Crapemyrtle Bark Scale: A New Threat for Crapemyrtles, a Popular Landscape Plant in the U.S.
Abstract
:1. Introduction
2. Taxonomy
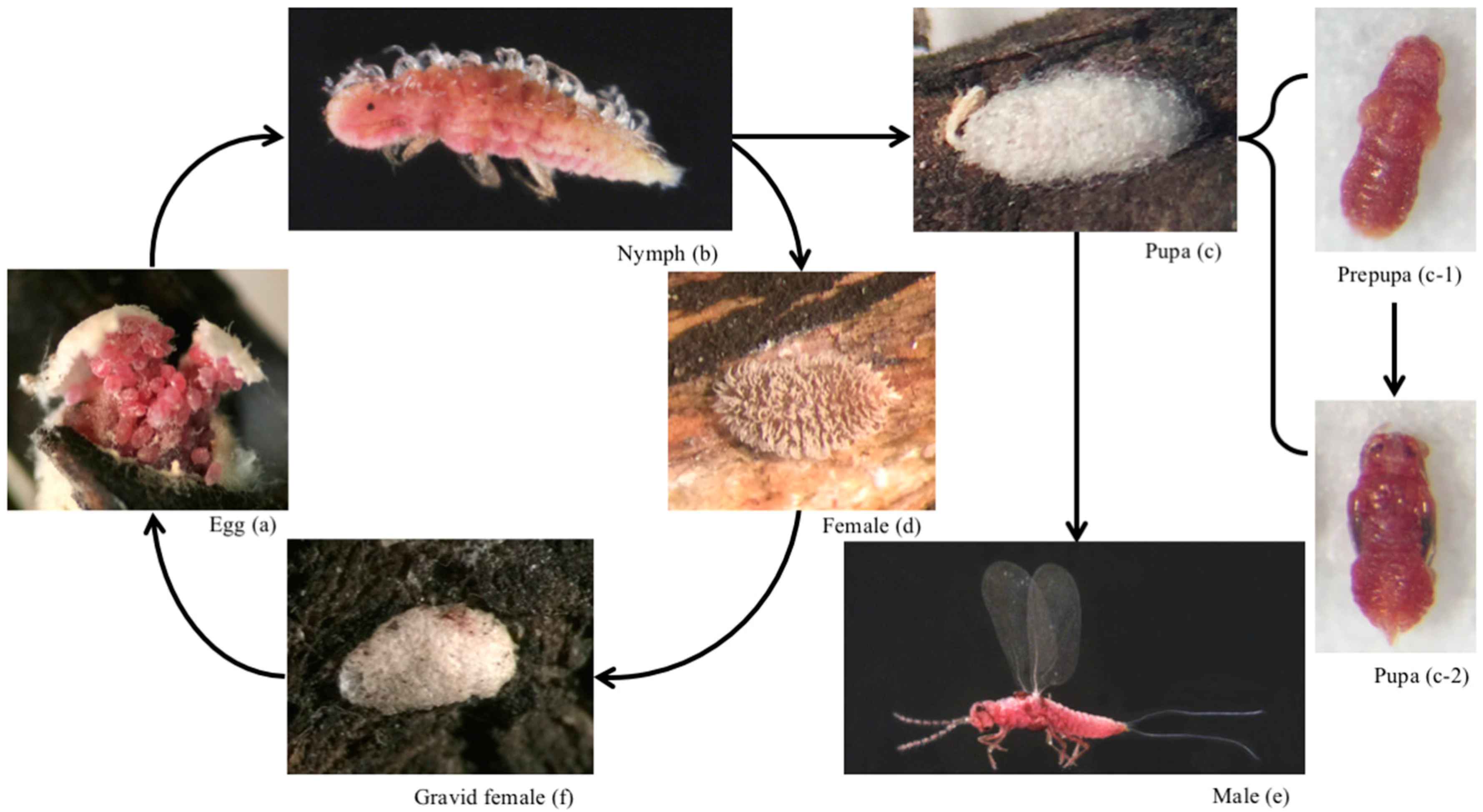
3. Biology
4. Host Range
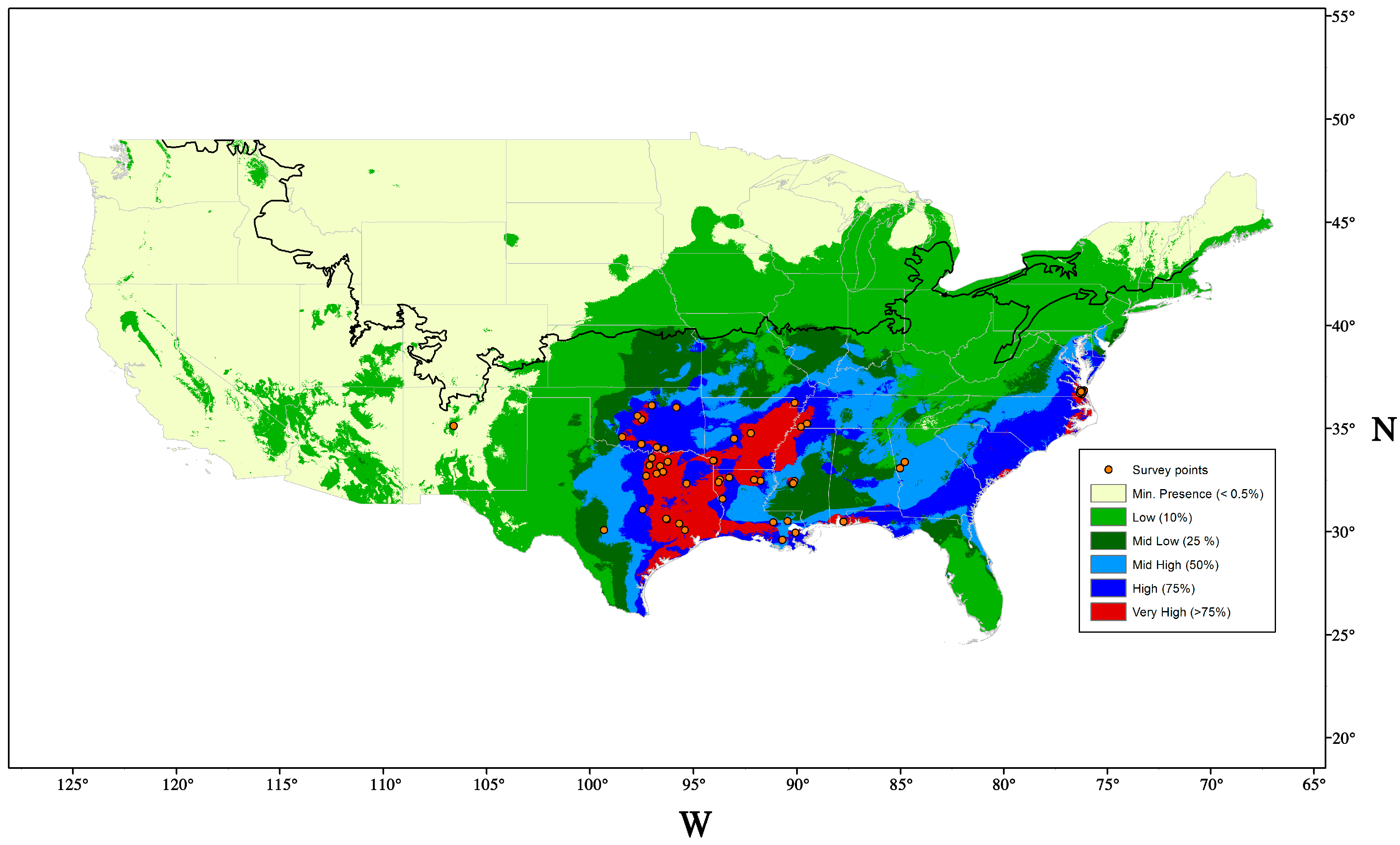
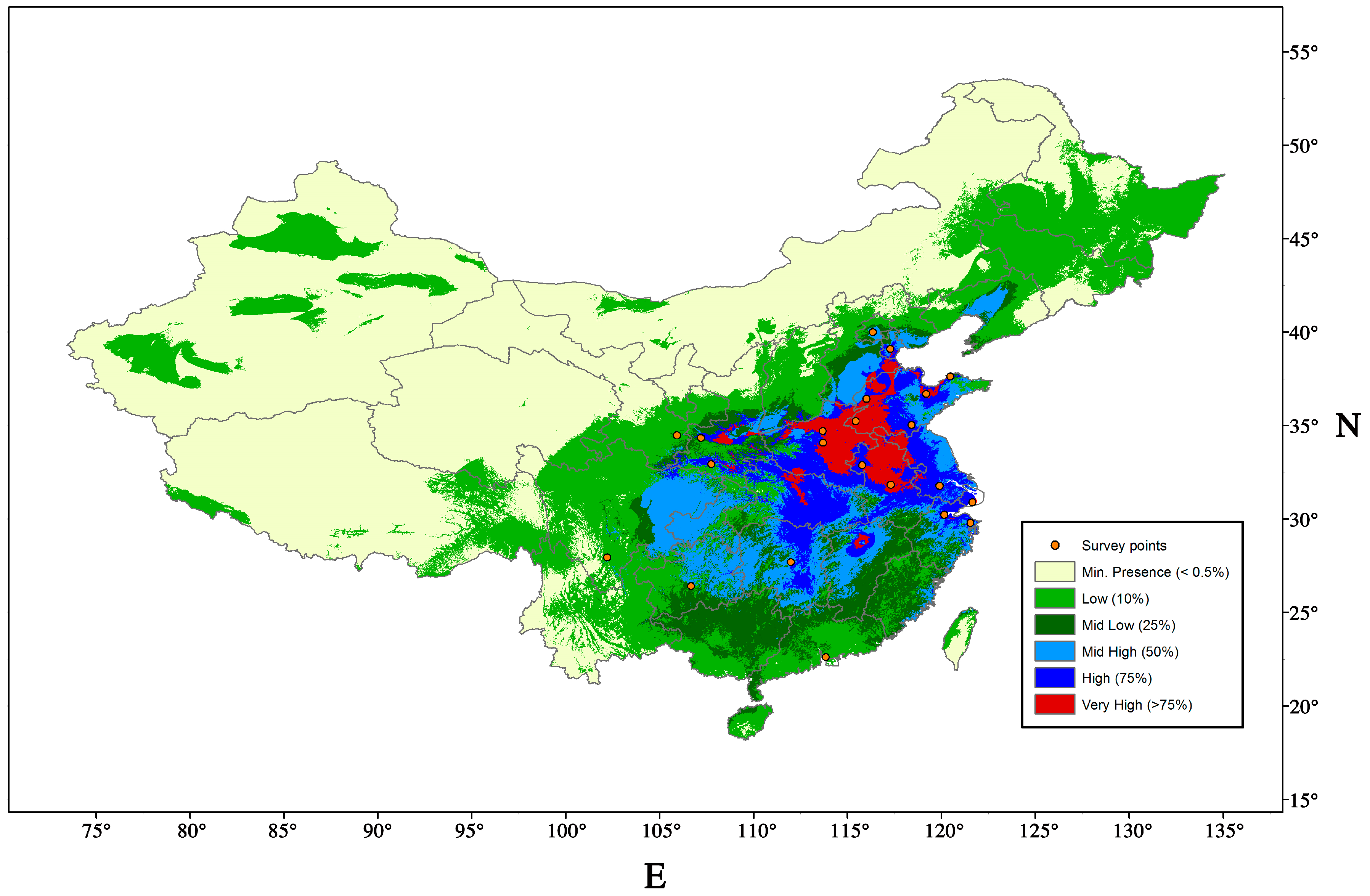
5. Distribution and Dispersal
6. Plant Damage and Economic Impact
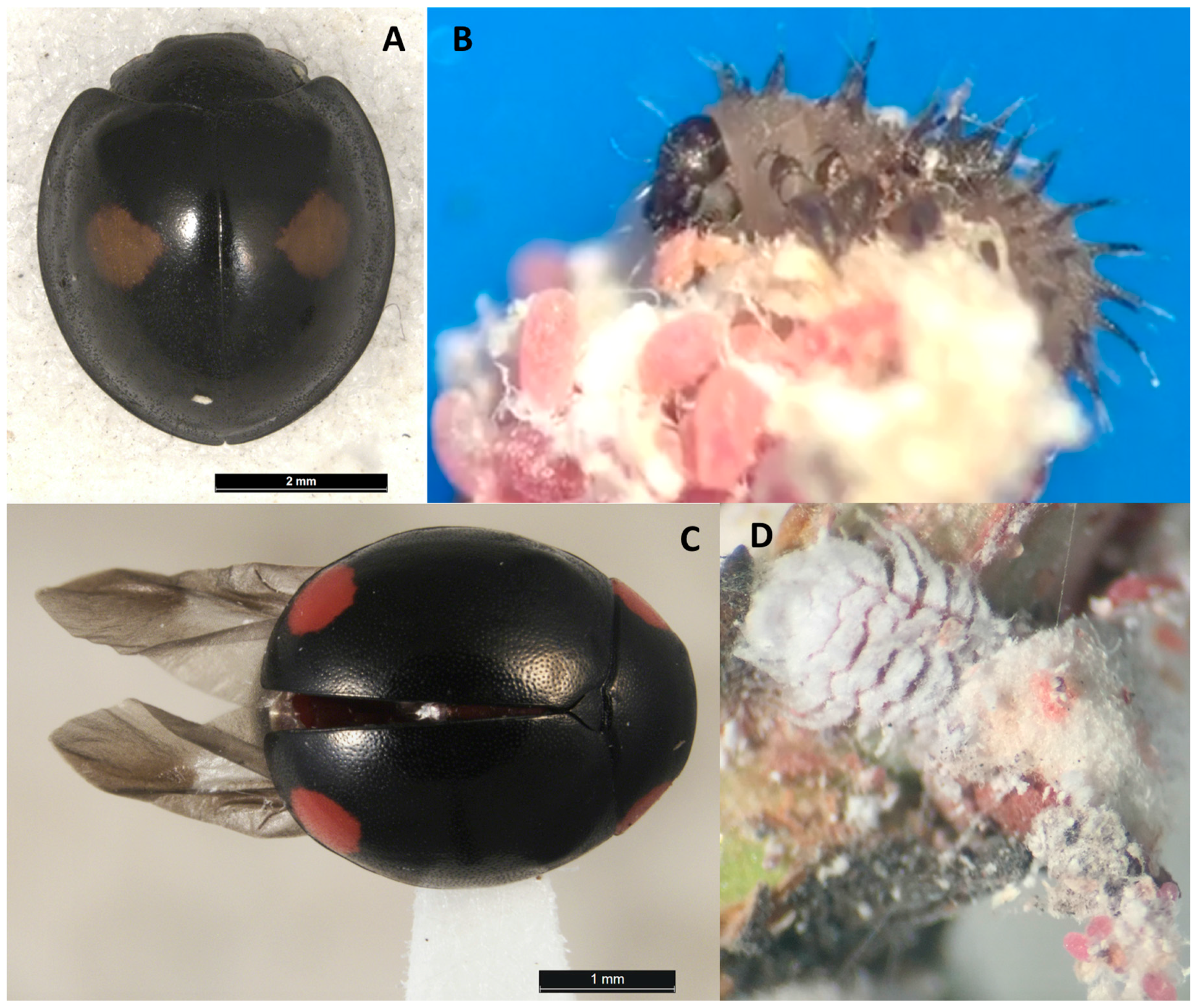
7. Natural Enemies
8. Current Management
9. Research Needed to Manage A. lagerstroemiae
9.1. Potential Distribution and Host Range
9.2. Plant Resistance
9.3. Biological Control
9.3.1. Classical Biological Control
9.3.2. Augmentative Biological Control
9.3.3. Conservation Biological Control
10. Conclusions
Supplemental Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Egolf, D.R.; Andrick, A.O. The Lagerstroemia Handbook/Checklist: A Guide to Crapemyrtle Cultivars; American Association of Botanical Gardens and Arboreta: Las Cruces, NM, USA, 1978; p. 9. [Google Scholar]
- Chappell, M.R.; Kristine, B.S.; Williams-Woodward, J.; Knox, G. Optimizing plant health and pest management of Lagerstroemia spp. HortScience 2012, 30, 161–172. [Google Scholar]
- 2014 Census of Horticultural Specialities. Available online: https://www.agcensus.usda.gov/Publications/2012/Online_Resources/Census_of_Horticulture_Specialties/ (accessed on 16 May 2016).
- Gu, M.; Merchant, M.; Robbins, J.; Hopkins, J. Crape Myrtle Bark Scale: A New Exotic Pest. Available online: https://www.eddmaps.org/cmbs/Resources/TAMUCrapemrytlebarkscaleEHT-049.pdf (accessed on 16 May 2016).
- Knox, G. Crapemyrtle in Florida. Available online: http://edis.ifas.ufl.edu/mg266 (accessed on 16 May 2016).
- Merchant, M.E.; Gu, M.; Robbins, J.; Vafaie, E.; Barr, N.; Tripodi, A.D.; Szalanski, A.L. Discovery and Spread of Eriococcus lagerstroemiae Kuwana (Hemiptera: Eriococcidae), a New Invasive Pest of Crape Myrtle, Lagerstroemia spp. Available online: http://bugwoodcloud.org/resource/pdf/ESAPosterDiscovAndSpread2014.pdf (accessed on 16 May 2016).
- Wang, Z.; Chen, Y.; Knox, G.W.; Diaz, R. Crape Myrtle Bark Scale. Available online: http://www.lsuagcenter.com/~/media/system/7/8/d/1/78d165df43ac0d4767607d88dadfb841/pub3440bugbizcrapemyrtlebarkscale_final.pdf (accessed on 16 May 2016).
- Miller, C. Top 9 Pest Reports in the Past Two Years. Available online: http://www.greenhousegrower.com/retailing/top-9-pest-reports-in-the-past-two-years/5/ (accessed on 17 May 2016).
- He, D.; Cheng, J.; Zhao, H.; Chen, S. Biological characteristic and control efficacy of Eriococcus lagerstroemiae. Chin. Bull. Entomol. 2008, 5, 34. (In Chinese) [Google Scholar]
- Jiang, N.; Xu, H. Observation on Eriococcus lagerstroemiae Kuwana. J. Anhui Agric. Univ. 1998, 25, 142–144. (In Chinese) [Google Scholar]
- Luo, Q.; Xie, X.; Zhou, L.; Wang, S.; Xu, Z. A study on the dynamics and biological characteristics of Eriococcus lagerstroemiae Kuwana population in Guiyang. Acta Entomol. Sin. 2000, 43, 35–41. (In Chinese) [Google Scholar]
- Ma, J. Occurrence and biological characteristics of Eriococcus lagerostroemiae Kuwana in Panxi district. South China Fruits 2011, 5, 3. (In Chinese) [Google Scholar]
- Zhang, Y. Effects of different insecticides on Eriococcus lagerstroemiae Kuwana (Hemiptera: Eriococcidae) in Changzhou district, Jiangsu. Hunan Agric. Sci. 2011, 14, 32–33. (In Chinese) [Google Scholar]
- Kozar, F.; Kaydan, M.B.; Konczne Benedicty, Z.; Szita, E. Acanthococcidae and Related Families of the Palaearctic Region; Plant Protection Institute, Centre for Agricultural Research, Hungarian Academy of Sciences: Budapest, Hungary, 2013; pp. 126–128. [Google Scholar]
- Miller, D.R. Selected scale insect groups (Hemiptera: Coccoidea) in the southern region of the United States. Fla. Entomol. 2005, 88, 482–501. [Google Scholar] [CrossRef]
- Miller, D.R.; Miller, G.L.; Hodges, G.S.; Davidson, J.A. Introduced scale insects (Hemiptera: Coccoidea) of the United States and their impact on U.S. agriculture. Proc. Entomol. Soc. Wash. 2005, 107, 123–158. [Google Scholar]
- Gullan, P.J.; Cook, L.G. Phylogeny and higher classification of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Zootaxa 2007, 1668, 413–425. [Google Scholar]
- Scalenet: Acanthococcus lagerstroemiae (Kuwana) (Hemiptera: Eriococcidae). Available online: http://scalenet.info/catalogue/eriococcus lagerstroemiae/ (accessed on 22 October 2016).
- Kondo, T.; Gullan, P.J.; Williams, D. Coccidology, the study of scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Corp. Cienc. Tecnol. Agropecu. 2008, 9, 55–61. [Google Scholar] [CrossRef]
- Gullan, P.J.; Kosztarab, M. Adaptations in scale insects. Annu. Rev. Entomol. 1997, 42, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.Y.; Park, S.D.; Choi, B.S.; Kwon, Y.J. Seasonal occurrence and chemical control effects of Eriococcus largerstroemiae Kuwana on persimmon trees. Korean J. Appl. Entomol. 1995, 34, 295–299. [Google Scholar]
- Gates, Z.; (Texas Forest Service, College Station, TX, USA). Personal communication, 2015.
- Hoy, J.M. Catalogue of family Eriococcidae. In A Catalogue of the Eriococcidae (Homoptera: Coccoidea) of the World; Owen, R.E., Ed.; New Zealand Department of Scientific and Industrial Research, Government Printer: Wellington, New Zealand, 1963; p. 99. [Google Scholar]
- Wang, T. Homoptera: Coccoidea: Pseudococcidae, Eriococcidae, Coccidae, Asterolecaniidae, Lecanodiaspididae, Cerococcidae, Aclerdidae; Science Press: Beijing, China, 2001; pp. 209–210. (In Chinese) [Google Scholar]
- Park, J.D.; Kim, Y.H.; Kim, S.S.; Park, I.S.; Kim, K.C. Seasonal occurrence, host preference and hatching behavior of Eriococcus lagerstroemiae. Korean J. Appl. Entomol. 1993, 32, 83–89. [Google Scholar]
- Son, J.K.; Park, C.G. Insect pest problems of sweet persimmon in Korea. In Proceedings of the IV International Symposium on Persimmon 833, Firenze, Faenza, Caserta, Italy, 8–13 November 2008; Bellini, E., Giordani, E., Eds.; Acta Hort: Santiago, Chile, 2008; pp. 325–330. [Google Scholar]
- Hua, L. List of Chinese Insects; Zhongshan (Sun Yat-Sen) University Press: Guangdong, China, 2000; p. 137. [Google Scholar]
- Kwon, G.M.; Park, K.T. Taxonomic reconsideration of Eriococcidae (Sternorrhyncha) occurring on the persimmon tree, Diospyros kaki Thunb. Korean J. Appl. Entomol. 2002, 41, 305–311. (In Korean) [Google Scholar]
- Knox, G. Gardening in the Panhandle: Crape Myrtle Bark Scale in China, and in the U.S.? Available online: http://nwdistrict.ifas.ufl.edu/hort/2014/03/18/crapemyrtle-bark-scale-in-china-and-in-the-u-s/ (accessed on 2 June 2016).
- Varshney, R.K. A Check List of the Scale Insects and Mealy Bugs of South Asia, Part 1; The Pooran Press: Calcutta, India, 1992; p. 152. [Google Scholar]
- Williams, D.J. The British and some other European Eriococcidae (Homoptera: Coccoidea). Bull. Br. Mus. Nat. Hist. Entomol. 1985, 51, 347–393. [Google Scholar]
- EDDMapS. Early Detection & Distribution Mapping System. Available online: https://www.eddmaps.org/distribution/usstate.cfm?sub=21613 (accessed on 29 September 2016).
- Carol, A.; University District Farmers Market, Seattle, WA, USA. Personal communication, 2016.
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Russo, N.J.; Cheah, C.A.S.-J.; Tingley, M.W. Experimental evidence for branch-to-bird transfer as a mechanism for avian dispersal of the hemlock woolly adelgid (Hemiptera: Adelgidae). Environ. Entomol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Magsig-Castillo, J.; Morse, J.; Walker, G.; Bi, J.; Rugman-Jones, P.; Stouthamer, R. Phoretic dispersal of armored scale crawlers (Hemiptera: Diaspididae). J. Econ. Entomol. 2010, 103, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Miller, L. NPAG Report Eriococcus lagerstroemiae Kuwana: Crapemyrtle Scale; New Pest Advisory Group: Raleigh, NC, USA, 2015. [Google Scholar]
- Merchant, M. Crape Myrtle Bark Scale Reduces Bloom. Available online: http://citybugs.tamu.edu/2014/08/14/crape-myrtle-bark-scale-reduces-bloom/ (accessed on 16 May 2016).
- Robbins, J.; Hopkins, J.; Merchant, M.; Gu, M. Crape Myrtle Bark Scale: A New Insect Pest. Available online: https://www.uaex.edu/publications/PDF/fsa-7086.pdf (accessed on 19 September 2015).
- Zhang, Y.Z.; Huang, D.W. Two new Encyrtid parasites (Hymenoptera: Chalcidoidea) from China. Orient. Insects 2001, 35, 311–319. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.-D.; Zhang, Y.Z. A taxonomic study of Chinese species of the insidiosus group of Metaphycus (Hymenoptera: Encyrtidae). ZooKeys 2014, 378, 49–81. [Google Scholar]
- Zeya, S.B.; Hayat, M. A review of the Indian species of Metaphycus (Hymenoptera: Encyrtidae). Orient. Insects 1993, 27, 185–209. [Google Scholar] [CrossRef]
- Hayat, M.; Alam, S.M.; Agarwal, M.M. Indian Insect Types IX: Taxonomic Survey of Encyrtid Parasites (Hymenoptera: Encyrtidae) in India; Aligarh Muslim University: Aligarh, India, 1975; p. 84. [Google Scholar]
- Yu, H.; (Beijing Forestry University, Beijing, China). Personal communication, 2016.
- Hendrickson, R.M.; Drea, J.J.; Rose, M. A distribution and establishment program for Chilocorus kuwanae (Silvestri) (Coleoptera: Coccinellidae) in the United States. Proc. Entomol. Soc. Wash. 1991, 93, 197–200. [Google Scholar]
- Vafaie, E. Crapemyrtle Bark Scale Efficacy Trials. Available online: http://sixleggedaggie.com/2014/09/10/crape-myrtle-bark-scale-efficacy-trial/ (accessed on 10 September 2016).
- Wang, Z.; Chen, Y.; Diaz, R. The Cactus Lady Beetle: A Voracious Predator of Scale Insects. Available online: http://entomology.lsu.edu/assets/thecactusladybeetle.pdf (accessed on 17 May 2016).
- DeBach, P.; Rosen, D. Armoured scale insects. In Studies in Biological Control; Delucchi, V.L., Ed.; Cambridge University Press: London, UK; New York, NY, USA, 1976; pp. 139–178. [Google Scholar]
- Hattinghl, V.; Samways, M. Physiological and behavioral characteristics of Chilocorus spp. (Coleoptera: Coccinellidae) in the laboratory relative to effectiveness in the field as biocontrol agents. J. Econ. Entomol. 1994, 87, 31–38. [Google Scholar] [CrossRef]
- Hill, M.; Allan, D.; Henderson, R.; Charles, J. Introduction of armoured scale predators and establishment of the predatory mite Hemisarcoptes coccophagus (Acari: Hemisarcoptidae) on latania scale, Hemiberlesia lataniae (Homoptera: Diaspididae) in kiwifruit shelter trees in new zealand. Bull. Entomol. Res. 1993, 83, 369–376. [Google Scholar] [CrossRef]
- Charles, J.; Hill, M.; Allan, D. Releases and recoveries of Chilocorus spp. (Coleoptera: Coccinellidae) and Hemisarcoptes spp. (Acari: Hemisarcoptidae) in kiwifruit orchards: 1987–1993. N. Z. J. Zool. 1995, 22, 319–324. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J. Control experiment on Eriococcus lagerstroemiae Kuwana in Guangdong area. Hubei For. Sci. Technol. 2012, 2, 26–27. (In Chinese) [Google Scholar]
- Zhang, Z. Biological characteristics of Eriococcus lagerstroemiae Kuwana and its chemcial cotnrol. Pract. For. Technol. 2010, 2, 32–34. (In Chinese) [Google Scholar]
- Kilpatrick, R. Crape Myrtle Bark Scale. Available online: http://www.lsuagcenter.com/portals/our_offices/parishes/bossier/features/forestry_wildlife/crape-myrtle-bark-scale (accessed on 2 June 2016).
- Nelms, B.; Louisiana State Unviersity Shreveport Campus, Shreveport, LA, USA. Personal communication, 2015.
- Chen, Y.; Stagg, J.; Louisiana State University Agriculture Center Hammond Research Station, Hammond, LA, USA. Personal observation, 2015.
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.; Overholt, W.A.; Cuda, J.; Pratt, P.D.; Fox, A. Temperature-dependent development, survival, and potential distribution of Ischnodemus variegatus (Hemiptera: Blissidae), a herbivore of west Indian marsh grass. Ann. Entomol. Soc. Am. 2008, 101, 604–612. [Google Scholar] [CrossRef]
- Zhao, L.; Jia, D.; Yuan, X.; Guo, Y.; Zhou, W.; Ma, R. Cold hardiness of the biological control agent, Agasicles hygrophila, and implications for its potential distribution. Biol. Control 2015, 87, 1–5. [Google Scholar] [CrossRef]
- Manrique, V.; Diaz, R.; Montemayor, C.; Serrano, D.; Cave, R.D. Temperature-dependent development and cold tolerance of Microtheca ochroloma (Coleoptera: Chrysomelidae), a pest of cruciferous crops in the southeastern United States. Ann. Entomol. Soc. Am. 2012, 105, 859–864. [Google Scholar] [CrossRef]
- Burgi, L.P.; Mills, N.J. Cold tolerance of the overwintering larval instars of light brown apple moth Epiphyas postvittana. J. Insect Physiol. 2010, 56, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, S.L.; Borchert, D.M.; Hall, D.G. Effect of low temperatures on mortality and oviposition in conjunction with climate mapping to predict spread of the root weevil Diaprepes abbreviatus and introduced natural enemies. Environ. Entomol. 2007, 36, 73–82. [Google Scholar] [CrossRef]
- Pollock, D. Pomegranate Interest Expands across California Agriculture. Available online: http://westernfarmpress.com/orchard-crops/pomegranate-interest-expands-across-california-agriculture?page=2 (accessed on 2 June 2016).
- Wapshere, A. A testing sequence for reducing rejection of potential biological control agents for weeds. Ann. Appl. Biol. 1989, 114, 515–526. [Google Scholar] [CrossRef]
- Diaz, R.; Manrique, V.; Munyaneza, J.E.; Sengoda, V.G.; Adkins, S.; Hendricks, K.; Roberts, P.D.; Overholt, W.A. Host specificity testing and examination for plant pathogens reveals that the gall-inducing psyllid calophya latiforceps is safe to release for biological control of brazilian peppertree. Entomol. Exp. Appl. 2015, 154, 1–14. [Google Scholar] [CrossRef]
- Heard, T. Host range testing of insects. In Biological Control of Weeds: Theory and Practical Application; Julien, M., White, G., Eds.; ACIAR Monograph Series: Canberra, Australia, 1997; pp. 77–82. [Google Scholar]
- Forno, W.; Heard, T. Compiling a plant list for testing the host range of agents. In Biological Control of Weeds: Theory and Practical Application; Julien, M., White, G., Eds.; ACIAR Monograph Series: Canberra, Australia, 1997; pp. 71–76. [Google Scholar]
- Wessels, F.J.; Cuda, J.P.; Johnson, M.T.; Pedrosa-Macedo, J.H. Host specificity of Tectococcus ovatus (Hemiptera: Eriococcidae), a potential biological control agent of the invasive strawberry guava, Psidium cattleianum (Myrtales: Myrtaceae), in Florida. BioControl 2007, 52, 439–449. [Google Scholar] [CrossRef]
- Stout, M.J. Reevaluating the conceptual framework for applied research on host-plant resistance. Insect Sci. 2013, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.J.; Mizell, R.F.; McAuslane, H.J. Host preference of the crapemyrtle aphid (Hemiptera: Aphididae) and host suitability of crapemyrtle cultivars. Environ. Entomol. 2009, 38, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, R.I.; Reinert, J.A.; McKenney, C.B. Differential resistance among crape myrtle (Lagerstroemia) species, hybrids, and cultivars to foliar feeding by adult flea beetles (Altica litigata). HortScience 2008, 43, 403–407. [Google Scholar]
- Aasen, E. Mckinney Is Crape Myrtle Central, with Thousands of Trees in Summer Bloom. Available online: http://www.dallasnews.com/news/community-news/mckinney/headlines/20120705-mckinney-is-crape-myrtle-central-with-thousands-of-trees-in-summer-bloom.ece (accessed on 16 June 2016).
- Lacomme, C. Strategies for altering plant traits using virus-induced gene silencing technologies. In Plant Gene Silencing: Methods and Protocols, 1st ed.; Mysore, K., Senthil-Kumar, M., Eds.; Humana Press: New York, NY, USA, 2015; pp. 25–41. [Google Scholar]
- Van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Hoddle, M.S. Classical biological control of arthropods in the 21st century. In Proceedings of the 1st International Symposium on Biological Control of Arthropods, Honolulu, HI, USA, 14–18 January 2002; USDA Forest Service: Morgantown, WV, USA, 2003; pp. 3–16. [Google Scholar]
- Naranjo, S.E.; Ellsworth, P.C.; Frisvold, G.B. Economic value of biological control in integrated pest management of managed plant systems. Annu. Rev. Entomol. 2015, 60, 621–645. [Google Scholar] [CrossRef] [PubMed]
- Bokonon-Ganta, A.H.; de Groote, H.; Neuenschwander, P. Socio-economic impact of biological control of mango mealybug in Benin. Agric. Ecosyst. Environ. 2002, 93, 367–378. [Google Scholar] [CrossRef]
- Myrick, S.; Norton, G.W.; Selvaraj, K.N.; Natarajan, K.; Muniappan, R. Economic impact of classical biological control of papaya mealybug in India. Crop Prot. 2014, 56, 82–86. [Google Scholar] [CrossRef]
- Kairo, M.T.K.; Pollard, G.V.; Peterkin, D.D.; Lopez, V.F. Biological control of the hibiscus mealybug, Maconellicoccus hirsutus green (Hemiptera: Pseudococcidae) in the caribbean. Integr. Pest Manag. Rev. 2000, 5, 241–254. [Google Scholar] [CrossRef]
- Agricultural Research Service (USDA). Plant Hardiness Zone Map in the U.S. Available online: http://planthardiness.ars.usda.gov/phzmweb/interactivemap.aspx (accessed on 22 October 2016).
- Van Lenteren, J.C.; Babendreier, D.; Bigler, F.; Burgio, G.; Hokkanen, H.M.T.; Kuske, S.; Loomans, A.J.M.; Menzler-Hokkanen, I.; Van Rijn, P.C.J.; Thomas, M.B.; et al. Environmental risk assessment of exotic natural enemies used in inundative biological control. BioControl 2003, 48, 3–38. [Google Scholar] [CrossRef]
- Chong, J.; Oetting, R.D. Specificity of Anagyrus sp. Nov. Nr. Sinope and Leptomastix dactylopii for six mealybug species. BioControl 2006, 52, 289–308. [Google Scholar] [CrossRef]
- Caltagirone, L.; Doutt, R. The history of the vedalia beetle importation to California and its impact on the development of biological control. Annu. Rev. Entomol. 1989, 34, 1–16. [Google Scholar] [CrossRef]
- Causton, C.E.; Lincango, M.P.; Poulsom, T.G. Feeding range studies of Rodolia cardinalis (Mulsant), a candidate biological control agent of Icerya purchasi Maskell in the Galapagos islands. Biol. Control 2004, 29, 315–325. [Google Scholar] [CrossRef]
- Sakthivel, N. Effectiveness of three introduced encyrtid parasitic wasps (Acerophagus papayae, Anagyrus loecki and Pseudleptomastix mexicana) against papaya mealybug, Paracoccus marginatus, infesting mulberry in Tamil Nadu. J. Biopestic. 2013, 6, 71–76. [Google Scholar]
- Wang, Z.; Department of Entomology, Louisiana State University, Baton Rouge, LA, USA. Personal observation, 2015.
- Mani, M.; Krishnamoorthy, A. Biological suppression of the mealybugs Planococcus citri (Risso), Ferrisia virgata (Cockerell) and Nipaecoccus viridis (Newstead) on pummelo with Cryptolaemus montrouzieri Mulsant in India. J. Biol. Control 2008, 22, 169–172. [Google Scholar]
- Eliopoulos, P.A.; Kontodimas, D.C.; Stathas, G.J. Temperature-dependent development of Chilocorus bipustulatus (Coleoptera: Coccinellidae). Environ. Entomol. 2010, 39, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Rosado, J.; Bacci, L.; Martins, J.; Silva, G.; Gontijo, L.; Picanço, M. Natural biological control of green scale (Hemiptera: Coccidae): A field life-table study. Biocontrol Sci. Technol. 2014, 24, 190–202. [Google Scholar] [CrossRef]
- Henderson, R.C.; Hill, M.G.; Wigley, P.J. Freeze-dried artificial diets for three species of Chilocorus ladybirds. N. Z. Entomol. 1992, 15, 83–87. [Google Scholar] [CrossRef]
- Clercq, P.D. Culture of natural enemies on factitious foods and artificial diets. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1133–1136. [Google Scholar]
- Dong, H.; Ellington, J.J.; Remmenga, M.D. An artificial diet for the lady beetle Harmonia axyridis pallas (Coleoptera: Coccinellidae). Southwest. Entomol. 2001, 26, 205–213. [Google Scholar]
- Ehler, L.E. Conservation biological control: Past, present, and future. In Conservation Biological Control; Barbosa, P., Ed.; Academic Press: San Diego, CA, USA, 1998; pp. 1–8. [Google Scholar]
- Reddy, P.P. Selective pesticides in IPM. In Sustainable Crop Protection under Protected Cultivation; Reddy, P.P., Ed.; Springer: Singapore, 2016; pp. 121–131. [Google Scholar]
- Roubos, C.R.; Rodriguez-Saona, C.; Isaacs, R. Mitigating the effects of insecticides on arthropod biological control at field and landscape scales. Biol. Control 2014, 75, 28–38. [Google Scholar] [CrossRef]
- Tang, S.; Tang, G.; Cheke, R.A. Optimum timing for integrated pest management: Modelling rates of pesticide application and natural enemy releases. J. Theor. Biol. 2010, 264, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, R.A.; Bethke, J.A. Impact of neonicotinoid insecticides on natural enemies in greenhouse and interiorscape environments. Pest Manag. Sci. 2011, 67, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Lin, R.; Fu, M.; Zhou, Y.; Zong, F.; Jiang, H.; Lv, N.; Piao, X.; Zhang, J.; Liu, Y. Impact of imidacloprid on life-cycle development of Coccinella septempunctata in laboratory microcosms. Ecotoxicol. Environ. Saf. 2014, 110, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Krischik, V.A.; Landmark, A.L.; Heimpel, G.E. Soil-applied imidacloprid is translocated to nectar and kills nectar-feeding Anagyrus pseudococci (Girault) (Hymenoptera: Encyrtidae). Environ. Entomol. 2007, 36, 1238–1245. [Google Scholar] [CrossRef]
- Smith, S.F.; Krischik, V.A. Effects of systemic imidacloprid on Coleomegilla maculata (Coleoptera: Coccinellidae). Environ. Entomol. 1999, 28, 1189–1195. [Google Scholar] [CrossRef]
- Rogers, M.; Krischik, V.A.; Martin, L. Effect of soil application of imidacloprid on survival of adult green lacewing, Chrysoperla carnea (Neuroptera: Chrysopidae), used for biological control in greenhouse. Biol. Control 2007, 42, 172–177. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Croft, B.A. Managing pesticide resistance in crop-arthropod complexes: Interactions between biological and operational factors. Environ. Entomol. 1982, 11, 1137–1144. [Google Scholar] [CrossRef]
- Acheampong, S.; Stark, J.D. Can reduced rates of pymetrozine and natural enemies control the cabbage aphid, Brevicoryne brassicae (Homoptera: Aphididae), on broccoli? Int. J. Pest Manag. 2004, 50, 275–279. [Google Scholar] [CrossRef]
- Hassan, S.A.; Van de Veire, M. Compatibility of pesticides with biological control agents. In Biocontrol in Protected Agriculture; Heinz, K.M., van Driesche, R.G., Parrella, M.P., Eds.; Ball Publishing: Batavia, IL, USA, 2004; pp. 129–147. [Google Scholar]


| Scientific Name | Common Name | Order | Family | Country | Reference |
|---|---|---|---|---|---|
| Anogeissus latifolia (Roxb. ex DC.) Wall. ex Guill. & Perr. | Axlewood | Myrtales | Combretaceae | Korea | [23] |
| Anogeissus sp. | − | Myrtales | Combretaceae | China | [24] |
| Buxus microphylla Sieb. et Zucc. | Korean Boxwood | Buxales | Buxaceae | Korea | [25] |
| Celtis sinensis Pers. | Chinese hackberry | Rosales | Cannabaceae | Korea | [25] |
| Dalbergia eremicola Polhill | Indian rosewood | Fabales | Fabaceae | Korea | [23] |
| Diospyros kaki Thunb. | Japanese persimmon | Ericales | Ebenaceae | Korea | [25,26] |
| Ficus carica L. | Edible fig | Rosales | Moraceae | Korea | [25] |
| Glochidion puberum (L.) Hutch | Needlebush | Malpighiales | Euphorbiaceae | China | [27] |
| Glycine max (L.) Merr. | Soybean | Fabales | Fabaceae | China | [27] |
| Ligustrum obtusifolium Sieb. et. Zucc. | Border privet | Lamiales | Oleaceae | − | [14] |
| Malus pumila Mill. | Paradise apple | Rosales | Rosaceae | China | [27] |
| Mallotus japonicus Muell. Arg. | Food wrapper plant | Malpighiales | Euphorbiaceae | Korea | [25,28] |
| Myrtus sp. | Myrtle | Myrtales | Myrtus | Hungary | [14] |
| Punica granatum L. | Pomegranate | Myrtales | Lythraceae | China and Korea | [25,27,28] |
| Pseudocydonia sinensis Schneid. | Chinese-quince | Rosales | Rosaceae | Korea | [26] |
| Rubus sp. | Brambles | Rosales | Rosaceae | Hungary | [14] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chen, Y.; Gu, M.; Vafaie, E.; Merchant, M.; Diaz, R. Crapemyrtle Bark Scale: A New Threat for Crapemyrtles, a Popular Landscape Plant in the U.S. Insects 2016, 7, 78. https://doi.org/10.3390/insects7040078
Wang Z, Chen Y, Gu M, Vafaie E, Merchant M, Diaz R. Crapemyrtle Bark Scale: A New Threat for Crapemyrtles, a Popular Landscape Plant in the U.S. Insects. 2016; 7(4):78. https://doi.org/10.3390/insects7040078
Chicago/Turabian StyleWang, Zinan, Yan Chen, Mengmeng Gu, Erfan Vafaie, Michael Merchant, and Rodrigo Diaz. 2016. "Crapemyrtle Bark Scale: A New Threat for Crapemyrtles, a Popular Landscape Plant in the U.S." Insects 7, no. 4: 78. https://doi.org/10.3390/insects7040078
APA StyleWang, Z., Chen, Y., Gu, M., Vafaie, E., Merchant, M., & Diaz, R. (2016). Crapemyrtle Bark Scale: A New Threat for Crapemyrtles, a Popular Landscape Plant in the U.S. Insects, 7(4), 78. https://doi.org/10.3390/insects7040078






