Assessing the Productivity of Colonies Headed by Preheated Honeybee Queens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Queen Rearing and Thermal Manipulation
2.2. Apicultural Practices
2.3. Measurement of Worker and Drone Brood Areas
2.4. Count of Adult Workers
2.5. Count of Swarm Queen Cells
2.6. Reporting Pollen Foraging Percentage per Hour
2.7. Count of Stored Pollen Cells
2.8. Weight of Pollen Loads
2.9. Statistical Analysis
3. Results
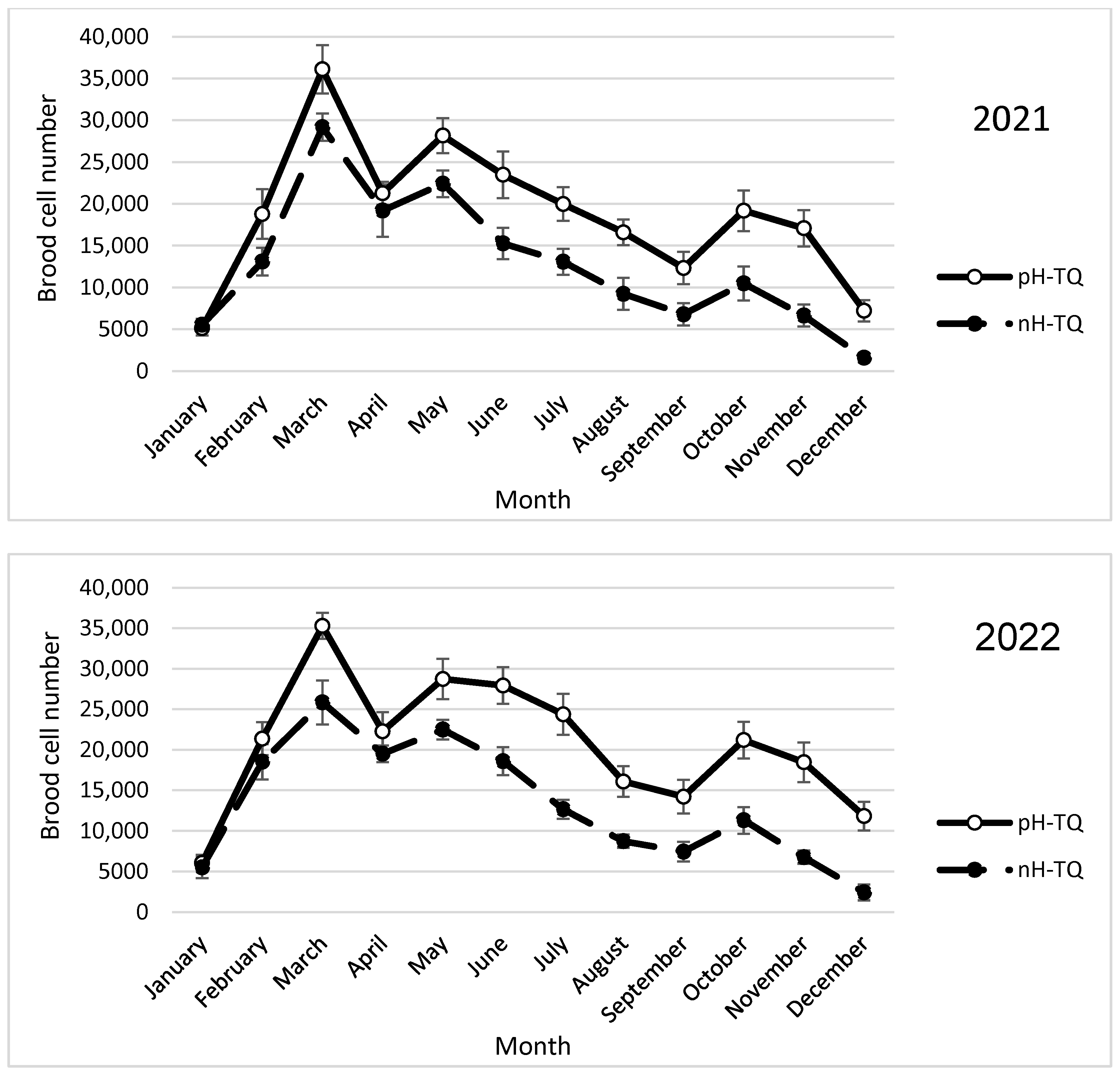
3.1. Brood-Rearing Activities
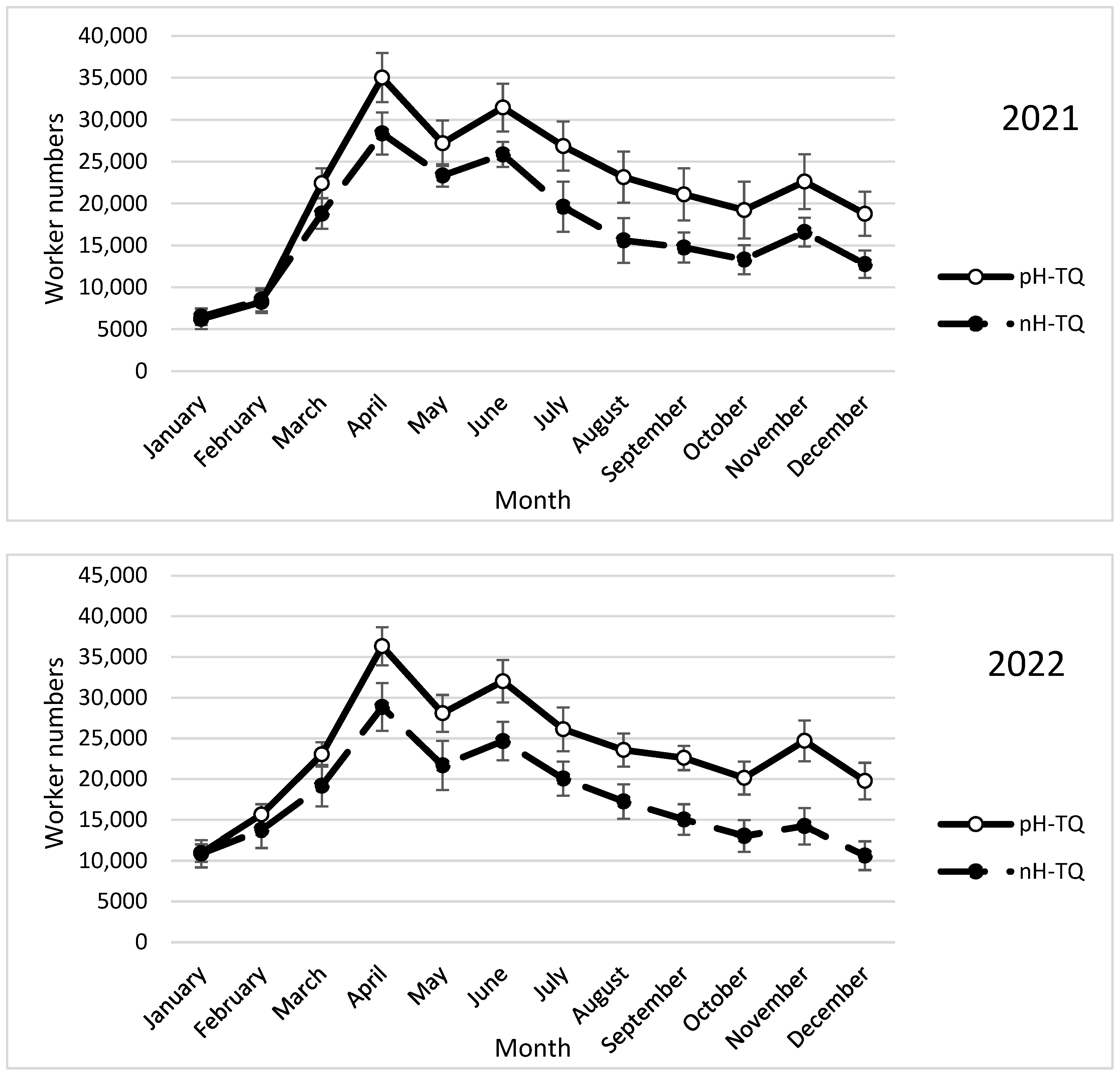
3.2. Honeybee Worker Population
3.3. Drone Brood Rearing
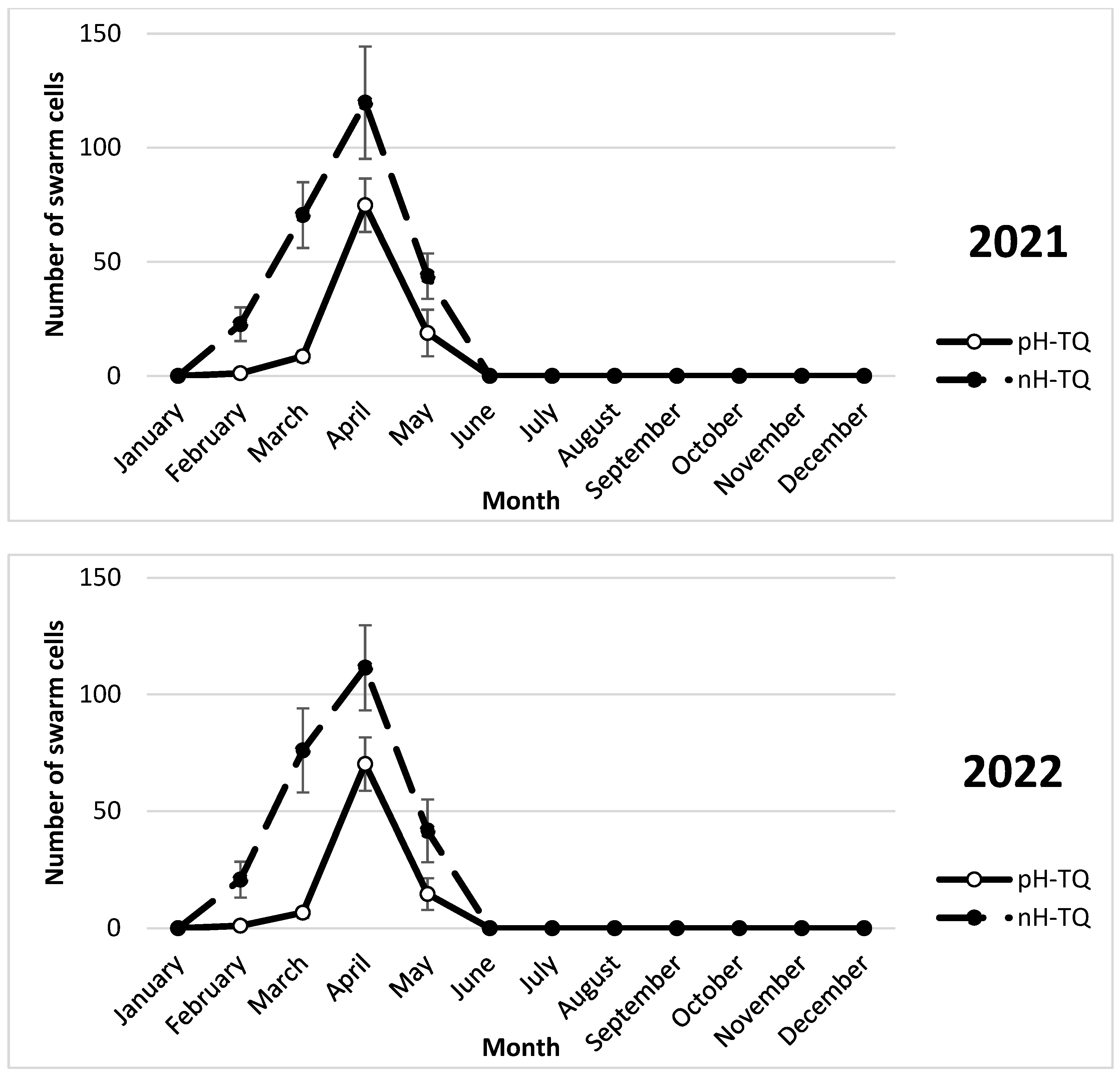
3.4. Swarm Cells
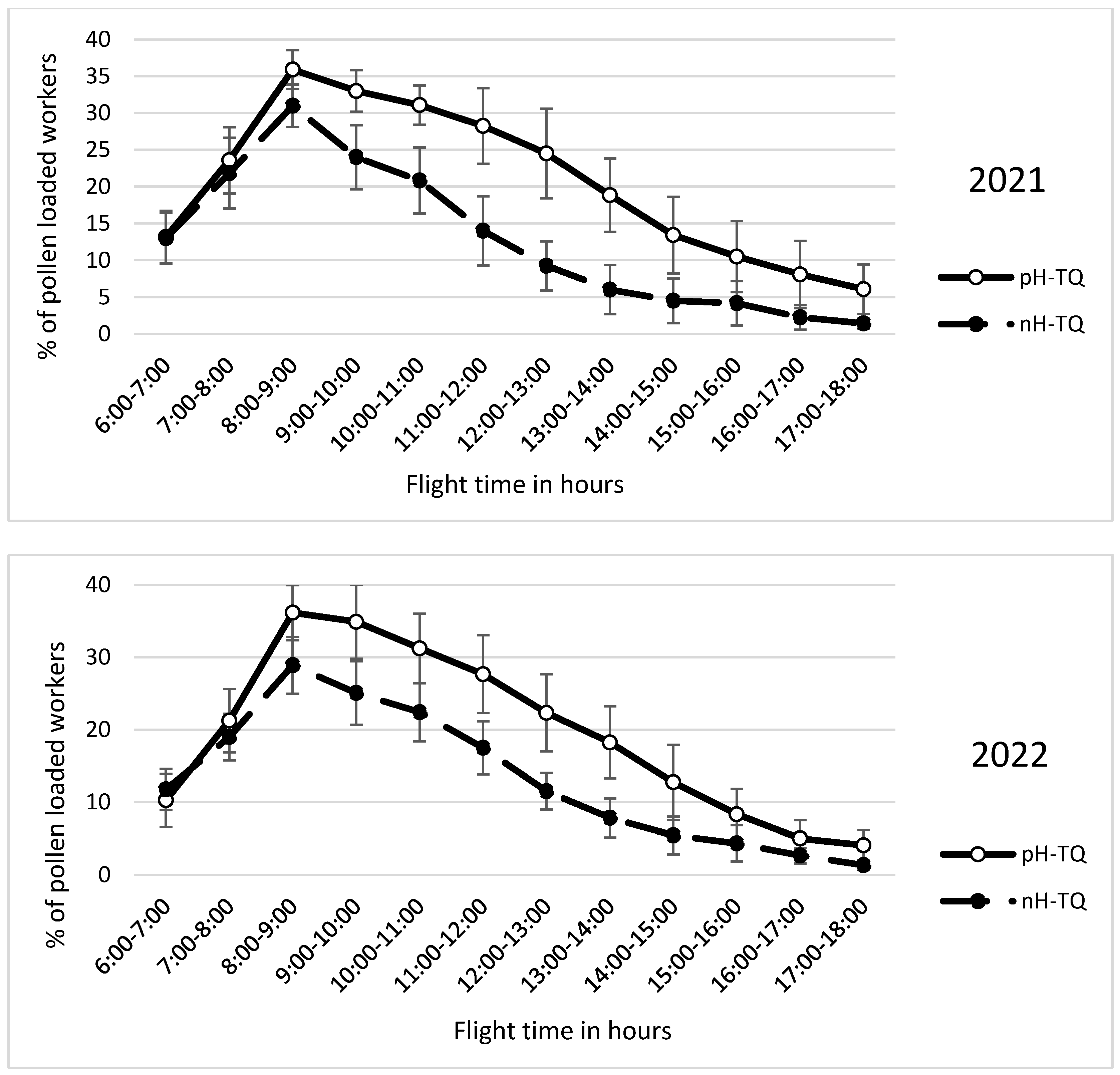
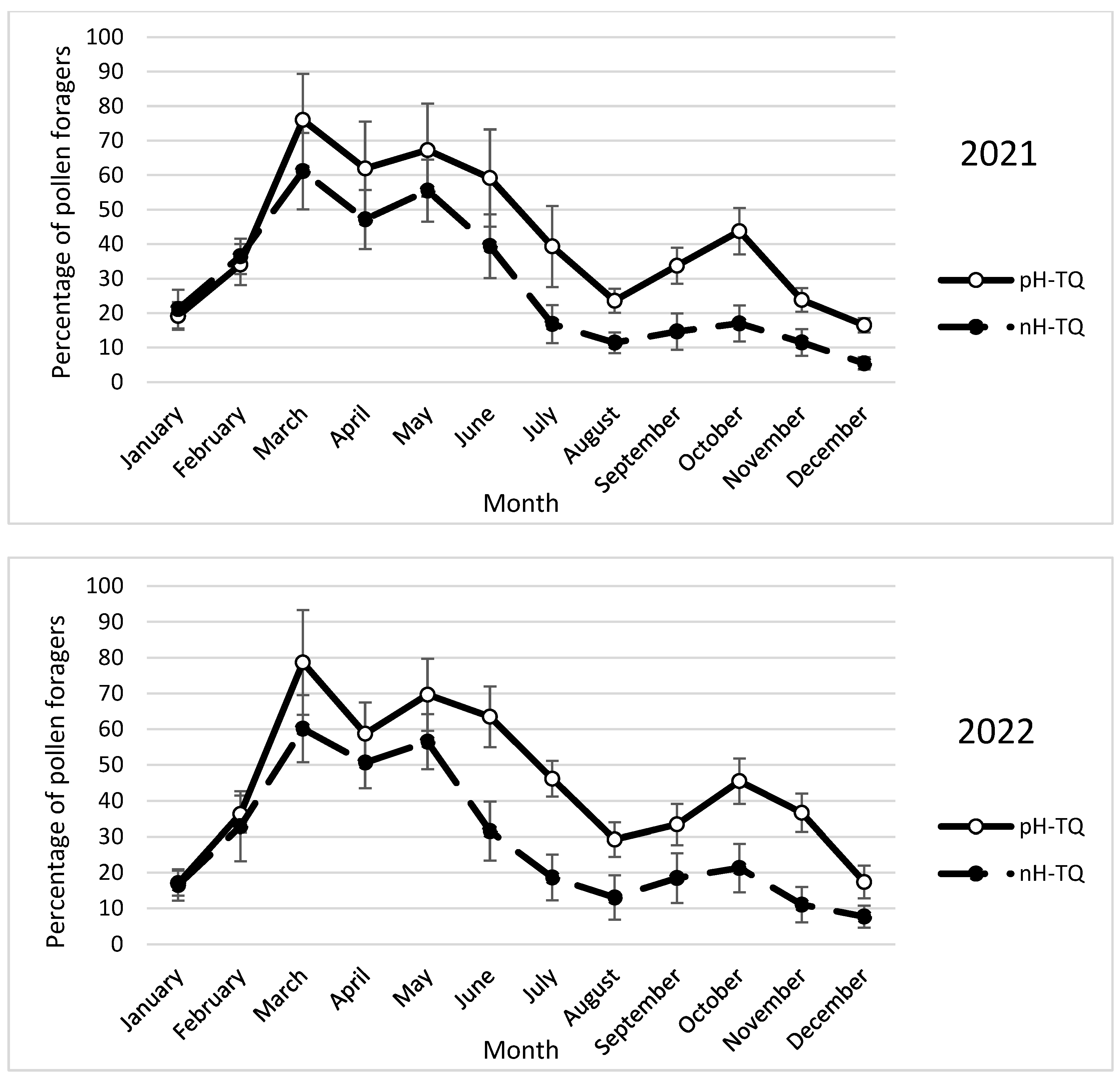
3.5. Pollen Foraging Percentage per Hour
3.6. Stored Pollen Cells
3.7. Monthly Percentage of Pollen Foragers
3.8. Weight of 10 Pollen Loads
4. Discussion
4.1. Effect of Preheat Hardening on the Survival of Honeybee Workers
4.2. Effect of Preheat Hardening on Brood Numbers
4.3. Changes Induced by Preheat Hardening on Honeybee Workers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| pH-TQ | Pre-heat-treated queens |
| nH-TQ | non-heat-treated queens |
| RH | Relative humidity |
References
- Vincent, C.; Hallman, G.; Panneton, B.; Fleurat-Lessard, F. Management of agricultural insects with physical control methods. Annu. Rev. Entomol. 2003, 48, 261–281. [Google Scholar] [CrossRef]
- Beckett, S.; Fields, P.; Subramanyam, B. Disinfestation of stored products and associated structures using heat. In Heat Treatments for Postharvest Pest Control: Theory and Practice; CABI: Wallingford, UK, 2007; pp. 182–237. [Google Scholar]
- Hansen, J.D.; Johnson, J.A.; Winter, D.A. History and use of heat in pest control: A review. Int. J. Pest. Manag. 2011, 57, 267–289. [Google Scholar] [CrossRef]
- Grondeau, C.; Samson, R.; Sands, D.C. A Review of Thermotherapy to Free Plant Materials from Pathogens, Especially Seeds from Bacteria. Crit. Rev. Plant Sci. 1994, 13, 57–75. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Zhang, Y.; Chen, Y.; Yan, F.; Han, L.; Ma, Y. Heat and cold therapy reduce pain in patients with delayed onset muscle soreness: A systematic review and meta-analysis of 32 randomized controlled trials. Phys. Ther. Sport 2021, 48, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Harbo, J.R. Heating adult honeybees to remove Varroa jacobsoni. J. Apic. Res. 2000, 39, 181–182. [Google Scholar] [CrossRef]
- Goras, G.; Tananaki, C.; Gounari, S.; Dimou, M.; Lazaridou, E.; Karazafiris, E.; Kanelis, D.; Liolios, V.; El Taj, H.F.; Thrasyvoulou, A. Hyperthermia non-chemical control strategy against varroa. J. Hell. Vet. Med. Soc. 2015, 66, 249–256. [Google Scholar] [CrossRef]
- Martin-Hernandez, R.; Meana, A.; Garcia-Palencia, P.; Marin, P.; Botias, C.; Garrido-Bailon, E.; Barrios, L.; Higes, M. Effect of temperature on the biotic potential of honeybee microsporidia. Appl. Environ. Microbiol. 2009, 75, 2554–2557. [Google Scholar] [CrossRef]
- McMenamin, A.J.; Daughenbaugh, K.F.; Flenniken, M.L. The Heat Shock Response in the Western Honeybee (Apis mellifera) is Antiviral. Viruses 2020, 12, 245. [Google Scholar] [CrossRef]
- Chidawanyika, F.; Terblanche, J.S. Rapid thermal responses and thermal tolerance in adult codling moth Cydia pomonella (Lepidoptera: Tortricidae). J. Insect Physiol. 2011, 57, 108–117. [Google Scholar] [CrossRef]
- Mutamiswa, R.; Chidawanyika, F.; Nyamukondiwa, C. Superior basal and plastic thermal responses to environmental heterogeneity in invasive exotic stemborer Chilo partellus Swinhoe over indigenous Busseola fusca (Fuller) and Sesamia calamistis Hampson. Physiol. Entomol. 2018, 43, 108–119. [Google Scholar] [CrossRef]
- Dalmon, A.; Peruzzi, M.; Le Conte, Y.; Alaux, C.; Pioz, M. Temperature-driven changes in viral loads in the honey bee Apis mellifera. J. Invertebr. Pathol. 2019, 160, 87–94. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Shen, J.; Long, D.; Feng, Y.; Su, W.; Xu, K.; Du, Y.; Jiang, Y. Tolerance and response of two honeybee species Apis cerana and Apis mellifera to high temperature and relative humidity. PLoS ONE 2019, 14, e0217921. [Google Scholar] [CrossRef]
- Ahamed, M.; Posgai, R.; Gorey, T.J.; Nielsen, M.; Hussain, S.M.; Rowe, J.J. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol. Appl. Pharmacol. 2010, 242, 263–269. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.; Wang, W.; Wang, G.; Li, Y. Transcriptome analysis reveals the early resistance of zebrafish larvae to oxidative stress. Fish Physiol. Biochem. 2022, 48, 1075–1089. [Google Scholar] [CrossRef]
- Elekonich, M.M. Extreme thermotolerance and behavioral induction of 70-kDa heat shock proteins and their encoding genes in honey bees. Cell Stress Chaperones 2009, 14, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Severson, D.W.; Erickson, E.H.; Williamson, J.L., Jr.; Aiken, J.M. Heat stress induced enhancement of heat shock protein gene activity in the honey bee (Apis mellifera). Experientia 1990, 46, 737–739. [Google Scholar] [CrossRef]
- Ramirez, L.; Luna, F.; Mucci, C.A.; Lamattina, L. Fast weight recovery, metabolic rate adjustment and gene-expression regulation define responses of cold-stressed honey bee brood. J. Insect Physiol. 2021, 128, 104178. [Google Scholar] [CrossRef] [PubMed]
- Degrandi-Hoffman, G.; Spivak, M.; Martin, J.H. Role of thermoregulation by nestmates on the development time of honey bee (Hymenoptera: Apidae) queens. Ann. Entomol. Soc. Am. 1993, 86, 165–172. [Google Scholar] [CrossRef]
- Tautz, J.; Maier, S.; Groh, C.; Rössler, W.; Brockmann, A. Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc. Natl. Acad. Sci. USA 2003, 100, 7343–7347. [Google Scholar] [CrossRef]
- Abou-Shaara, H.; Owayss, A.A.; Ibrahim, Y.; Basuny, N. A review of the impacts of temperature and relative humidity on various activities of honey bees. Insectes Sociaux 2017, 64, 455–463. [Google Scholar] [CrossRef]
- Chuda-Mickiewicz, B.; Samborski, J. The quality of honey bee queens from queen cells incubated at different temperatures. Acta Sci. Pol. Zootech. 2015, 14, 25–32. [Google Scholar]
- Medina, R.G.; Paxton, R.J.; Hernandez-Sotomayor, S.M.T.; Pech-Jimenez, C.; Medina-Medina, L.A.; Quezada-Euan, J.J.G. Heat stress during development affects immunocompetence in workers, queens and drones of Africanized honey bees (Apis mellifera L.) (Hymenoptera: Apidae). J. Therm. Biol. 2020, 89, 102541. [Google Scholar] [CrossRef] [PubMed]
- Langowska, A.; Zawilak, M.; Sparks, T.H.; Glazaczow, A.; Tomkins, P.W.; Tryjanowski, P. Long-term effect of temperature on honey yield and honeybee phenology. Int. J. Biometeorol. 2017, 61, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Helliwell, P.; Beekman, M.; Maleszka, R.; Oldroyd, B.P. The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005, 191, 1121–1129. [Google Scholar] [CrossRef]
- Alqarni, A.S. Tolerance of summer temperature in imported and indigenous honeybee Apis mellifera L. races in central Saudi Arabia. Saudi J. Biol. Sci. 2006, 13, 123–127. [Google Scholar] [CrossRef]
- Blažytė-Čereškienė, L.; Vaitkevičienė, G.; Venskutonytė, S.; Būda, V. Honey bee foraging in spring oilseed rape crops under high ambient temperature conditions. Zemdirbyste 2010, 79, 61–70. [Google Scholar]
- Le Conte, Y.; Navajas, M. Climate change: Impact on honey bee populations and diseases. Revue Scientif. Techn. 2008, 27, 499–510. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F.; Al-Ghamdi, A.A.; Mohamed, A.A. Tolerance of two honeybee races to various temperature and relative humidity gradients. Environ. Exp. Biol. 2012, 10, 133–138. [Google Scholar]
- Stabentheiner, A.; Kovac, H.; Brodschneider, R. Honeybee colony thermoregulation–regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE 2010, 5, e8967. [Google Scholar] [CrossRef]
- Southwick, E.E.; Moritz, R.F.A. Social control of air ventilation in colonies of honey bees, Apis mellifera. J. Insect Physiol. 1987, 33, 623–626. [Google Scholar] [CrossRef]
- Nicolson, S.W. Water homeostasis in bees, with the emphasis on sociality. J. Exp. Biol. 2009, 212, 429–434. [Google Scholar] [CrossRef]
- Stabentheiner, A.; Kovac, H.; Mandl, M.; Käfer, H. Coping with the cold and fighting the heat: Thermal homeostasis of a superorganism, the honeybee colony. J. Comp. Physiol. 2021, 207, 337–351. [Google Scholar] [CrossRef]
- Starks, P.T.; Gilley, D.C. Heat shielding: A novel method of colonial thermoregulation in honey bees. Naturwissenschaften 1999, 86, 438–440. [Google Scholar] [CrossRef]
- Al-Ghzawi, A.A.A.; Zaitoun, S. Origin and Rearing Season of Honeybee Queens Affect Some of Their Physiological and Reproductive Characteristics. Entomol. Res. 2008, 38, 139–148. [Google Scholar] [CrossRef]
- Laidlaw, H.H.; Page, R.E., Jr. Queen Rearing and Bee Breeding, 1st ed.; Wicwas Press: Cheshire, CT, USA, 1997; p. 224. [Google Scholar]
- Gerig, L. Lehrgang zur erfassung der volksstarke. Schweiz. Bienenztg. 1983, 106, 199–204. [Google Scholar]
- Ruttner, H. Technical recommendations for methods of evaluating performance of bee colonies. In Controlled Mating and Selection of the Honey Bee; Ruttner, F., Ed.; Apimondia: Bucharest, Romania, 1972; pp. 87–92. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing (Computer Software); R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Camazine, S. The regulation of pollen foraging by honey bees: How foragers assess the colony's need for pollen. Behav. Ecol. Sociobiol. 1993, 32, 265–272. [Google Scholar] [CrossRef]
- Bilisik, A.; Cakmak, I.; Bicakci, A.; Malyer, H. Seasonal variation of collected pollen loads of honeybees (Apis mellifera L. anatoliaca). Grana 2008, 47, 70–77. [Google Scholar] [CrossRef]
- Ghosh, S.; Jeon, H.; Jung, C. Foraging behaviour and preference of pollen sources by honey bee (Apis mellifera) relative to protein contents. J. Ecol. Environ. 2020, 44, 4. [Google Scholar] [CrossRef]
- Tan, K.; Yang, S.; Wang, Z.-W.; Radloff, S.E.; Oldroyd, B.P. Differences in foraging and broodnest temperature in the honey bees Apis cerana and A. mellifera. Apidologie 2012, 43, 618–623. [Google Scholar] [CrossRef]
- Cooper, P.D.; Schaffer, W.M.; Buchmann, S.L. Temperature Regulation of Honey Bees (Apis mellifera) Foraging in the Sonoran Desert. J. Exp. Biol. 1985, 114, 1–15. [Google Scholar] [CrossRef]
- Heinrich, B. Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia 1979, 40, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.R. Heat Transfer and Body Temperature in Honey Bee (Hymenoptera: Apidae) Drones and Workers. Environ. Entomol. 1991, 20, 1627–1635. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Thorp, R.W. The collection of pollen by bees. Plant Syst. Evol. 2000, 222, 211–223. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Alaux, C.; Le Conte, Y.; Odoux, J.F.; Pioz, M.; Vaissiere, B.E.; Belzunces, L.P.; Decourtye, A. Variations in the Availability of Pollen Resources Affect Honey Bee Health. PLoS ONE 2016, 11, e0162818. [Google Scholar] [CrossRef]
- Mattila, H.R.; Otis, G.W. Influence of pollen diet in spring on development of honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 2006, 99, 604–613. [Google Scholar] [CrossRef]
- Zaitoun, S.T.; Al-Ghzawi, A.-M.; Shannag, H.K. Population dynamics of the Syrian Honeybee, Apis mellifera syriaca, under semi-arid Mediterranean conditions. Zool. Middle East 2000, 21, 129–132. [Google Scholar] [CrossRef]
- Jeffree, E.P. Observations on the Decline and Growth of Honey Bee Colonies1. J. Econ. Entomol. 1955, 48, 723–726. [Google Scholar] [CrossRef]
- Roberts, S.P.; Harrison, J.F. Mechanisms of thermal stability during flight in the honeybee Apis mellifera. J. Exp. Biol. 1999, 202, 1523–1533. [Google Scholar] [CrossRef]
- Perez, R.; Aron, S. Adaptations to thermal stress in social insects: Recent advances and future directions. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1535–1553. [Google Scholar] [CrossRef]
- Colinet, H.; Lee, S.F.; Hoffmann, A. Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosoph melanogaster. FEBS J. 2010, 277, 174–185. [Google Scholar] [CrossRef]
- Al-Ghzawi, A.A.A.; Al-Zghoul, M.B.; Zaitoun, S.; Al-Omary, I.M.; Alahmad, N.A. Dynamics of Heat Shock Proteins and Heat Shock Factor Expression During Heat Stress in Daughter Workers in Pre-Heat-Treated (Rapid Heat Hardening) Apis mellifera Mother Queens. J. Therm. Biol. 2022, 104, 103194. [Google Scholar] [CrossRef]
- Zaitoun, S.T.; Al-Ghzawi, A.A.; Al-Zghoul, M.B.; Al-Omary, I.M.; Al-Sabi, M.N.S. Effects of Rapid Heat Hardening of Honeybee Queens (Apis mellifera) During the Larval Stage on the Oxidative Response of Their Workers During Heat Stress. Stresses 2025, 5, 32. [Google Scholar] [CrossRef]
- Yahav, S.; Plavnik, I. Effect of early-stage thermal conditioning and food restriction on performance and thermotolerance of male broiler chickens. Br. Poult. Sci. 1999, 40, 120–126. [Google Scholar] [CrossRef]
- Yahav, S.; McMurtry, J.P. Thermotolerance acquisition in broiler chickens by temperature conditioning early in life--the effect of timing and ambient temperature. Poult. Sci. 2001, 80, 1662–1666. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Finstad, A.G. Linking embryonic temperature with adult reproductive investment in Atlantic salmon Salmo salar. Mar. Ecol. Prog. Ser. 2014, 515, 217–226. [Google Scholar] [CrossRef]
- Gray, E.M. Thermal acclimation in a complex life cycle: The effects of larval and adult thermal conditions on metabolic rate and heat resistance in Culex pipiens (Diptera: Culicidae). J. Insect Physiol. 2013, 59, 1001–1007. [Google Scholar] [CrossRef]
- Sgro, C.M.; Terblanche, J.S.; Hoffmann, A.A. What Can Plasticity Contribute to Insect Responses to Climate Change? Annu. Rev. Entomol. 2016, 61, 433–451. [Google Scholar] [CrossRef]
- Stevens, D.J. Pupal development temperature alters adult phenotype in the speckled wood butterfly, Pararge aegeria. J. Therm. Biol. 2004, 29, 205–210. [Google Scholar] [CrossRef]
- Chuche, J.; Thiery, D. Egg incubation temperature differently affects female and male hatching dynamics and larval fitness in a leafhopper. Ecol Evol. 2012, 2, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, Z.; Wang, Q.; Zhang, F.; Liu, T.-X. Exposing eggs to high temperatures affects the development, survival and reproduction of Harmonia axyridis. J. Therm. Biol. 2014, 39, 40–44. [Google Scholar] [CrossRef]
- Ottenheim, M.M.; Volmer, A.D. Wing length plasticity in Eristalis arbustorum (Diptera: Syrphidae). Neth. J. Zool. 1999, 49, 15–27. [Google Scholar] [CrossRef]
- Andersen, D.H.; Pertoldi, C.; Scali, V.; Loeschcke, V. Heat stress and age induced maternal effects on wing size and shape in parthenogenetic Drosophila mercatorum. J. Evol. Biol. 2005, 18, 884–892. [Google Scholar] [CrossRef]
- Baudier, K.M.; Mudd, A.E.; Erickson, S.C.; O'Donnell, S. Microhabitat and body size effects on heat tolerance: Implications for responses to climate change (army ants: Formicidae, Ecitoninae). J. Anim. Ecol. 2015, 84, 1322–1330. [Google Scholar] [CrossRef]
- Wendt, C.F.; Verble-Pearson, R. Critical thermal maxima and body size positively correlate in red imported fire ants, Solenopsis invicta. Southwest. Nat. 2016, 61, 79–83. [Google Scholar] [CrossRef]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef]
- Willmer, P.; Finlayson, K. Big bees do a better job: Intraspecific size variation influences pollination effectiveness. J. Pollinat. Ecol. 2014, 14, 244–254. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Skandalis, D.A.; Darveau, C.A. Morphological and physiological idiosyncrasies lead to interindividual variation in flight metabolic rate in worker bumblebees (Bombus impatiens). Physiol. Biochem. Zool. 2012, 85, 657–670. [Google Scholar] [CrossRef]
- Billardon, F.; Darveau, C.A. Flight energetics, caste dimorphism and scaling properties in the bumblebee, Bombus impatiens. J. Exp. Biol. 2019, 222, jeb187807. [Google Scholar] [CrossRef]
- Darveau, C.A.; Hochachka, P.W.; Welch, K.C.; Roubik, D.W., Jr.; Suarez, R.K. Allometric scaling of flight energetics in Panamanian orchid bees: A comparative phylogenetic approach. J. Exp. Biol. 2005, 208, 3581–3591. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.; Reim, C.; Blanckenhorn, W.U. Size-dependent insect flight energetics at different sugar supplies. Biol. J. Linn. Soc. 2013, 108, 565–578. [Google Scholar] [CrossRef]
- Wos, G.; Willi, Y. Thermal Acclimation in Arabidopsis lyrata: Genotypic Costs and Transcriptional Changes. J. Evol. Biol. 2018, 31, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Lee, R.E.; Denlinger, D.L. Cold shock and heat shock: A comparison of the protection generated by brief pretreatment at less severe temperatures. Physiol. Entomol. 1991, 16, 19–26. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Ali, H.; Iqbal, J.; Owayss, A.A.; Smith, B.H. Expression of heat shock proteins in adult honey bee (Apis mellifera L.) workers under hot-arid subtropical ecosystems. Saudi J. Biol. Sci. 2019, 26, 1372–1376. [Google Scholar] [CrossRef]
- Malmendal, A.; Overgaard, J.; Bundy, J.G.; Sorensen, J.G.; Nielsen, N.C.; Loeschcke, V.; Holmstrup, M. Metabolomic Profiling of Heat Stress: Hardening and Recovery of Homeostasis in Drosophila. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R205–R212. [Google Scholar] [CrossRef]
- Robert, J. Evolution of heat shock protein and immunity. Dev. Comp. Immunol. 2003, 27, 449–464. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Lavilla, N.; García-Reina, A.; De la Rúa, P. Mild thermal stress does not negatively affect immune gene expression in the bumblebee Bombus terrestris. Apidologie 2021, 52, 163–173. [Google Scholar] [CrossRef]
- Larsen, A.; Reynaldi, F.J.; Guzmán-Novoa, E. Fundaments of the honey bee (Apis mellifera) immune system. Review. Rev. Mex. Cienc. Pecu. 2019, 10, 705–728. [Google Scholar] [CrossRef]
- Corona, M.; Robinson, G.E. Genes of the Antioxidant System of the Honey Bee: Annotation and Phylogeny. Insect Mol. Biol. 2006, 15, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.B.; Colle, D.; Moreira, E.L.G.; Santos, A.A.; Hort, M.A.; Santos, K.; Oses, J.P.; Razzera, G.; Farina, M. Probucol Protects Neuronal Cells Against Peroxide-Induced Damage and Directly Activates Glutathione Peroxidase-1. Mol. Neurobiol. 2020, 57, 3245–3257. [Google Scholar] [CrossRef]
- Aurori, C.M.; Buttstedt, A.; Dezmirean, D.S.; Marghitas, L.A.; Moritz, R.F.; Erler, S. What Is the Main Driver of Ageing in Long-Lived Winter Honeybees: Antioxidant Enzymes, Innate Immunity, or Vitellogenin? J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Paleolog, J.; Wilde, J.; Miszczak, A.; Gancarz, M.; Strachecka, A. Antioxidation Defenses of Apis mellifera Queens and Workers Respond to Imidacloprid in Different Age-Dependent Ways: Old Queens Are Resistant, Foragers Are Not. Animals 2021, 11, 1246. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ghzawi, A.A.-M.; Zaitoun, S.T.; Al-Sabi, M.N.S.; Mazari, S.S.; Al-Omari, I.M.; Altalhi, M.S. Assessing the Productivity of Colonies Headed by Preheated Honeybee Queens. Insects 2025, 16, 858. https://doi.org/10.3390/insects16080858
Al-Ghzawi AA-M, Zaitoun ST, Al-Sabi MNS, Mazari SS, Al-Omari IM, Altalhi MS. Assessing the Productivity of Colonies Headed by Preheated Honeybee Queens. Insects. 2025; 16(8):858. https://doi.org/10.3390/insects16080858
Chicago/Turabian StyleAl-Ghzawi, Abd Al-Majeed, Shahera Talat Zaitoun, Mohammad Nafi Solaiman Al-Sabi, Salem Saleh Mazari, Ilham Mustafa Al-Omari, and Maqbool Saed Altalhi. 2025. "Assessing the Productivity of Colonies Headed by Preheated Honeybee Queens" Insects 16, no. 8: 858. https://doi.org/10.3390/insects16080858
APA StyleAl-Ghzawi, A. A.-M., Zaitoun, S. T., Al-Sabi, M. N. S., Mazari, S. S., Al-Omari, I. M., & Altalhi, M. S. (2025). Assessing the Productivity of Colonies Headed by Preheated Honeybee Queens. Insects, 16(8), 858. https://doi.org/10.3390/insects16080858







