Pathogenicity of Tolypocladium spp. Against Plutella xylostella: Effects on Immune Enzyme Activities and Gene Expression Profile
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomaterials and Their Culture Conditions
2.2. Molecular Identification of Fungal Strains
2.3. Phylogenetic Analysis and Multiple Sequence Alignment
2.4. Evaluation of Fungal Growth and Sporulation Capacity
2.5. Environmental Stress Tolerance Assays
2.5.1. Thermotolerance Assay
2.5.2. UV Resistance Assay
2.6. Virulence Bioassays Against P. xylostella
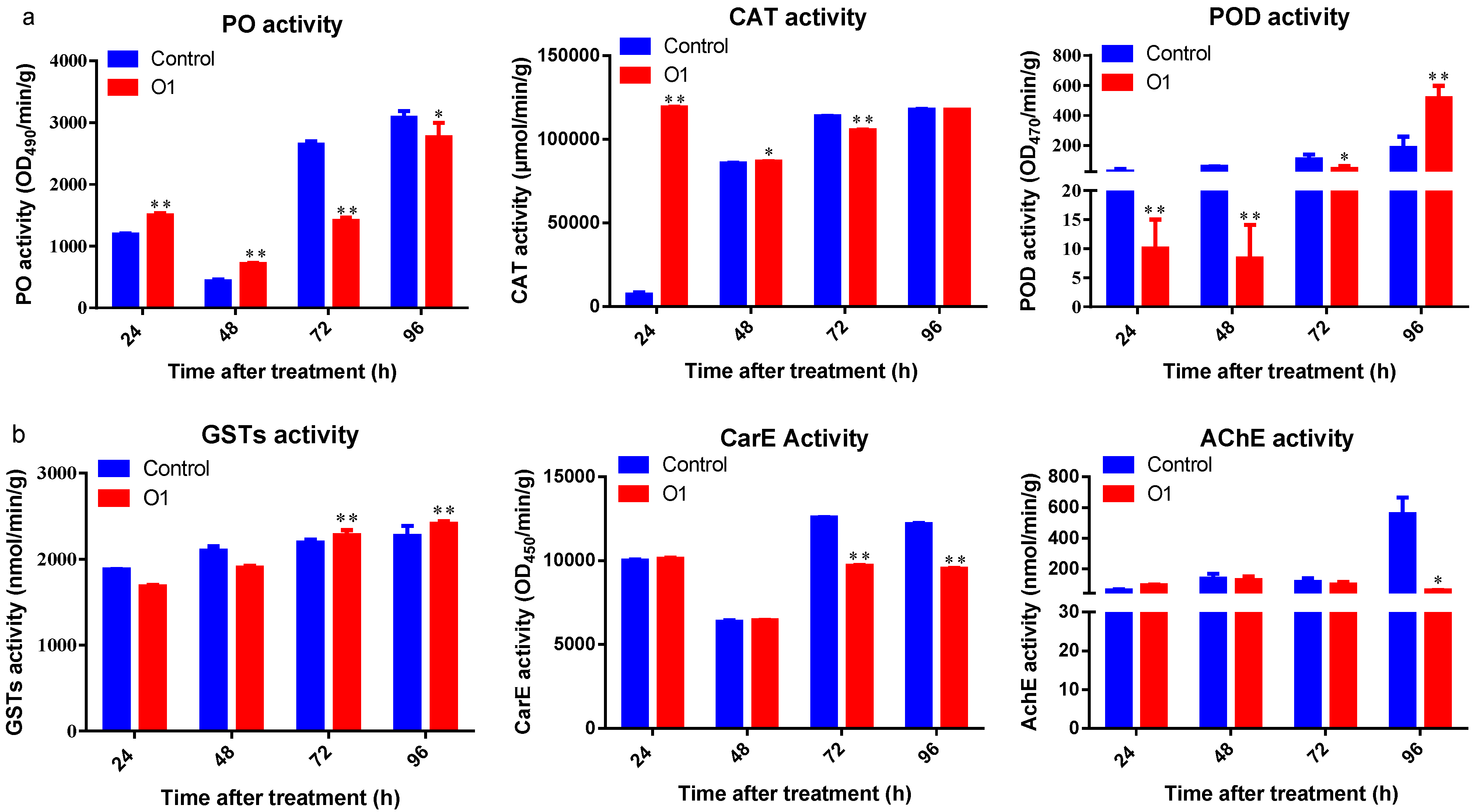
2.7. Determination of Protective and Detoxification Enzyme Activities in P. xylostella
2.7.1. Sample Preparation
2.7.2. Enzyme Activity Assays
2.8. Analysis of Immune Signaling Pathway-Related Gene Expression in P. xylostella
2.9. Statistical Analysis
3. Results
3.1. Molecular Identification and Morphological Characterization of Tolypocladium
3.2. Strain Growth and Sporulation Capacity
3.3. Tolerance of Spores Produced by Toluypocladium spp. to Thermal and UV Stress
3.4. Virulence of Four Tolypocladium Strains Against P. xylostella
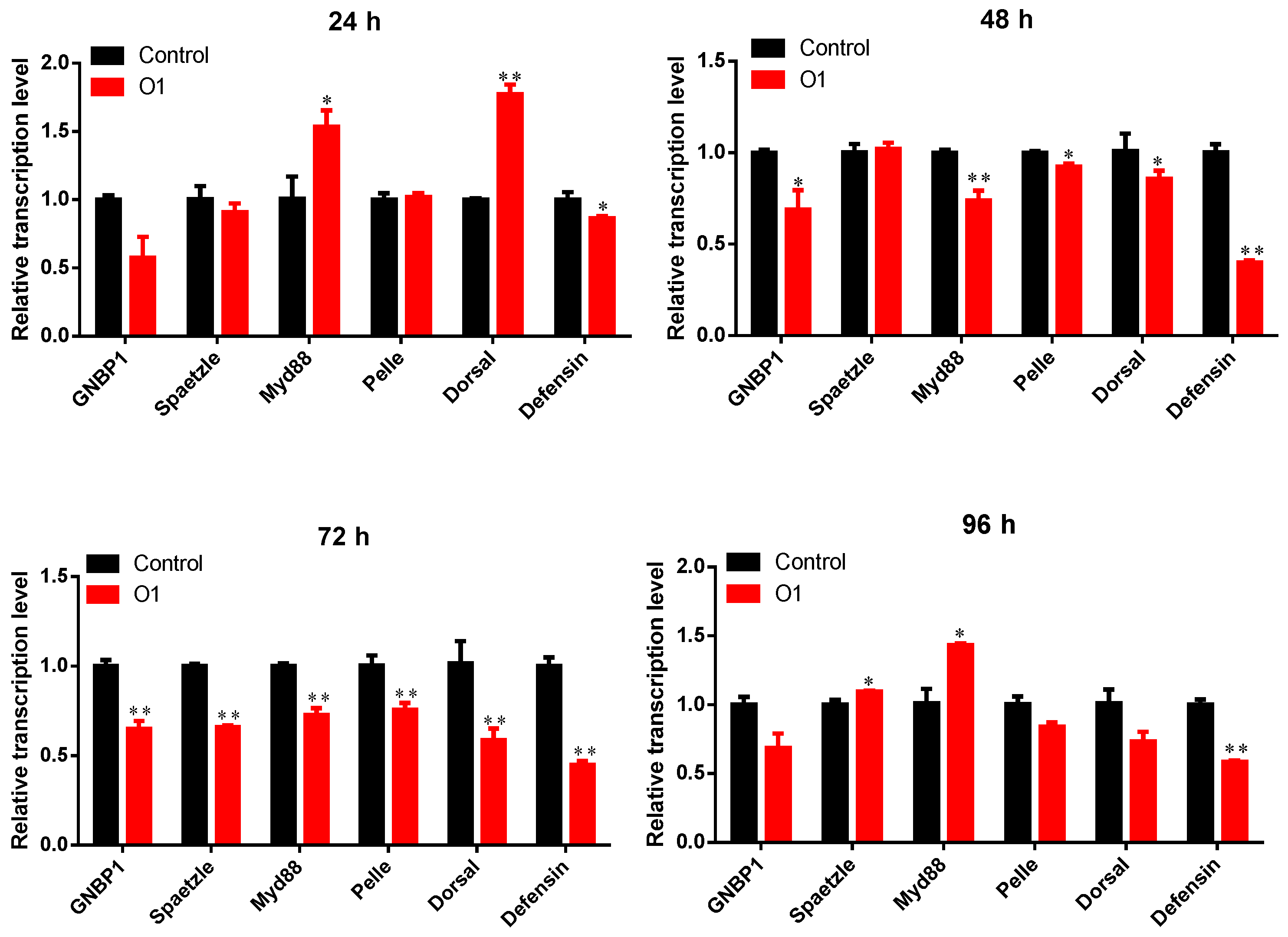
3.5. Effects of Tolypocladium Infection on Toll Pathway Immune Gene Expression in P. xylostella
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gams, W. Tolypocladium, eine Hyphomycetengattung mit geschwollenen Phialiden. Persoonia 1971, 6, 185–191. [Google Scholar]
- Barron, G.L. Fungal parasites of rotifers: A new Tolypocladium with underwater conidiation. Can. J. Bot. 1980, 58, 439–442. [Google Scholar] [CrossRef]
- Barron, G.L. Two new fungal parasites of bdelloid rotifers. Can. J. Bot. 1981, 59, 1449–1455. [Google Scholar] [CrossRef]
- Barron, G.L. Structure and biology of a new Tolypocladium attacking bdelloid rotifers. Can. J. Bot. 1983, 61, 2566–2569. [Google Scholar] [CrossRef]
- Gams, M.M.D. Proposal to reject Pachybasium niveum Rostr. in order to retain the name Tolypocladium inflatum W. Gams for the fungus that produces Cyclosporin. Taxon 1994, 43, 660–661. [Google Scholar] [CrossRef]
- Gams, W. Report of the committee for fungi: 12. Taxon 2005, 54, 520–522. [Google Scholar] [CrossRef]
- Bissett, J. Notes on Tolypocladium and related genera. Can. J. Bot. 1983, 61, 1311–1329. [Google Scholar] [CrossRef]
- Lam, T.N.C.; Goettel, M.S.; Soares, G.G. Host records for the entomopathogenic Hyphomycete Tolypocladium cylindrosporum. Fla. Entomol. 1988, 71, 86. [Google Scholar] [CrossRef]
- Scholte, E.J.; Knols, B.G.; Samson, R.A.; Takken, W. Entomopathogenic fungi for mosquito control: A review. J. Insect Sci. 2004, 4, 19. [Google Scholar] [CrossRef]
- Humber, R.A.; Hansen, K.S. ARSEF, USDA-ARS Collection of Entomopathogenic Fungal Culture. 2015. Available online: https://www.ars.usda.gov/ARSUserFiles/80620520/ARSEFpdfs/Catalog%20all.pdf (accessed on 20 September 2024).
- Soares, G.G.J.; Pinnock, D.E.; Samson, R.A. Tolypocladium, a new fungal pathogen of mosquito larvae with promise for use in microbial control. In Proceedings and Papers of the Forty Seventh Annual Conference of the California Mosquito and Vector Control Association, Inc. January 28–31, 1979, held at the Airport Marina Hotel, Burlingame, California; CMVCA Press: Visalia, CA, USA, 1980; pp. 51–54. [Google Scholar]
- Weiser, J.; Pillai, J.S. Tolypocladium cylindrosporum [Deuteromycetes, Moniliaceae] a new pathogen of mosquito larvae. Entomophaga 1981, 26, 357–361. [Google Scholar] [CrossRef]
- Goettel, M.S. Studies on bioassay of the entomopathogenic hyphomycete fungus Tolypocladium cylindrosporum in mosquitoes. J. Am. Mosq. Control Assoc. 1987, 3, 561–567. [Google Scholar]
- Li, Z.L. Research on new species of Tolypocladium and cyclosporine production in China. Mycosystema 1988, 7, 93–98. (In Chinese) [Google Scholar] [CrossRef]
- Bushley, K.E.; Raja, R.; Jaiswal, P.; Cumbie, J.S.; Nonogaki, M.; Boyd, A.E.; Owensby, C.A.; Knaus, B.J.; Elser, J.; Miller, D.; et al. The Genome of Tolypocladium inflatum: Evolution, organization, and expression of the cyclosporin biosynthetic gene cluster. PLoS Genet. 2013, 9, e1003496. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.S.; You, S.J.; Huang, S.M.; Liu, T.S.; Xie, D.D.; You, M.S. Spatiotemporal dynamics of genetic variation in populations of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), in China. Acta Entomol. Sin. 2019, 62, 624–633. (In Chinese) [Google Scholar] [CrossRef]
- Lei, Y.Y.; Lü, L.H.; He, Y.R.; Chen, D.H. Correlation between biological characteristics of Beauveria bassiana and its virulence to Plutella xylostella. Chin. J. Biol. Control 2010, 2, 143–148. (In Chinses) [Google Scholar] [CrossRef]
- Yin, Y.Q.; Chen, A.D.; Li, X.Y.; Zhao, X.Q. Research status and prospects of Plutella xylostella in Yunnan Province. Yunnan Agric. Sci. Technol. 2018, A01, 4. (In Chinses) [Google Scholar]
- Wei, S.J.; Shi, B.C.; Gong, Y.J.; Jin, G.H.; Chen, X.X.; Meng, X.F. Genetic structure and demographic history reveal migration of the diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) from the southern to northern regions of China. PLoS ONE 2013, 8, e59654. [Google Scholar] [CrossRef]
- Lu, L.H.; He, Y.R.; Pang, X.F. Effects of cruciferous vegetables on experimental population of diamondback moth Plutella xylostella. Chin. J. Appl. Ecol. 2003, 10, 1732–1734. (In Chinese) [Google Scholar]
- Yang, X.F.; Zhou, X.X.; Zheng, X.L. Research progress in insect lmmunity response induced by fungi. Guizhou Agric. Sci. 2008, 36, 73–96. (In Chinese) [Google Scholar] [CrossRef]
- Kanost, M.R.; Gorman, M.J. Phenoloxidases in insect immunity. In Insect Immunology; Academic Press: Cambridge, MA, USA, 2008; pp. 69–96. [Google Scholar] [CrossRef]
- Zambon, R.A.; Nandakumar, M.; Vakharia, V.N.; Wu, L.P. The Toll pathway is important for an antiviral response in Drosophila. Proc. Natl. Acad. Sci. USA 2005, 102, 7257–7262. [Google Scholar] [CrossRef]
- Valanne, S.; Wang, J.H.; Rämet, M. The Drosophila Toll signaling pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef]
- Tang, W.Y.; Qiang, F.Q.; Xing, C.C.; Dong, Y.G.; Zhang, Y.M. Research progress of adverse environmental stress on antioxidant enzymes in insects. Guizhou Agric. Sci. 2016, 44, 75–79. (In Chinese) [Google Scholar] [CrossRef]
- Tang, W.Y.; Xing, C.C.; Dong, Y.G.; Wang, X.; Zhang, Y.M. Changes in the activities of antioxidant enzymes in Spodoptera litura (Lepidoptera: Noctuidae) larvae infected by the fungus Nomuraea rileyi. Acta Entomol. Sin. 2015, 58, 526–534. (In Chinese) [Google Scholar] [CrossRef]
- Tunsoy, B.S.; Ozalp, P. Combined effects of pyriproxyfen and Bacillus thuringiensis on antioxidant activity of hemolymph, midgut and fat body of Galleria mellonella larvae. Fresenius Environ. Bull. 2016, 25, 1660–1665. [Google Scholar]
- Wang, J.; Zhang, K.; Zhang, X.; Liu, L.; Ning, S.Y.; Chen, C.; Wan, Y. Isolation, identification and diversity analysis of entomogenous fungi in Qinba Moutain. J. Northwest A F Univ. 2021, 49, 122–129. (In Chinese) [Google Scholar] [CrossRef]
- Feng, M.G. Replacement of probability analysis techniques with Time-Dose-Mortality models. Chin. J. Appl. Entomol. 1998, 4, 233–237. (In Chinese) [Google Scholar]
- Zhu, H.; Wang, Y.; Zhou, X.M. Characteristics of mRNA relative expression of ABCG 2 in Plutella xylostella and the effect of indoxacarb on its expression. Plant Prot. 2015, 41, 117–121. (In Chinese) [Google Scholar] [CrossRef]
- Niu, H.T.; Luo, W.C.; Zong, J.P.; Wei, S.J.; Wang, H.Y.; Pan, Z.X. Realized heritability of resistance to butene-fipronil in diamondback moth, Plutella xylostella. Acta Phytophylacica Sin. 2008, 35, 165–168. (In Chinese) [Google Scholar] [CrossRef]
- Luo, Y.J.; Wu, W.W.; Yang, Z.B.; Pu, E.T.; Guo, Z.X.; Yin, K.S.; He, C.X. Advances in insecticide resistance of diamondback moth (Plutella Xylostella L.) in Yunnan. J. Yunnan Univ. 2008, 30, 178–182. (In Chinese) [Google Scholar]
- Shen, F.Y. Research progress on insecticide resistance management in Diamondback moth. J. Hebei Agric. Sci. 2010, 14, 58–60. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Zhang, H.H.; Wu, H.; Zhu, H. Pathogenecity and infectation differentiations of Beauveria bassiana against Bemisia tabaci and Plutella xylostella. Chin. J. Biol. Control 2016, 32, 728–734. (In Chinese) [Google Scholar] [CrossRef]
- Yang, F.; Liu, C.L.; Wang, S.; Liu, L.; Li, X.M. Identification of Metarhizium pingshaense and assessing potentiality of bio-control application. Plant Prot. 2018, 44, 199–205. (In Chinese) [Google Scholar] [CrossRef]
- Tan, A.J.; Ou, X.; Liu, A.Y. Study on pathogenicity of Paecilomyces cicadae to Plutella xylostella. XianDai NongYe KeJi 2009, 15, 369–373. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Q.E.; Xu, J.H.; Feng, M.G. Comparative virulence of Zoophthora radicans isolates from different hosts against Plutella xylostella. Acta Entomol. Sin. 2003, 46, 447–453. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.C.; Zhang, Z.Z.; Li, Z.L.; Wang, Y. Research Progress of Tolypocladium in Ophiocordycipitaceae. J. Fungal Res. 2020, 9, 54–62. (In Chinese) [Google Scholar]
- Borel, J.F.; Feurer, C.; Gubler, H.U.; Stähelin, H. Biological effects of cyclosporin A: A new antilymphocytic agent. Agents Actions 1994, 43, 179–186. [Google Scholar] [CrossRef]
- Survase, S.A.; Shaligram, N.S.; Pansuriya, R.C.; Annapure, U.S.; Singhal, R.S. A novel medium for the enhanced production of cyclosporin A by Tolypocladium inflatum MTCC 557 using solid state fermentation. Microbiol. Biotechnol. 2009, 19, 462–467. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Cai, X.; Yu, X.; Liu, X.; Cao, Y.; Che, Y. Isolation and characterization of aphidicolin and chlamydosporol derivatives from Tolypocladium inflatum. J. Nat. Prod. 2011, 74, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Krasnoff, S.B.; Gupta, S. Efrapeptin production by Tolypocladium fungi (Deuteromycotina: Hyphomycetes): Intra- and interspecific variation. J. Chem. Ecol. 1992, 18, 1727. [Google Scholar] [CrossRef] [PubMed]
- Kneifel, H.; König, W.A.; Loeffler, W.; Müller, R. Ophiocordin, an antifungal antibiotic of Cordyceps ophioglossoides. Arch. Microbiol. 1977, 113, 121. [Google Scholar] [CrossRef]
- Myrand, V.; Buffet, J.P.; Guertin, C. Susceptibility of cabbage maggot larvae (Diptera: Anthomyiidae) to hypocreales entomopathogenic fungi. J. Econ. Entomol. 2015, 108, 34–44. [Google Scholar] [CrossRef]
- Rocha, L.F.; Sousa, N.A.; Rodrigues, J.; Catão, A.M.; Marques, C.S.; Fernandes, É.K.; Luz, C. Efficacy of Tolypocladium cylindrosporum against Aedes aegypti eggs, larvae and adults. J. Appl. Microbiol. 2015, 119, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.D.; Rasanayagam, S.; Moody, S.R.; Hatcher, P.E.; Ayers, P.G. The role of interactions between trophic levels in determining the effects of UV-B on terrestrial ecosystems. Plant Ecol. 1997, 128, 296–308. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Braga, G.U.L.; Anderson, A.J.; Roberts, D.W. Variability in conidial thermotolerance of Metarhizium anisopliae isolates from different geographic origins. J. Invertebr. Pathol. 2005, 88, 116–125. [Google Scholar] [CrossRef]
- Santos, M.P.; Dias, L.P.; Ferreira, P.C.; Pasin, L.A.A.P.; Rangel, D.E.N. Cold activity and tolerance of the entomopathogenic fungus Tolypocladium spp. to UV-B irradiation and heat. J. Invertebr. Pathol. 2011, 108, 209–213. [Google Scholar] [CrossRef]
- Ouedraogo, R.M.; Cusson, M.; Goettel, M.S.; Brodeur, J. Inhibition of fungal growth in thermoregulating locusts, Locusta migratoria, infected by the fungus Metarhizium anisopliae var acridum. J. Invertebr. Pathol. 2003, 82, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bolter, C.J.; Chefurka, W. Extramitochondrial release of hydrogen peroxide from insect and mouse liver mitochondria using the respiratory inhibitors phosphine, myxothiazol, and antimycin and spectral analysis of inhibited cytochromes. Arch. Biochem. Biophys. 1990, 278, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef]
- Wen, Y.Y.; He, Z.; Xu, T.; Jiao, Y.; Liu, X.; Wang, Y.F.; Yu, X.Q. Ingestion of killed bacteria activates antimicrobial peptide genes in Drosophila melanogaster and protects flies from septic infection. Dev. Comp. Immunol. 2019, 95, 10–18. [Google Scholar] [CrossRef]
- Lu, A.R.; Zhang, Q.L.; Zhang, J.; Yang, B.; Wu, K.; Xie, W.; Luan, Y.X.; Ling, E.J. Insect prophenoloxidase: The view beyond immunity. Front. Physiol. 2014, 5, 252. [Google Scholar] [CrossRef]
- Jiang, H.B.; Vilcinskas, A.; Kanost, M.R. Immunity in Lepidopteran Insects. Adv. Exp. Med. Biol. 2010, 708, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Schmidt, S.L.; Christensen, B.M. The antibacterial innate immune response by the mosquito Aedes aegypti is mediated by hemocytes and independent of Gram type and pathogenicity. Microbes Infect. 2004, 6, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Zafar, J.; Shoukat, R.F.; Zhang, Y.; Freed, S.; Xu, X.; Jin, F. Metarhizium Anisopliae challenges immunity and demography of Plutella xylostella. Insects 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Abarna, V.P.; Muthuswami, M.; Jeyarajan, N.S.; Johnson, T.E.Y.S.; Manikanda, B.N.; Anita, B.; Jeya, S.S.D. Investigating the enzymatic response of Plutella xylostella L. (Lepidoptera: Plutellidae) to Metarhizium anisopliae (Metschnikoff) Sorokin (Ascomycota: Hypocreales) Infection: A Comprehensive study. Asia-Pac. Entomol. 2024, 27, 102321. [Google Scholar] [CrossRef]
- Niu, Y.; Xue, J.L.; Xie, Y.P.; Zhao, Y.Y. Changes of superoxide dismutase and catalase in Dendrolimus tabulaeformis infected by Beauveria bassiana. Chin. J. Appl. Environ. Biol. 2005, 11, 182–186. (In Chinese) [Google Scholar] [CrossRef]
- Li, H.P.; Huang, D.Z.; Su, X.Y.; Zheng, J.W.; Wang, X.H. Change of activities of SOD, CAT and POD in Apriona germari larvae infected by Beauveria bassiana. Acta Sericologica Sin. 2007, 33, 634–636. (In Chinese) [Google Scholar] [CrossRef]
- Shi, G.L.; Wang, Y.N.; Wang, H.L.; Zhao, L.L.; Liu, S.Q.; Cao, H.; Yu, T.Q.; Lu, P. Effects of Tagetes erecta extracts on glutathione S-transferase and protease activities and protein content in Tetranychus viennensis. Chin. J. Appl. Ecol. 2007, 18, 400–404. (In Chinese) [Google Scholar] [CrossRef]



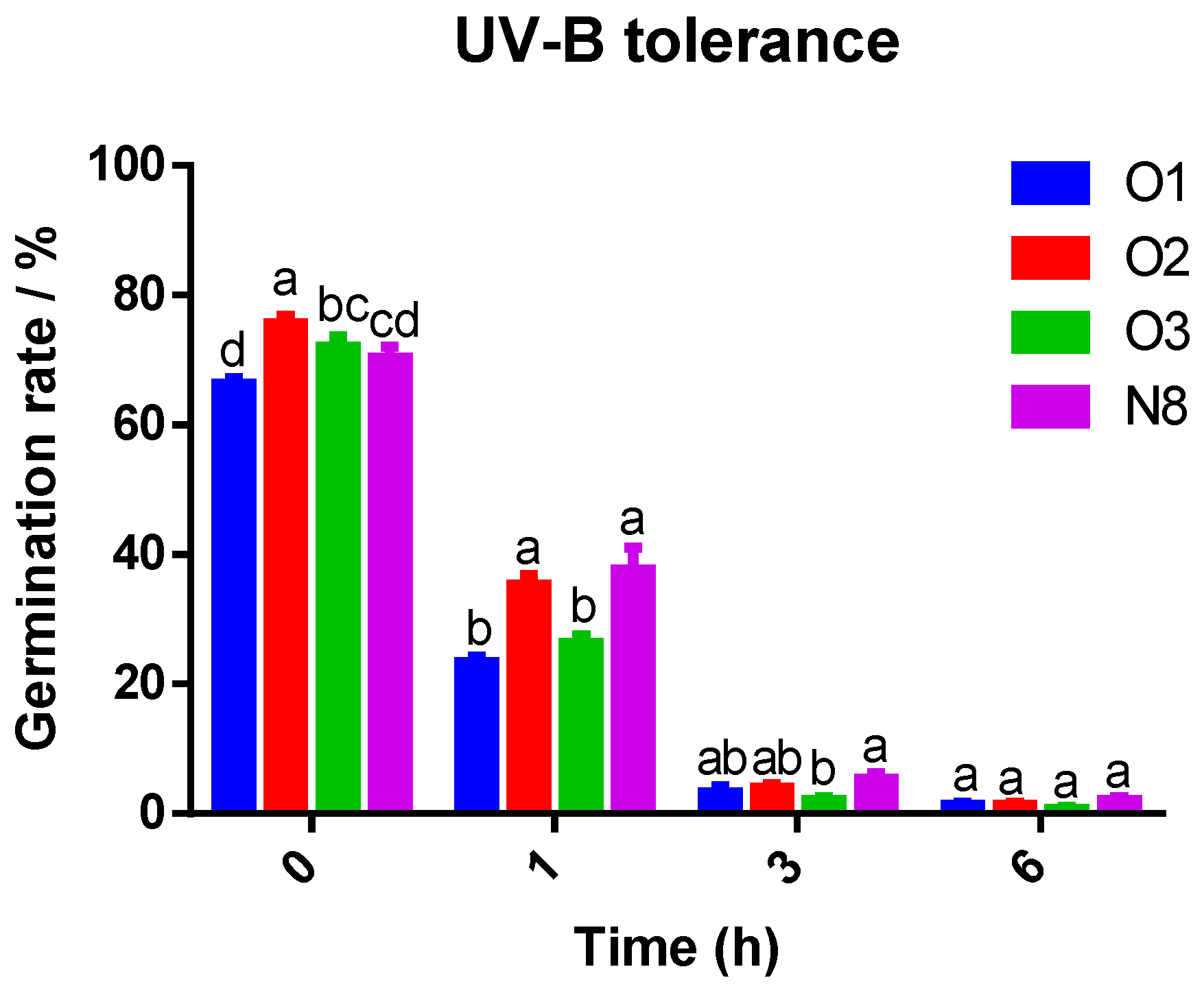


| 45 °C Heat Stress | Germinate Rates % | |||
|---|---|---|---|---|
| Time (min) | N8-SF-04092 | O1-SF-04630 | O2-SF-04927 | O3-SF-04931 |
| 0 | (56.67 ± 1.96) aA | (25.67 ± 0.98) aC | (63 ± 1.89) aA | (32.33 ± 1.52) aB |
| 10 | (54.33 ± 1.44) aA | (23.33 ± 2.33) aB | (53.33 ± 1.19) bA | (26.67 ± 0.27) aB |
| 30 | (52.33 ± 3.07) aB | (23 ± 0.47) aC | (62.33 ± 1.19) aA | (29.33 ± 1.19) aC |
| 60 | (54.67 ± 2.23) aA | (22.67 ± 0.54) aC | (42.33 ± 1.19) cB | (28 ± 0.94) aC |
| 120 | (53 ± 1.7) aA | (23 ± 0.94) aC | (46.67 ± 0.72) cB | (27.67 ± 1.44) aC |
| 300 | (48.33 ± 2.88) aA | (15 ± 0.94) bC | (46 ± 2.62) dA | (27 ± 2.94) aB |
| Treatment | Days After Treatment (Day) | ||||||
|---|---|---|---|---|---|---|---|
| Spores/mL | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| N8-SF-04092 | (1.67 ± 1.36)% a | (3.33 ± 1.36)% b | (11.67 ± 2.72)% b | (40 ± 4.08)% ab | (70 ± 6.24)% a | (85 ± 6.24)% a | (96.67 ± 2.72)% a |
| O1-SF-04630 | (3.33 ± 2.72)% a | (16.67 ± 5.93)% a | (28.33 ± 4.91)% a | (51.67 ± 3.6)% a | (71.67 ± 1.36)% a | (90 ± 2.36)% a | (98.33 ± 1.36)% a |
| O2-SF-04927 | (0 ± 0)% a | (1.67 ± 1.36)% b | (3.33 ± 2.72)% b | (20 ± 0)% c | (40 ± 2.36)% b | (60 ± 4.08)% b | (70 ± 4.71)% b |
| O3-SF-04931 | (0 ± 0)% a | (1.67 ± 1.36)% b | (11.67 ± 1.36)% b | (36.67 ± 4.91)% b | (71.67 ± 7.2)% a | (88.33 ± 3.6)% a | (93.33 ± 3.6)% a |
| Control | (0 ± 0)% a | (0 ± 0)% b | (0 ± 0)% b | (0 ± 0)% d | (1.67 ± 1.36)% c | (1.67 ± 1.36)% c | (3.33 ± 1.36)% c |
| Treatment Strain | LT50 (days) (95% Fiducial Limits) | LT90 (days) (95% Fiducial Limits) | Probit Equation | X2 | p Value |
|---|---|---|---|---|---|
| O1 | 3.89 (3.44–4.34) a | 6.03 (5.43–6.97) a | y = −2.336 + 0.6x | 0.556 | 0.990 |
| O2 | 5.71 (5.23–6.34) b | 8.01 (7.18–9.55) b | y = −3.183 + 0.557x | 1.4 | 0.924 |
| O3 | 4.45 (4.11–4.89) a | 6.19 (5.69–6.96) a | y = −3.416 + 0.759x | 1.716 | 0.887 |
| N8 | 4.43 (4.03–4.84) a | 6.21 (5.68–7.03) a | y = −3.202 + 0.723x | 0.703 | 0.983 |
| Treatment Strain | LC50 (Spores mL−1) (95% Fiducial Limits) | LC90 (Spores mL−1) (95% Fiducial Limits) | Probit Equation | X2 | p Value |
|---|---|---|---|---|---|
| O1 | 2.79 × 104 (0.12 × 104–1.36 × 105) a | 3.31 × 108 (0.11 × 108–4.65 × 10815) a | y = −2.94 + 4.537x | 4.45 | 0.217 |
| O2 | 1.43 × 105 (7.24 × 102–1.86 × 109) a | 2.98 × 1021 (1.86 × 1010–9.55 × 1044) a | y = −1.473 + 2.068x | 0.491 | 0.921 |
| O3 | 4.37 × 104 (0.09 × 104–2.74 × 105) a | 1.08 × 1010 (0.01 × 1010–1.69 × 1030) a | y = −2.55 + 3.826x | 3.567 | 0.312 |
| N8 | 5.58 × 104 (0.34 × 104–2.74 × 105) a | 1.37 × 109 (0.03 × 109–5.87 × 1017) a | y = −3.048 + 4.506x | 4.826 | 0.185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, N.; Zhang, Z.; Chang, B.H.; Qiao, Z.; Liu, F.; Nong, X.; Wang, K. Pathogenicity of Tolypocladium spp. Against Plutella xylostella: Effects on Immune Enzyme Activities and Gene Expression Profile. Insects 2025, 16, 859. https://doi.org/10.3390/insects16080859
Cai N, Zhang Z, Chang BH, Qiao Z, Liu F, Nong X, Wang K. Pathogenicity of Tolypocladium spp. Against Plutella xylostella: Effects on Immune Enzyme Activities and Gene Expression Profile. Insects. 2025; 16(8):859. https://doi.org/10.3390/insects16080859
Chicago/Turabian StyleCai, Ni, Zhigang Zhang, Babar Hussain Chang, Zhijun Qiao, Fang Liu, Xiangqun Nong, and Kaimei Wang. 2025. "Pathogenicity of Tolypocladium spp. Against Plutella xylostella: Effects on Immune Enzyme Activities and Gene Expression Profile" Insects 16, no. 8: 859. https://doi.org/10.3390/insects16080859
APA StyleCai, N., Zhang, Z., Chang, B. H., Qiao, Z., Liu, F., Nong, X., & Wang, K. (2025). Pathogenicity of Tolypocladium spp. Against Plutella xylostella: Effects on Immune Enzyme Activities and Gene Expression Profile. Insects, 16(8), 859. https://doi.org/10.3390/insects16080859







