Using Leaf-Derived Materials to Stop Common Bed Bugs (Cimex lectularius L.) in Their Tracks
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bed Bugs, Plants, and Leaf-Derived Trapping Material
2.1.1. Bed Bugs
2.1.2. Bean Plants and Leaf-Derived Trapping Material
2.2. The Effects of Nymphal Stages and Sex on Bed Bug Trapping
2.3. Effects of Surface Type and Orientation (Horizontal vs. Vertical) on Bed Bug Trapping
2.4. Verification of Loss of Trapping Ability in Fresh Leaves
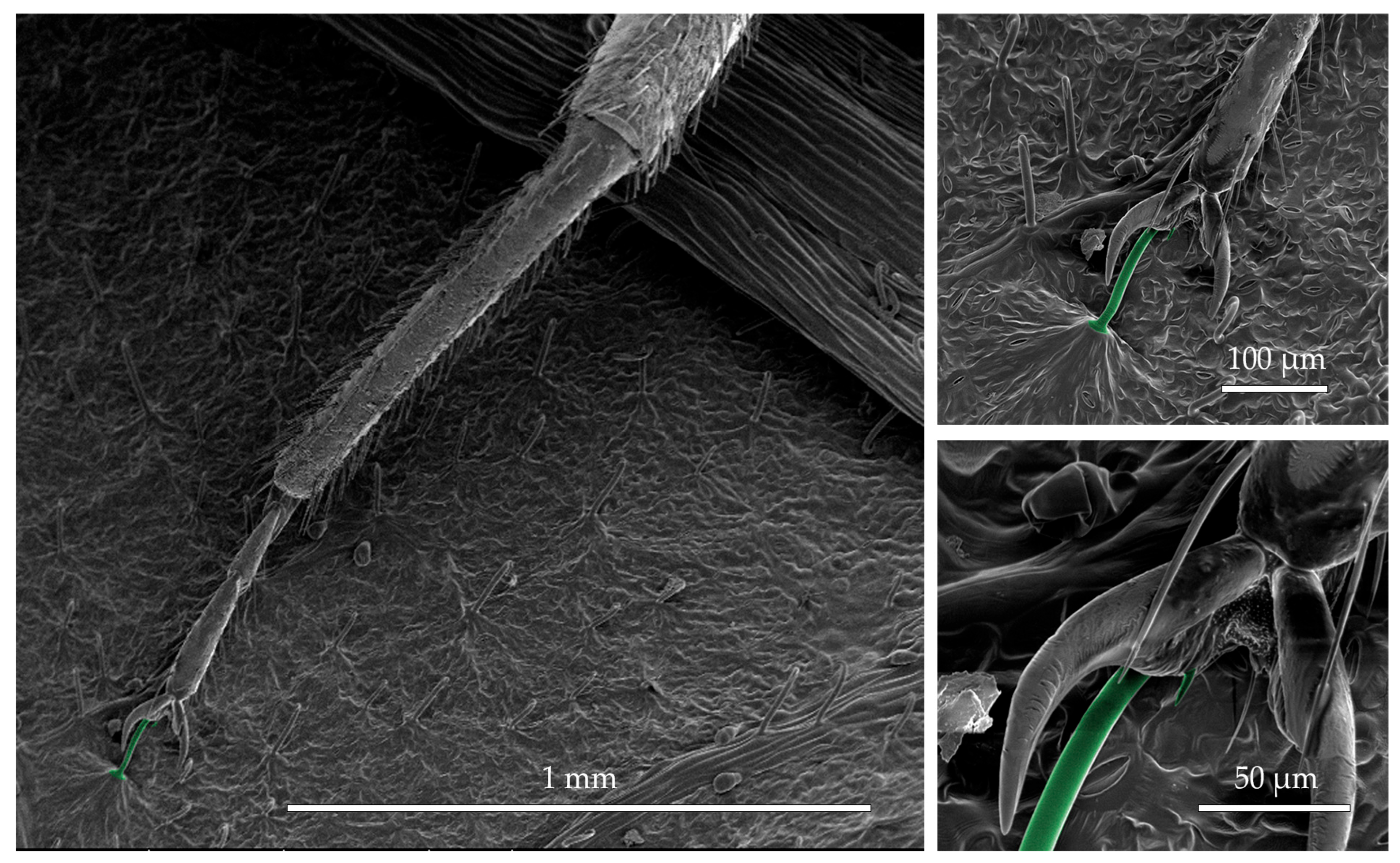
2.5. Scanning Electron Microscopy of Bed Bugs on Leaf-Derived Trapping Material
2.6. Statistical Analysis
3. Results
3.1. The Effects of Life Stages and Sex on Bed Bug Trapping
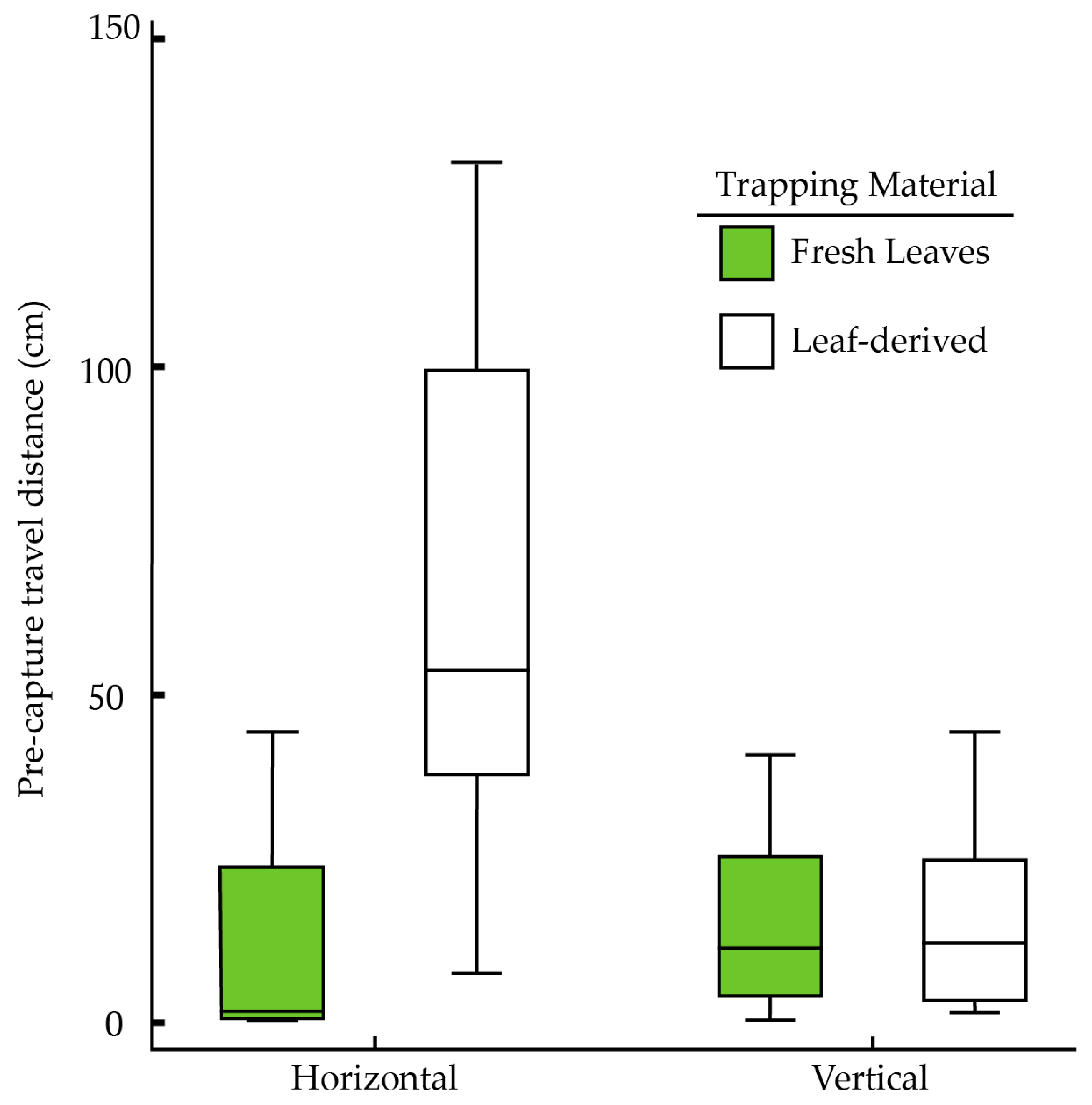
3.2. Effects of Surface Type and Orientation (Horizontal vs. Vertical) on Trapping Bed Bugs
3.3. Verification of Loss of Trapping Ability in Fresh Leaves
3.4. Scanning Electron Microscopy of Bed Bugs on Leaf-Derived Trapping Material
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usinger, R.L. Monograph of Cimicidae (Hemiptera, Heteroptera); Entomological Society of America: College Park, MD, USA, 1966; 585p. [Google Scholar]
- Doggett, S.L.; Dwyer, D.E.; Penas, P.F.; Russell, R.C. Bed Bugs: Clinical Relevance and Control Options. Clin. Microbiol. Rev. 2012, 25, 164–192. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.C. Magnitude and Spread of Bed Bugs (Cimex lecturalius) Throughout Ohio (USA) Revealed by Surveys of Pest Management Industry. Insects 2021, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Hasnaoui, B.; Bérenger, J.M.; Delaunay, P.; Diarra, A.Z.; Ndiaye, E.I.; M’madi, S.A.; Masotti, N.; Sevestre, J.; Parola, P. Survey of Bed Bug Infestations in Homeless Shelters in Southern France. Sci. Rep. 2023, 13, 12557. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Zumelzu, C.; Sereno, D.; Marteau, A.; Brun, S.; Jan, J.; Izri, A. Bed Bugs (Hemiptera, Cimicidae): A Global Challenge for Public Health and Control Management. Diagnostics 2023, 13, 2281. [Google Scholar] [CrossRef]
- Ashcroft, R.; Seko, Y.; Chan, L.F.; Dere, J.; Kim, J.; McKenzie, K. The Mental Health Impact of Bed Bug Infestations: A Scoping Review. Int. J. Public Health 2015, 60, 827–837. [Google Scholar] [CrossRef]
- Sheele, J.M.; Pritt, B.S.; Libertin, C.R.; Wysokinska, E.M. Bed Bugs are Associated with Anemia. Am. J. Emerg. Med. 2021, 46, 482–488. [Google Scholar] [CrossRef]
- Koganemaru, R.; Miller, D.M. The Bed Bug Problem: Past, Present, and Future Control Methods. Pestic. Biochem. Physiol. 2013, 106, 177–189. [Google Scholar] [CrossRef]
- Richards, L.; Boase, C.; Gezan, S.; Cameron, M. Are Bed Bug Infestations on the Increase within Greater London. J. Environ. Health Res. 2009, 9, 17–24. [Google Scholar]
- Hwang, S.W.; Svoboda, T.J.; De Jong, I.J.; Kabasele, K.J.; Gogosis, E. Bed bug Infestations in an Urban Environment. Emerg. Infect. Dis. 2005, 11, 533. [Google Scholar] [CrossRef]
- Doggett, S.L.; Lee, C.-Y. Historical and Contemporary Control Options against Bed Bugs, Cimex spp. Annu. Rev. Entomol. 2023, 68, 169–190. [Google Scholar] [CrossRef]
- Romero, A.; Potter, M.F.; Potter, D.A.; Haynes, K.F. Insecticide Resistance in the Bed Bug: A Factor in the Pest’s Sudden Resurgence? J. Med. Entomol. 2007, 44, 175–178. [Google Scholar] [CrossRef]
- Doggett, S.L.; Russell, R.C.; Robinson, W.; Bajomi, D. The Resurgence of Bed Bugs, Cimex spp. (Hemiptera: Cimicidae) in Australia. In Proceedings of the Sixth International Conference on Urban Pests, Budapest, Hungary, 13–16 July 2008; pp. 407–425. [Google Scholar]
- Fourie, J.; Crafford, D. The Bed Bug Resurgence in Africa. In Advances in the Biology and Management of Modern Bed Bugs; Wiley: Hoboken, NJ, USA, 2018; pp. 87–94. [Google Scholar]
- Criado, P.R.; Junior, W.B.; Criado, R.F.J.; e Silva, R.V.; Vasconcellos, C. Bedbugs (Cimicidae infestation): The Worldwide Renaissance of an Old Partner of Human Kind. Braz. J. Infect. Dis. 2011, 15, 74–80. [Google Scholar]
- Potter, M.F. The History of Bed Bug Management. Am. Entomol. 2011, 57, 14–25. [Google Scholar] [CrossRef]
- Dang, K.; Doggett, S.L.; Veera Singham, G.; Lee, C.Y. Insecticide Resistance and Resistance Mechanisms in Bed Bugs, Cimex spp. (Hemiptera: Cimicidae). Parasites Vectors 2017, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Romero, A. Moving from the Old to the New: Insecticide Research on Bed Bugs since the Resurgence. Insects 2011, 2, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Hill, A.L.; Rehmann, C.R.; Levy, M.Z. Dynamics of Bed Bug Infestations and Control under Disclosure Policies. Proc. Natl. Acad. Sci. USA 2019, 116, 6473–6481. [Google Scholar] [CrossRef]
- Doggett, S.L.; Orton, C.J.; Lilly, D.G.; Russell, R.C. Bed Bugs: The Australian Response. Insects 2011, 2, 96–111. [Google Scholar] [CrossRef]
- Wang, C.; Singh, N.; Zha, C.; Cooper, R. Bed Bugs: Prevalence in Low-income Communities, Resident’s Reactions, and Implementation of a Low-cost Inspection Protocol. J. Med. Entomol. 2016, 53, 639–646. [Google Scholar] [CrossRef]
- Schneider, D. They’re Back: Municipal Responses to the Resurgence of Bed Bug Infestations. J. Am. Plan. Assoc. 2019, 85, 96–113. [Google Scholar] [CrossRef]
- Gressier, A.; Galakhoff, N.; Thuillier, P.; Kerlan, V.; Cogulet, V.; Cosse, M.; Daniel, L.; Canevet, M.; Cabon, S.; Le Grand, A. Bed Bug Infestation in a French University Hospital: Control Strategy, Financial Impact and Perspectives. J. Hosp. Infect. 2022, 126, 81–86. [Google Scholar] [CrossRef]
- Meisyara, D.; Guswenrivo, I.; Veera Singham, G. Perception, Attitudes, and Knowledge on Infestation and Management of Bed Bugs in Major Cities of Indonesia: A Cross-sectional Online Survey. PLoS ONE 2023, 18, e0288682. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Sutherland, A.; Gouge, D.; Spafford, H.; Nair, S.; Lewis, V.; Choe, D.-H.; Li, S.; Young, D. Pest Management Strategies for Bed Bugs (Hemiptera: Cimicidae) in Multiunit Housing: A Literature Review on Field Studies. J. Integr. Pest Manag. 2017, 8, 13. [Google Scholar] [CrossRef]
- Crawley, S.E.; Borden, J.H. Detection and Monitoring of Bed Bugs (Hemiptera: Cimicidae): Review of the Underlying Science, Existing Products and Future Prospects. Pest Manag. Sci. 2021, 77, 5334–5346. [Google Scholar] [CrossRef] [PubMed]
- Szyndler, M.W.; Haynes, K.F.; Potter, M.F.; Corn, R.M.; Loudon, C. Entrapment of Bed Bugs by Leaf Trichomes Inspires Microfabrication of Biomimetic Surfaces. J. R. Soc. Interface 2013, 10, 20130174. [Google Scholar] [CrossRef]
- Richardson, H.H. The Action of Bean Leaves Against the Bedbug. J. Econ. Entomol. 1943, 36, 543–545. [Google Scholar] [CrossRef]
- The Regents of the University of California. Surfaces Incorporating Treated Leaves for Physical Capture of Arthropods. Patent Application PCT/US2024/042239, 14 August 2024.
- Dallmann, C.J.; Dürr, V.; Schmitz, J. Motor Control of an Insect Leg during Level and Incline Walking. J. Exp. Biol. 2019, 222, jeb188748. [Google Scholar] [CrossRef]
- Weihmann, T.; Blickhan, R. Comparing Inclined Locomotion in a Ground-living and a Climbing Ant Species: Sagittal Plane Kinematics. J. Comp. Physiol. A 2009, 195, 1011–1020. [Google Scholar] [CrossRef]
- Goldman, D.I.; Chen, T.S.; Dudek, D.M.; Full, R.J. Dynamics of Rapid Vertical Climbing in Cockroaches Reveals a Template. J. Exp. Biol. 2006, 209, 2990–3000. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. Comparing Multiple Proportions: The Marascuillo [Sic] Procedure. Available online: https://www.itl.nist.gov/div898/handbook/prc/section4/prc474.htm (accessed on 22 January 2025).
- Cochran, W.G. Some Methods for Strengthening the Common χ2 Tests. Biometrics 1954, 10, 417–451. [Google Scholar] [CrossRef]
- Armitage, P. Tests for Linear Trends in Proportions and Frequencies. Biometrics 1955, 11, 375–386. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practices of Statistics in Biological Research; W.H. Freeman: New York, NY, USA, 1995. [Google Scholar]
- Ievinsh, G. Water Content of Plant Tissues: So Simple That Almost Forgotten? Plants 2023, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.S.; Rajendren, S.V.; Bartos, I.; Marka, S.; Mann, R.S. Kinematic Responses to Changes in Walking Orientation and Gravitational Load in Drosophila melanogaster. PLoS ONE 2014, 9, e109204. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Peterson, D.; Schmitt, J.; Gravish, N.; Clark, J.E. Impact of Slope on Dynamics of Running and Climbing. Bioinspir. Biomim. 2020, 15, 056005. [Google Scholar] [CrossRef] [PubMed]
- Duch, C.; Pflüger, H.J. Motor Patterns for Horizontal and Upside Down Walking and Vertical Climbing in the Locust. J. Exp. Biol. 1995, 198, 1963–1976. [Google Scholar] [CrossRef]
- Dai, Z.; Gorb, S.N.; Schwarz, U. Roughness-dependent Friction Force of the Tarsal Claw System in the Beetle Pachnoda marginata (Coleoptera, Scarabaeidae). J. Exp. Biol. 2002, 205, 2479–2488. [Google Scholar] [CrossRef]
- Dwyer-Joyce, R.; Voigt, D.; Reinhardt, K. Surface Wettability Affects Attachment of Male Bed Bugs Cimex lectularius to Rough Perspex Substrates. Physiol. Entomol. 2024, 50, 96–104. [Google Scholar] [CrossRef]
- Arzt, E.; Gorb, S.; Spolenak, R. From Micro to Nano Contacts in Biological Attachment Devices. Proc. Natl. Acad. Sci. USA 2003, 100, 10603–10606. [Google Scholar] [CrossRef]
- Gorb, S. Attachment Devices of Insect Cuticle; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Maass, A.; Hirn, U. Long Term Curl of Printing Paper due to Ink Solvent Migration. Mater. Des. 2024, 237, 112593. [Google Scholar] [CrossRef]
- Hottel, B.A.; Pereira, R.M.; Gezan, S.A.; Qing, R.; Sigmund, W.M.; Koehler, P.G. Climbing Ability of the Common Bed Bug (Hemiptera: Cimicidae). J. Med. Entomol. 2015, 52, 289–295. [Google Scholar] [CrossRef]
- Hinson, K.R.; Reukov, V.; Benson, E.P.; Zungoli, P.A.; Bridges, W.C.; Ellis, B.R.; Song, J. Climbing Ability of Teneral and Sclerotized Adult Bed Bugs and Assessment of Adhesive Properties of the Exoskeletal Fluid using Atomic Force Microscopy. PLoS ONE 2017, 12, e0189215. [Google Scholar] [CrossRef]
- Campbell, B.E.; Koehler, P.G.; Buss, L.J.; Baldwin, R.W. Recent Documentation of the Tropical Bed Bug (Hemiptera: Cimicidae) in Florida since the Common Bed Bug Resurgence. Fla. Entomol. 2016, 99, 549–551. [Google Scholar] [CrossRef]
- Zulaikha, Z.; Hassan, A.A. A Survey on the Infestation Levels of Tropical Bed Bugs in Peninsular Malaysia: Current Updates and Status on Resurgence of Cimex hemipterus (Hemiptera: Cimicidae). Asian Pac. J. Trop. Dis. 2016, 6, 40–45. [Google Scholar] [CrossRef]
- Davies, T.; Field, L.; Williamson, M. The Re-emergence of the Bed Bug as a Nuisance Pest: Implications of Resistance to the Pyrethroid Insecticides. Med. Vet. Entomol. 2012, 26, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Billen, J.; Doggett, S.L.; Lee, C.-Y. Differences in Climbing Ability of Cimex lectularius and Cimex hemipterus (Hemiptera: Cimicidae). J. Econ. Entomol. 2017, 110, 1179–1186. [Google Scholar] [CrossRef]
- Bustamante, J., Jr.; Liu, P.; Campbell, K.; Sutherland, A.M.; Choe, D.-H.; Loudon, C. A Novel Leaf-derived Trapping Material is more Effective at Capturing Common Bed Bugs (Hemiptera: Cimicidae) than Selected Commercial Monitoring Devices. Insects 2025, 16, 362. [Google Scholar] [CrossRef]

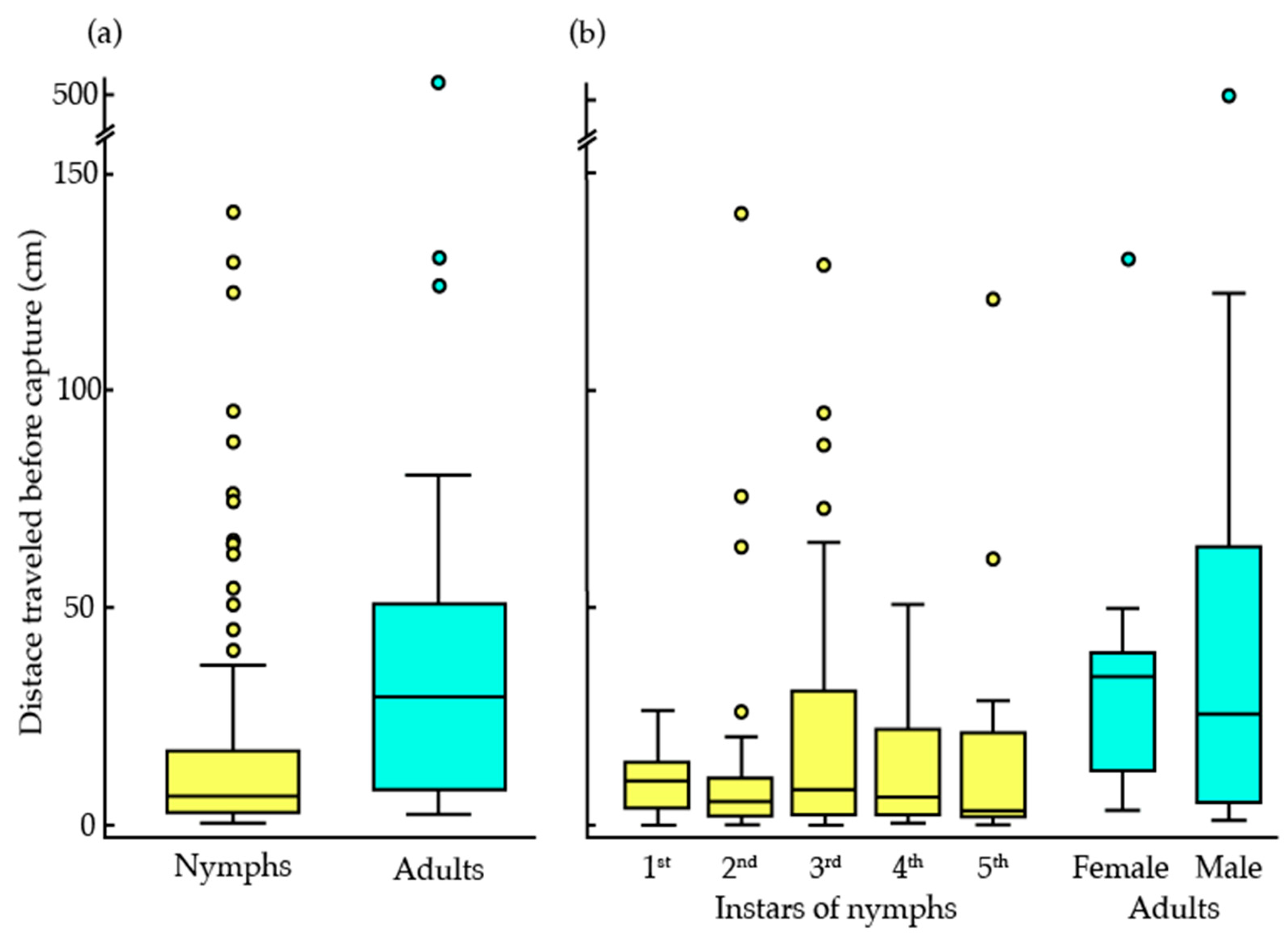
| Life Stage | Bugs Trapped (% Trapped, Trapped/Total Number) |
|---|---|
| All Bugs | 68% (95/140) |
| 1st Instar | 92% (11/12) |
| 2nd Instar | 100% (23/23) |
| 3rd Instar | 95% (20/21) |
| 4th Instar | 71% (15/21) |
| 5th Instar | 67% (14/21) |
| Adult (females) | 24% (5/21) |
| Adult (males) | 52% (11/21) |
| Orientation | Fresh Leaves (% Trapped/Total Number) | Leaf-Derived Trapping Material (% Trapped/Total Number) |
|---|---|---|
| Horizontal | 92% (11/12) | 67% (8/12) |
| Vertical | 67% (8/12) | 67% (8/12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Bustamante, J., Jr.; Campbell, K.; Sutherland, A.M.; Choe, D.-H.; Loudon, C. Using Leaf-Derived Materials to Stop Common Bed Bugs (Cimex lectularius L.) in Their Tracks. Insects 2025, 16, 786. https://doi.org/10.3390/insects16080786
Liu P, Bustamante J Jr., Campbell K, Sutherland AM, Choe D-H, Loudon C. Using Leaf-Derived Materials to Stop Common Bed Bugs (Cimex lectularius L.) in Their Tracks. Insects. 2025; 16(8):786. https://doi.org/10.3390/insects16080786
Chicago/Turabian StyleLiu, Patrick, Jorge Bustamante, Jr., Kathleen Campbell, Andrew M. Sutherland, Dong-Hwan Choe, and Catherine Loudon. 2025. "Using Leaf-Derived Materials to Stop Common Bed Bugs (Cimex lectularius L.) in Their Tracks" Insects 16, no. 8: 786. https://doi.org/10.3390/insects16080786
APA StyleLiu, P., Bustamante, J., Jr., Campbell, K., Sutherland, A. M., Choe, D.-H., & Loudon, C. (2025). Using Leaf-Derived Materials to Stop Common Bed Bugs (Cimex lectularius L.) in Their Tracks. Insects, 16(8), 786. https://doi.org/10.3390/insects16080786








