Simple Summary
The diamondback moth, Plutella xylostella (L.) (DBM), is highly widespread and the most destructive pest of plants belonging to the Brassicaceae family. Traps equipped with synthetic sex attractant (SSA) of DBM are widely used to monitor the pest population dynamics worldwide. In addition, successful results have been achieved in using SSA-based traps to control DBM populations through mass trapping. However, not only SSA but also light-emitting diodes (LEDs) can be used in traps both for monitoring and for controlling DBM populations, significantly reducing the use of insecticides. We present the results of field trials using traps equipped with SSA and UV LEDs, indicating a powerful synergistic interaction between these attraction means for capturing DBM adults.
Abstract
Many cases have been described where the combination of semiochemicals and light sources in traps cause an increase in adult insect attraction. In this context, we tested different treatments using Delta plastic traps to catch DBM adults: (1) dispensers containing DBM SSA; (2) UV (365–370 nm) LEDs; (3) a combination of a dispenser containing DBM SSA and LEDs (SSA + LED); and (4) no lures (Control). The trials were conducted in northwestern Russia (the vicinity of St. Petersburg) during the period of 2022–2024 on cabbage crops. The results showed a highly significant interaction between SSA and LEDs with respect to their attractiveness to male DBM adults, as evidenced by an average 15-fold increase in DBM captures after the traps containing SSA were equipped with a second lure, an LED. This article discusses the prospects for using the identified synergistic effect of interaction between SSA and LEDs to enhance the catch of DBM adults for practical purposes, such as improving monitoring and developing more effective mass-trapping technologies.
1. Introduction
The diamondback moth (DBM) Plutella xylostella (L.) (Lepidoptera: Plutellidae) is a highly widespread and most dangerous pest of plants from the Brassicaceae family, including broccoli, Brussels sprouts, cabbage, Chinese cabbage, cauliflower, collard, kale, kohlrabi, mustard, radish, and watercress, in Europe, Asia, Africa, America, and Australia [1,2,3,4,5,6,7,8,9], though there is no consensus of the place of the DBM’s origin [10,11,12,13]. In addition to the cultivated, wild-growing, and weedy plants of the Brassicaceae family [1,14,15], the DBM attacks some plants from other botanical families [16,17,18]. Due to its exceptionally high migration activity, the DBM can be found almost anywhere where its host plants grow, and due to climate warming, the harmful activity of this pest is expected to further increase [19,20]. So, the DBM is deservedly considered one of the most difficult insect pests in terms of plant protection and, consequently, one of the most harmful [7,14,21]. For instance, at the end of the 20th century, annual losses from the DBM were estimated at USD 1 billion worldwide [11], and by the end of the first decade of the 21st century, the harm caused by this insect is estimated to be in the range of USD 4–5 billion, according to the most conservative estimates [22].
In Russia, the frequency of DBM outbreaks has greatly increased over the past decade. The geographic range of these outbreaks has also expanded, and they now periodically occur not only in different regions of the European but also in the Asian part of the country (in Siberia), although at the end of the last century, this species was considered only to be a secondary pest capable of causing damage to cabbage crops, only in some years [23,24,25]. The increase in DBM harmfulness in Russia was apparently initiated by the following: (1) climate warming (there is an earlier appearance of the pest in the fields, as well as an increase in the number of generations per season), (2) the expansion of acreage under cabbage crops and primarily under rapeseed, and (3) the spread of minimal tillage, which creates conditions for the accumulation of a wintering stock of the pest [25,26,27]. Thus, in recent years, the harm of the DBM in Russia has increased by several orders of magnitude, and outbreaks of the pest have been observed every 4–6 years. Consequently, this insect is now considered one of the worst pests for cabbage crops in the Russian Federation and its neighboring countries (Belarus, Kazakhstan, etc.) [23].
In Russia, as in other countries, DBM control is based mainly on the use of a wide range of insecticides. Preparations containing active substances from a variety of chemical classes, such as pyrethroids, neonicotinoids, organophosphorus compounds, avermectins, chitin synthesis inhibitors, juvenile hormone analogues, alkaloids, and carbamates, are used, and the effectiveness of many insecticides is ensured by the interaction of two and sometimes even three toxicants [28]. The intensive use of insecticides in Russia, as it is everywhere in the world, leads to breeding resistant DBM populations [23,29]. Currently, 642 cases of resistance have been recorded worldwide in various populations of the DBM to 101 different insecticides [30], and reports of new resistance cases are becoming more frequent in the literature [31,32,33,34,35,36,37]. As a result, the difficulty of controlling the DBM is determined not only by its extremely high migratory activity [11,38,39,40,41,42,43,44,45] but also by its ability to rapidly develop resistance to almost all insecticides, both chemical and biological [1,33,46,47,48,49,50,51]. Therefore, improving DBM management in most countries includes developing and implementing a range of measures, including not only insecticide use but also biological control, host plant resistance, habitat management, regulation of planting period, push–pull strategies, trap cropping, intercropping, crop rotation, genetic control, etc., that try to consolidate into integrated systems [1,3,8,52,53,54,55,56,57,58]. One of the most important elements of these systems is undoubtedly monitoring of the pest [59,60,61,62].
The composition and properties of the female sex pheromone in the DBM have been studied since the 1970s [63,64,65,66,67]. The main components of this semiochemical were first identified as (Z)-11-hexadecenal and (Z)-11-hexadecenyl acetate [68,69], then (Z)-11-hexadecen-1-ol [70]; finally, a minor component, (Z)-9-tetradecenyl acetate [71], was discovered. Later, it was determined that the composition of the DBM sex pheromone varies within the insect’s range, and accordingly, to ensure maximum attractiveness, the composition of the synthetic sex attractant (SSA) for a specific population needs to be clarified [72,73,74,75]. For the DBM found in Russia, the most attractive composition includes (Z)-11-hexadecenal and (Z)-11-hexadecenyl acetate in a ratio of 10:90, with (Z)-11-hexadecen-1-ol added as a minor constituent (0.99% of the total composition) [76].
Traps equipped with DBM SSA are widely used for pest monitoring [60,77,78,79,80,81,82,83,84,85,86,87]. The composition of the SSA, designed for pest monitoring in Russia, has undergone detailed field testing, during which the concentration of active substances and the dispenser type were optimized [76,88]. Currently, two commercial organizations distribute DBM SSA in Russia: JSC Shchelkovo Agrochem (https://betaren.ru/) and Pherotrap LLC (https://pherotrap.ru/).
In addition to monitoring, semiochemicals are increasingly used as a tool for controlling insect pest populations [89]. Thus, to control the DBM, SSA can be used to achieve the following: (1) male disorientation during mating [90,91,92,93,94,95,96,97,98,99], (2) mass capture of adults in order to gain male annihilation [69,100,101,102,103,104,105,106], and (3) the spread of entomopathogenic microorganisms in the pest population by an autodissemination process [107,108,109]. Pilot trials in Russia also have shown promising results using SSA to control the DBM [110].
Although pheromone monitoring is a simple, low-cost, and fairly accurate method for detecting and counting most insect pest species, it is known that sex pheromones in Lepidoptera typically attract only males, while harmful offspring are produced by females [111]. Therefore, it is not surprising that sometimes the density of DBM adults captured by SSA-based traps does not correlate with the density of larvae feeding on plants [60]. For this reason, other attractive semiochemicals are being tried in addition to SSA to lure not only male but also female DBMs. Volatile organic compounds isolated from DBM host plants are among the most attractive to both sexes [112,113,114,115].
Even though DBM adults are capable of flying during the daytime, most of their flying activity begins at dusk and continues at night [116,117,118,119,120]. Moreover, under controlled conditions it was found that adult movement is strongly dependent on the illumination in their surroundings: it is almost instantly suppressed by light simulating daylight and activated almost immediately after darkness [121]. Accordingly, DBM adult activity during twilight and night is characterized by the phenomenon of positive phototaxis, which is inherent in both sexes. Therefore, capturing moths with light traps remains relevant for this species [122,123,124,125,126]. Until recently, the use of light traps for trapping the DBM and other microlepidoptera species was hindered by the bulkiness of their design and dependence on power sources [40,127]. However, thanks to the advent of LED technology, this situation has improved dramatically in recent years [126,128,129,130]. In comparison with gas-discharge and incandescent lamps, LEDs have a number of advantages: they are distinguished by a significantly longer service life, low energy consumption, higher luminous efficiency, adjustable color temperature, compactness, low heating, resistance to mechanical damage, and low maintenance costs [131,132,133,134]. And most important, LEDs are able to provide large insect captures in comparison with light sources of previous generations [135,136,137,138].
It has been shown that not only SSA but also LEDs can be used in traps both for monitoring and for suppressing DBM populations by mass-trapping adults, which significantly reduces insecticide treatments [139,140]. Given the relatively low selectivity of insect species for light attraction, low-intensity LEDs are currently considered the most promising for trapping harmful species. In fact, low-intensity LEDs exhibit a low level of danger to non-target entomofauna since traps equipped with these LEDs have the following characteristics: (1) they are easily placed in the habitats of target insect pests due to their compact size, and (2) their attractiveness is limited by rather short distances [141,142,143,144,145].
Taking into account the real possibility of achieving synergy, researchers are increasingly paying attention to combining different baits for insect trapping. In particular, it has been found that a mixture of semiochemicals of plant origin with DBM SSA not only can provide more effective monitoring but also guarantees an environmentally safe reduction in pest populations [146,147]. As for evaluating the potential of combining both SSA and LEDs in traps as a way to enhance the attractiveness of traps for DBM adults, no such work has yet been conducted. To date, only a few articles have compared DBM catches in traps equipped with either LEDs or SSA individually [140,148,149].
The purpose of this study is to conduct field trials of traps equipped with both SSA and UV LEDs to quantify effects of the interaction of these lures for capturing DBM adults.
2. Materials and Methods
2.1. Site of Experimentation
The trapping trials were conducted in the vicinity of St. Petersburg (northwestern Russia) at the experimental field of the Pushkin and Pavlovsk Laboratories of the N. I. Vavilov All-Russian Institute of Plant Genetic Resources (VIR) (PPL VIR) in the city of Pushkin (59°42′51″ N, 30°23′47″ E) (Figure 1) in 2022–2024.
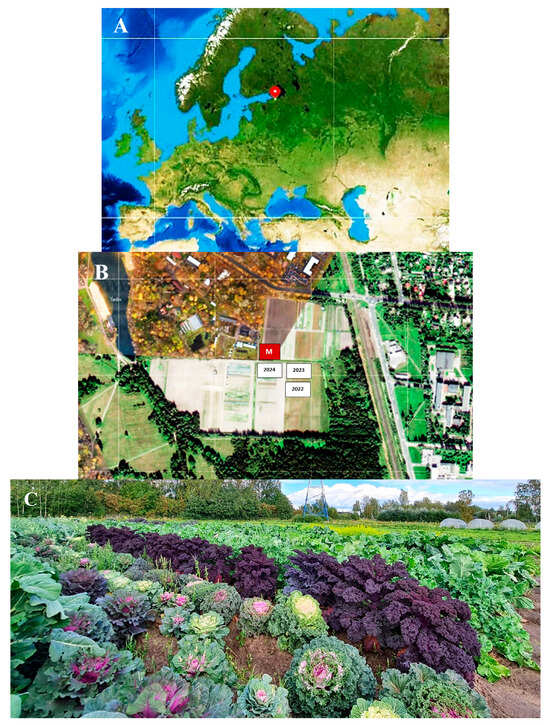
Figure 1.
Area under experimental study at PPL VIR (Pushkin, near St. Petersburg, northwestern Russia). (A) Geographical location of the place of work (the vicinity of St. Petersburg). (B) Google map of the part of the city of Pushkin where the cabbage collection was grown and where field trials of traps equipped with various baits for catching DBM adults were carried out. M—meteorological station; 2022, 2023, and 2024—plots occupied by cabbage in 2022, 2023, and 2024. (C) General view of the site where the VIR world collection of cabbage was grown and where the traps for catching DBM adults were placed.
The site on which samples of the VIR world collection of cabbage were grown in 2022–2024 had an area of at least 1500 m2. The area had previously been planted with squash, pattypan, and pumpkin. The collection material of the garden cabbage crops (white cabbage, red cabbage, leafy cabbage, Brussels sprouts, cauliflower, and broccoli), represented by different varieties and hybrids, was grown with a seedling method. As a rule, seedlings were planted in the ground in the first week of June. There were 20 plants per plot; row spacing was 70 cm; distance between plants within the plot was 60 cm. Three days before planting the seedlings in the ground, they were treated with Syngenta Aktara VDG (250 g/kg) to control cabbage flies, Delia radicum (L.) and flea beetles, and Phyllotreta spp. In 2022, 35 days after the seedlings were planted in the ground, a single treatment of the site was carried out with Syngenta Proklame VG (50 g/L) controlling a complex of lepidopteran pests. In 2023, 16 days after planting the seedlings in the ground, the site was treated once with Aktara VDG to control cabbage flies and flea beetles. In 2024, 16 days after planting the seedlings, the site was treated once with Keminova A/S Danadim Expert CE (400 g/L). Weeding, loosening, and fertilization with ammonium nitrate as well as row-by-row plant processing were also carried out during the growing season of the cabbage crop.
Figure 2 illustrates the meteorological conditions on the territory of the PPL VIR during the summer months of 2022–2024 based on data obtained at the meteorological station (see Figure 1).
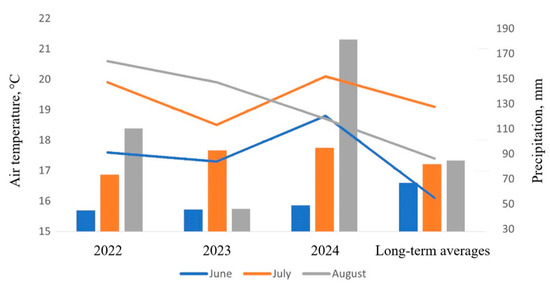
Figure 2.
Meteorological conditions in the summer months of 2022–2024 in the territory of the PPL VIR, St. Petersburg. Lines: average monthly air temperatures, °C. Columns: monthly precipitation, mm.
2.2. Traps and Insect Baits
To carry out field trials to assess the attractiveness of a set of baits for DBM adults, we used Delta traps manufactured according to utility model patents RU 195732 U1 and RU 220753 U1 by the Innovation Center RIKSO LLC (St. Petersburg, Russia) (https://companium.ru/id/1037800125241-ic-rikso, accessed on 20 August 2025) (Figure 3). In the upper part of each trap made of transparent plastic, a removable cassette was installed, which contained a power supply (six AA 1.2 V batteries with a capacity of 2200 mAh each), a photosensor, a board with two low-intensity LEDs, control unit in the form of an Attiny 25 V microcontroller with a recorded LED control program and photoresistor connected to it, an illumination programming button, and an electric throttle. The removable cassette was easily installed in the trap and could be easily changed if necessary. Batteries were recharged using a solar panel mounted on one of the side faces of the trap. Considering that the DBM adult is actively attracted to UV radiation in the range of 365–400 nm [126,139,140], two low-intensity LEDs manufactured by Oumurui (Shenzhen, China) with a wavelength of 365–370 nm which radiated light in opposite directions from each other were installed in each trap. The rated power of each LED was 3 W, the electric current was 40 mA, and the estimated luminous flux in the UV range was 25–35 lm each. The LED power supply was controlled by an Attiny 25 V microcontroller. The program loaded into it provided control of the brightness of the LED glow by using the following: (1) pulse power supply with a frequency of 30 kHz; (2) software setting of the brightness of the LEDs; (3) switching on and off the LED at a preset (programmable) illumination level; (4) minimization of thermal energy loss of power sources; (5) disconnection of batteries to prevent their deep discharge; and (6) determination and display of battery charge level.
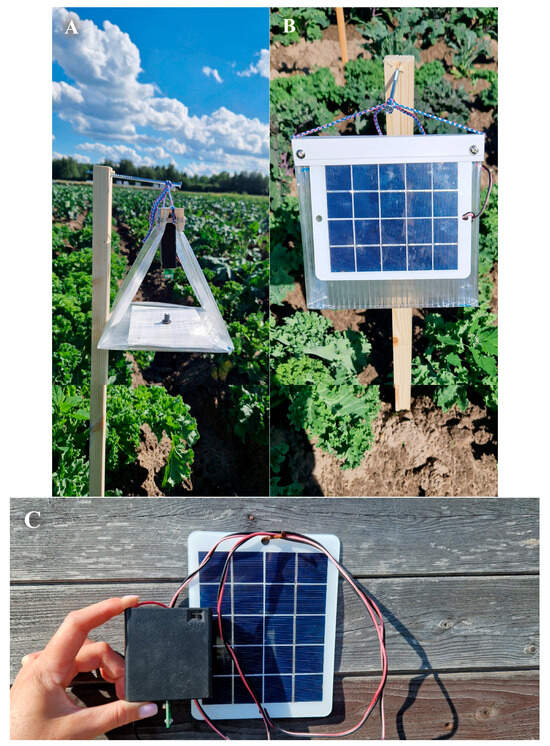
Figure 3.
The Delta trap used for catching DBM adults. (A) SSA + LED experiment option, full-face view. A cassette with LEDs is installed at the top of the trap, and an SSA dispenser is installed at the bottom. (B) The same, profile view. A solar battery is fixed on the side of the trap. (C) The LED cassette and solar battery are shown separately from the trap body.
Previously, it was experimentally demonstrated that an LED power supply using high-frequency pulsed current contributes to a highly significant increase in DBM collection by light traps [150]. This result was achieved due to the psychophysical effects of flicker fusion at high frequency [151] and visible persistence [152], although the energy consumption when powering LEDs with pulsed and direct current was the same (Patent RU 220753 U1). The frequency of a pulsed LED power supply (and, accordingly, flicker) equal to 30 kHz was chosen based on the desire to minimize the size of the construction, the capabilities of microcontroller, and the time of switching on/off transients of LEDs, as well as an analysis of the literature on the effects of flickering lights on insect behavior [150,153].
A commercial product from Pherotrap LLC (Moscow, Russia) (https://pherotrap.ru/) was used as the source of SSA in the traps, namely, rubber dispensers impregnated with DBM SSA (a mixture of (Z)-11-hexadecenal and (Z)-11-hexadecenyl acetate in a ratio of 10:90 to which as a minor component (0.99% of the total composition) (Z)-11-hexadecene-1-ol was added). To trap the attracted insects, sticky cardboard manufactured by the same company was placed at the bottom of each trap. The SSA dispenser was placed in the center of the sticky cardboard. As a result, direct exposure of the light radiation from the LEDs on the SSA dispenser in the trap was excluded, which ensured the correct assessment of the effect of the interaction of SSA and UV radiation on insect attractiveness while using both baits in one trap.
The automatic switching on of the LEDs in the traps was adjusted to the actual observed illumination level during sunset, and the switching off was set for illumination during sunrise; these times were recorded annually before the start of trap trials in early June.
2.3. Experimental Design
In the trials, the following baits were tested in traps: (1) dispensers with SSA; (2) LEDs; (3) a combination of a dispenser with SSA and LEDs (SSA + LED); and (4) no bait in the trap (Control). The traps were placed on wooden stakes at a height of 60 cm above the ground and at a distance no closer than ten meters from each other and from the edge of the cabbage plot, in three randomized blocks (Figure 4). Traps were installed immediately after planting cabbage seedlings in the ground. The catches of DBM adults by traps were recorded until the second week of September over three seasons (2022–2024). Before the first DBM adult appeared in the traps, they were checked daily; subsequent counts were carried out at least twice weekly. During inspection of the traps, DBM adults caught were counted and removed from the sticky cardboard, and the sex of each individual was determined based on the external structure of their genitalia [154]. The sticky cardboard was replaced as it became dirty, and the SSA dispenser was changed once a month.

Figure 4.
Delta traps placed on wooden stakes in a cabbage plot at no closer than ten meters from each other.
2.4. Statistical Analysis
It is well known that obtaining resulting data close to 0 in particular experimental variants (usually the control) is a problem when performing parametric statistical analysis [155]. Since even after transforming the data of DBM captures according to the recommendation [156], a significant portion of the distributions of captured insects did not meet the requirements for correct implementation of ANOVA (normality of distributions according to the Shapiro–Wilk test and uniform variances according to the Levene and Cochran criteria), non-parametric Kruskal–Wallis and Mood’s Median tests were used for statistical analysis. After proving the validity of the bait effect, the significance of differences between the averages was evaluated according to the Wilcoxon–Mann–Whitney pairwise test, post hoc Bonferroni-corrected for multiple comparisons at 5% significant level (α = 0.05). To assess the effects of the interaction of the attractive properties of UV LED and SSA, the Adjusted Rank Transform Test was used [157], which is recommended for verifying the validity of interactions between factors with non-normal distributions [158,159,160,161].
To determine if the ranking of DBM adult capture distributions across various treatments consistently follows a monotonic pattern, we employed both Friedman and Page [162] tests (the latter was assessed to prove the significance of the linearity effect in ranking). To visualize the variation in DBM captures using traps with different treatments during a weekly period, diagrams were made both over variations for each year and over all three years together (in the form of a box-and-whisker plot). The quantitative assessment of increased DBM trapping resulting from combining two baits (SSA and LED) is characterized by a multiplicity of increases in moth collection due to retrofitting with second bait (LED) of traps previously equipped with SSA, as this bait is used practically constantly for trapping DBM adults, e.g., [163,164]; therefore, only such an indicator allows a comparison of the results obtained with the literature data. To perform all the abovementioned work, MS Excel 2024, Statgraphics Centurion 19, and Tibco Statistica 14 were used.
3. Results
3.1. Statistics of DBM Adult Captures Using Delta Traps with Different Baits
The results of capturing DBM adults at the experimental site of the VIR cabbage collection using traps with four treatments (Figure 5) are presented in Table 1. During a period of three years (2022–2024), a total of 14,655 DBM adults were captured, with the vast majority being males (98.5%). The SSA + LED traps captured almost three times more DBMs than LED alone and almost 13 times more than SSA alone (Table 1).

Figure 5.
Randomly selected photos of sticky cardboard containing insects captured, which were in traps with different treatments for the same three days. (A) Dispenser with SSA. (B) LED. (C) SSA + LED. (D) Without lure (Control).

Table 1.
Captures of DBM adults using Delta traps equipped with four combinations of lures (PPL VIR, Pushkin, 2022–2024).
The results of statistical analysis of data on the catches of DBM adults using traps equipped with different baits for each year and a total of three years’ worth of trials are presented in Table 2. These data indicate a statistically significant difference (Kruskal–Wallis and Mood’s Median tests) in average capture rates between males and females using different baits. It is important to note that there was a highly significant synergistic interaction between the SSA and LED baits in terms of their attraction to DBM males, as determined by the Adjusted Rank Transformation Test (F = 8.56, p < 0.001). As for the females, the SSA did not appear to be attractive, as expected, while the LEDs were attractive but approximately 55 times less than for males on average over 3 years of trials. Consequently, the interaction effect of SSA and LED on females was statistically insignificant (F = 1.39, ns) in contrast to males. However, if the catches of males and females are considered in total, i.e., without taking into account the sex of the captured adults, then the synergy effect of SSA and LED attractions can be proven at a high level of significance (F = 7.27, p < 0.01). This is not surprising, since males are attracted to traps many times more often than females: in three years, the proportion of females captured in traps with different treatments was estimated at only about 1.5% (Table 1).

Table 2.
Comparison of DBM adult captures (per seven days) using Delta traps baited with four combinations of lures (with or without SSA and UV LED).
3.2. Monotonic Relationship Among the Distributions of DBM Catches with Different Treatments in Traps
To more accurately characterize the variation in DBM adult trapping trials, it is important to assess whether the order of DBM adult capture distributions across different treatments remains consistent over time. For this purpose, we used the averages across three randomized blocks of DBM adult captures during the 2022–2024 growing seasons (Figure 6) for analysis using the Friedman test. This test clearly validated the robust statistical differences between treatment rankings based on DBM captures: χ2 values were 30.28 in 2022, 30.51 in 2023, and 31.61 in 2024 (df = 3, p < 0.000001 in all cases). Furthermore, we used the Page test [162] to verify the consistent monotonicity of DBM adult catch distributions across the four treatments, i.e., effect of linearity in rankings. First, according to the gradations of the distributions of trapping treatments by quartiles, non-outlier ranges, and outliers of captured moths (Figure 7), each treatment was assigned a rank from 1 to 4: 1 for Control, 2 for SSA, 3 for LED, and 4 for SSA + LED. In the result, the Page test confirmed a highly significant linear component in the ordering of the four treatments over time: L = 375 in 2022, L = 379 in 2023, and L = 378 in 2024 (n = 4, m = 13, and p < 0.001 in each analysis).
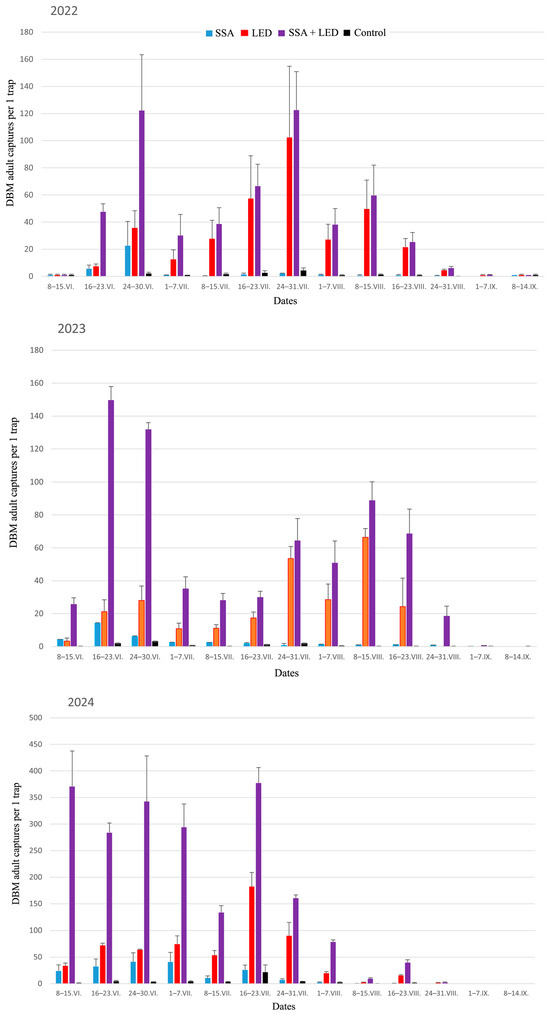
Figure 6.
Dynamics of DBM adult (males + females) captures (x ± SE) with the four different treatments over a weekly period from 8 June to 14 September each year in 2022–2024. SSA = synthetic sex attractant, LED = light-emitting diode, and Control is traps equipped with neither of these.
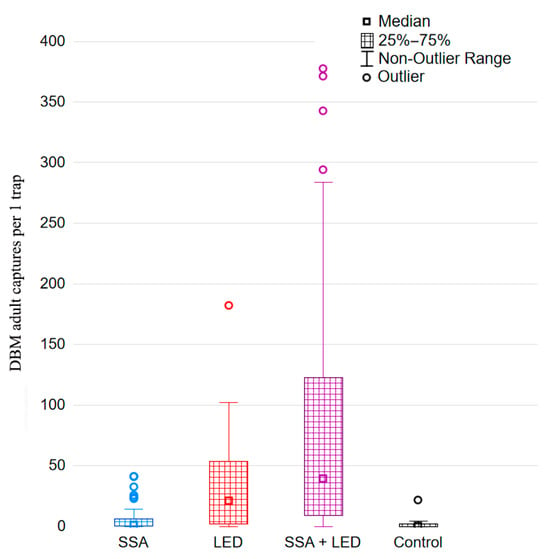
Figure 7.
Box-and-whisker plot of DBM adult (males + females) captures with the four different treatments over a weekly period in each of the three years (2022–2024). SSA = synthetic sex attractant, LED = light-emitting diode, and Control is traps equipped with neither of these.
3.3. The Increase in the Number of DBM Adults Collected by Traps Due to the Interaction of Bait Attractiveness
As mentioned above, the increase in insect trapping as a result of combining two baits in a trap was usually characterized in publications by a multiplicity of increases in insect captures due to the retrofitting with additional bait of traps, which were already equipped with the main bait conventionally used for trapping target species. The main bait normally used to catch DBM adults is SSA [60,82,83,84,85,86,87]. Therefore, in order to compare our results with the literature data, we estimated the effect of retrofitting SSA-based traps with LED as the second bait on an increase in DBM adult captures (Table 3). If we use data on whole-moth captures from traps equipped with SSA, LED, and SSA + LED (Table 1 and Table 2) to quantify the synergistic effect, the multiplicity of adult collection increases ranges from 11.2 to 19.2 when retrofitting SSA-based traps with LED bait. On average, this gives a 15.1-fold increase (Table 3).

Table 3.
The multiplicity of the increase in DBM adult captures after retrofitting SSA-based traps with LEDs as the second bait.
3.4. Correlation Analysis of DBM Adult Captures Using Traps with Different Baits
The findings of the correlational analysis (Table 4) primarily suggest that the traps equipped with SSA and LED exhibit distinct patterns in the population dynamics of DBM adults. Secondly, the data presented in Table 4 underscore the consistency in the manifestation of statistically significant correlations between the numbers of captured DBM adults in traps with SSA and LED on the one hand and in SSA + LED-equipped traps on the other hand. This observation implies that employing traps equipped with SSA + LED can provide a comprehensive overview of pest population dynamics, which differs from that obtained by using SSA or LED traps individually (Figure 6), making it a valuable tool for monitoring purposes.

Table 4.
Matrix of Pearson correlation coefficients for the results of capturing DBM adults using Delta traps with different treatments in 2022–2024.
4. Discussion
Insect traps, which come in a variety of designs, have long been widely used in plant protection practices, both as a monitoring tool and as a means of controlling crop pests [165]. Traps typically use various methods of attraction to manipulate the behavior of insects by influencing their sensory systems [166,167]. The use of these traps to manipulate pests’ behavior fully aligns with the principle of using approaches that prioritize the safety of the environment [168].
In accordance with the specifics of the effect on the sensory organs of insects, the means of attraction are usually divided into attractants of a chemical (semiochemicals) or physical (semiophysicals) nature [169]. Among semiochemicals, a diverse range of compounds have been identified that control a wide array of insect behaviors, with SSA compounds, specifically providing intraspecific communication, most frequently employed in plant protection practices [170,171]. Semiophysicals, on the other hand, encompass a variety of distinct signals, such as sound impulses and vibrations [169], although light radiation is predominantly employed for the purposes of pest management [172].
The joint functioning within traps of attraction tools with diverse modalities holds promise, offering two key advantages: (1) a substantial enhancement in the dependability of parent–offspring regression relationships as a predictive framework for monitoring models when low population occurs, and (2) a substantial reduction in labor expenses for mass fishing operations due to a diminished requirement for traps to achieve the desired level of pest reduction. Consequently, the integration of attractants with various modalities within traps, resulting in a multimodal influence on insect behavior, offers the potential for improved trap efficacy both in monitoring tasks and in pest management efforts [172]. Since the mid-20th century, scientists have been endeavoring to enhance the effectiveness of traps by integrating light signals with specific odorous lures. This approach has proven successful in capturing males of various moth species, such as cabbage looper, Trichoplusia ni (Hbn.) [173,174], tobacco hawk moth, Manduca sexta (L.) [175,176,177], and tobacco budworm, Chloridea virescens (F.) [178], when virgin females were placed into a specially designed chamber within the light trap.
The data presented in this paper strongly suggests that when SSA and LED signals are combined, they can produce a synergistic enhancement in the capture of DBM males using Delta traps. As a result of retrofitting SSA-based traps with LEDs as an additional lure, catches of DBM adults showed an average 15-fold increase (Table 3).
The findings presented in this study are of interest for the following reasons: (i) their contribution to our knowledge of ranges in interactions between different types of insect lures, (ii) their potential applications in improving DBM monitoring, and (iii) the possible enhancement of the efficiency of mass-trapping techniques to control the pest.
4.1. Interactions of Baits with Various Insect Attraction Modalities
There is a large amount of evidence in the literature for the increased efficiency of using a combination of various attractants—semiochemicals (pheromones, allomones, kairomones, and synomones) and semiophysicals (primarily light radiation) to trap harmful insects, which achieves an increase in attraction [169,172,179]. For example, when traps were equipped with a combined bait of SSA and LEDs, it was possible to increase the catch of sweet potato weevil males, Cylas formicarius (Fabr.), almost five-fold [164]. In addition, traps equipped with UV-emitting lamps and SSA attracted six times more cabbage looper (Trichoplusia ni) males than traps with UV lamps alone [163], and the combination in traps of UV LED with semiochemicals resulted in a 2–12-fold increase in catches of adults of four harmful Lepidoptera species, including orchard codling moth, Cydia pomonella (L.), oriental fruit moth, Grapholita molesta (Busck), oblique banded leafroller, Choristoneura rosaceana (Har.), and eye-spotted budmoth, Spilonota ocellana (Den. et Schiff.). Interestingly, when low-intensity LEDs were used alone for trapping, no insects were attracted [145]. The increase in attractiveness when combining semiochemicals and semiophysical attractants has also been shown to be rather synergistic for some other insect species, including red flour beetle, Tribolium castaneum (Herbst) [180], cigarette beetle, Lasioderma serricorne (F.) [181], brown marmorated stink bug, Halyomorpha halys (Stål) [182,183], western flower thrips, Frankliniella occidentalis Perg. [184], midnight woodwasp, Sirex noctilio F. [185], and several species of sink flies from the Psychodidae family [186,187]. In some cases, there were other effects of the interaction between light and chemical signals, including additive, non-additive, and even antagonistic ones [188,189,190,191]. Although the latter are rarely mentioned in the literature, a comparative analysis of published information on the scale of increase in insect trapping as a result of combining semiochemicals and light radiation shows that the highest recorded synergy effect is a nine-fold increase in captures of the tomato leafminer, Tuta absoluta (Meyrick) [192]. However, the data presented in this article suggests that the current record now belongs to the DBM, with a 15-fold increase in catches after SSA traps were retrofitted with LEDs.
4.2. Prospects for Combining SSA and LED in Traps for DBM Monitoring
In the field of pest management, insect traps have long been used primarily to monitor harmful insects [193]. Until now, light traps continue to be widely used for ecological and faunal research, as well as for studying insect distribution patterns and phenology, providing long-term data on population dynamics across various regions worldwide [194,195,196,197,198]. However, in the context of routine phytosanitary surveillance for insect pests in agriculture, light traps have been superseded by pheromone traps since the 1970s and 1980s due to their inferior specificity, higher cost, and limited mobility [199,200,201]. At present, owing to the exceptional properties of LED technology, there is a trend towards an increasing use of light traps for monitoring different agricultural pests. This trend is gaining momentum due to the efficiency of LED-based solutions in this field [137,138,202].
It is well known that pheromone-based products exhibit a wide range of unique properties, including selectivity of action, high efficacy, low toxicity, high volatility, and short persistence [203,204,205,206,207]. However, the degree of attractiveness of these products to insects varies significantly depending on various factors, such as weather or climatic conditions, the specific composition of the pheromonal attractant, trap design and manufacturing techniques, characteristics of insect reproductive behavior, and the state of the target pest population [111,207,208,209,210,211,212,213,214]. Similarly, the attractiveness of light-based stimuli for insects also varies significantly depending on many factors and especially on the level of natural illumination [209,215,216,217,218,219,220]. Also, since pheromone and light traps are equipped with attraction devices with fundamentally different modalities, their attractiveness varies in a completely different way. So, it has been repeatedly recommended to use both types of traps together to achieve a more accurate prediction of the population dynamics of the number of harmful insect species [209,221,222,223,224,225]. It is likely that similar results can be achieved by combining light and pheromone baits within a single trap, especially because in the case of synergistic or even additive effects of the lures, in addition to increased reliability of monitoring, sometimes one can also expect an increase in the negative impact of the traps on the density of pest populations [145,164,180,181,182,183,184]. As for the DBM, given the exceptional additive interaction of LED and SSA baits in terms of attractiveness to the adults of this species, the advantages of monitoring the pest using traps equipped with a combination of these baits seem quite evident. Furthermore, as the results of the correlation analysis (Table 4) demonstrate a high level of consistency in statistically significant associations between the numbers of captured DBM adults in traps with SSA or LED on the one hand and SSA + LED on the other hand, the utilization of traps equipped with SSA + LED allows us to anticipate obtaining a more comprehensive understanding of the population dynamics of the pest, which is displayed differently by traps equipped with either SSA or LEDs.
4.3. The Potential of Combining SSA and LED for Mass Trapping of DBM Adults
In addition to monitoring, SSAs are used as a means to control the number of harmful insect species, which is achieved through the use of various technologies such as mass trapping, attract-and-kill, mating disruption, and push–pull. Of these, the first two techniques, which are similar in principle, have certain advantages over the latter two, as they are more cost-effective, requiring much smaller amounts of pheromone-based substances [111,171,204]. Although mating disruption technology occupies a leading position throughout the world in terms of its application due to its high efficacy and adaptability [207,226,227,228], mass-trapping technology also holds promising prospects [229]. For instance, while attract-and-kill systems that utilize gel drops require insects to come into direct contact with the odor source [230], traps designed for mass trapping typically do not necessitate such behavior of the target object [204]. Although pheromones that attract individuals of both sexes of a harmful species have a stronger negative impact on population dynamics, SSA attracting only males can also suppress pest reproduction due to the fact that male removal prevents mating with females, thus preventing the development of offspring that damage plants. Despite some challenges associated with the implementation of mass-trapping techniques using SSA [172], a significant number of successful examples have been documented in the literature, both in standalone use and in combination with other control approaches [111,204].
The literature contains a wealth of data that suggests the efficiency of employing DBM SSA as a tool for controlling the pest through the implementation of mass-trapping techniques. This approach effectively depletes the male population, leading to delays, reductions, and avoidance of fertilization in females, ultimately resulting in a decrease in the density of larvae that feed on plants, which, in turn, reduces plant damage and promotes crop growth [100,101,102,103,104,105,106].
One of the first attempts to assess the feasibility of using pheromone traps as a method for large-scale DBM trapping was the testing of SSA-equipped Pherocon® 1CP traps in Canada in the late 1970s [69]. Experiments conducted in India in the late 1980s demonstrated that sticky Delta traps equipped with SSA, installed in experimental cabbage fields (six traps per field), caught between 11 and 13 thousand DBM males per season, resulting in a significant reduction of plant damage caused by the pest and, consequently, a substantial increase in cabbage yield [100]. Subsequently, Chinese researchers found that the use of three SSA-based traps per 180 m2 greenhouse ensured the capture of more than 1800 DBM adults per season, allowing for the achievement of 70% efficiency in suppressing the DBM population, albeit under conditions of low pest egg density (one to three eggs per plant) [101]. In a mountainous region of China, traps containing SSA were shown to catch between 11 and 70 moths daily, significantly reducing the population of the DBM and preventing the need for insecticide treatments to protect cabbage [103]. Experiments conducted in Vietnam evaluated the efficiency of three types of traps and two SSA combinations, demonstrating the potential for mass trapping of DBM adults in the tropics. In these experiments, the maximum catches of male moths (up to 170 or more individuals per day) were obtained using traps in the form of plastic basins filled with water [102]. The efficiency of mass trapping as a standalone method to control the DBM was assessed over two seasons in India on cabbage, also using SSA-based traps in the form of basins filled with water. In the first season, 675 moths were captured by 21 traps in the experimental cabbage field (i.e., an average of 32.14 individuals per trap), and in the second season, 13,935 moths were captured by 24 traps (an average of 580.60 individuals per trap). As a result, the maximum reduction in the number of DBM larvae and pupae on the experimental plants compared to the control was observed in plots where moth trapping was used: in the first season, this resulted in a decrease of 73.96% in the number of larvae and pupae compared to the control, while in plots where conventional farming methods were used (five insecticide treatments per season), the decrease was only 46.11%. In the second season, mass moth trapping resulted in a 51.42% decrease in larvae and pupae compared to the control, whereas using conventional farming methods resulted in a population decrease of only 39.04%. The largest percentage reduction in the DBM population compared to the control was observed during mass trapping as a standalone method. This was largely due to the significant increase in the population of the DBM parasitoid Cotesia vestalis (Hal.) as opposed to plots where conventional farming methods were used. Based on calculations using the data obtained, it was recommended that traps should be placed in fields at a density of 40 per acre (98.8 per one ha) for mass trapping as a standalone technique [104]. As part of the standardization of using SSA-based traps for mass trapping of DBMs in India, it was shown that the maximum number of DBM adults (15,394 moths) was trapped in a cabbage field with a density of 24 traps per acre (i.e., 59.3 per ha). Since SSA traps actively attract DBM adults for 60 days and during this time farmers typically apply 8–10 insecticide treatments to cabbage, each trap installed on the field prevents 4–5 chemical insecticide applications per acre throughout the year [105]. Experiments conducted in Costa Rica with water-filled SSA-based traps placed at a density of 50 traps per one ha confirmed their high efficacy in controlling the DBM compared to insecticide treatments, as they provided a profit of USD 1240 per one ha [231]. Another publication presenting the results of studies conducted not only in Costa Rica but also in Nicaragua convincingly confirms the high efficacy of using SSA-based traps not only for monitoring but also for mass trapping of DBM males, which recommends placing traps with SSA at a density of 60 per ha in cabbage fields [106]. Therefore, the test results for SSA-based traps conducted under a diverse range environments indicate that the mass trapping of DBM males on cabbage fields at a density between 50 and about 100 traps per ha has the potential to reduce DBM populations to a level comparable to or even surpassing that achieved through the use of chemical insecticides.
The use of light traps as an alternative to chemical control of harmful insects predates the development of pheromone traps by several decades, dating back to the 1920–1930s, and often, their use proved to be highly effective [232,233]. Despite some advantages of light traps over SSA-equipped traps, such as their ability to attract a wider range of species, their trapping of not only males but also females, their longer lifespan, and other benefits, they were eventually superseded by pheromone traps in the 1970s due to the superior specificity, affordability, and portability of SSAs-based traps [234]. However, recent advancements in LED technology have sparked renewed interest in the utilization of light traps for pest management [125,130,137,234].
Experiments conducted in Indonesia using LED traps for mass trapping demonstrated their effectiveness in controlling not only the DBM but also other pests such as cabbage webworm, Crocidolomia pavonana (F.), and tobacco cutworm, Spodoptera litura (F.). The experiments were carried out using UV-emitting LED (365–385 nm) traps, which were designed as water-filled containers. Experimental cabbage plots were divided in half: one group of plots had light traps installed, and the other group did not. Plants in the first group of plots (with light traps installed) were periodically inspected for pest presence, and if the density of DBM larvae or C. pavonana eggs exceeded a harm threshold, the plants were treated with insecticide. Plants in the second (control) group of plots (without light traps) were sprayed with insecticide twice weekly invariably. DBM, C. binotalis, and S. litura adults were detected in traps installed on cabbage plots as early as four days after planting the cabbage seedlings. The number of DBM adults averaged 25 individuals per trap at this time, and 46–50 days after planting it exceeded 50 individuals per trap. The results obtained indicate that plants on plots with light traps needed only two insecticide sprays throughout the entire six-month test period, since the density of harmful species’ preimaginal stages on these plants was maintained below the harm thresholds. This reduced the number of insecticide treatments by 81.82% compared to the control, and the yield of cabbage on these plots was equivalent to the yield in the control. Therefore, the use of LED traps to protect cabbage from lepidopteran pests has proven to be economically profitable [139]. Further studies carried out in Indonesia aimed to compare the effectiveness of LED-equipped traps, DBM SSA, and SSA of C. pavonana in protecting cabbage from lepidopteran pests. The results showed that LED traps started attracting moth adults earlier than traps with either type of SSA and caught them more effectively. As a result, plots protected with LED traps had lower densities of harmful larvae and higher yields (1.75 kg per plant) than plots protected with SSA traps (1.18 kg per plant with DBM SSA and 0.92 kg per plant with SSA of C. pavonana) [140]. In the articles cited above, there is no information regarding the sex of moths caught in light traps. However, even if light traps in experiments conducted in Indonesia caught almost exclusively males, as in trials conducted in St. Petersburg (see Table 1), their negative impact on the DBM population was significantly greater than that of traps equipped with SSA, which was confirmed by comparing pest densities on plants and crop yields.
Based on the analysis of published materials, it can be concluded that SSA-based traps designed to capture exclusively males can significantly reduce the DBM population. With regard to LED-based trapping systems for DBM mass trapping, these appear to hold even greater potential in controlling pest harmful activity. However, it is clear that LED-based traps are more expensive than traps with SSA. Despite this, considering the great increase in DBM adult captures achieved by retrofitting SSA-based traps with LEDs (estimated at a 15-fold increase), it seems likely that a mass-trapping approach utilizing SSA + LED traps could effectively suppress DBM populations at a relatively lower cost. This is because the number of SSA + LED-based traps per hectare of cabbage cultivation required would be significantly lower than that needed when using SSA alone to effectively control the pest population.
It should be noted that the level of SSA and LED interaction, estimated by a 15-fold increase in trap catchability, was obtained under conditions of high latitudes. In these areas, highly varying natural illumination at night during the season prevents the LEDs from achieving maximum attraction of night-flying insects. Hence, at lower latitudes with their consistently low levels of natural illumination at night, the level of SSA and LED interaction may be even stronger. However, it is evident that through the use of SSA + LED traps, an effective reduction in DBM populations can be achieved when fewer traps are deployed per hectare compared to traditional SSA traps (which are deployed at 50–100 per hectare). This will further enhance the profitability of mass-trapping efforts for DMB management.
5. Conclusions
Trials of traps equipped with four treatments (SSA, UV LED, SSA + UV LED, Control) and installed in cabbage fields were conducted in the vicinity of St. Petersburg, Russia, for three years (2022–2024). The trials were successful in demonstrating a very high level of synergy between SSA and UV LEDs in attracting DBM adults, resulting in a 15-fold increase in DBM adult (mostly represented by males) catches. Analysis of published data indicates that this effect is likely the most substantial of those reported in the literature. In addition to its monitoring value, the combination of SSA + LED, which is highly attractive to DBM adults, makes it promising for use in mass-trapping technology.
6. The Patents Employed in the Production of Traps
Miltsen, A.A.; Grushevaya, I.V.; Kononchuk, I.V.; Malysh, Y.M.; Tokarev, Y.S.; Frolov, A.N. Light trap for insect monitoring. Utility model patent RU 195732 U1, 2020-02-04.
Zakharova, Y.A.; Miltsen, A.A.; Frolov, A.N. LED insect trap with pulse power supply. Utility model patent RU 220753 U1, 2023-10-02.
Author Contributions
Conceptualization, A.N.F.; methodology, A.N.F. and Y.A.Z.; investigation, Y.A.Z.; data analysis, A.N.F. and Y.A.Z.; data interpretation, A.N.F. and Y.A.Z.; writing—original draft preparation, A.N.F.; writing—review and editing, A.N.F. and Y.A.Z.; project administration, A.N.F.; funding acquisition, A.N.F. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by the Ministry of Science and Higher Education of the Russian Federation (Project 122032900135-0) and partially provided by the Russian Science Foundation (Project No. 22-26-00199).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to Alexander A. Miltsen (VIZR), Anna M. Artemyeva (VIR), and Eugene E. Radchenko (VIR) for their versatile, multifaceted, and invaluable assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef]
- Li, Z.; Feng, X.; Liu, S.S.; You, M.; Furlong, M.J. Biology, ecology, and management of the diamondback moth in China. Annu. Rev. Entomol. 2016, 61, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.P.; Singh, H.; Kumar, S.; Kumar, V.; Singh, G.; Singh, S.N. Diamondback moth, Plutella xylostella (Linnaeus)(Insecta: Lepidoptera: Plutellidae) a major insect of cabbage in India: A review. J. Entomol. Zool. Stud. 2018, 6, 1394–1399. [Google Scholar]
- Philips, C.R.; Fu, Z.; Kuhar, T.P.; Shelton, A.M.; Cordero, R.J. Natural history, ecology, and management of diamondback moth (Lepidoptera: Plutellidae), with emphasis on the United States. J. Integr. Pest Manag. 2014, 5, D1–D11. [Google Scholar] [CrossRef]
- Fathipour, Y.; Mirhosseini, M.A. Diamondback moth (Plutella xylostella) management. In Integrated Management of Insect Pests on Canola and Other Brassica Oilseed Crops; Reddy, G.V.P., Ed.; CABI: Wallingford, UK, 2017; pp. 13–43. [Google Scholar]
- Kumar, A.; Tiwari, G.; Singh, A.K. IPM practices for insect pests of major vegetable crops: An overview. Pharm. Innov. J. 2022, 11, 1728–1734. [Google Scholar]
- Mason, P. Plutella xylostella (diamondback moth). In CABI Compendium; CABI International: Wallingford, UK, 2022; p. 42318. [Google Scholar]
- Paudel, A.; Yadav, P.K.; Karna, P. Diamondback moth Plutella xylostella (Linnaeus, 1758)(Lepidoptera: Plutellidae); a real menace to crucifers and its integrated management tactics. Turk. J. Agric. Food Sci. Technol. 2022, 10, 2504–2515. [Google Scholar] [CrossRef]
- Elimem, M.; Kalboussi, M.; Lahfef, C.; Mhamdi, N.; Limem-Sellemi, E.; Hammami, A.; Koubaa, A.; Rouz, S. The diamondback moth in Tunisia: Risk analysis, and influence of biotic and meteorological parameters on its population dynamics. Biologia 2023, 78, 1035–1045. [Google Scholar] [CrossRef]
- Hardy, J.E. Plutella maculipennis, Curt., its natural and biological control in England. Bull. Entomol. Res. 1938, 29, 343–372. [Google Scholar] [CrossRef]
- Talekar, N.S.; Shelton, A.M. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 1993, 38, 275–301. [Google Scholar] [CrossRef]
- Kfir, R. Origin of the diamondback moth (Lepidoptera: Plutellidae). Ann. Entomol. Soc. Am. 1998, 91, 164–167. [Google Scholar] [CrossRef]
- Liu, S.S.; Wang, X.G.; Guo, S.J.; He, J.H.; Shi, Z.H. Seasonal abundance of the parasitoid complex associated with the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) in Hangzhou, China. Bull. Entomol. Res. 2000, 90, 221–231. [Google Scholar] [CrossRef]
- Sarfraz, M.; Dosdall, L.M.; Keddie, B.A. Diamondback moth—Host plant interactions: Implications for pest management. Crop Prot. 2006, 25, 625–639. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Hossain, M.R.; Park, J.I.; Kim, H.R.; Nou, I.S. Glucosinolate profiles in cabbage genotypes influence the preferential feeding of diamondback moth (Plutella xylostella). Front. Plant Sci. 2017, 8, 1244. [Google Scholar] [CrossRef] [PubMed]
- Löhr, B.; Gathu, R. Evidence of adaptation of diamondback moth, Plutella xylostella (L.), to pea, Pisum sativum L. Int. J. Trop. Insect Sci. 2002, 22, 161–173. [Google Scholar] [CrossRef]
- Sarfraz, R.M.; Dosdall, L.M.; Keddie, B.A. Performance of the specialist herbivore Plutella xylostella (Lepidoptera: Plutellidae) on Brassicaceae and non-Brassicaceae species. Can. Entomol. 2010, 142, 24–35. [Google Scholar] [CrossRef]
- Yang, F.Y.; Chen, J.H.; Ruan, Q.Q.; Wang, B.B.; Jiao, L.; Qiao, Q.X.; He, W.Y.; You, M.S. Fitness comparison of Plutella xylostella on original and marginal hosts using age-stage, two-sex life tables. Ecol. Evol. 2021, 11, 9765–9775. [Google Scholar] [CrossRef] [PubMed]
- Marchioro, C.A.; Foerster, L.A. Modelling reproduction of Plutella xylostella L. (Lepidoptera: Plutellidae): Climate change may modify pest incidence levels. Bull. Entomol. Res. 2012, 102, 489–496. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Walter, G.H.; Furlong, M.J. Predicting the impacts of climate change on the biological control of Plutella xylostella by Diadegma semiclausum. Agric. For. Entomol. 2023, 25, 251–260. [Google Scholar] [CrossRef]
- De Bortoli, S.A.; Polanczyk, R.A.; Vacari, A.M.; De Bortoli, C.P.; Duarte, R.T. Plutella xylostella (Linnaeus, 1758)(Lepidoptera: Plutellidae): Tactics for integrated pest management in Brassicaceae. In Weed and Pest Control—Conventional and New Challenges; Soloneski, S., Larramendy, M., Eds.; IntechOpen: Rijeka, Croatia, 2013; pp. 31–51. [Google Scholar]
- Zalucki, M.P.; Shabbir, A.; Silva, R.; Adamson, D.; Liu, S.S.; Furlong, M.J. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): Just how long is a piece of string? J. Econ. Entomol. 2012, 105, 1115–1129. [Google Scholar] [CrossRef]
- Andreeva, I.V.; Shatalova, E.I.; Khodakova, A.V. The diamondback moth Plutella xylostella: Ecological and biological aspects, harmfulness, population control. Plant Prot. News 2021, 104, 28–39. [Google Scholar] [CrossRef]
- Shpanev, A.M. New cases of massive reproduction of Plutella xylostella. Plant Prot. Quarantine 2021, 4, 27–30. [Google Scholar] [CrossRef]
- Shpanev, A.M. Development and harmfulness of the cabbage moth Plutella xylostella (L.) (Lepidoptera, Plutellidae) on spring rape crops in Leningrad Province. Entmol. Rev. 2023, 103, 123–130. [Google Scholar] [CrossRef]
- Kholod, A.S.; Korenyuk, E.F. Plutella maculipennis—The threat to the rape crops in the Omsk Region. Plant Prot. Quarantine 2016, 5, 32–33. [Google Scholar]
- Tuleeva, A.K.; Sarmanova, R.S. Pests of spring rape in Akmola Province. Plant Prot. Quarantine 2019, 12, 20. [Google Scholar]
- Agro XXI. Handbook of Pesticides 2025. Available online: https://www.agroxxi.ru/goshandbook (accessed on 10 June 2025).
- Emelyanov, D.A.; Sukhoruchenko, G.I.; Ivanova, G.P. Resistance of the diamondback moth Plutella xylostella L. to insecticides used in the North-Western Region of Russia. In 5th All-Russian Congress on Plant Protection, Book of Abstracts, Proceedings of the Congress on Plant Protection, St. Petersburg, Russia, 16–19 April 2024; VIZR: St Petersburg, Russia, 2024; p. 153. [Google Scholar]
- Mota-Sanchez, D.; Wise, J.C. The Arthropod Pesticide Resistance Database. Michigan State University. 2024. Available online: http://www.pesticideresistance.org (accessed on 10 June 2025).
- Bhandari, K.B.; Torrance, P.; Huffman, E.; Bennett, J.; Riley, D.G. Insecticide resistance in diamondback moth (Lepidoptera: Plutellidae) in Georgia. J. Entomol. Sci. 2020, 55, 416–420. [Google Scholar] [CrossRef]
- Uesugi, R.; Jouraku, A.; Sukonthabhirom Na Pattalung, S.; Hinomoto, N.; Kuwazaki, S.; Kanamori, H.; Katayose, Y.; Sonoda, S. Origin, selection, and spread of diamide insecticide resistance allele in field populations of diamondback moth in east and southeast Asia. Pest Manag. Sci. 2021, 77, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Banazeer, A.; Afzal, M.B.S.; Hassan, S.; Ijaz, M.; Shad, S.A.; Serrão, J.E. Status of insecticide resistance in Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) from 1997 to 2019: Cross-resistance, genetics, biological costs, underlying mechanisms, and implications for management. Phytoparasitica 2022, 50, 465–485. [Google Scholar] [CrossRef]
- Shehzad, M.; Bodlah, I.; Siddiqui, J.A.; Bodlah, M.A.; Fareen, A.G.E.; Islam, W. Recent insights into pesticide resistance mechanisms in Plutella xylostella and possible management strategies. Environ. Sci. Pollut. Res. 2023, 30, 95296–95311. [Google Scholar] [CrossRef]
- Shen, X.J.; Cao, L.J.; Chen, J.C.; Ma, L.J.; Wang, J.X.; Hoffmann, A.A.; Wei, S.J. A comprehensive assessment of insecticide resistance mutations in source and immigrant populations of the diamondback moth Plutella xylostella (L.). Pest Manag. Sci. 2023, 79, 569–583. [Google Scholar] [CrossRef]
- Chang, C.C.; Dai, S.M.; Chen, C.Y.; Huang, L.H.; Chen, Y.H.; Hsu, J.C. Insecticide resistance and characteristics of mutations related to target site insensitivity of diamondback moths in Taiwan. Pestic. Biochem. Physiol. 2024, 203, 106001. [Google Scholar] [CrossRef] [PubMed]
- Oplopoiou, M.; Elias, J.; Slater, R.; Bass, C.; Zimmer, C.T. Characterization of emamectin benzoate resistance in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. 2024, 80, 498–507. [Google Scholar] [CrossRef] [PubMed]
- French, R.A.; White, J.H. The diamond-back moth outbreak of 1958. Plant Pathol. 1960, 9, 77–84. [Google Scholar] [CrossRef]
- Lokki, J.; Malmström, K.K.; Suomalainen, E. Migration of Vanessa cardui and Plutella xylostella (Lepidoptera) to Spitsbergen in the summer 1978. Not. Entomol. 1978, 58, 121–123. [Google Scholar]
- Chapman, J.W.; Reynolds, D.R.; Smith, A.D.; Riley, J.R.; Pedgley, D.E.; Woiwod, I.P. High-altitude migration of the diamondback moth Plutella xylostella to the UK: A study using radar, aerial netting, and ground trapping. Ecol. Entomol. 2002, 27, 641–650. [Google Scholar] [CrossRef]
- Hopkinson, R.F.; Soroka, J.J. Air trajectory model applied to an in-depth diagnosis of potential diamondback moth infestations on the Canadian Prairies. Agric. For. Meteorol. 2010, 150, 1–11. [Google Scholar] [CrossRef]
- Wei, S.J.; Shi, B.C.; Gong, Y.J.; Jin, G.H.; Chen, X.X.; Meng, X.F. Genetic structure and demographic history reveal migration of the diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) from the southern to northern regions of China. PLoS ONE 2013, 8, e59654. [Google Scholar] [CrossRef]
- Fu, X.; Xing, Z.; Liu, Z.; Ali, A.; Wu, K. Migration of diamondback moth, Plutella xylostella, across the Bohai Sea in northern China. Crop Prot. 2014, 64, 143–149. [Google Scholar] [CrossRef]
- Chen, M.Z.; Cao, L.J.; Li, B.Y.; Chen, J.C.; Gong, Y.J.; Yang, Q.; Schmidt, T.L.; Yue, L.; Zhu, J.Y.; Li, H.; et al. Migration trajectories of the diamondback moth Plutella xylostella in China inferred from population genomic variation. Pest Manag. Sci. 2021, 77, 1683–1693. [Google Scholar] [CrossRef]
- Yang, F.; Wang, P.; Zheng, M.; Hou, X.Y.; Zhou, L.L.; Wang, Y.; Si, S.Y.; Wang, X.P.; Chapman, J.W.; Wang, Y.M.; et al. Physiological and behavioral basis of diamondback moth Plutella xylostella migration and its association with heat stress. Pest Manag. Sci. 2024, 80, 1751–1760. [Google Scholar] [CrossRef]
- Sarfraz, M.; Keddie, B.A. Conserving the efficacy of insecticides against Plutella xylostella (L.) (Lep., Plutellidae). J. Appl. Entomol. 2005, 129, 149–157. [Google Scholar] [CrossRef]
- Santos, V.C.; de Siqueira, H.A.A.; da Silva, J.E.; de Farias, M.J.D.C. Insecticide resistance in populations of the diamondback moth, Plutella xylostella (L.)(Lepidoptera: Plutellidae), from the state of Pernambuco, Brazil. Neotrop. Entomol. 2011, 40, 264–270. [Google Scholar] [CrossRef]
- Xia, X.; Zheng, D.; Zhong, H.; Qin, B.; Gurr, G.M.; Vasseur, L.; Lin, H.; Bai, J.; He, W.; You, M. DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS ONE 2013, 8, e68852. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Shen, J.; Mao, K.; You, H.; Li, J. Susceptibility of field populations of the diamondback moth, Plutella xylostella, to a selection of insecticides in Central China. Pestic. Biochem. Physiol. 2016, 132, 38–46. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Shen, J.; Li, D.; Wan, H.; You, H.; Li, J. Cross-resistance and biochemical mechanisms of resistance to indoxacarb in the diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2017, 140, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, S.; Seram, D. Insecticidal resistance in diamondback moth (Plutella xylostella): A review. Pharm. Innov. J. 2022, 11, 958–962. [Google Scholar]
- Reddy, G.V.P. Comparative effect of integrated pest management and farmers standard pest control practice for managing insect pests on cabbage (Brassica spp.). Pest. Manag. Sci. 2011, 67, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, M.; Farooq, M.; Nasim, W.; Akram, W.; Khan, F.Z.A.; Jaleel, W.; Zhu, X.; Yin, H.; Li, S.; Fahad, S.; et al. Environment polluting conventional chemical control compared to an environmentally friendly IPM approach for control of diamondback moth, Plutella xylostella (L.), in China: A review. Environ. Sci. Pollut. Res. 2017, 24, 14537–14550. [Google Scholar] [CrossRef] [PubMed]
- Machekano, H.; Mvumi, B.M.; Nyamukondiwa, C. Diamondback moth, Plutella xylostella (L.) in Southern Africa: Research trends, challenges and insights on sustainable management options. Sustainability 2017, 9, 91. [Google Scholar] [CrossRef]
- Li, Z.; Furlong, M.J.; Yonow, T.; Kriticos, D.J.; Bao, H.; Yin, F.; Lin, Q.; Feng, X.; Zalucki, M.P. Management and population dynamics of diamondback moth (Plutella xylostella): Planting regimes, crop hygiene, biological control and timing of interventions. Bull. Entomol. Res. 2019, 109, 257–265. [Google Scholar] [CrossRef]
- Jamtsho, T.; Banu, N.; Kinley, C. Critical review on past, present and future scope of diamondback moth management. Plant Arch. 2021, 21, 1199–1210. [Google Scholar] [CrossRef]
- Mayanglambam, S.; Singh, K.D.; Rajashekar, Y. Current biological approaches for management of crucifer pests. Sci. Rep. 2021, 11, 11831. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Majumder, S.; Halder, J.; Kedar, S.C.; Singh, V. Sustainable pest management in cabbage using botanicals: Characterization, effectiveness and economic appraisal. J. Plant Dis. Prot. 2024, 131, 113–130. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Guerrero, A. Pheromone-based integrated pest management to control the diamondback moth Plutella xylostella in cabbage fields. Pest Manag. Sci. 2000, 56, 882–888. [Google Scholar] [CrossRef]
- Miluch, C.E.; Dosdall, L.M.; Evenden, M.L. The potential for pheromone-based monitoring to predict larval populations of diamondback moth, Plutella xylostella (L.), in canola (Brassica napus L.). Crop Prot. 2013, 45, 89–97. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Zhang, S.; Xu, B.; Zhang, Y.; Zalucki, M.P.; Wu, Q.; Yin, X. Population dynamics of the diamondback moth, Plutella xylostella (L.), in northern China: The effects of migration, cropping patterns and climate. Pest Manag. Sci. 2018, 74, 1845–1853. [Google Scholar] [CrossRef]
- Pandey, R.; Behera, S.R.; Mohapatra, A.; Pradhan, P.P. Integrated pest management strategies for controlling diamondback moth (DBM) in cruciferous vegetables. AgriTech Today 2023, 1, 9–11. [Google Scholar]
- Chow, Y.S.; Chiu, S.C.; Chien, C.C. Demonstration of a sex pheromone of the diamondback moth (Lepidoptera: Plutellidae). Ann. Entomol. Soc. Am. 1974, 67, 510–512. [Google Scholar] [CrossRef]
- Chow, Y.S.; Lin, Y.M.; Hsu, C.L. Sex pheromone of the diamondback moth (Lepidoptera: Plutellidae). Bull. Instit. Zool. Acad. Sin. 1977, 16, 99–105. [Google Scholar]
- Koshihara, T.; Yamada, H.; Tamaki, Y.; Ando, T. Field attractiveness of the synthetic sex-pheromone of the diamondback moth, Plutella xylostella (L.). Appl. Entomol. Zool. 1978, 13, 138–141. [Google Scholar] [CrossRef]
- Ando, T.; Koshihara, T.; Yamada, H.; Vu, M.H.; Takahashi, N.; Tamaki, Y. Electroantennogram activities of sex pheromone analogues and their synergistic effect on field attraction in the diamondback moth. Appl. Entomol. Zool. 1979, 14, 362–364. [Google Scholar] [CrossRef]
- Kawasaki, K. Effects of ratio and amount of the two sex-pheromonal components of the diamondback moth on male behavioral response. Appl. Entomol. Zool. 1984, 19, 436–442. [Google Scholar] [CrossRef]
- Tamaki, Y.; Kawasaki, K.; Yamada, H.; Koshihara, T.; Osaki, N.; Ando, T.; Yoshida, S.; Kakinohana, H. (Z)-11-Hexadecenal and (Z)-11-hexadecenyl acetate: Sex-pheromone components of the diamondback moth (Lepidoptera: Plutellidae). Appl. Entomol. Zool. 1977, 12, 208–210. [Google Scholar] [CrossRef]
- Chisholm, M.D.; Underhill, E.W.; Steck, W.F. Field trapping of the diamondback moth Plutella xylostella using synthetic sex attractants. Environ. Entomol. 1979, 8, 516–518. [Google Scholar] [CrossRef]
- Koshihara, T.; Yamada, H. Activity of the female sex pheromone of diamondback moth, Plutella xylostella (L.), and analogue. Jpn. J. Appl. Entomol. Zool. 1980, 24, 6–12. [Google Scholar] [CrossRef]
- Chisholm, M.D.; Steck, W.F.; Underhill, E.W.; Palaniswamy, P. Field trapping of diamondback moth Plutella xylostella using an improved four-component sex attractant blend. J. Chem. Ecol. 1983, 9, 113–118. [Google Scholar] [CrossRef]
- Zilahi-Balogh, G.M.; Angerilli, N.P.; Borden, J.H.; Meray, M.; Tulung, M.; Sembel, D. Regional differences in pheromone responses of diamondback moth in Indonesia. Int. J. Pest Manag. 1995, 41, 201–204. [Google Scholar] [CrossRef]
- Suckling, D.M.; Gibb, A.R.; Daly, J.M.; Rogers, D.J.; Walker, G.P. Improving the pheromone lure for diamondback moth. N. Z. Plant Prot. 2002, 55, 182–187. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.W.; Boo, K.S. Sex pheromone composition of the diamondback moth, Plutella xylostella (L) in Korea. J. Asia-Pac. Entomol. 2005, 8, 243–248. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lee, S.; Choi, K.S.; Jeon, H.Y.; Boo, K.S. Sex pheromone production and response in Korean populations of the diamondback moth, Plutella xylostella. Entomol. Exp. Appl. 2007, 124, 293–298. [Google Scholar] [CrossRef]
- Bobreshova, I.Y.; Ryabchinskaya, T.A.; Stulov, S.V.; Pyatnova, Y.B.; Karakotov, S.D. Method of pheromone monitoring of the diamondback moth (Plutella xylostella L.), a dangerous pest of rapeseed. Agrochemistry 2020, 7, 68–75. [Google Scholar]
- Baker, P.B.; Shelton, A.M.; Andaloro, J.T. Monitoring of diamondback moth (Lepidoptera: Yponomeutidae) in cabbage with pheromones. J. Econ. Entomol. 1982, 75, 1025–1028. [Google Scholar] [CrossRef]
- Shirai, Y.; Nakamura, A. Relationship between the number of wild males captured by sex pheromone trap and the population density estimated from a mark-recapture study in the diamondback moth, Plutella xylostella (L.)(Lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 1995, 30, 543–549. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Guerrero, A. Optimum timing of insecticide applications against diamondback moth Plutella xylostella in cole crops using threshold catches in sex pheromone traps. Pest Manag. Sci. 2001, 57, 90–94. [Google Scholar] [CrossRef]
- Walker, G.P.; Wallace, A.R.; Bush, R.; MacDonald, F.H.; Suckling, D.M. Evaluation of pheromone trapping for prediction of diamondback moth infestations in vegetable brassicas. N. Z. Plant Prot. 2003, 56, 180–184. [Google Scholar] [CrossRef]
- Sulifoa, J.B.; Ebenebe, A.A. Evaluation of pheromone trapping of diamondback moth (Plutella xylostella) as a tool for monitoring larval infestations in cabbage crops in Samoa. South Pacific J. Nat. Appl. Sci 2007, 25, 43–46. [Google Scholar] [CrossRef]
- Evenden, M.L.; Gries, R. Assessment of commercially available pheromone lures for monitoring diamondback moth (Lepidoptera: Plutellidae) in canola. J. Econ. Entomol. 2010, 103, 654–661. [Google Scholar] [CrossRef]
- Nofemela, R.S. The ability of synthetic sex pheromone traps to forecast Plutella xylostella infestations depends on survival of immature stages. Entomol. Exp. Appl. 2010, 136, 281–289. [Google Scholar] [CrossRef]
- Miluch, C.E.; Dosdall, L.M.; Evenden, M.L. Factors influencing male Plutella xylostella (Lepidoptera: Plutellidae) capture rates in sex pheromone-baited traps on canola in western Canada. J. Econ. Entomol. 2014, 107, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Tacain, J.; Parpal, F.; Abbate, S.; Silva, H.; Ribeiro, A.; Heguaburu, V. Synthesis and field evaluation of the sex pheromone of Plutella xylostella (L.)(Lepidoptera: Plutellidae) in canola (Brassica napus L.). Agrocienc. Urug. 2016, 20, 61–67. [Google Scholar] [CrossRef]
- Wainwright, C.; Jenkins, S.; Wilson, D.; Elliott, M.; Jukes, A.; Collier, R. Phenology of the diamondback moth (Plutella xylostella) in the UK and provision of decision support for Brassica growers. Insects 2020, 11, 118. [Google Scholar] [CrossRef]
- Akbari, S.; Jalalizand, A.; Mahmoudi, E. Effective factors in the use of sex pheromone traps on the capture rate of Plutella xylostella. Res. Crop Ecophysiol. 2020, 15, 111–115. [Google Scholar]
- Semerenko, S.A.; Bushneva, N.A. Application of pheromone traps on the spring rapeseed for the account of diamondback moth. Oil Crops 2018, 4, 172–177. [Google Scholar] [CrossRef]
- Guerrero, A.; Reddy, G.V. Chemical communication in insects: New advances in integrated pest management strategies. Insects 2023, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, M.D.; Underhill, E.W.; Palaniswamy, P.; Gerwing, V.J. Orientation disruption of male diamondback moths (Lepidoptera: Plutellidae) to traps baited with synthetic chemicals or female moths in small field plots. J. Econ. Entomol. 1984, 77, 157–160. [Google Scholar] [CrossRef]
- Chow, Y.S. Disruption effect of the synthetic sex pheromone and its analogues on diamondback moth. In Diamondback Moth and Other Crucifer Pests, Proceedings of the Second International Workshop. Tainan, Taiwan, 10–14 December 1990; Talker, N.S., Ed.; Asian Vegetable Research and Development Center (AVRDC) Publication: Shanhua, Taiwan, 1992; pp. 105–108. [Google Scholar]
- Ohbayashi, N.; Shimizu, K.; Nagata, K. Control of diamondback moth using synthetic sex pheromones. In Diamondback Moth and Other Crucifer Pests, Proceedings of the Second International Workshop. Tainan, Taiwan, 10–14 December 1990; Talker, N.S., Ed.; Asian Vegetable Research and Development Center (AVRDC) Publication: Shanhua, Taiwan, 1992; pp. 99–104. [Google Scholar]
- Ohno, T.; Asayama, T.; Ichikawa, K. Evaluation of communication disruption method using synthetic sex pheromone to suppress diamondback moth infestations. In Diamondback Moth and Other Crucifer Pests, Proceedings of the Second International Workshop. Tainan, Taiwan, 10–14 December 1990; Talker, N.S., Ed.; Asian Vegetable Research and Development Center (AVRDC) Publication: Shanhua, Taiwan, 1992; pp. 109–114. [Google Scholar]
- McLaughlin, J.R.; Mitchell, E.R.; Kirsch, P. Mating disruption of diamondback moth (Lepidoptera: Plutellidae) in cabbage: Reduction of mating and suppression of larval populations. J. Econ. Entomol. 1994, 87, 1198–1204. [Google Scholar] [CrossRef]
- Mitchell, E.R.; Hu, G.Y.; Okine, J.; McLaughlin, J.R. Mating disruption of diamondback moth (Lepidoptera: Plutellidae) and cabbage looper (Lepidoptera: Noctuidae) in cabbage using a blend of pheromones emitted from the same dispenser. J. Entomol. Sci. 1997, 32, 120–137. [Google Scholar] [CrossRef]
- Schroeder, P.C.; Shelton, A.M.; Ferguson, C.S.; Hoffmann, M.P.; Petzoldt, C.H. Application of synthetic sex pheromone for management of diamondback moth, Plutella xylostella, in cabbage. Entomol. Exp. Appl. 2000, 94, 243–248. [Google Scholar] [CrossRef]
- Mitchell, E.R. Promising new technology for managing diamondback moth (Lepidoptera: Plutellidae) in cabbage with pheromone. J. Environ. Sci. Health B 2002, 37, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Fang, Y.L.; Zhang, Z.N. Synthesis and assessment of attractiveness and mating disruption efficacy of sex pheromone microcapsules for the diamondback moth, Plutella xylostella (L.). Chin. Sci. Bull. 2007, 52, 1365–1371. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, S.F.; Yao, J.L.; Xu, B.Y.; Wang, S.L.; Zhang, Y.J. Management of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) by mating disruption. Insect Sci. 2012, 19, 643–648. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Urs, K.C.D. Mass trapping of diamondback moth Plutella xylostella in cabbage fields using synthetic sex pheromones. Int. Pest Control 1997, 39, 125–126. [Google Scholar]
- Hou, Y.; Pang, X.; Liang, G.; You, M. Control effect of Plutella xylostella with synthetic sex pheromone. Chin. J. Biol. Control 2001, 17, 121–125. [Google Scholar]
- Wang, X.P.; Le, V.T.; Fang, Y.L.; Zhang, Z.N. Trap effect on the capture of Plutella xylostella (Lepidoptera: Plutellidae) with sex pheromone lures in cabbage fields in Vietnam. Appl. Entomol. Zool. 2004, 39, 303–309. [Google Scholar] [CrossRef]
- Wang, X.P.; Zhang, Z.N.; Lei, C.L.; Zhao, Y.C.; Wu, D.X. Mass trapping and control efficacy on the diamondback moth, Plutella xylostella (L.) with synthetic sex pheromone lures at high altitudes in Hubei. Acta Entomol. Sin. 2004, 47, 135–140. [Google Scholar]
- Topagi, S.C.; Bhanu, K.R.M.; Kumar, C.T.A. Mass trapping technique using pheromones: A standalone method for management of diamondback moth, Plutella xylostella (Linnaeus)(Plutellidae: Lepidoptera) in cabbage. Int. J. Appl. Sci. Eng. 2018, 15, 211–232. [Google Scholar]
- Syed, I.; Mutthuraju, G.P.; Doddabasappa, B.; Sudarshan, G.K.; Sahida, A.; Chakravarthy, A.K. Standardization of height and density of pheromone traps for mass trapping diamond back moth, Plutella xylostella (L.) in cabbage. J. Entomol. Zool. Stud. 2019, 7, 1049–1052. [Google Scholar]
- Gonzalez, F.; Rodríguez, C.; Oehlschlager, C. Economic benefits from the use of mass trapping in the management of diamondback moth, Plutella xylostella, in Central America. Insects 2023, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Pell, J.K.; Macauly, E.D.M.; Wilding, N. A pheromone trap for dispersal of the pathogen Zoophthora radicans Brefeld. (Zygomycetes: Entomophthorales) amongst populations of the diamondback moth, Plutella xylostella L. (Lepidoptera; Yponomeutidae). Biocontrol Sci. Technol. 1993, 3, 315–320. [Google Scholar] [CrossRef]
- Vickers, R.A.; Furlong, M.J.; White, A.; Pell, J.K. Initiation of fungal epizootics in diamondback moth populations within a large field cage: Proof of concept for auto-dissemination. Entomol. Exp. Appl. 2004, 111, 7–17. [Google Scholar] [CrossRef]
- Furlong, M.J.; Pell, J.K.; Choo, O.P.; Rahman, S.A. Field and laboratory evaluation of a sex pheromone trap for the autodissemination of the fungal entomopathogen Zoophthora radicans (Entomophthorales) by the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Bull. Entomol. Res. 1995, 85, 331–337. [Google Scholar] [CrossRef]
- Bushneva, N.A.; Semerenko, S.A. Control of diamondback moth males (Plutella xylostella L.) in crops of spring rapeseed using pheromones. Oil Crops 2023, 4, 68–74. [Google Scholar] [CrossRef]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Guerrero, A. Behavioral responses of the diamondback moth, Plutella xylostella, to green leaf volatiles of Brassica oleracea subsp. capitata. J. Agric. Food Chem. 2000, 48, 6025–6029. [Google Scholar] [CrossRef]
- Dai, J.; Deng, J.; Du, J. Development of bisexual attractants for diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) based on sex pheromone and host volatiles. Appl. Entomol. Zool. 2008, 43, 631–638. [Google Scholar] [CrossRef]
- Yan, X.Z.; Ma, L.; Li, X.F.; Chang, L.; Liu, Q.Z.; Song, C.F.; Zhao, J.Y.; Qie, X.T.; Deng, C.P.; Wang, C.Z.; et al. Identification and evaluation of cruciferous plant volatiles attractive to Plutella xylostella L. (Lepidoptera: Plutellidae). Pest Manag. Sci. 2023, 79, 5270–5282. [Google Scholar] [CrossRef] [PubMed]
- Chidwala, N.; Chilumpha, G.; Makhwira, A.; Namandwa, B.; Zhou, Q.; Li, W.; Yu, T.; Nasser, R.; Mo, J. Study on the trapping effects of Brassica allelochemicals on Plutella xylostella adults. Afr. J. Agric. Res. 2024, 20, 323–336. [Google Scholar] [CrossRef]
- Harcourt, D.G. Biology of the diamondback moth, Plutella maculipennis (Curt.)(Lepidoptera: Plutellidae), in Eastern Ontario. II. Life-history, behaviour, and host relationships. Can. Entomol. 1957, 89, 554–564. [Google Scholar] [CrossRef]
- Goodwin, S.; Danthanarayana, W. Flight activity of Plutella xylostella (L.) (Lepidoptera: Yponomeutidae). Aust. J. Entomol. 1984, 23, 235–240. [Google Scholar] [CrossRef]
- Couty, A.; Van Emden, H.; Perry, J.N.; Hardie, J.; Pickett, J.A.; Wadhams, L.J. The roles of olfaction and vision in host-plant finding by the diamondback moth, Plutella xylostella. Physiol. Entomol. 2006, 31, 134–145. [Google Scholar] [CrossRef]
- Nowinszky, L.; Mészáros, Z.; Puskás, J. The beginning and the end of the insects’ flight towards the light according to different environmental lightings. Appl. Ecol. Environ. Res. 2008, 6, 137–145. [Google Scholar] [CrossRef]
- Wang, D.; Yang, G.; Chen, W. Diel and circadian patterns of locomotor activity in the adults of diamondback moth (Plutella xylostella). Insects 2021, 12, 727. [Google Scholar] [CrossRef]
- Tyler, C.J.; Mahajan, S.; Smith, L.; Okamoto, H.; Wijnen, H. Adult diel locomotor behaviour in the agricultural pest Plutella xylostella reflects temperature-driven and light-repressed regulation rather than coupling to circadian clock gene rhythms. Insects 2025, 16, 182. [Google Scholar] [CrossRef]
- Williams, C.B. An analysis of four years captures of insects in a light trap. Part I. General survey; sex proportion; phenology; and time of flight. Trans. R. Entomol. Soc. London 1939, 89, 79–131. [Google Scholar] [CrossRef]
- Robinson, H.S. On the behaviour of night-flying insects in the neighbourhood of a bright source of light. Proc. R. Entomol. Soc. Lond. 1952, 27, 13–21. [Google Scholar] [CrossRef]
- Nowinszky, L. The Handbook of Light Trapping; Savaria University Press: Szombathely, Hungary, 2003; p. 276. [Google Scholar]
- Shimoda, M.; Honda, K.I. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 2013, 48, 413–421. [Google Scholar] [CrossRef]
- Yun, C.N.; Maeng, I.S.; Yang, S.H.; Hwang, U.J.; Kim, K.N.; Kim, K.C.; Ho, K.C.; Ri, C.S.; Yang, H.S.; Jang, S.H. Evaluating the phototactic behavior responses of the diamondback moth, Plutella xylostella, to some different wavelength LED lights in laboratory and field. J. Asia-Pac. Entomol. 2023, 26, 102080. [Google Scholar] [CrossRef]
- Harcourt, D.G.; Cass, L.M. A controlled-interval light trap for Microlepidoptera. Can. Entomol. 1958, 90, 617–622. [Google Scholar] [CrossRef]
- Cho, K.S.; Lee, H.S. Visual preference of diamondback moth, Plutella xylostella, to light-emitting diodes. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 681–684. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.M.; Lee, S.G.; Lee, H.S. Attractive effects efficiency of LED trap on controlling Plutella xylostella adults in greenhouse. J. Appl. Biol. Chem. 2014, 57, 255–257. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.S. Phototactic behavioral response of agricultural insects and stored-product insects to light-emitting diodes (LEDs). Appl. Biol. Chem. 2017, 60, 137–144. [Google Scholar] [CrossRef]
- Bourget, C.M. An introduction to light-emitting diodes. HortScience 2008, 43, 1944–1946. [Google Scholar] [CrossRef]
- Bessho, M.; Shimizu, K. Latest trends in LED lighting. Electron. Commun. Jpn. 2012, 95, 1–7. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Theagarajan, R.; Narayanaswamy, L.M.; Choudhary, P. Introduction to light-emitting diodes: Principles, fundamentals, advantages, limitations, and applications. In Nonthermal Light-Based Technologies in Food Processing; Sunil, C.K., Goyal, M.R., Birwal, P., Mahendran, R., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2025; pp. 361–385. [Google Scholar]
- Cohnstaedt, L.W.; Gillen, J.I.; Munstermann, L.E. Light-emitting diode technology improves insect trapping. J. Am. Mosq. Control. Assoc. 2008, 24, 331–334. [Google Scholar] [CrossRef]
- Infusino, M.; Brehm, G.; Di Marco, C.; Scalercio, S. Assessing the efficiency of UV LEDs as light sources for sampling the diversity of macro-moths (Lepidoptera). Eur. J. Entomol. 2017, 114, 25–33. [Google Scholar] [CrossRef]
- Kim, K.N.; Huang, Q.Y.; Lei, C.L. Advances in insect phototaxis and application to pest management: A review. Pest Manag. Sci. 2019, 75, 3135–3143. [Google Scholar] [CrossRef]
- van Deijk, J.R.; Wever, R.; van der Heide, S.R.; Boers, J.; van Deijl, I.H.J.; van Grunsven, R.H.A. UV-LEDs outperform actinics for standalone moth monitoring. J. Insect Conserv. 2024, 28, 959–968. [Google Scholar] [CrossRef]
- Prabaningrum, L.; Moekasan, T.K. Use of light trap for controlling cabbage pests. IOP Conf. Ser. Earth Environ. Sci. 2021, 752, 012027. [Google Scholar] [CrossRef]
- Tarigan, R.; Asgar, A.; Moekasan, T.K.; Prabaningrum, L.; Hutabarat, R.C.; Marpaung, A.E.; Rosliani, R.; Karo, B.B.; Aryani, D.S. The potential of light and pheromone traps for controlling main pests in cabbage. AIP Conf. Proc. 2024, 2957, 090034. [Google Scholar] [CrossRef]
- Truxa, C.; Fiedler, K. Attraction to light—From how far do moths (Lepidoptera) return to weak artificial sources of light? Eur. J. Entomol. 2012, 109, 77–84. [Google Scholar] [CrossRef]
- Merckx, T.; Slade, E.M. Macro-families differ in their attraction to light: Implications for light-trap monitoring programmes. Insect Conserv. Diver. 2014, 7, 453–461. [Google Scholar] [CrossRef]
- Balamurugan, R.; Kandasamy, P. Effectiveness of portable solar-powered light-emitting diode insect trap: Experimental investigation in a groundnut field. J. Asia Pac. Entomol. 2021, 24, 1024–1032. [Google Scholar] [CrossRef]
- Niermann, J.; Brehm, G. The number of moths caught by light traps is affected more by microhabitat than the type of UV lamp used in a grassland habitat. Eur. J. Entomol. 2022, 119, 36–42. [Google Scholar] [CrossRef]
- Knight, A.L.; Preti, M.; Basoalto, E.; Fuentes-Contreras, E. Increasing catches of adult moth pests (Lepidoptera: Tortricidae) in pome fruit with low-intensity LED lights added to sex pheromone/kairomone lure-baited traps. J. Appl. Entomol. 2023, 147, 843–856. [Google Scholar] [CrossRef]
- Li, P.; Zhu, J.; Qin, Y. Enhanced attraction of Plutella xylostella (Lepidoptera: Plutellidae) to pheromone-baited traps with the addition of green leaf volatiles. J. Econ. Entomol. 2012, 105, 1149–1156. [Google Scholar] [CrossRef]
- Chi, D.T.; Le Thi, H.; Le, V.V.; Thy, T.T.; Yamamoto, M.; Ando, T. Mass trapping of the diamondback moth (Plutella xylostella L.) by a combination of its sex pheromone and allyl isothiocyanate in cabbage fields in southern Vietnam. J. Pestic. Sci. 2024, 49, 15–21. [Google Scholar] [CrossRef]
- Suresh, S.; Chandrasekaran, S.; Babu, P.C. Studies on pheromone and light trap for the attraction of diamondback moth Plutella xylostella. South Indian Hortic. 1989, 37, 353–354. [Google Scholar]
- Zakharova, Y.A.; Frolov, A.N.; Artemyeva, A.M. Monitoring of the diamondback moth (Plutella xylostella L.) on the Brassica oleracea L. collection in the vicinity of St. Petersburg. Proc. Appl. Bot. Genet. Breed. 2022, 183, 219–228. [Google Scholar] [CrossRef]
- Zakharova, Y.A.; Miltsen, A.A.; Frolov, A.N. High-frequency pulsed power supply of LEDs as a way to increase collections of night-flying insects with light traps using diamondback moth, Plutella xylostella (L.) (Lepidoptera, Plutellidae) as an example. Entomol. Obozr. 2025, 104, 51–77. [Google Scholar]
- Umeton, D.; Read, J.C.A.; Rowe, C. Unravelling the illusion of flicker fusion. Biol. Lett. 2017, 13, 20160831. [Google Scholar] [CrossRef]
- Di Lollo, V.; Hogben, J.H. Suppression of visible persistence as a function of spatial separation between inducing stimuli. Percept. Psychophys. 1987, 41, 345–354. [Google Scholar] [CrossRef]
- Inger, R.; Bennie, J.; Davies, T.; Gaston, K. Potential biological and ecological effects of flickering artificial light. PLoS ONE 2014, 9, e98631. [Google Scholar] [CrossRef] [PubMed]
- Justus, K.A.; Mitchell, B.K. Reproductive morphology, copulation, and inter-populational variation in the diamondback moth, Plutella xylostella (L.)(Lepidoptera: Plutellidae). Int. J. Insect Morphol. Embryol. 1999, 28, 233–246. [Google Scholar] [CrossRef]
- Reeve, J.D.; Strom, B.L. Statistical problems encountered in trapping studies of Scolytids and associated insects. J. Chem. Ecol. 2004, 30, 1575–1590. [Google Scholar] [CrossRef]
- Roelofs, W.L.; Cardé, R.T. Responses of Lepidoptera to synthetic sex pheromone chemicals and their analogues. Annu. Rev. Entomol. 1977, 22, 377–405. [Google Scholar] [CrossRef]
- Sawilowsky, S.S. Nonparametric tests of interaction in experimental design. Rev. Educ. Res. 1990, 60, 91–126. [Google Scholar] [CrossRef]
- Peterson, K. Six modifications of the aligned rank transform test for interaction. J. Mod. Appl. Stat. Methods 2002, 1, 13. [Google Scholar] [CrossRef]
- Leys, C.; Schumann, S. A nonparametric method to analyze interactions: The adjusted rank transform test. J. Exp. Soc. Psychol. 2010, 46, 684–688. [Google Scholar] [CrossRef]
- Feys, J. New nonparametric rank tests for interactions in factorial designs with repeated measures. J. Mod. Appl. Stat. Methods 2016, 15, 6. [Google Scholar] [CrossRef]
- Thongteeraparp, A. The comparison of nonparametric statistical tests for interaction effects in factorial design. Decis. Sci. Lett. 2019, 8, 309–316. [Google Scholar] [CrossRef]
- Page, E.B. Ordered hypotheses for multiple treatments: A significance test for linear ranks. J. Am. Stat. Assoc. 1963, 58, 216–230. [Google Scholar] [CrossRef]
- Gentry, C.R.; Davis, D.R. Weather: Influence on catches of adult cabbage loopers in traps baited with BL only or with BL plus synthetic sex pheromone. Environ. Entomol. 1973, 2, 1074–1077. [Google Scholar] [CrossRef]
- McQuate, G.T. Green light synergistally enhances male sweetpotato weevil response to sex pheromone. Sci. Rep. 2014, 4, 4499. [Google Scholar] [CrossRef]
- Muirhead-Thompson, R.C. Trap Responses of Flying Insects: The Influence of Trap Design on Capture Efficiency; Academic Press: London, UK, 1991; p. 287. [Google Scholar]
- Foster, S.P.; Harris, M.O. Behavioral manipulation methods for insect pest management. Annu. Rev. Entomol. 1997, 42, 123–146. [Google Scholar] [CrossRef]
- Roitberg, B.D. Why pest management needs behavioral ecology and vice versa. Entomol. Res. 2007, 37, 14–18. [Google Scholar] [CrossRef]
- Dent, D.; Binks, R.H. Insect Pest Management, 3rd ed.; CAB International: Oxfordshire, UK, 2020; p. 380. [Google Scholar]
- Nieri, R.; Anfora, G.; Mazzoni, V.; Stacconi, M.M.R. Semiochemicals, semiophysicals and their integration for the development of innovative multi-modal systems for agricultural pests’ monitoring and control. Entomol. Gen. 2022, 42, 167–183. [Google Scholar] [CrossRef]
- Agelopoulos, N.; Birkett, M.A.; Hick, A.J.; Hooper, A.M.; Pickett, J.A.; Pow, E.M.; Smart, L.E.; Smiley, D.W.M.; Wadhams, L.J.; Woodcock, C.M. Exploiting semiochemicals in insect control. Pest. Sci. 1999, 55, 225–235. [Google Scholar] [CrossRef]
- El-Ghany, N.M.A. Semiochemicals for controlling insect pests. J. Plant Prot. Res. 2019, 59, 1–11. [Google Scholar] [CrossRef]
- Frolov, A.N. Controlling the behavior of harmful insects: Light and chemical signals and their combined action. Entomol. Rev. 2022, 102, 782–819. [Google Scholar] [CrossRef]
- Henneberry, T.J.; Howland, A.F. Response of male cabbage loopers to black-light with or without the presence of the female sex pheromone. J. Econ. Entomol. 1966, 59, 623–626. [Google Scholar] [CrossRef]
- Henneberry, T.J.; Howland, A.F.; Wolf, W.W. Combinations of blacklight and virgin females as attractants to cabbage looper moths. J. Econ. Entomol. 1967, 60, 152–156. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Lawson, F.R.; Peace, B. Attraction of blacklight traps baited with virgin female tobacco hornworm moths. J. Econ. Entomol. 1966, 59, 809–811. [Google Scholar] [CrossRef]
- Cantelo, W.W.; Smith, J.S. Attraction of tobacco horn worm moths to blacklight traps baited with virgin females. J. Econ. Entomol. 1971, 64, 1511–1514. [Google Scholar] [CrossRef]
- Cantelo, W.W.; Smith, J.S.; Baumhover, A.H.; Stanley, J.M.; Henneberry, T.J. Suppression of an isolated population of the tobacco hornworm, with blacklight traps unbaited or baited with virgin female moths. Environ. Entomol. 1972, 1, 253–258. [Google Scholar] [CrossRef]
- Hendricks, D.E. Use of virgin female tobacco budworms to increase catch of males in blacklight traps and evidence that trap location and wind influence catch. J. Econ. Entomol. 1968, 61, 1581–1585. [Google Scholar] [CrossRef]
- Gross, J.; Franco, J.C. Novel trends on semiochemicals and semiophysicals for insect science and management. Entomol. Gen. 2022, 42, 163–165. [Google Scholar] [CrossRef]
- Duehl, A.J.; Cohnstaedt, L.W.; Arbogast, R.T.; Teal, P.E.A. Evaluating light attraction to increase trap efficiency for Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2011, 104, 1430–1435. [Google Scholar] [CrossRef]
- Miyatake, T.; Yokoi, T.; Fuchikawa, T.; Korehisa, N.; Kamura, T.; Nanba, K.; Ryouji, S.; Kamioka, N.; Hironaka, M.; Osada, M.; et al. Monitoring and detecting the cigarette beetle (Coleoptera: Anobiidae) using ultraviolet (LED) direct and reflected lights and/or pheromone traps in a laboratory and a storehouse. J. Econ. Entomol. 2016, 109, 2551–2560. [Google Scholar] [CrossRef]
- Rice, K.B.; Cullum, J.P.; Wiman, N.G.; Hilton, R.; Leskey, T.C. Halyomorpha halys (Hemiptera: Pentatomidae) response to pyramid traps baited with attractive light and pheromonal stimuli. Fla. Entomol. 2017, 100, 449–453. [Google Scholar] [CrossRef]
- Rondoni, G.; Chierici, E.; Marchetti, E.; Nasi, S.; Ferrari, R.; Conti, E. Improved captures of the invasive brown marmorated stink bug, Halyomorpha halys, using a novel multimodal trap. Insects 2022, 13, 527. [Google Scholar] [CrossRef] [PubMed]
- Otieno, J.A.; Stukenberg, N.; Weller, J.; Poehling, H.M. Efficacy of LED-enhanced blue sticky traps combined with the synthetic lure Lurem-TR for trapping of western flower thrips (Frankliniella occidentalis). J. Pest Sci. 2018, 91, 1301–1314. [Google Scholar] [CrossRef]
- Sarvary, M.A.; Cooperband, M.F.; Hajek, A.E. The importance of olfactory and visual cues in developing better monitoring tools for Sirex noctilio (Hymenoptera: Siricidae). Agr. Forest Entomol. 2015, 17, 29–35. [Google Scholar] [CrossRef]
- Mann, R.S.; Kaufman, P.E.; Butler, J.F. Lutzomyia spp. (Diptera: Psychodidae) response to olfactory attractant- and light emitting diode-modified Mosquito Magnet X (MMX) traps. J. Med. Entomol. 2009, 46, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.A.; Rebêlo, J.M.M.; Carneiro, B.F.; Castro, M.P.P.; de Sousa de Almeida, M.; Ponte, I.S.; Aguiar, J.V.C.; Silva, F.S. Exploiting the synergistic effect of kairomones and light-emitting diodes on the attraction of phlebotomine sand flies to light traps in Brazil. J. Med. Entomol. 2019, 56, 1441–1445. [Google Scholar] [CrossRef]
- Delisle, J.; West, R.J.; Bowers, W.W. The relative performance of pheromone and light traps in monitoring the seasonal activity of both sexes of the eastern hemlock looper, Lambdina fiscellaria fiscellaria. Entomol. Exp. Appl. 1998, 89, 87–98. [Google Scholar] [CrossRef]
- Sambaraju, K.R.; Phillips, T.W. Responses of adult Plodia interpunctella (Hübner)(Lepidoptera: Pyralidae) to light and combinations of attractants and light. J. Insect Behav. 2008, 21, 422–439. [Google Scholar] [CrossRef]
- Frolov, A.N.; Zakharova, Y.A.; Malysh, S.M. Through twilight to the light: A new sight of variability in codling moth behavioral reactions. Plant Prot. News 2024, 107, 40–74. [Google Scholar] [CrossRef]
- Zhukovskaya, M.I.; Grushevaya, I.V.; Miltsen, A.A.; Selitskaya, O.G.; Shchenikova, A.V.; Frolov, A.N.; Tóth, M. To attract a moth: Wind tunnel and field testing of plant odor and light stimuli and their combination for Ostrinia nubilalis. Acta Phytopathol. Entomol. Hung. 2024, 59, 108–120. [Google Scholar] [CrossRef]
- Pezhman, H.; Saeidi, K. Effectiveness of various solar light traps with and without sex pheromone for mass trapping of tomato leaf miner (Tuta absoluta) in a tomato field. Not. Sci. Biol. 2018, 10, 475–484. [Google Scholar] [CrossRef]
- Frost, S.W. Light traps for insect collections, survey, and control. Pa. Agr. Exp. Stn. Bull. 1952, 550, 32. [Google Scholar]
- Blomberg, O.; Itämies, J.; Kuusela, K. Insect catches in a blended and a black light-trap in northern Finland. Oikos 1976, 27, 57–63. [Google Scholar] [CrossRef]
- Maelzer, D.A.; Zalucki, M.P. Analysis of long-term light-trap data for Helicoverpa spp. (Lepidoptera: Noctuidae) in Australia: The effect of climate and crop host plants. Bull. Entomol. Res. 1999, 89, 455–463. [Google Scholar] [CrossRef]
- Szentkirályi, F. Fifty years-long insect survey in Hungary: T. Jeremy’s contribution to light-trapping. Acta Zool. Acad. Sci. Hung. 2002, 48, 85–105. [Google Scholar]
- Southwood, T.R.E.; Henderson, P.A.; Woiwod, I.P. Stability and change over 67 years—The community of Heteroptera as caught in a light-trap at Rothamsted, UK. Eur. J. Entomol. 2003, 100, 557–561. [Google Scholar] [CrossRef]
- Hufnagel, L.; Gimesi, L. The possibilities of biodiversity monitoring based on Hungarian light trap networks. Appl. Ecol. Environ. Res. 2010, 8, 223–239. [Google Scholar]
- Oloumi-Sadeghi, H.; Showers, W.B.; Reed, G.L. European corn borer: Lack of synchrony of attraction to sex pheromone and capture in light traps. J. Econ. Entomol. 1975, 68, 663–667. [Google Scholar] [CrossRef]
- Starratt, A.N.; McLeod, D.G.R. Influence of pheromone trap age on capture of the European com borer. Environ. Entomol. 1976, 5, 1008–1010. [Google Scholar] [CrossRef]
- Fletcher-Howell, G.; Ferro, D.N.; Butkewich, S. Pheromone and blacklight trap monitoring of adult European corn borer (Lepidoptera: Pyralidae) in western Massachusetts. Environ. Entomol. 1983, 12, 531–534. [Google Scholar] [CrossRef]
- Kammar, V.; Rani, A.T.; Kumar, K.P.; Chakravarthy, A.K. Light trap: A dynamic tool for data analysis, documenting, and monitoring insect populations and diversity. In Innovative Pest Management Approaches for the 21st Century: Harnessing Automated Unmanned Technologies; Chakravarthy, A.K., Ed.; Springer: Singapore, 2020; pp. 137–163. [Google Scholar]
- Cardé, R.T.; Minks, A.K. Insect Pheromone Research: New Directions; Chapman & Hall: New York, NY, USA, 1997; p. 684. [Google Scholar]
- El-Sayed, A.M.; Suckling, D.M.; Wearing, C.H.; Byers, J.A. Potential of mass trapping for long-term pest management and eradication of invasive species. J. Econ. Entomol. 2006, 99, 1550–1564. [Google Scholar] [CrossRef] [PubMed]
- Čokl, A.A.; Millar, J.G. Manipulation of insect signaling for monitoring and control of pest insects. In Biorational Control of Arthropod Pests; Ishaaya, I., Horowitz, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 279–316. [Google Scholar]
- Yew, J.Y.; Chung, H. Insect pheromones: An overview of function, form, and discovery. Prog. Lipid Res. 2015, 59, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.H.; George, J.; Reddy, G.V.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484. [Google Scholar] [CrossRef]
- Webster, R.P.; Charlton, R.E.; Schal, C.; Cardé, R.T. High-efficiency pheromone trap for the European corn borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 1986, 79, 1139–1142. [Google Scholar] [CrossRef]
- Dent, D.R.; Pawar, C.S. The influence of moonlight and weather on catches of Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) in light and pheromone traps. Bull. Entomol. Res. 1988, 78, 365–377. [Google Scholar] [CrossRef]
- Mitchell, E.R.; Agee, H.R.; Heath, R.R. Influence of pheromone trap color and design on capture of male velvetbean caterpillar and fall armyworm moths (Lepidoptera: Noctuidae). J. Chem. Ecol. 1989, 15, 1775–1784. [Google Scholar] [CrossRef]
- Kondo, A.; Tanaka, F.; Sugie, H.; Hokyou, N. Analysis of some biological factors affecting differential pheromone trap efficiency between generations in the rice stem borer moth, Chilo suppressalis (Walker)(Lepidoptera: Pyralidae). Appl. Entomol. Zool. 1993, 28, 503–511. [Google Scholar] [CrossRef]
- Kehat, M.; Anshelevich, L.; Dunkelblum, E.; Fraishtat, P.; Greenberg, S. Sex pheromone traps for monitoring the codling moth: Effect of dispenser type, field aging of dispenser, pheromone dose and type of trap on male captures. Entomol. Exp. Appl. 1994, 70, 55–62. [Google Scholar] [CrossRef]
- Suckling, D.M. Issues affecting the use of pheromones and other semiochemicals in orchards. Crop Prot. 2000, 19, 677–683. [Google Scholar] [CrossRef]
- Pélozuelo, L.; Frérot, B. Monitoring of European corn borer with pheromone-baited traps: Review of trapping system basics and remaining problems. J. Econ. Entomol. 2007, 100, 1797–1807. [Google Scholar]
- Bowden, J. An analysis of factors affecting catches of insects in light-traps. Bull. Entomol. Res. 1982, 72, 535–556. [Google Scholar] [CrossRef]
- Yela, J.L.; Holyoak, M. Effects of moonlight and meteorological factors on light and bait trap catches of noctuid moths (Lepidoptera: Noctuidae). Environ Entomol 1997, 26, 1283–1290. [Google Scholar] [CrossRef]
- Intachat, J.; Woiwod, I.P. Trap design for monitoring moth biodiversity in tropical rainforests. Bull. Entomol. Res. 1999, 89, 153–163. [Google Scholar] [CrossRef]
- Butler, L.; Kondo, V.; Barrows, E.M.; Townsend, E.C. Effects of weather conditions and trap types on sampling for richness and abundance of forest Macrolepidoptera. Environ. Entomol. 1999, 28, 795–811. [Google Scholar] [CrossRef]
- Bates, A.J.; Sadler, J.P.; Everett, G.; Grundy, D.; Lowe, N.; Davis, G.; Baker, D.; Bridge, M.; Clifton, J.; Freestone, R.; et al. Assessing the value of the Garden Moth Scheme citizen science dataset: How does light trap type affect catch? Entomol. Exp. Appl. 2013, 146, 386–397. [Google Scholar] [CrossRef]
- Jonason, D.; Franzén, M.; Ranius, T. Surveying moths using light traps: Effects of weather and time of year. PLoS ONE 2014, 9, e92453. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.D.; Walgenbach, J.F.; Kennedy, G.G. Comparison of black light and pheromone traps for monitoring Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in tomato. J. Agric. Entomol. 1992, 9, 17–24. [Google Scholar]
- Srivastava, C.; Pimbert, M.; Reed, W. Monitoring of Helicoverpa (= Heliothis) armigera (Hübner) moths with light and pheromone traps in India. Int. J. Trop. Insect Sci. 1992, 13, 205–210. [Google Scholar] [CrossRef]
- Baker, G.; Tann, C.; Fitt, G. A tale of two trapping methods: Helicoverpa spp. (Lepidoptera, Noctuidae) in pheromone and light traps in Australian cotton production systems. Bull. Entomol. Res. 2011, 101, 9–23. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Nowinszky, L.; Szeőke, K. Different catching series from light and pheromone trapping of Helicoverpa armigera (Lepidoptera: Noctuidae). Biologia 2016, 71, 818–823. [Google Scholar] [CrossRef]
- Mangrio, G.Q.; Gilal, A.A.; Rajput, L.B.; Hajano, J.U.D.; Gabol, A.H. Performance of pheromone and light traps in monitoring and management of tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). J. Saudi Soc. Agric. Sci. 2023, 22, 288–297. [Google Scholar] [CrossRef]
- Miller, J.R.; Gut, L.J. Mating disruption for the 21st century: Matching technology with mechanism. Environ. Entomol. 2015, 44, 427–453. [Google Scholar] [CrossRef]
- Ioriatti, C.; Lucchi, A. Semiochemical strategies for tortricid moth control in apple orchards and vineyards in Italy. J. Chem. Ecol. 2016, 42, 571–583. [Google Scholar] [CrossRef]
- Lance, D.R.; Leonard, D.S.; Mastro, V.C.; Walters, M.L. Mating disruption as a suppression tactic in programs targeting regulated lepidopteran pests in US. J. Chem. Ecol. 2016, 42, 590–605. [Google Scholar] [CrossRef]
- Byers, J.A. Modelling female mating success during mass trapping and natural competitive attraction of searching males or females. Entomol. Exp. Appl. 2012, 145, 228–237. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Suckling, D.M. Development of an attracticide against light brown apple moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 1999, 92, 853–859. [Google Scholar] [CrossRef]
- González-Fuentes, F.; Narváez-Niño, S.; Rodríguez-Chinchilla, C.; Vargas-Martínez, A.; González-Herrera, A. Trampeo masivo de Plutella xylostella: Una alternativa ambiental y económicamente beneficiosa para su control en Costa Rica. Revis. Bionatura 2023, 8, 6. [Google Scholar] [CrossRef]
- Cantelo, W.W. Blacklight traps as control agents: An appraisal. Bull. Entomol. Soc. Am. 1974, 20, 279–282. [Google Scholar] [CrossRef]
- Hienton, T.E. Summary of Investigations of Electric Insect Traps; Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1974; p. 136.
- Rhainds, M. Mass trapping lepidopteran pests with light traps, with focus on tortricid forest pests: What if? Insects 2024, 15, 267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).