Living Control Systems: Exploring a Teleonomic Account of Behavior in Apis mellifera
Simple Summary
Abstract
1. Introduction
1.1. Living Control Systems: Exploring a Teleonomic Account of Behavior in Apis mellifera
1.2. Perceptual Control Theory
1.3. Current Experiment
2. Materials and Methods
2.1. Subjects
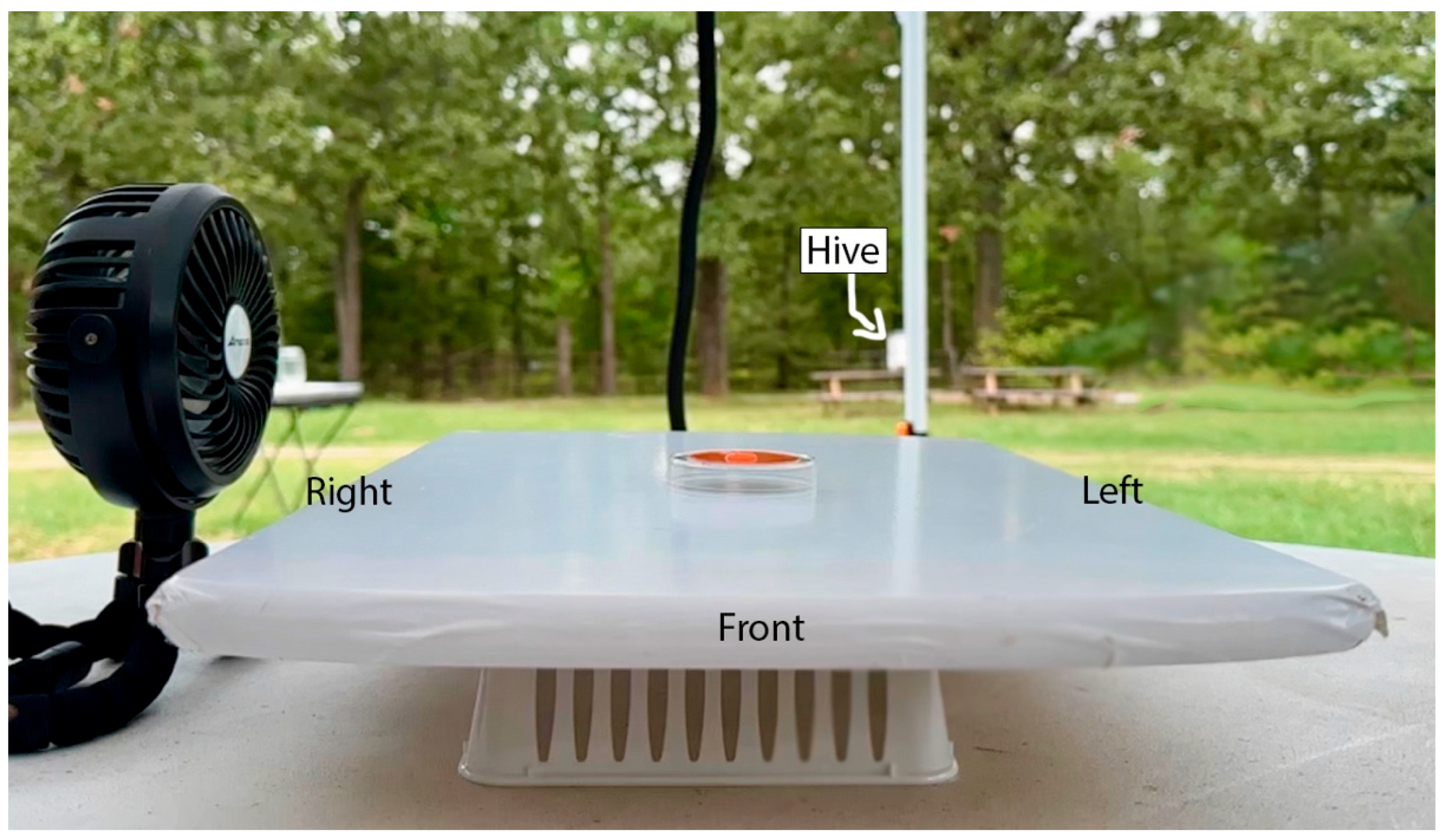
2.2. Apparatus
2.3. Procedures
2.4. Behavioral Coding and Dependent Variables
3. Results
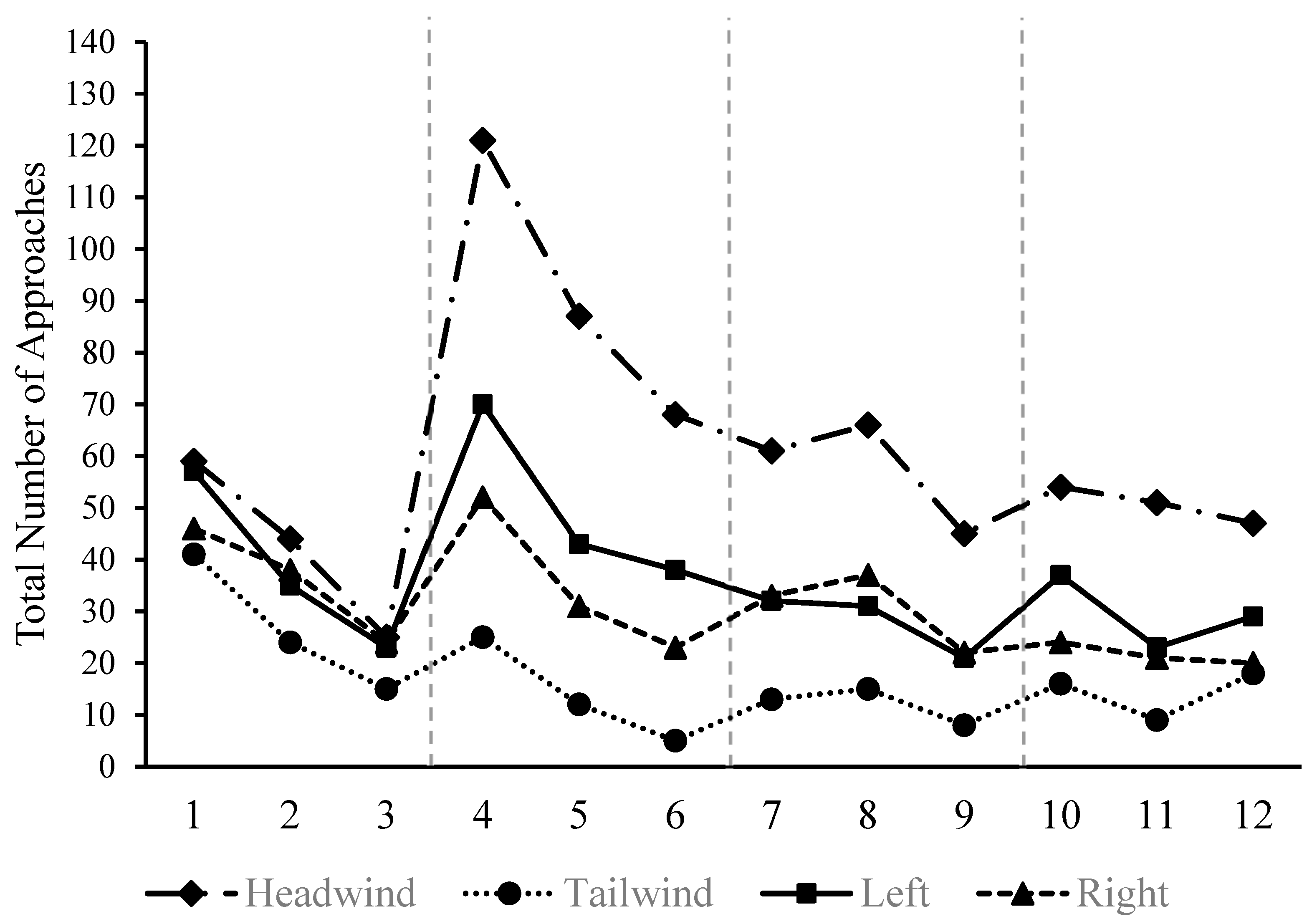
3.1. Successful Landings
3.2. Approaches and Direction
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medial Physiology, 14th ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Marken, R.S. The Study of Living Control Systems: A Guide to Doing Research on Purpose; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Yin, H.H. Restoring purpose in behavior. In Computational and Robotic Models of the Hierarchical Organization of Behavior; Springer: Berlin/Heidelberg, Germany, 2013; pp. 319–347. [Google Scholar] [CrossRef]
- Arocha, J.F. Scientific realism and the issue of variability in behavior. Theory Psychol. 2021, 31, 375–398. [Google Scholar] [CrossRef]
- Powers, W.T. Behavior: The Control of Perception, 1st ed.; Aldine Publishing Company: Chicago, IL, USA, 1973. [Google Scholar]
- Runkel, P.J. Casting Nets and Testing Specimens: Two Grand Methods of Psychology; Praeger Publishers: Westport, CT, USA, 2007. [Google Scholar]
- Powers, W.T.; Clark, R.K.; McFarland, R.L. A general feedback theory of human behavior: Part I. Percept. Mot. Ski. 1960, 11, 71–88. [Google Scholar] [CrossRef]
- Powers, W.T.; Clark, R.K.; McFarland, R.L. A general feedback theory of human behavior: Part II. Percept. Mot. Ski. 1960, 11, 309–323. [Google Scholar] [CrossRef]
- Powers, W.T. Quantitative analysis of purposive systems: Some spadework at the foundations of scientific psychology. Psychol. Rev. 1978, 85, 417–435. [Google Scholar] [CrossRef]
- Ashby, W.R. An Introduction to Cybernetics; Chapman & Hall: London, UK, 1956. [Google Scholar] [CrossRef]
- Mead, M. Cybernetics of Cybernetics. In American Society for Cybernetics, Purposive Systems, Proceedings of the First Annual Symposium of the American Society for Cybernetics, Gaithersburg, MD, USA, 26–27 October 1967; Foerster, H.V., Ed.; Spartan Books: Washington, DC, USA, 1968. [Google Scholar]
- Rosenblueth, A.; Wiener, N.; Bigelow, J. Behavior, purpose and teleology. Philos. Sci. 1943, 10, 18–24. [Google Scholar] [CrossRef]
- Wiener, N. Cybernetics; John Wiley: New York, NY, USA, 1948. [Google Scholar]
- Brembs, B. The brain as a dynamically active organ. Biochem. Biophys. Res. Commun. 2021, 564, 55–69. [Google Scholar] [CrossRef]
- Marken, R.S. You say you had a revolution: Methodological foundations of closed-loop psychology. Rev. Gen. Psychol. 2009, 13, 137–145. [Google Scholar] [CrossRef]
- Shaffer, D.M.; Marken, R.S.; Dolgov, I.; Maynor, A.B. Chasin’choppers: Using unpredictable trajectories to test theories of object interception. Atten. Percept. Psychophys. 2013, 75, 1496–1506. [Google Scholar] [CrossRef]
- Bell, H.C.; Pellis, S.M. A cybernetic perspective on food protection in rats: Simple rules can generate complex and adaptable behavior. Anim. Behav. 2011, 82, 659–666. [Google Scholar] [CrossRef]
- Bell, H.C. Control in Living Systems: An Exploration of the Cybernetic Properties of Interactive Behaviour. Doctoral Dissertation, Department of Neuroscience, University of Lethbridge, Lethbridge, AB, Canada, 2018. [Google Scholar]
- Bell, H.C. Behavioral variability in the service of constancy. Int. J. Comp. Psychol. 2014, 27, 338–360. [Google Scholar] [CrossRef]
- Powers, W.T. A feedback model for behavior: Analysis of a rat experiment. Behav. Sci. 1971, 16, 558–563. [Google Scholar] [CrossRef]
- Hendriksma, H.P.; Hsiung, K.K.; Broccard-Bell, H.C.; Nieh, J.C. Aggression between invasive hymenopterans in Southern California: Scutellata-hybrids versus German yellowjackets. J. Apic. Res. 2023, 64, 147–149. [Google Scholar] [CrossRef]
- Albert, D.J. What’s on the mind of a jellyfish? A review of behavioural observations on Aurelia sp. jellyfish. Neurosci. Biobehav. Rev. 2011, 35, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Sugiura, S.; Chiba, S. Prey-tracking behavior in the invasive terrestrial planarian Platydemus manokwari (Platyhelminthes, Tricladida). Naturwissenschaften 2010, 97, 997–1002. [Google Scholar] [CrossRef]
- Pellis, S.M.; Bell, H.C. Unraveling the dynamics of dyadic interactions: Perceptual control in animal contests. In The Interdisciplinary Handbook of Perceptual Control Theory; Academic Press: Cambridge, MA, USA, 2020; pp. 75–99. [Google Scholar] [CrossRef]
- Menzel, R.; Erber, J. Learning and memory in bees. Sci. Am. 1978, 239, 102–110. [Google Scholar] [CrossRef]
- Mujagic, S.; Erber, J. Sucrose acceptance and different forms of associative learning of the honey bee (Apis mellifera L.) in the field and laboratory. Front. Behav. Neurosci. 2010, 4, 46. [Google Scholar] [CrossRef]
- Rodriguez, S.D.; Wincheski, R.J.; Jones, I.T.; Jesus-Soto, D.; Michael, G.; Fletcher, S.J.; Pretends Eagle, T.J.; Grice, J.W.; Abramson, C.I. Some phenomena of the cap-pushing response in honey bees (Apis mellifera spp.). J. Comp. Psychol. 2023, 137, 178. [Google Scholar] [CrossRef]
- Wincheski, R.; Jones, I.T.; Rodriguez, S.; Soto, M.; Fletcher, S.; Eagle, T.; Grice, J.; Abramson, C. Training Honey Bees (Apis mellifera) to Push a Cap: Shaping, Observational Learning, and Memory. Int. J. Comp. Psychol. 2023, 36, 1–10. [Google Scholar] [CrossRef]
- Grice, J.W. Observation Oriented Modeling: Analysis of Cause in the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Grice, J.W. Drawing inferences from randomization tests. Personal. Individ. Differ. 2021, 179, 110931. [Google Scholar] [CrossRef]
- Duan, B.; Xu, X.S. How to break a fever: A feedback circuit for body temperature control. Neuron 2019, 103, 179–181. [Google Scholar] [CrossRef]
- Mitchell, D.; Fuller, A.; Snelling, E.P.; Tattersall, G.J.; Hetem, R.S.; Maloney, S.K. Revisiting concepts of thermal physiology: Understanding negative feedback and set-point in mammals, birds, and lizards. Biol. Rev. 2025, 100, 1317–1346. [Google Scholar] [CrossRef]
- Tan, C.L.; Knight, Z.A. Regulation of body temperature by the nervous system. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.; Srinivasan, M.V. Visual regulation of ground speed and headwind compensation in freely flying honey bees (Apis mellifera L.). J. Exp. Biol. 2006, 209, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, W.; Rothe, U.; Feller, P.; Jungmann, R. Flight of the honey bee: III. Flight metabolic power calculated from gas analysis, thermoregulation and fuel consumption. J. Comp. Physiol. B 1989, 158, 729–737. [Google Scholar] [CrossRef]
- Srinivasan, M.V.; Zhang, S.W.; Lehrer, M.; Collett, T.S. Honey bee navigation en route to the goal: Visual flight control and odometry. J. Exp. Biol. 1996, 199, 237–244. [Google Scholar] [CrossRef]
- Baird, E.; Srinivasan, M.V.; Zhang, S.; Cowling, A. Visual control of flight speed in honeybees. J. Exp. Biol. 2005, 208, 3895–3905. [Google Scholar] [CrossRef]
- Baird, E.; Boeddeker, N.; Ibbotson, M.R.; Srinivasan, M.V. A universal strategy for visually guided landing. Proc. Natl. Acad. Sci. USA 2013, 110, 18686–18691. [Google Scholar] [CrossRef]
- Srinivasan, M.V.; Zhang, S.; Altwein, M.; Tautz, J. Honeybee navigation: Nature and calibration of the “odometer”. Science 2000, 287, 851–853, Erratum in Science 2000, 287, 1756. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K. Oscillators and servomechanisms in orientation and navigation, and sometimes in cognition. Proc. R. Soc. B 2022, 289, 20220237. [Google Scholar] [CrossRef]
- Frigg, R. Models and Theories, a Philosophical Inquiry; Routledge: Abingdon, UK, 2023. [Google Scholar] [CrossRef]
- Grice, J.W.; Jones, I.T. Iconic models in science and psychology. Theory Psychol. 2024, 34, 815–835. [Google Scholar] [CrossRef]
- Harré, R. Modeling: Gateway to the Unknown Studies in Multidisciplinarity; Rothbart, D., Ed.; Elselvier: Amsterdam, The Netherlands, 2004; Volume 1. [Google Scholar] [CrossRef]
- Ayala, F.J. Teleological explanations in evolutionary biology. Philos. Sci. 1970, 37, 1–15. [Google Scholar] [CrossRef]
- Mayr, E. The idea of teleology. J. Hist. Ideas 1992, 53, 117–135. [Google Scholar] [CrossRef]
- Wright, L. Teleological Explanations: An Etiological Analysis of Goals and Functions; University of California Press: Berkeley, CA, USA, 1976. [Google Scholar] [CrossRef]
- Wallace, W.A. The Modeling of Nature: Philosophy of Science and Philosophy of Nature in Synthesis; The Catholic University of America Press: Washington, DC, USA, 1996. [Google Scholar]
- Marken, R.S. The dancer and the dance: Methods in the study of living control systems. Psychol. Methods 1997, 2, 436–446. [Google Scholar] [CrossRef]
- Tolman, E.C. A cognition motivation model. Psychol. Rev. 1952, 59, 389. [Google Scholar] [CrossRef]
- Hull, C.L. Principles of Behavior: An Introduction to Behavior Theory; Appleton-Century: New York, NY, USA, 1943. [Google Scholar]
- Spence, K.W.; Spence, J.T. Psychology of Learning and Motivation; Academic Press: Cambridge, MA, USA, 1967; Volume 1. [Google Scholar]
- Buehlmann, C.; Mangan, M.; Graham, P. Multimodal interactions in insect navigation. Anim. Cogn. 2020, 23, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; De Marco, R.J.; Menzel, R. Learning reward expectations in honey bees. Learn. Mem. 2007, 14, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, C.E.; Cook, P.; Hill, P.S.; Orozco, B.S.; Abramson, C.I.; Wells, H. Nectar quality perception by honey bees (Apis mellifera ligustica). J. Comp. Psychol. 2013, 127, 341. [Google Scholar] [CrossRef] [PubMed]
- Abramson, C.I. Why the study of comparative psychology is important to neuroscientists. Front. Behav. Neurosci. 2023, 16, 1095033. [Google Scholar] [CrossRef]
- Jones, I.T. On The Origins of Behavior: Restoring Teleological Accounts of Behavior. Doctoral Dissertation, Oklahoma State University, Stillwater, OK, USA, 1875. Available online:https://www.proquest.com/openview/e0756339d6c32f99b9b855df57002139/1? (accessed on 6 July 2025).


| Trial | Group | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 1 | F | F | L | L | R | R |
| 2 | F | F | L | L | R | R |
| 3 | F | F | L | L | R | R |
| 4 | F | F | L | L | R | R |
| 5 | F | F | L | L | R | R |
| 6 | F | F | L | L | R | R |
| 7 | L | R | F | R | F | L |
| 8 | L | R | F | R | F | L |
| 9 | L | R | F | R | F | L |
| 10 | R | L | R | F | L | F |
| 11 | R | L | R | F | L | F |
| 12 | R | L | R | F | L | F |
| Case # | Trials 1–3 | Trials 4–12 | ||||
|---|---|---|---|---|---|---|
| Trials | PCC | c-Value | Trials | PCC | c-Value | |
| 1 | 3 | 100.00 | 0.123 | 9 | 100.00 | 0.002 |
| 2 | 3 | 100.00 | 0.126 | 9 | 100.00 | 0.002 |
| 3 | 3 | 100.00 | 0.124 | 9 | 100.00 | 0.002 |
| 4 | 3 | 100.00 | 0.124 | 9 | 100.00 | 0.002 |
| 5 | 3 | 100.00 | 0.125 | 9 | 100.00 | 0.003 |
| 6 | 3 | 100.00 | 0.126 | 9 | 100.00 | 0.001 |
| 7 | 3 | 100.00 | 0.125 | 9 | 100.00 | 0.002 |
| 8 | 3 | 100.00 | 0.124 | 9 | 100.00 | 0.001 |
| 9 | 3 | 100.00 | 0.124 | 9 | 100.00 | 0.002 |
| 10 | 3 | 100.00 | 0.125 | 9 | 100.00 | 0.002 |
| 11 | 3 | 100.00 | 0.126 | 8 | 0.00 | 1 |
| 12 | 3 | 100.00 | 0.125 | 9 | 100.00 | 0.002 |
| 13 | 3 | 100.00 | 0.126 | 9 | 100.00 | 0.002 |
| 14 | 3 | 100.00 | 0.125 | 9 | 100.00 | 0.001 |
| Total | 42 | 100.00 | <0.001 | 125 | 93.60 | <0.0001 |
| Case # | Trials 1–3 | Trials 4–12 | ||||
|---|---|---|---|---|---|---|
| Trials | PCC | c-Value | Trials | PCC | c-Value | |
| 1 | 3 | 66.67 | 0.51 | 9 | 77.78 | 0.09 |
| 2 | 3 | 0 | 1 | 9 | 66.67 | 0.26 |
| 3 | 3 | 0 | 1 | 9 | 88.89 | 0.02 |
| 4 | 2 | 0 | 1 | 9 | 66.67 | 0.26 |
| 5 | 3 | 33.33 | 0.87 | 8 | 50.00 | 0.64 |
| 6 | 3 | 66.67 | 0.50 | 9 | 77.78 | 0.09 |
| 7 | 3 | 66.67 | 0.51 | 9 | 66.67 | 0.25 |
| 8 | 3 | 0 | 1 | 8 | 75.00 | 0.15 |
| 9 | 3 | 0 | 1 | 8 | 62.50 | 0.37 |
| 10 | 3 | 0 | 1 | 9 | 66.67 | 0.25 |
| 11 | 3 | 33.33 | 0.87 | 7 | 42.86 | 0.77 |
| 12 | 3 | 0 | 1 | 9 | 66.67 | 0.25 |
| 13 | 3 | 0 | 1 | 9 | 77.78 | 0.09 |
| 14 | 3 | 33.33 | 0.88 | 9 | 77.78 | 0.09 |
| Total | 41 | 21.95 | 1.00 | 121 | 69.42 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, I.T.; Grice, J.W.; Abramson, C.I. Living Control Systems: Exploring a Teleonomic Account of Behavior in Apis mellifera. Insects 2025, 16, 848. https://doi.org/10.3390/insects16080848
Jones IT, Grice JW, Abramson CI. Living Control Systems: Exploring a Teleonomic Account of Behavior in Apis mellifera. Insects. 2025; 16(8):848. https://doi.org/10.3390/insects16080848
Chicago/Turabian StyleJones, Ian T., James W. Grice, and Charles I. Abramson. 2025. "Living Control Systems: Exploring a Teleonomic Account of Behavior in Apis mellifera" Insects 16, no. 8: 848. https://doi.org/10.3390/insects16080848
APA StyleJones, I. T., Grice, J. W., & Abramson, C. I. (2025). Living Control Systems: Exploring a Teleonomic Account of Behavior in Apis mellifera. Insects, 16(8), 848. https://doi.org/10.3390/insects16080848







