streams. Biol. Invasions 2015, 17, 1163–1179. [Google Scholar] [CrossRef]
- Adkins, J.K.; Rieske, L.K. Benthic collector and grazer communities are threatened by hemlock woolly adelgid-induced eastern hemlock loss. Forests 2015, 6, 2719–2738. [Google Scholar] [CrossRef]
- Willacker, J.J., Jr.; Sobczak, W.V.; Colburn, E.A. Stream macroinvertebrate communities in paired hemlock and deciduous watersheds. Northeast. Nat. 2009, 101–112. [Google Scholar] [CrossRef]
- Yorks, T.E.; Leopold, D.J.; Raynal, D.J. Effects of Tsuga canadensis mortality on soil water chemistry and understory vegetation: Possible consequences of an invasive insect herbivore. Can. J. For. Res. 2003, 33, 1525–1537. [Google Scholar] [CrossRef]
- Cessna, J.F.; Nielsen, C. Influences of Hemlock Woolly Adelgid–Induced Stand-Level Mortality on Nitrogen Cycling and Stream Water Nitrogen Concentrations in Southern Pennsylvania. Castanea 2012, 77, 127–135. [Google Scholar] [CrossRef]
- Cobb, R.C.; Orwig, D.A. Changes in decomposition dynamics in hemlock forests impacted by hemlock woolly adelgid: Restoration and conservation of hemlock ecosystem function. In Proceedings of the Fourth Symposium on hemlock woolly adelgid in the eastern United States, Hartford, CT, USA, 12–14 February 2008; Onken, B., Reardon, R., Eds.; USDA Forest Service: Morgantown, WV, USA, 2008; pp. 157–167. [Google Scholar]
- Stadler, B.; Müller, T.; Orwig, D. The ecology of energy and nutrient fluxes in hemlock forests invaded by hemlock woolly adelgid. Ecology 2006, 87, 1792–1804. [Google Scholar] [CrossRef]
- Vendettuoli, J.F.; Orwig, D.A.; Krumins, J.A.; Waterhouse, M.D.; Preisser, E.L. Hemlock woolly adelgid alters fine root bacterial abundance and mycorrhizal associations in eastern hemlock. For. Ecol. Manag. 2015, 339, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Schaeffer, R.N.; Wilson, C.M.; Radville, L.; Barrett, M.; Whitney, E.; Roitman, S.; Miller, E.R.; Wolfe, B.E.; Thornber, C.S.; Orians, C.M.; et al. Individual and non-additive effects of exotic sap-feeders on root functional and mycorrhizal traits of a shared conifer host. Funct. Ecol. 2017, 31, 2024–2033. [Google Scholar] [CrossRef]
- Wilson, C.M.; Schaeffer, R.N.; Hickin, M.L.; Rigsby, C.M.; Sommi, A.F.; Thornber, C.S.; Orians, C.M.; Preisser, E.L. Chronic impacts of invasive herbivores on a foundational forest species: A whole-tree perspective. Ecology 2018, 99, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Orwig, D.A.; Boucher, P.; Paynter, I.; Saenz, E.; Li, Z.; Schaaf, C. The potential to characterize ecological data with terrestrial laser scanning in Harvard Forest, MA. Interface Focus 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hwang, T.; Schaaf, C.L.; Orwig, D.A.; Boose, E.; Munger, J.W. Increased water yield due to the hemlock woolly adelgid infestation in New England. Geophys. Res. Lett. 2017, 44, 2327–2335. [Google Scholar] [CrossRef]
- Daley, M.J.; Phillips, N.G.; Pettijohn, C.; Hadley, J.L. Water use by eastern hemlock (Tsuga canadensis) and black birch (Betula lenta): Implications of effects of the hemlock woolly adelgid. Can. J. For. Res. 2007, 37, 2031–2040. [Google Scholar] [CrossRef]
- Morin, R.S.; Liebhold, A.M.; Gottschalk, K.W. Anisotropic spread of hemlock woolly adelgid in the eastern United States. Biological Invasions 2009, 11, 2341–2350. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Preisser, E.L.; Porter, A.; Elkinton, J.; Waller, L.A.; Carlin, B.P.; Ellison, A.M. Ecological boundary detection using Bayesian areal wombling. Ecology 2010, 91, 3448–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, A.M.; Gregoire, T.G. A geographically variable model of hemlock woolly adelgid spread. Biological Invasions 2007, 9, 369–382. [Google Scholar] [CrossRef]
- Paradis, A. Population Dynamics of the Hemlock Woolly Adelgid (Hemiptera: Adelgidae). Ph.D. Thesis, University of Massachusetts, Amherst, MA, USA, 2011. [Google Scholar]
- Stoetzel, M.B.; Onken, B.; Reardon, R.; Lashomb, J. History of the introduction of Adelges tsugae based on voucher specimens in the Smithsonian Institute National Collection of Insects. In Proceedings of the Hemlock Woolly Adelgid in the Eastern United States Symposium, New Brunswick, NY, USA, 5–7 February 2002. [Google Scholar]
- USDA Forest Service, Northern Research Station. Alien Forest Pest Explorer. 2018. Available online: https://foresthealth.fs.usda.gov/portal/Flex/APE (accessed on 21 October 2018).
- Albani, M.; Moorcroft, P.R.; Ellison, A.M.; Orwig, D.A.; Foster, D.R. Predicting the impact of hemlock woolly adelgid on carbon dynamics of eastern United States forests. Can. J. For. Res. 2010, 40, 119–133. [Google Scholar] [CrossRef]
- Moorcroft, P.R.; Hurtt, G.; Pacala, S.W. A method for scaling vegetation dynamics: The ecosystem demography model (ED). Ecol. Monogr. 2001, 71, 557–586. [Google Scholar] [CrossRef]
- Finzi, A.C.; Raymer, P.C.L.; Giasson, M.-A.; Orwig, D.A. Net primary production and soil respiration in New England hemlock forests affected by the hemlock woolly adelgid. Ecosphere 2014, 5, art98. [Google Scholar] [CrossRef] [Green Version]
- Munger, W.; Hadley, J. Net Carbon Exchange of an Old-Growth Hemlock Forest at Harvard Forest HEM Tower Since 2000; Harvard Forest Data Archive HF103; Harvard Forest: Petersham, MA, USA, 2017. [Google Scholar] [CrossRef]
- Raymer, P.C.L.; Orwig, D.A.; Finzi, A.C. Hemlock loss due to the hemlock woolly adelgid does not affect ecosystem C storage but alters its distribution. Ecosphere 2013, 4, art63. [Google Scholar] [CrossRef]
- Crowley, K.F.; Lovett, G.M.; Arthur, M.A.; Weathers, K.C. Long-term effects of pest-induced tree species change on carbon and nitrogen cycling in northeastern US forests: A modeling analysis. For. Ecol. Manag. 2016, 372, 269–290. [Google Scholar] [CrossRef]
- Krebs, J.; Pontius, J.; Schaberg, P.G. Modeling the impacts of hemlock woolly adelgid infestation and presalvage harvesting on carbon stocks in northern hemlock forests. Can. J. For. Res. 2017, 47, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Ignace, D.D.; Fassler, A.; Bellemare, J. Decline of a foundation tree species due to invasive insects will trigger net release of soil organic carbon. Ecosphere 2018, 9, e02391. [Google Scholar] [CrossRef]
- Foster, D.R.; Zebryk, T.; Schoonmaker, P.; Lezberg, A. Post-settlement history of human land-use and vegetation dynamics of a Tsuga canadensis (hemlock) woodlot in central New England. J. Ecol. 1992, 773–786. [Google Scholar] [CrossRef]
- Ellison, A.M.; Barker-Plotkin, A.A.; Foster, D.R.; Orwig, D.A. Experimentally testing the role of foundation species in forests: The Harvard Forest Hemlock Removal Experiment. Methods Ecol. Evol. 2010, 1, 168–179. [Google Scholar] [CrossRef]
- Meehl, G.A.; Covey, C.; Delworth, T.; Latif, M.; McAvaney, B.; Mitchell, J.F.; Stouffer, R.J.; Taylor, K.E. The WCRP CMIP3 multimodel dataset: A new era in climate change research. Bull. Am. Meteorol. Soc. 2007, 88, 1383–1394. [Google Scholar] [CrossRef]
- McClure, M.S. Density-Dependent Feedback and Population Cycles in Adelges tsugae (Homoptera: Adelgidae) on Tsuga canadensis. Environ. Entomol. 1991, 20, 258–264. [Google Scholar] [CrossRef]
- Gómez, S.; Gonda-King, L.; Orians, C.M.; Orwig, D.A.; Panko, R.; Radville, L.; Soltis, N.; Thornber, C.S.; Preisser, E.L. Interactions between invasive herbivores and their long-term impact on New England hemlock forests. Biol. Invasions 2015, 17, 661–673. [Google Scholar] [CrossRef]
- McClure, M. Dispersal of the scale Fiorinia externa (Homoptera: Diaspididae) and effects of edaphic factors on its establishment on hemlock. Environ. Entomol. 1977, 6, 539–544. [Google Scholar] [CrossRef]
- McClure, M. Parasitism of the scale insect Fiorinia externa (Homoptera: Diaspididae) by Aspidiotiphagus citrinus (Hymenoptera: Eulophidae) in a hemlock forest: Density-dependence. Environ. Entomol. 1977, 6, 551–555. [Google Scholar] [CrossRef]
- Preisser, E.; Elkinton, J. Exploitative competition between invasive herbivores benefits a native host plant. Ecology 2008, 89, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- Miller-Pierce, M.; Preisser, E. Asymmetric priority effects influence the success of invasive forest insects. Ecol. Entomol. 2012, 37, 350–358. [Google Scholar] [CrossRef]
- Gómez, S.; Gonda-King, L.; Orians, C.; Preisser, E.L. Competitor avoidance drives within-host feeding site selection in a passively-dispersed herbivore. Ecol. Entomol. 2014, 39, 10–16. [Google Scholar] [CrossRef]
- Lany, N.K.; Zarnetske, P.L.; Schliep, E.M.; Schaeffer, R.N.; Orians, C.; Orwig, D.A.; Preisser, E.L. Asymmetric biotic interactions and abiotic niche differences revealed by a dynamic joint species distribution model. Ecology 2018, 99, 1018–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duveneck, M.J.; Thompson, J.R. Climate change imposes phenological trade-offs on forest net primary productivity. J. Geophys. Res. Biogeosci. 2017, 122, 2298–2313. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.G. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellison, A.M.; Orwig, D.A.; Fitzpatrick, M.C.; Preisser, E.L. The Past, Present, and Future of the Hemlock Woolly Adelgid (Adelges tsugae) and Its Ecological Interactions with Eastern Hemlock (Tsuga canadensis) Forests. Insects 2018, 9, 172. https://doi.org/10.3390/insects9040172
Ellison AM, Orwig DA, Fitzpatrick MC, Preisser EL. The Past, Present, and Future of the Hemlock Woolly Adelgid (Adelges tsugae) and Its Ecological Interactions with Eastern Hemlock (Tsuga canadensis) Forests. Insects. 2018; 9(4):172. https://doi.org/10.3390/insects9040172
Chicago/Turabian StyleEllison, Aaron M., David A. Orwig, Matthew C. Fitzpatrick, and Evan L. Preisser. 2018. "The Past, Present, and Future of the Hemlock Woolly Adelgid (Adelges tsugae) and Its Ecological Interactions with Eastern Hemlock (Tsuga canadensis) Forests" Insects 9, no. 4: 172. https://doi.org/10.3390/insects9040172
APA StyleEllison, A. M., Orwig, D. A., Fitzpatrick, M. C., & Preisser, E. L. (2018). The Past, Present, and Future of the Hemlock Woolly Adelgid (Adelges tsugae) and Its Ecological Interactions with Eastern Hemlock (Tsuga canadensis) Forests. Insects, 9(4), 172. https://doi.org/10.3390/insects9040172




