Differences in Diversity of Collembola Communities Between Primary and Secondary Forests and Driving Factors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Procedure
2.3. Morphospecies Identification
2.4. Environmental Variables
2.5. Statistical Analysis
3. Results
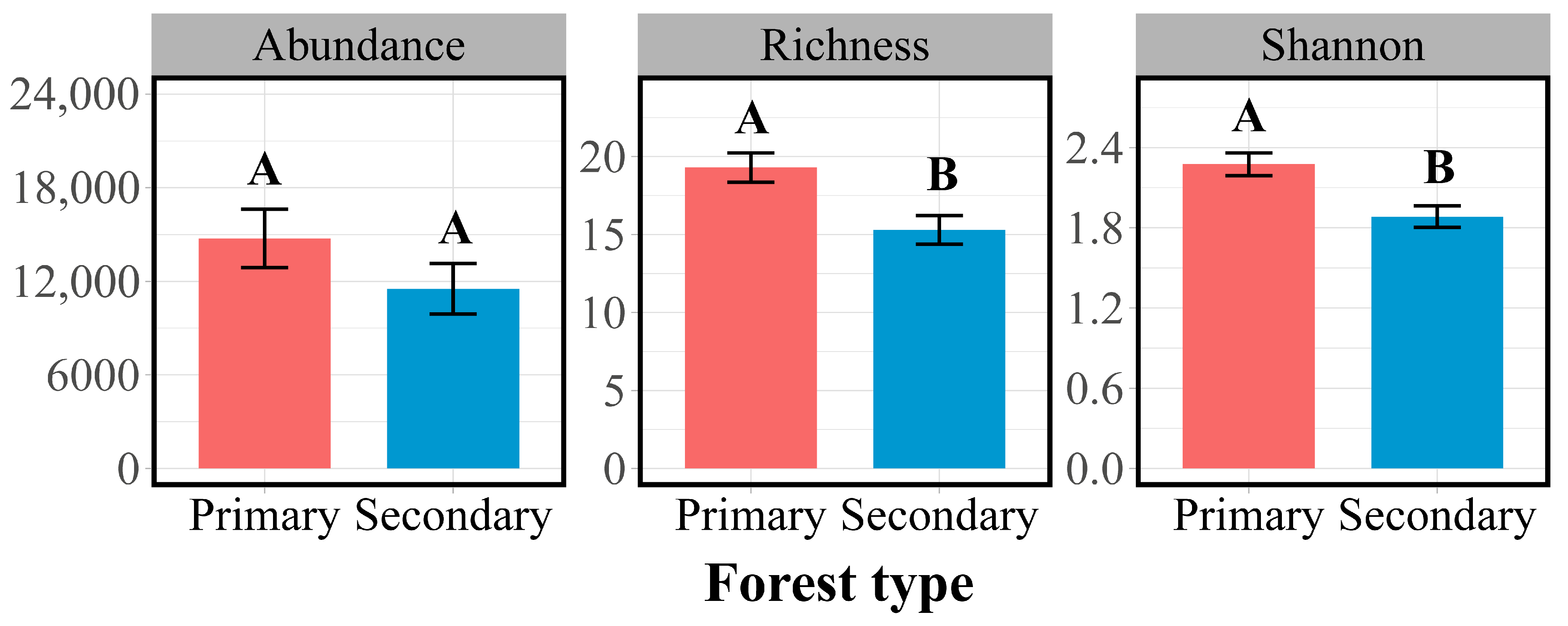
3.1. Litter and Soil Properties and Collembola Species Diversity
3.2. Community Composition of Collembola
3.3. The Drivers of the Collembola Diversity and Species Composition
4. Discussion
4.1. Differences in Collembolan Diversity Between Primary and Secondary Forests
4.2. Differences in Collembolan Community Composition Between Primary and Secondary Forests
4.3. Relationship with Environmental Variables in Primary and Secondary Forests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.A.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary Forests Are Irreplaceable for Sustaining Tropical Biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Kormos, C.; Mackey, B.; Smith, R.; Young, V.; Rao, M. Primary Forests, Ecosystem Integrity & Climate Change; IUCN: Gland Switzerland, 2023; pp. 1–4. [Google Scholar]
- Frey, S.J.K.; Hadley, A.S.; Johnson, S.L.; Schulze, M.; Jones, J.A.; Betts, M.G. Spatial Models Reveal the Microclimatic Buffering Capacity of Old-Growth Forests. Sci. Adv. 2016, 2, e1501392. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.L.; Edwards, D.P.; Galbraith, D. Increasing Human Dominance of Tropical Forests. Science 2015, 349, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef]
- Potapov, A.M.; Beaulieu, F.; Birkhofer, K.; Bluhm, S.L.; Degtyarev, M.I.; Devetter, M.; Goncharov, A.A.; Gongalsky, K.B.; Klarner, B.; Korobushkin, D.I.; et al. Feeding Habits and Multifunctional Classification of Soil-Associated Consumers from Protists to Vertebrates. Biol. Rev. 2022, 97, 1057–1117. [Google Scholar] [CrossRef]
- Murdjoko, A.; Brearley, F.Q.; Ungirwalu, A.; Djitmau, D.A.; Benu, N.M.H.; Penelitian, P.; Hayati, K.; Papua, U.; Gunung, J.; Amban, S.; et al. Secondary Succession after Slash-and-Burn Cultivation in Papuan Lowland Forest, Indonesia. Forests 2022, 13, 434. [Google Scholar] [CrossRef]
- Liu, Z.; Zuo, Y.; Feng, G. Primary Forests Harbour More Bird Taxonomic, Phylogenetic and Functional Diversity than Secondary and Plantation Forests in the Pantropics. J. Biogeogr. 2024, 51, 2338–2355. [Google Scholar] [CrossRef]
- Bradford, M.A.; Tordoff, G.M.; Eggers, T.; Hefin Jones, T.; Newington Bradford, J.E.; Bradford, M.A.; Tordoff, G.M.; Eggers, T.; Jones, T.H.; Newington, J.E. Microbiota, Fauna, and Mesh Size Interactions in Litter Decomposition. Oikos 2002, 99, 317–323. [Google Scholar] [CrossRef]
- Slade, E.M.; Riutta, T. Interacting Effects of Leaf Litter Species and Macrofauna on Decomposition in Different Litter Environments. Basic Appl. Ecol. 2012, 13, 423–431. [Google Scholar] [CrossRef]
- de Oliveira, T.; Hättenschwiler, S.; Handa, I.T. Snail and Millipede Complementarity in Decomposing Mediterranean Forest Leaf Litter Mixtures. Funct. Ecol. 2010, 24, 937–946. [Google Scholar] [CrossRef]
- Castillo-Avila, C.; Castillo-Figueroa, D.; Posada, J.M. Drivers of Soil Fauna Communities along a Successional Gradient in Upper Andean Tropical Forests. Soil Biol. Biochem. 2025, 202, 109692. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, S.; Chen, X.; Chen, H.Y.H. Plant Diversity Increases the Abundance and Diversity of Soil Fauna: A Meta-Analysis. Geoderma 2022, 411, 115694. [Google Scholar] [CrossRef]
- Fujii, K.; Shibata, M.; Kitajima, K.; Ichie, T.; Kitayama, K.; Turner, B.L. Plant–Soil Interactions Maintain Biodiversity and Functions of Tropical Forest Ecosystems. Ecol. Res. 2018, 33, 149–160. [Google Scholar] [CrossRef]
- Bitencourt, B.S.; da Silva, P.G.; Morato, E.F.; de Lima, Y.G. Dung Beetle Responses to Successional Stages in the Amazon Rainforest. Biodivers. Conserv. 2019, 28, 2745–2761. [Google Scholar] [CrossRef]
- Höfer, H.; Hanagarth, W.; Garcia, M.; Martius, C.; Franklin, E.; Römbke, J.; Beck, L. Structure and Function of Soil Fauna Communities in Amazonian Anthropogenic and Natural Ecosystems. Eur. J. Soil Biol. 2001, 37, 229–235. [Google Scholar] [CrossRef]
- McGee, K.M.; Porter, T.M.; Wright, M.; Hajibabaei, M. Drivers of Tropical Soil Invertebrate Community Composition and Richness across Tropical Secondary Forests Using DNA Metasystematics. Sci. Rep. 2020, 10, 18429. [Google Scholar] [CrossRef]
- Skubała, P.; Marzec, A. Importance of Different Types of Beech Dead Wood for Soil Microarthropod Fauna. Pol. J. Ecol. 2013, 61, 545–560. [Google Scholar]
- Takeda, H. Dynamics and Maintenance of Collembolan Community Structure in a Forest Soil System. Popul. Ecol. 1987, 29, 291–346. [Google Scholar] [CrossRef]
- Müller, J.; Brandl, R.; Brändle, M.; Förster, B.; de Araujo, B.C.; Gossner, M.M.; Ladas, A.; Wagner, M.; Maraun, M.; Schall, P.; et al. LiDAR-Derived Canopy Structure Supports the More-Individuals Hypothesis for Arthropod Diversity in Temperate Forests. Oikos 2018, 127, 814–824. [Google Scholar] [CrossRef]
- González del Pliego, P.; Scheffers, B.R.; Basham, E.W.; Woodcock, P.; Wheeler, C.; Gilroy, J.J.; Medina Uribe, C.A.; Haugaasen, T.; Freckleton, R.P.; Edwards, D.P. Thermally Buffered Microhabitats Recovery in Tropical Secondary Forests Following Land Abandonment. Biol. Conserv. 2016, 201, 385–395. [Google Scholar] [CrossRef]
- Klimes, P.; Idigel, C.; Rimandai, M.; Fayle, T.M.; Janda, M.; Weiblen, G.D.; Novotny, V. Why Are There More Arboreal Ant Species in Primary than in Secondary Tropical Forests? J. Anim. Ecol. 2012, 81, 1103–1112. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.; Ai, N.; Tuo, X.; Zhang, Z.; Gao, R.; Qin, J.; Yuan, C. Characteristics of Soil Macrofauna and Its Coupling Relationship with Environmental Factors in the Loess Area of Northern Shaanxi. Sustainability 2022, 14, 2484. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Ding, S.; Yan, P.; Zhang, P.; Ding, S. Environmental Effects on Taxonomic Turnover in Soil Fauna across Multiple Forest Ecosystems in East Asia. Insects 2022, 13, 1103. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Q.; Zhang, Y.; Yan, L.; Cui, D.; Xu, L. Effects of Natural Forest Conversion and Plantation Tree Species Composition on Soil Macrofauna Communities in Northeast China Mountains. J. For. Res. 2023, 34, 1475–1489. [Google Scholar] [CrossRef]
- Hardt Ferreira dos Santos, V.A.; Modolo, G.S.; Ferreira, M.J. How Do Silvicultural Treatments Alter the Microclimate in a Central Amazon Secondary Forest? A Focus on Light Changes. J. Environ. Manag. 2020, 254, 109816. [Google Scholar] [CrossRef]
- Viketoft, M. Determinants of Small-Scale Spatial Patterns: Importance of Space, Plants and Abiotics for Soil Nematodes. Soil Biol. Biochem. 2013, 62, 92–98. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, F.; Wei, J.; Wang, W.; Wu, Z.; Xu, D.; Haider, F.U.; Li, X.; Dong, Y. Ecological Restoration Increases the Diversity of Understory Vegetation in Secondary Forests: An Evidence from 90 Years of Forest Closures. Forests 2024, 15, 1642. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of the Springtails (Insecta, Collembola); Oxford University Press: Oxford, UK, 1997; ISBN 9780198540847. [Google Scholar]
- Siddiky, M.R.K.; Schaller, J.; Caruso, T.; Rillig, M.C. Arbuscular Mycorrhizal Fungi and Collembola Non-Additively Increase Soil Aggregation. Soil Biol. Biochem. 2012, 47, 93–99. [Google Scholar] [CrossRef]
- Deharveng, L. Recent Advances in Collembola Systematics. Pedobiologia 2004, 48, 415–433. [Google Scholar] [CrossRef]
- Van Straalen, N.M.; Kraak, M.H.S.; Denneman, C.A.J. Soil Microarthropods as Indicators of Soil Acidification and Forest Decline in the Veluwe Area, the Netherlands. Pedobiologia 1988, 32, 47–56. [Google Scholar] [CrossRef]
- Ponge, J.F.; Gillet, S.; Dubs, F.; Fedoroff, E.; Haese, L.; Sousa, J.P.; Lavelle, P. Collembolan Communities as Bioindicators of Land Use Intensification. Soil Biol. Biochem. 2003, 35, 813–826. [Google Scholar] [CrossRef]
- Cassagne, N.; Gauquelin, T.; Bal-Serin, M.C.; Gers, C. Endemic Collembola, Privileged Bioindicators of Forest Management. Pedobiologia 2006, 50, 127–134. [Google Scholar] [CrossRef]
- Sławski, M.; Sławska, M. Seven Decades of Spontaneous Forest Regeneration after Large-Scale Clear-Cutting in Białowieża Forest Do Not Ensure the Complete Recovery of Collembolan Assemblages. Forests 2019, 10, 948. [Google Scholar] [CrossRef]
- Addison, J.A.; Trofymow, J.A.; Marshall, V.G. Abundance, Species Diversity, and Community Structure of Collembola in Successional Coastal Temperate Forests on Vancouver Island, Canada. Appl. Soil Ecol. 2003, 24, 233–246. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, A.; Zhou, Z.; Chen, T.W.; Pang, X.; Scheu, S. Driving Mechanisms of Taxonomic and Functional Community Composition of Collembola during Subalpine Succession. Geoderma 2025, 453, 117156. [Google Scholar] [CrossRef]
- Setälä, H.; Marshall, V.G.; Trofymow, J.A. Influence of Micro- and Macro-Habitat Factors on Collembolan Communities in Douglas-Fir Stumps during Forest Succession. Appl. Soil Ecol. 1995, 2, 227–242. [Google Scholar] [CrossRef]
- Daghighi, E.; Koehler, H.; Kesel, R.; Filser, J. Long-Term Succession of Collembola Communities in Relation to Climate Change and Vegetation. Pedobiologia 2017, 64, 25–38. [Google Scholar] [CrossRef]
- Jucevica, E.; Melecis, V. Global Warming Affect Collembola Community: A Long-Term Study. Pedobiologia 2006, 50, 177–184. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Mo, J.; Gilliam, F.S.; Zhou, G.; Luo, Y.; Zhang, W.; Huang, J. Divergent Responses of Soil Buffering Capacity to Long-Term N Deposition in Three Typical Tropical Forests with Different Land-Use History. Environ. Sci. Technol. 2015, 49, 4072–4080. [Google Scholar] [CrossRef]
- Balaguer-Romano, R.; De Cáceres, M.; Espelta, J.M. Second-Growth Forests Exhibit Higher Sensitivity to Dry and Wet Years than Long-Existing Ones. Ecosystems 2025, 28, 6. [Google Scholar] [CrossRef]
- Wang, T. Landscape of Heilongjiang Twin Mountain National Forest Park Research on Pattern and Network Optimization. Northeast Forestry University: Harbin, China, 2018; Volume 6. [Google Scholar]
- Hongqiang, Z.; Zhiyong, G.; Geng, L.; Chunyan, J.; Dongdong, Z.; Xiangdong, Z.; Suhui, C.; Zhixia, M. Winter Bed-Site Selection by Roe Deer (Capreolus capreolus) in Huangnihe Nature Reserve. Acta Ecol. Sin. 2013, 33, 2054–2061. [Google Scholar] [CrossRef]
- Kun, W. Research on Ecotourism of Forest Zone in Northeast East Area—For Example the Ecotourism Zones in Fangzheng and Maoershan; Northeast Forestry University: Harbin, China, 2004; Volume 4. [Google Scholar]
- Yin, W. Pictorial Keys to Soil Animals of China; Science Press: Beijing, China, 1998; p. 727. [Google Scholar]
- Jordana, R. Synopses on Palaearctic Collembola—Capbryinae & Entomobryini. Soil Org. 2012, 84, 1–390. [Google Scholar]
- Bellinger, P.F.; Christiansen, K.A.; Janssens, F. 1996–2024. Checklist of the Collembola of the World. Available online: http://www.collembola.org (accessed on 31 October 2024).
- Cornelissen, J.H.C.; Quested, H.M.; Van Logtestijn, R.S.P.; Pérez-Harguindeguy, N.; Gwynn-Jones, D.; Díaz, S.; Callaghan, T.V.; Press, M.C.; Aerts, R. Foliar PH as a New Plant Trait: Can It Explain Variation in Foliar Chemistry and Carbon Cycling Processes among Subarctic Plant Species and Types? Oecologia 2006, 147, 315–326. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. INEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (Hill Numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. vegan: Community Ecology Package. R Package Vegan Version 2.6-10. CRAN: Contributed Packages 2025. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 31 October 2024).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing Hierarchical and Variation Partitioning in Multiple Regression and Canonical Analyses Using the Rdacca.Hp R Package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Ma, C.; Yin, X.; Xu, H.; Tao, Y. Responses of Soil Collembolans to Vegetation Restoration in Temperate Coniferous and Broad-Leaved Mixed Forests. Journal of Forestry Research. 2020, 31, 2333–2345. [Google Scholar] [CrossRef]
- Boonrotpong, S.; Sotthibandhu, S.; Pholpunthin, C. Species Composition of Dung Beetles in the Primary and Secondary Forests at Ton Nga Chang Wildlife Sanctuary. Sci. Asia 2004, 30, 59–65. [Google Scholar] [CrossRef]
- Hethcoat, M.G.; King, B.J.; Castiblanco, F.F.; Ortiz-Sepúlveda, C.M.; Achiardi, F.C.P.; Edwards, F.A.; Medina, C.; Gilroy, J.J.; Haugaasen, T.; Edwards, D.P. The Impact of Secondary Forest Regeneration on Ground-Dwelling Ant Communities in the Tropical Andes. Oecologia 2019, 191, 475–482. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Y.; Potapov, A.M.; Ram Bhusal, D.; Qiang, W.; Wang, M.; Pang, X. Changes in Diversity and Functional Groups of Soil Mite Communities Are Associated with Properties of Food Resources along a Subalpine Secondary Succession. Geoderma 2023, 432, 116395. [Google Scholar] [CrossRef]
- Wang, M.; Yu, B.; Shen, Z.; Zhao, L.; Zhang, J.; Cui, Y.; Fan, Z.; Zu, W.; Dai, G.; Zhang, W.; et al. Changes in Soil and Litter Properties Differentially Influence Soil Nematode Communities across Three Successional Stages in Two Contrasting Forests. Land Degrad Dev. 2023, 34, 3196–3207. [Google Scholar] [CrossRef]
- Wang, M.; Shao, Y.; Zhang, W.; Yu, B.; Shen, Z.; Fan, Z.; Zu, W.; Dai, G.; Fu, S. Secondary Succession Increases Diversity and Network Complexity of Soil Microbial Communities in Subtropical and Temperate Forests. Catena 2025, 249, 108662. [Google Scholar] [CrossRef]
- Karaman, S.; Vargovitsh, R.S. Deep Troglomorphy: New Arrhopalitidae (Collembola: Symphypleona) of Different Life Forms from the Snezhnaya Cave System in the Caucasus. Diversity 2022, 14, 678. [Google Scholar] [CrossRef]
- Chang, G.D.; Son, M.; Beak, S.A.; Lee, T.G.; Keum, E.; Choi, Y.; Park, K.H. Correlation between Environmental Factors and Springtail Species (Hexapoda: Collembola) Inhabiting Jangamgul Cave of Korea. J. Asia Pac. Biodivers. 2022, 15, 336–344. [Google Scholar] [CrossRef]
- Simões, M.H.; Souza-Silva, M.; Lopes Ferreira, R. Cave Physical Attributes Influencing the Structure of Terrestrial Invertebrate Communities in Neotropics. Subterr. Biol. 2015, 16, 103–121. [Google Scholar] [CrossRef]
- Chauvat, M.; Trap, J.; Perez, G.; Delporte, P.; Aubert, M. Assemblages of Collembola across a 130-Year Chronosequence of Beech Forest. Soil Org. 2011, 83, 405–418. [Google Scholar]
- Sømme, L. Ecology of Cryptopygus Sverdrupi (Insecta: Collembola) from Dronning Maud Land, Antarctica. Polar Biol. 1986, 6, 179–184. [Google Scholar] [CrossRef]
- Zhang, B.; Chang, L.; Ni, Z.; Callaham, M.A.; Sun, X.; Wu, D. Effects of Land Use Changes on Winter-Active Collembola in Sanjiang Plain of China. Appl. Soil Ecol. 2014, 83, 51–58. [Google Scholar] [CrossRef]
- Fountain, M.T.; Hopkin, S.P. Folsomia Candida (Collembola): A “Standard” Soil Arthropod. Annu. Rev. Entomol. 2005, 50, 201–222. [Google Scholar] [CrossRef]
- Strong, W.L. Biased Richness and Evenness Relationships within Shannon–Wiener Index Values. Ecol. Indic. 2016, 67, 703–713. [Google Scholar] [CrossRef]
- Zutta, B.R.; Salinas, N.; Cosio, E.G.; Tito, R.; Aragón, S.; Nina-Quispe, A.; Roman-Cuesta, R.M. Satellite-Derived Forest Canopy Greenness Shows Differential Drought Vulnerability of Secondary Forests Compared to Primary Forests in Peru. Environ. Res. Lett. 2023, 18, 064004. [Google Scholar] [CrossRef]
- Li, H.; Ye, D.; Wang, X.; Settles, M.L.; Wang, J.; Hao, Z.; Zhou, L.; Dong, P.; Jiang, Y.; Ma, Z.S. Soil Bacterial Communities of Different Natural Forest Types in Northeast China. Plant Soil 2014, 383, 203–216. [Google Scholar] [CrossRef]
- Walker, T.W.; Syers, J.K. The Fate of Phosphorus during Pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Dalling, J.W.; Heineman, K.; Lopez, O.R.; Wright, S.J.; Turner, B.L. Nutrient Availability in Tropical Rain Forests: The Paradigm of Phosphorus Limitation. In Tropical Tree Physiology; Tree Physiology; Springer: Cham, Switzerland, 2016; pp. 261–273. [Google Scholar] [CrossRef]
- Camenzind, T.; Hättenschwiler, S.; Treseder, K.K.; Lehmann, A.; Rillig, M.C. Nutrient Limitation of Soil Microbial Processes in Tropical Forests. Ecol. Monogr. 2018, 88, 4–21. [Google Scholar] [CrossRef]
- Mueller, K.E.; Eissenstat, D.M.; Hobbie, S.E.; Oleksyn, J.; Jagodzinski, A.M.; Reich, P.B.; Chadwick, O.A.; Chorover, J. Tree Species Effects on Coupled Cycles of Carbon, Nitrogen, and Acidity in Mineral Soils at a Common Garden Experiment. Biogeochemistry 2012, 111, 601–614. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Modrzynski, J.; Mrozinski, P.; Hobbie, S.E.; Eissenstat, D.M.; Chorover, J.; Chadwick, O.A.; Hale, C.M.; Tjoelker, M.G. Linking Litter Calcium, Earthworms and Soil Properties: A Common Garden Test with 14 Tree Species. Ecol. Lett. 2005, 8, 811–818. [Google Scholar] [CrossRef]
- Chagnon, M.; Pare, D. Relationships between Soil Chemistry, Microbial Biomass and the Collembolan Fauna of Southern Québec Sugar Maple Stands. Écoscience 2000, 7, 307–316. [Google Scholar] [CrossRef]
- Stange, E.E.; Ayres, M.P. Climate Change Impacts: Insects. In Encyclopedia of Life Sciences (eLS); John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar] [CrossRef]
- Meehan, M.L.; Song, Z.; Lumley, L.M.; Cobb, T.P.; Proctor, H. Soil Mites as Bioindicators of Disturbance in the Boreal Forest in Northern Alberta, Canada: Testing Taxonomic Sufficiency at Multiple Taxonomic Levels. Ecol. Indic. 2019, 102, 349–365. [Google Scholar] [CrossRef]





| Environmental Factor | Site | Depth | Forest | Summary of ANOVA Results | ||
|---|---|---|---|---|---|---|
| Primary | Secondary | F Test | p Value | |||
| Total N (%) | Fangzheng | Litter | 1.07 ± 0.29 a | 1.66 ± 0.27 b | 10.802 | 0.011 |
| Soil | 0.61 ± 0.18 | 0.92 ± 0.4 | 2.437 | 0.157 | ||
| Huangnihe | Litter | 1.2 ± 0.13 a | 1.57 ± 0.26 b | 7.797 | 0.023 | |
| Soil | 0.6 ± 0.23 | 0.38 ± 0.05 | 3.973 | 0.081 | ||
| Total C (%) | Fangzheng | Litter | 44.77 ± 1.44 | 44.77 ± 1.68 | 0.200 | 0.667 |
| Soil | 7.32 ± 2.49 | 12.11 ± 6.55 | 2.290 | 0.169 | ||
| Huangnihe | Litter | 41.83 ± 8.95 | 44.65 ± 0.46 | 0.848 | 0.384 | |
| Soil | 7.9 ± 3.53 | 5.16 ± 0.94 | 2.138 | 0.182 | ||
| Total P (g/kg) | Fangzheng | Litter | 4.39 ± 0.63 a | 5.49 ± 0.59 b | 8.030 | 0.022 |
| Soil | 1.42 ± 0.27 | 1.77 ± 0.98 | 0.581 | 0.468 | ||
| Huangnihe | Litter | 3.12 ± 0.37 a | 3.75 ± 0.36 b | 7.396 | 0.026 | |
| Soil | 1.8 ± 0.51 | 1.59 ± 0.3 | 0.575 | 0.470 | ||
| pH | Fangzheng | Litter | 5.72 ± 0.13 | 5.88 ± 0.19 | 1.980 | 0.197 |
| Soil | 5.52 ± 0.31 | 5.2 ± 0.19 | 3.879 | 0.084 | ||
| Huangnihe | Litter | 5.5 ± 0.12 | 5.96 ± 0.82 | 0.001 | 0.972 | |
| Soil | 5.07 ± 0.26 | 5.26 ± 0.36 | 0.979 | 0.352 | ||
| C/N ratio | Fangzheng | Litter | 45.3 ± 16.17 a | 27.51 ± 4.18 b | 8.219 | 0.021 |
| Soil | 11.88 ± 0.92 | 12.85 ± 1.58 | 1.419 | 0.268 | ||
| Huangnihe | Litter | 34.48 ± 5.36 | 29.02 ± 4.52 | 2.792 | 0.133 | |
| Soil | 12.87 ± 2.35 | 13.02 ± 0.62 | 0.019 | 0.894 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Xie, Z.; Li, Y.; Wan, Z.; Shi, H.; Wang, L.; Ji, Q.; Wang, Z.; Wu, D. Differences in Diversity of Collembola Communities Between Primary and Secondary Forests and Driving Factors. Insects 2025, 16, 853. https://doi.org/10.3390/insects16080853
Zheng M, Xie Z, Li Y, Wan Z, Shi H, Wang L, Ji Q, Wang Z, Wu D. Differences in Diversity of Collembola Communities Between Primary and Secondary Forests and Driving Factors. Insects. 2025; 16(8):853. https://doi.org/10.3390/insects16080853
Chicago/Turabian StyleZheng, Mingxin, Zhijing Xie, Yueying Li, Zhuoma Wan, Haozhe Shi, Liping Wang, Qiaoqiao Ji, Zhaojun Wang, and Donghui Wu. 2025. "Differences in Diversity of Collembola Communities Between Primary and Secondary Forests and Driving Factors" Insects 16, no. 8: 853. https://doi.org/10.3390/insects16080853
APA StyleZheng, M., Xie, Z., Li, Y., Wan, Z., Shi, H., Wang, L., Ji, Q., Wang, Z., & Wu, D. (2025). Differences in Diversity of Collembola Communities Between Primary and Secondary Forests and Driving Factors. Insects, 16(8), 853. https://doi.org/10.3390/insects16080853






