Impact of Enzymatically Treated Substrate on Yellow Mealworm Development and Composition
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Stock
2.2. Bioassay 1: Larvae Growth and Development on Selected Feed Mixtures
- Wheat bran (control feed) (WB).
- Enzymatically hydrolysed wheat straw pretreated with steam explosion 100% (WES).
- Enzymatically hydrolysed wheat straw pretreated by the organosolv method 100% (WEO).
- Enzymatically hydrolysed cup plant pretreated by the organosolv method 100% (CEO).
2.3. Bioassay 2: Larvae Chemical Composition and Development
2.4. Chemical Analyses of Feed and Insect Depending on the Type of Diet
2.4.1. Analyses of Feed Materials
2.4.2. Analyses of Insect Composition
2.5. Statistical Analysis
3. Results
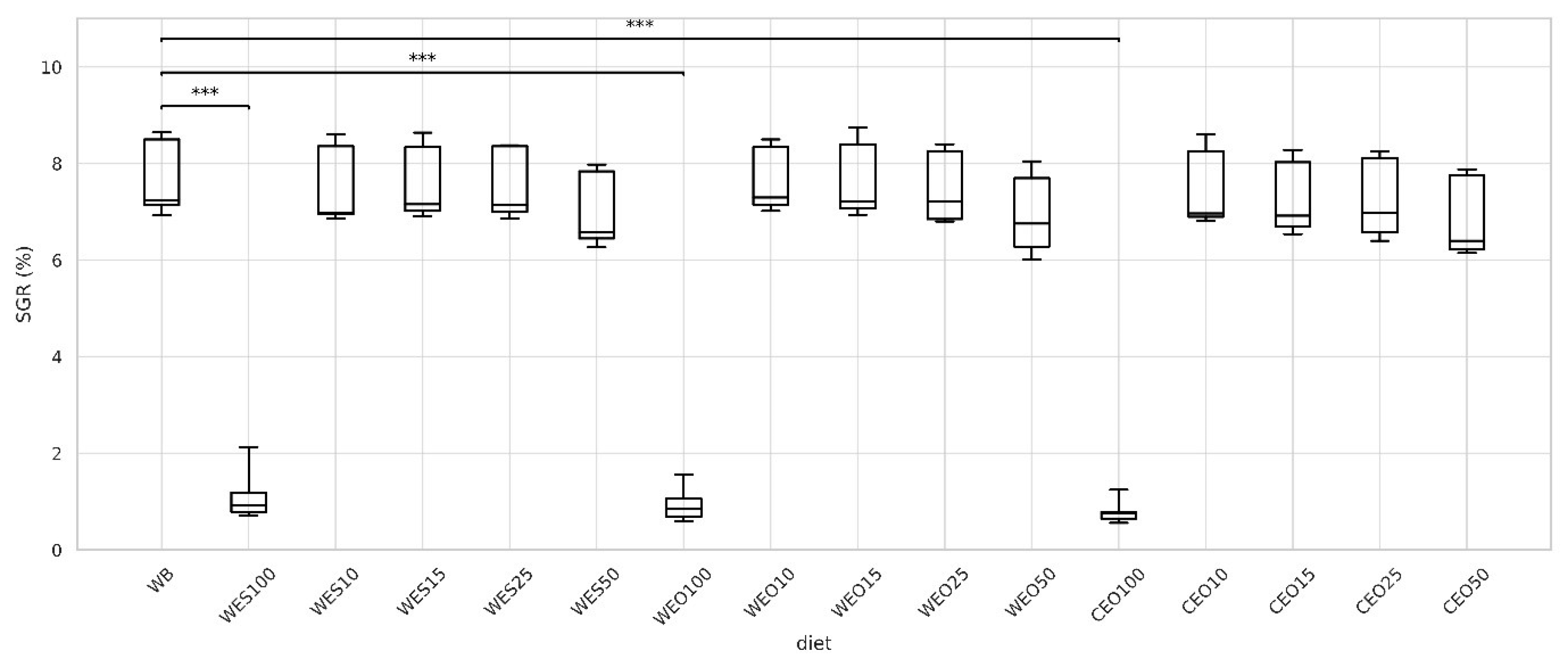
3.1. Bioassay 1
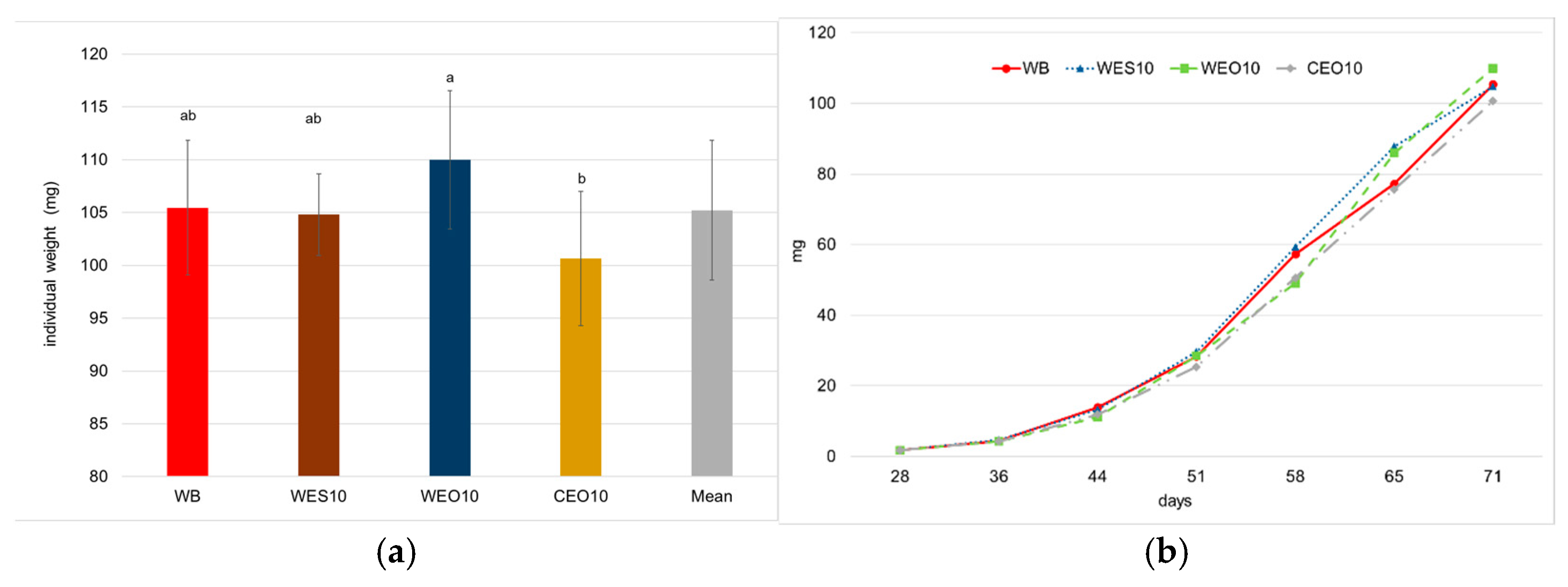
3.2. Bioassay 2
3.2.1. Growth and Development
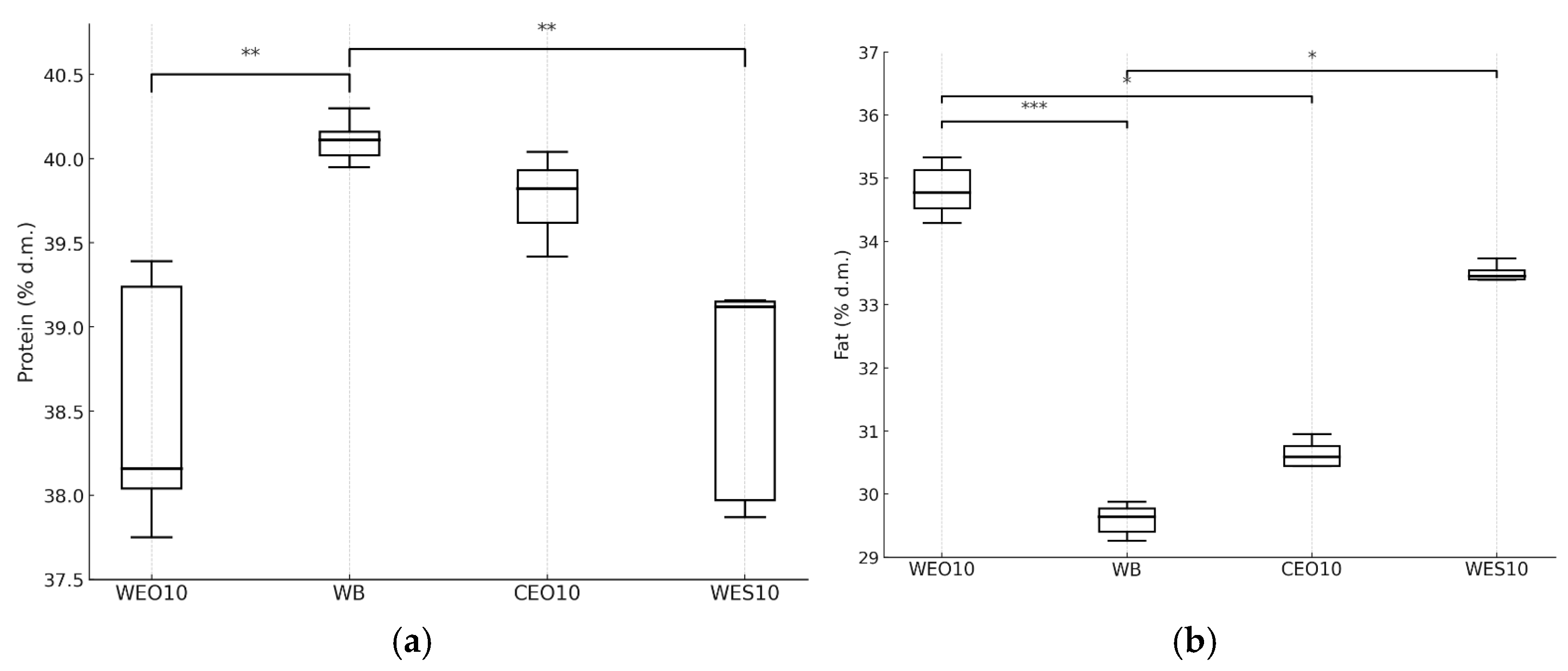
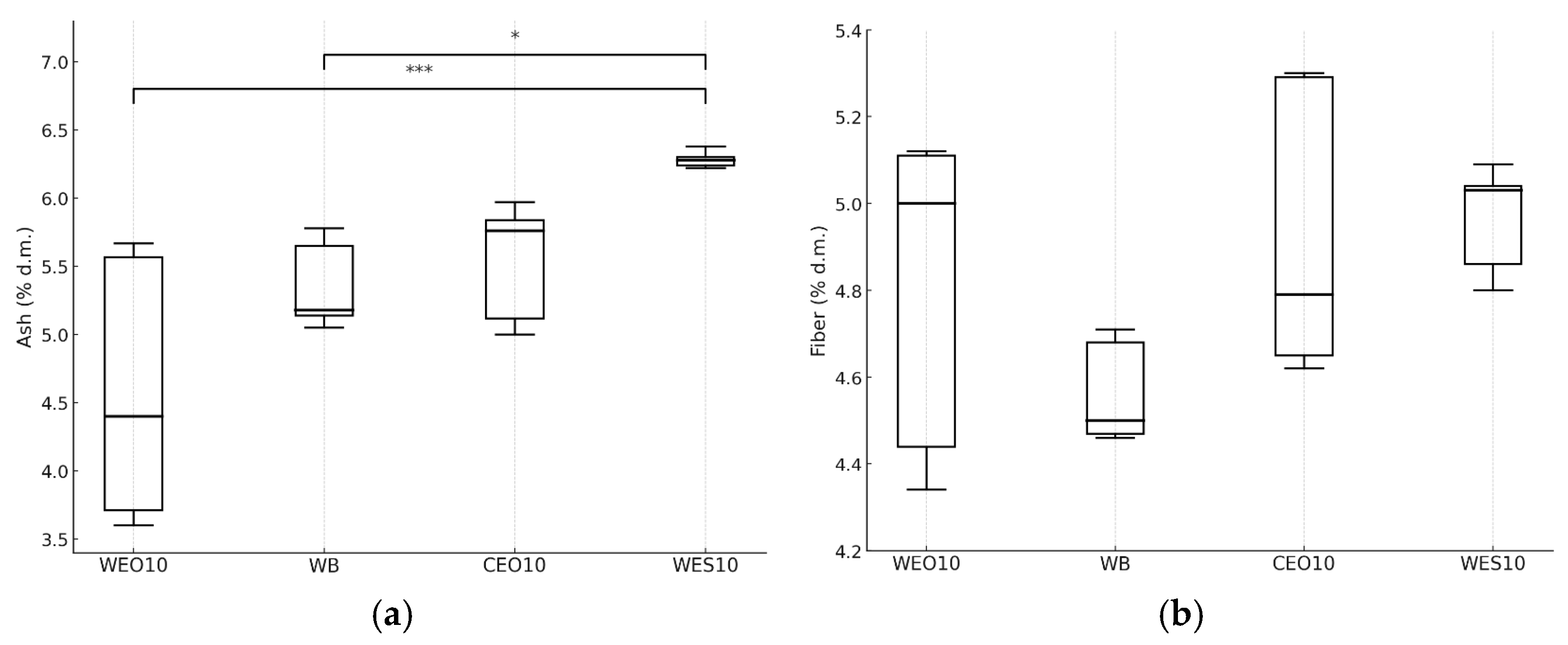
3.2.2. Insect Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, R.P.; Boruah, A.; Khan, A.; Thilagam, P.; Akanksha; Sivakumar, S.; Dhapola, P.; Singh, B.V. Exploring the Significance of Insects in Ecosystems: A Comprehensive Examination of Entomological Studies. Int. J. Environ. Clim. Change 2023, 13, 1243–1252. [Google Scholar] [CrossRef]
- Stork, N.E. How Many Species of Insects and Other Terrestrial Arthropods Are There on Earth? Annu. Rev. Entomol. 2024, 44, 39. [Google Scholar] [CrossRef] [PubMed]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The Potential Role of Insects as Feed: A Multi-Perspective Review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Scudder, G.G.E. The Importance of Insects. In Insect Biodiversity; Wiley: Hoboken, NJ, USA, 2017; pp. 9–43. [Google Scholar]
- Losey, J.E.; Vaughan, M. The Economic Value of Ecological Services Provided by Insects. Bioscience 2006, 56, 311–323. [Google Scholar] [CrossRef]
- Zhang, E.; Ji, X.; Ouyang, F.; Lei, Y.; Deng, S.; Rong, H.; Deng, X.; Shen, H. A Minireview of the Medicinal and Edible Insects from the Traditional Chinese Medicine (TCM). Front. Pharmacol. 2023, 14, 1125600. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Osei-Owusu, J.; Asante, K.; Dofuor, A.K.; Boateng, B.O.; Debrah, S.K.; Ninsin, K.D.; Siddiqui, S.A.; Chia, S.Y. Insects as Food and Medicine: A Sustainable Solution for Global Health and Environmental Challenges. Front. Nutr. 2023, 10, 1113219. [Google Scholar] [CrossRef]
- Alper Akçay, A. The Use of Insect Pigment in Art Works. Insects 2024, 15, 519. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Madureira, A.R.; Pintado, M.E. The Potential of Insects as Food Sources—A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3642–3652. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Jankielsohn, A. The Importance of Insects in Agricultural Ecosystems. Adv. Entomol. 2018, 6, 62–73. [Google Scholar] [CrossRef]
- Chowdhury, S.; Dubey, V.K.; Choudhury, S.; Das, A.; Jeengar, D.; Sujatha, B.; Kumar, A.; Kumar, N.; Semwal, A.; Kumar, V. Insects as Bioindicator: A Hidden Gem for Environmental Monitoring. Front. Environ. Sci. 2023, 11, 1146052. [Google Scholar] [CrossRef]
- Parikh, G.; Rawtani, D.; Khatri, N. Insects as an Indicator for Environmental Pollution. Environ. Claims J. 2021, 33, 161–181. [Google Scholar] [CrossRef]
- Pruszyński, S. The Progress in Research and Application of Entomophagous Insects in Integrated Pest Management (IPM). Prog. Plant Prot. 2013, 53, 2013. [Google Scholar] [CrossRef][Green Version]
- Hung, K.L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The Worldwide Importance of Honey Bees as Pollinators in Natural Habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Czachorowski, S.; Peni, D. Will Yellow Mealworm Become a Source of Safe Proteins for Europe? Agriculture 2020, 10, 233. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J. Anthropo-Entomophagy: Cultures, Evolution and Sustainability. Entomol. Res. 2009, 39, 271–288. [Google Scholar] [CrossRef]
- Siemianowska, E.; Kosewska, A.; Aljewicz, M.; Skibniewska, K.A.; Polak-Juszczak, L.; Jarocki, A.; Jędras, M. Larvae of Mealworm (Tenebrio molitor L.) as European Novel Food. Agric. Sci. 2013, 4, 287–291. [Google Scholar] [CrossRef]
- Brandon, A.M.; Gao, S.H.; Tian, R.; Ning, D.; Yang, S.S.; Zhou, J.; Wu, W.M.; Criddle, C.S. Biodegradation of Polyethylene and Plastic Mixtures in Mealworms (Larvae of Tenebrio molitor) and Effects on the Gut Microbiome. Environ. Sci. Technol. 2018, 52, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Stasiak, M.; Li, L.; Xie, B.; Fu, Y.; Gidzinski, D.; Dixon, M.; Liu, H. Rearing Tenebrio Molitor in BLSS: Dietary Fiber Affects Larval Growth, Development, and Respiration Characteristics. Acta Astronaut. 2016, 118, 130–136. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kosewska, A.; Kosewska, O.; Purwin, C.; Lipiński, K.; Ciesielski, S. Polyethylene, Polystyrene and Lignocellulose Wastes as Mealworm (Tenebrio molitor L.) Diets and Their Impact on the Breeding Condition, Biometric Parameters, Metabolism, and Digestive Microbiome. Sci. Total Environ. 2022, 832, 154758. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kosewska, A.; Ciesielski, S.; Kosewska, O. Changes in the Gut Microbiome and Enzymatic Profile of Tenebrio molitor Larvae Biodegrading Cellulose, Polyethylene and Polystyrene Waste. Environ. Pollut. 2020, 256, 113265. [Google Scholar] [CrossRef] [PubMed]
- Kosewska, O.; Kosewska, A.; Przemieniecki, S.; Sienkiewicz, S. Alternative Ways of Foamed Polystyrene Recycling Using Insects as an Element of Sustainable Development. In Proceedings of the 2019 International Conference “Economic Science for Rural Development”, Jelgava, Latvia, 8 May 2019; pp. 45–52. [Google Scholar]
- Yang, S.S.; Chen, Y.-d.; Zhang, Y.; Zhou, H.M.; Ji, X.Y.; He, L.; Xing, D.F.; Ren, N.Q.; Ho, S.H.; Wu, W.M. A Novel Clean Production Approach to Utilize Crop Waste Residues as Co-Diet for Mealworm (Tenebrio molitor) Biomass Production with Biochar as Byproduct for Heavy Metal Removal. Environ. Pollut. 2019, 252, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Chen, C.L.; Wang, J.Y. Reducing Sugar-Producing Bacteria from Guts of Tenebrio molitor Linnaeus (Yellow Mealworm) for Lignocellulosic Waste Minimization. Microbes Environ. 2011, 26, 354–359. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.U.; Liu, X.; Yang, Q.; Zheng, L.; Li, W.; Cai, M.; Li, Q.; Zhang, J.; Yu, Z. Insect Biorefinery: A Green Approach for Conversion of Crop Residues into Biodiesel and Protein. Biotechnol Biofuels 2017, 10, 304. [Google Scholar] [CrossRef]
- Dessie, W.; Luo, X.; He, F.; Liao, Y.; Duns, G.J.; Qin, Z. Lignin Valorization: A Crucial Step towards Full Utilization of Biomass, Zero Waste and Circular Bioeconomy. Biocatal. Agric. Biotechnol. 2023, 51, 102777. [Google Scholar] [CrossRef]
- Ghosh, V. Nanotechnological Advancements for Enhancing Lignocellulosic Biomass Valorization. In Valorization of Biomass Wastes for Environmental Sustainability; Springer Nature: Cham, Switzerland, 2024; pp. 99–113. [Google Scholar]
- Crane, D.P.; Ogle, D.H.; Shoup, D.E. Use and Misuse of a Common Growth Metric: Guidance for Appropriately Calculating and Reporting Specific Growth Rate. Rev. Aquac. 2020, 12, 1542–1547. [Google Scholar] [CrossRef]
- PN-ISO 5498:1996; Agricultural Food Products—Determination of Crude Fibre Content—General Method. Polish Committee for Standardization: Warsaw, Poland, 1996.
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- PN-EN ISO 12966-4:2015; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 4: Determination by Capillary Gas Chromatography. Polish Committee for Standardization: Warsaw, Poland, 2015.
- PN-EN ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. Polish Committee for Standardization: Warsaw, Poland, 2017.
- PN-EN ISO 13903:2006; Animal Feeding Stuffs—Determination of Amino Acids Content. Polish Committee for Standardization: Warsaw, Poland, 2006.
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Manditsera, F.A.; Luning, P.A.; Fogliano, V.; Lakemond, C.M.M. Effect of Domestic Cooking Methods on Protein Digestibility and Mineral Bioaccessibility of Wild Harvested Adult Edible Insects. Food Res. Int. 2019, 121, 404–411. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Saura-Martínez, J.; Montalbán, A.; Manzano-Nicolás, J.; Taboada-Rodríguez, A.; Hernández, F.; Marín-Iniesta, F. A Treatment for Rice Straw and Its Use for Mealworm (Tenebrio molitor L.) Feeding: Effect on Insect Performance and Diet Digestibility. Insects 2024, 15, 631. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yuan, T.; Zhou, W.; Wu, B.; Zhou, Y.; Xiao, N. Effects of Straw Structure and Component on Feeding Efficiency of Yellow Mealworm for Insect Protein Production. Bioresour. Technol. 2024, 414, 131630. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Ottoboni, M. Substrate as Insect Feed for Bio-Mass Production. J. Insects Food Feed 2021, 7, 585–596. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Aljewicz, M.; Stolarski, M.J. Influence of Different Diets on Growth and Nutritional Composition of Yellow Mealworm. Foods 2022, 11, 3075. [Google Scholar] [CrossRef] [PubMed]
- Cereals Market Data on Production, Price and Trade for Wheat, Barley, Maize, Oats, Rye, Sorghum, Triticale and Other Cereals. Available online: https://agridata.ec.europa.eu/extensions/DataPortal/cereals.html (accessed on 9 July 2025).
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Peni, D. Growth Potential of Yellow Mealworm Reared on Industrial Residues. Agriculture 2020, 10, 599. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Gold, M.; Bosch, G.; Guillaume, J.B.; Rumbos, C.I.; Naser El Deen, S.; Sandrock, C.; Bellezza Oddon, S.; Athanassiou, C.G.; Cambra-López, M.; et al. Bugbook: Nutritional Requirements for Edible Insect Rearing. J. Insects Food Feed, 2025; 1–20, published online ahead of print. [Google Scholar] [CrossRef]
- Said, S.M.; El Defrawy, B.M. Effect of Different Diets on Some Larval Body Characteristics and Food Utilization Efficiency of Tenebrio molitor (Coleoptera: Tenebrionidae). Menoufia J. Plant Prot. 2022, 7, 9–17. [Google Scholar] [CrossRef]
- Montalbán, A.; Martínez-Miró, S.; Schiavone, A.; Madrid, J.; Hernández, F. Growth Performance, Diet Digestibility, and Chemical Composition of Mealworm (Tenebrio molitor L.) Fed Agricultural By-Products. Insects 2023, 14, 824. [Google Scholar] [CrossRef]
- Theron, W.; Teke, G.M.; Diedericks, D.; Van Rensburg, E.; Görgens, J.F. Steam Pre-Treatment of Sugarcane Bagasse and Wheat Straw as a Cleaner Feedstock for Black Soldier Fly Larvae Rearing. J. Insects Food Feed. 2023, 10, 585–599. [Google Scholar] [CrossRef]
- Kröncke, N.; Benning, R. Influence of Dietary Protein Content on the Nutritional Composition of Mealworm Larvae (Tenebrio molitor L.). Insects 2023, 14, 261. [Google Scholar] [CrossRef]
- Iyapo, K.A.; Adewole, A.M.; Sule, S.O.; Oluwatobi, A.S.; Olofintoye, B.O. Nutritional Composition Profile of Mealworm Larvae (Tenebrio molitor) Reared on Different Substrate: A Potential Protein Substitute for Fishmeal. Niger. J. Entomol. 2024, 40, 92–100. [Google Scholar] [CrossRef]
- Jajić, I.; Popović, A.; Urošević, M.; Krstović, S.; Petrović, M.; Guljaš, D. Chemical Composition of Mealworm Larvae (Tenebrio molitor) Reared in Serbia. Contemp. Agric. 2019, 68, 23–27. [Google Scholar] [CrossRef]
- Adámková, A.; Adámek, M.; Mlček, J.; Borkovcová, M.; Bednářová, M.; Kouřimská, L.; Skácel, J.; Vítová, E. Welfare of the mealworm (Tenebrio molitor) breeding with regard to nutrition value and food safety. Potravin. Slovak J. Food Sci. 2017, 11, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Deruytter, D.; Rumbos, C.I.; Adamaki-Sotiraki, C.; Tournier, L.; Ageorges, V.; Coudron, C.L.; Yakti, W.; Ulrichs, C.; Spranghers, T.; Berrens, S.; et al. Make It a Standard? The Creation and Variability Assessment of a Consensus Standard Protocol for Tenebrio molitor Larvae Feeding Trials. J. Insects Food Feed. 2024, 11, 1023–1033. [Google Scholar] [CrossRef]
- Eggink, K.M.; Lund, I.; Pedersen, P.B.; Hansen, B.W.; Dalsgaard, J. Biowaste and By-Products as Rearing Substrates for Black Soldier Fly (Hermetia illucens) Larvae: Effects on Larval Body Composition and Performance. PLoS ONE 2022, 17, e0275213. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Mattioli, S.; Rondoni, G.; Bosco, A.D.; Servili, M.; Castellini, C.; Conti, E. Characterisation of Fatty Acid Profiles of Tenebrio molitor Larvae Reared on Diets Enriched with Edible Oils. J. Insects Food Feed. 2022, 8, 901–912. [Google Scholar] [CrossRef]
- Bonomini, M.G.; Prandi, B.; Caligiani, A. Black Soldier Fly (Hermetia illucens L.) Whole and Fractionated Larvae: In Vitro Protein Digestibility and Effect of Lipid and Chitin Removal. Food Res. Int. 2024, 196, 115102. [Google Scholar] [CrossRef]







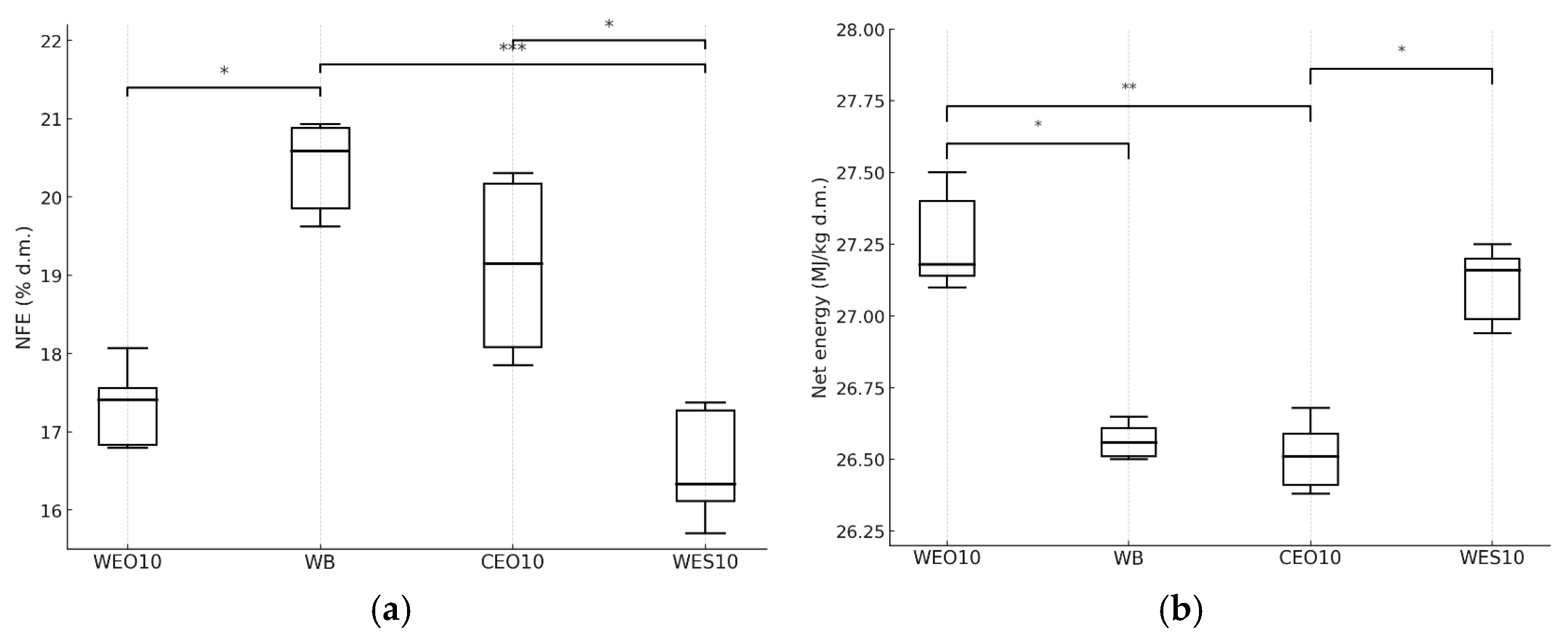
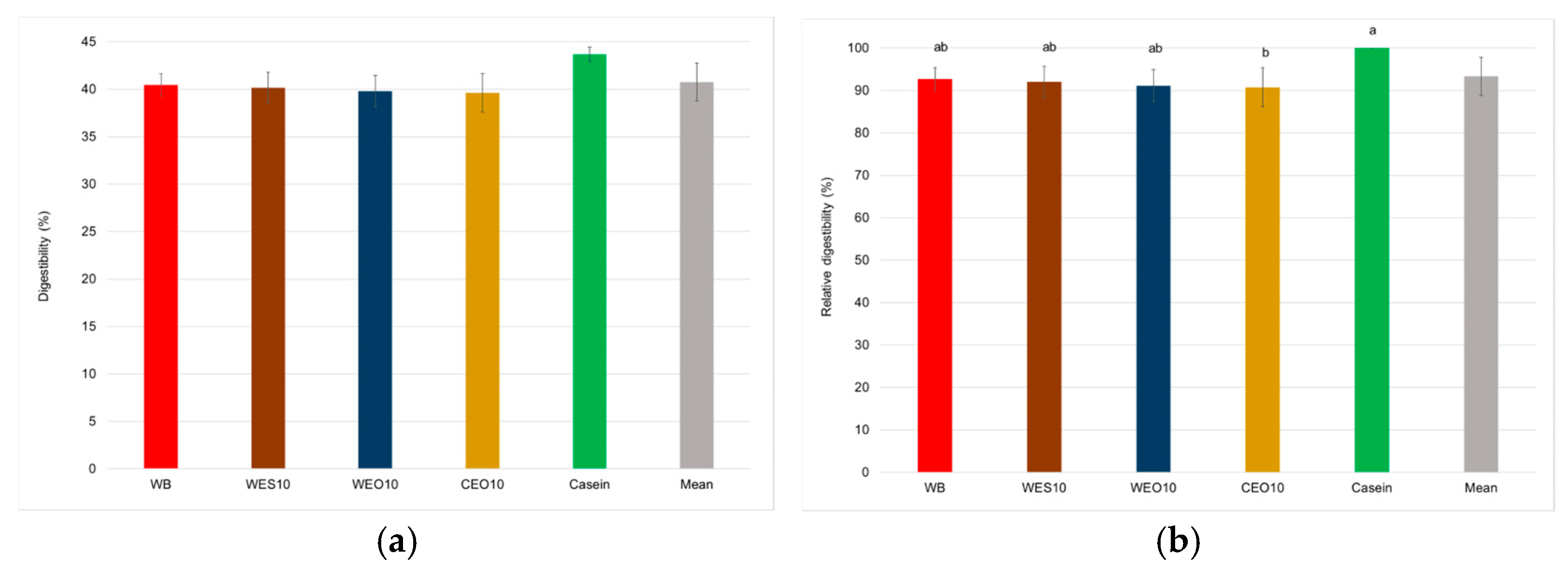
| Diet | Moisture (%) | Ash (% d.m.) | Protein (% d.m.) | Fat (% d.m.) | Fibre (% d.m.) | NFE (% d.m.) |
|---|---|---|---|---|---|---|
| WB | 12.1 ± 0.02 | 5.56 ± 0.05 | 17.6 ± 0.10 | 3.57 ± 0.03 | 7.95 ± 0.49 | 65.3 ± 0.51 |
| WES100 | 11.2 ± 0.03 | 6.07 ± 0.01 | 4.48 ± 0.02 | 1.02 ± 0.07 | 24.7 ± 0.44 | 63.7 ± 0.52 |
| WEO100 | 10.5 ± 0.02 | 2.44 ± 0.01 | 2.18 ± 0.06 | 0.30 ± 0.11 | 41.7 ± 0.10 | 53.4 ± 0.14 |
| CEO100 | 4.26 ± 0.02 | 5.66 ± 0.03 | 2.42 ± 0.00 | 1.01 ± 0.02 | 59.2 ± 0.41 | 31.7 ± 0.41 |
| WES10 | 12.0 | 5.61 | 16.3 | 3.31 | 9.62 | 65.2 |
| WES15 | 12.0 | 5.64 | 15.6 | 3.19 | 10.5 | 65.1 |
| WES25 | 11.9 | 5.7 | 14.3 | 2.9 | 12.1 | 64.9 |
| WES50 | 11.7 | 5.8 | 11.0 | 2.3 | 16.3 | 64.5 |
| WEO10 | 12.0 | 5.25 | 16.07 | 3.24 | 11.3 | 64.1 |
| WEO15 | 11.9 | 5.1 | 15.3 | 3.1 | 13.0 | 63.5 |
| WEO25 | 11.7 | 4.8 | 13.8 | 2.8 | 16.4 | 62.3 |
| WEO50 | 11.3 | 4.0 | 9.9 | 1.9 | 24.8 | 59.3 |
| CEO10 | 11.3 | 5.57 | 16.09 | 3.31 | 13.1 | 61.9 |
| CEO15 | 10.9 | 5.6 | 15.3 | 3.2 | 15.6 | 60.3 |
| CEO25 | 10.2 | 5.6 | 13.8 | 2.9 | 20.8 | 56.9 |
| CEO50 | 8.2 | 5.6 | 10.0 | 2.3 | 33.6 | 48.5 |
| Feature | N | df | H | p-Value |
|---|---|---|---|---|
| Survival | 9 | 15 | 85.50127 | ≤0.001 |
| Individual weight | 9 | 15 | 123.0147 | ≤0.001 |
| Total biomass | 9 | 15 | 117.5094 | ≤0.001 |
| SGR | 9 | 15 | 82.63662 | ≤0.001 |
| FCR | 9 | 15 | 62.60062 | ≤0.001 |
| Feature | N | df | F | p-Value |
|---|---|---|---|---|
| Survival | 4 | 3 | 2.562 | 0.128 |
| Final individual weight | 4 | 3 | 5.06 | 0.004 |
| Total biomass | 4 | 3 | 15.78 | 0.001 |
| SGR | 4 | 3 | 5.0 | 0.004 |
| Feature | N | df | H | p-Value |
|---|---|---|---|---|
| Crude protein | 6 | 3 | 18.99 | <0.001 |
| Crude fat | 6 | 3 | 21.60 | <0.001 |
| Crude ash | 6 | 3 | 16.09 | 0.001 |
| Crude fibre | 6 | 3 | 7.41 | 0.059 |
| Nitrogen-free extract | 6 | 3 | 19.42 | <0.001 |
| Feature | N | df | F | p-Value |
|---|---|---|---|---|
| Protein digestibility | 3 | 3 | 3.66 | 0.044 |
| Relative digestibility | 3 | 3 | 3.85 | 0.038 |
| Amino acid composition: | ||||
| Thr | 4 | 3 | 6.90 | 0.006 |
| Cys | 4 | 3 | 16.63 | <0.001 |
| Met | 4 | 3 | 71.60 | <0.001 |
| Tyr | 4 | 3 | 3.96 | 0.036 |
| Phe | 4 | 3 | 13.50 | <0.001 |
| His | 4 | 3 | 3.51 | 0.049 |
| Arg | 4 | 3 | 4.65 | 0.022 |
| Trp | 4 | 3 | 10.47 | 0.001 |
| Fatty acid composition: | ||||
| C12:0 | 4 | 3 | 14.72 | 0.001 |
| C14:0 | 4 | 3 | 26.48 | <0.001 |
| C16:1 | 4 | 3 | 623.7 | <0.001 |
| C17:0 | 4 | 3 | 111.5 | <0.001 |
| C18:0 | 4 | 3 | 25.72 | <0.001 |
| C18:1 | 4 | 3 | 148.9 | <0.001 |
| C18:2 | 4 | 3 | 109.94 | <0.001 |
| C18:3 | 4 | 3 | 25.68 | <0.001 |
| MUFA | 4 | 3 | 167.4 | <0.001 |
| PUFA | 4 | 3 | 99.81 | <0.001 |
| Feed/Fatty Acid | C 12:0 | C 14:0 | C 15:0 | C 16:0 | C 16:1 | C 17:0 | C 17:1 | C 18:0 | C 18:1 | C 18:2 | C 18:3 | C 20:0 | C 20:1 | SFA | MUFA | PUFA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WB | 0.16 c | 2.24 b | 0.13 a | 17.7 | 1.17 d | 0.11 a | 0.10 | 2.47 a | 39.6 d | 34.9 a | 1.29 a | 0.08 | 0.10 | 22.9 | 40.9 d | 36.1 a |
| WEO10 | 0.20 a | 2.99 a | 0.10 b | 17.8 | 1.61 a | 0.06 b | 0.08 | 1.94 c | 45.3 a | 28.8 c | 0.98 b | 0.07 | 0.08 | 23.2 | 47.1 a | 29.8 c |
| CEO10 | 0.17 abc | 2.54 b | 0.13 a | 18.1 | 1.39 c | 0.11 a | 0.05 | 2.22 b | 42.2 c | 31.8 b | 1.09 b | 0.06 | 0.08 | 23.4 | 43.7 c | 32.9 b |
| WES10 | 0.18 ab | 2.95 a | 0.11 b | 18.3 | 1.50 b | 0.08 c | 0.08 | 2.21 b | 43.8 b | 29.6 c | 0.99 b | 0.07 | 0.09 | 24.0 | 45.4 b | 30.6 c |
| Mean | 0.18 | 2.68 | 0.12 | 18.0 | 1.42 | 0.09 | 0.08 | 2.21 | 42.7 | 31.3 | 1.09 | 0.07 | 0.09 | 23.4 | 44.3 | 32.4 |
| Diet/Amino Acid | Asp | Thr | Ser | Glu | Pro | Gly | Ala | Cys | Val | Met | Ile | Leu | Tyr | Phe | His | Lys | Arg | Trp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WB | 7.03 | 2.81 b | 3.58 | 7.31 | 4.93 | 3.97 | 6.02 | 2.54 b | 4.65 | 0.42 a | 3.25 | 7.93 | 5.89 a | 2.31a | 3.41 | 4.03 a | 3.99 a | 0.59 a |
| WEO10 | 7.15 | 2.95 ab | 3.74 | 7.60 | 5.14 | 4.11 | 6.06 | 2.89 a | 4.77 | 0.37 b | 3.24 | 7.94 | 5.59 ab | 2.04 b | 3.20 | 3.80 ab | 3.69 ab | 0.49 b |
| CEO10 | 7.27 | 3.07 a | 3.87 | 7.46 | 5.21 | 4.19 | 5.99 | 2.54 b | 4.92 | 0.40 a | 3.21 | 7.74 | 5.48 b | 2.14 b | 3.21 | 3.72 b | 3.57 b | 0.46 b |
| WES10 | 7.17 | 2.92 ab | 3.81 | 7.48 | 5.06 | 4.05 | 6.34 | 2.53 b | 4.57 | 0.32 c | 3.30 | 7.85 | 5.65 ab | 2.06 b | 3.24 | 3.90 ab | 3.73 ab | 0.41 b |
| Mean | 7.15 | 2.93 | 3.75 | 7.46 | 5.08 | 4.08 | 6.10 | 2.63 | 4.73 | 0.38 | 3.25 | 7.87 | 5.65 | 2.14 | 3.26 | 3.86 | 3.74 | 0.49 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyżaniak, M.; Kosewska, O.; Białoskórski, P.; Warmiński, K.; Stolarski, M.J.; Graban, Ł.; Lajszner, W.; Sikorski, Ł.; Wilke, A.; Eisele, T. Impact of Enzymatically Treated Substrate on Yellow Mealworm Development and Composition. Insects 2025, 16, 842. https://doi.org/10.3390/insects16080842
Krzyżaniak M, Kosewska O, Białoskórski P, Warmiński K, Stolarski MJ, Graban Ł, Lajszner W, Sikorski Ł, Wilke A, Eisele T. Impact of Enzymatically Treated Substrate on Yellow Mealworm Development and Composition. Insects. 2025; 16(8):842. https://doi.org/10.3390/insects16080842
Chicago/Turabian StyleKrzyżaniak, Michał, Olga Kosewska, Przemysław Białoskórski, Kazimierz Warmiński, Mariusz J. Stolarski, Łukasz Graban, Waldemar Lajszner, Łukasz Sikorski, Andreas Wilke, and Thomas Eisele. 2025. "Impact of Enzymatically Treated Substrate on Yellow Mealworm Development and Composition" Insects 16, no. 8: 842. https://doi.org/10.3390/insects16080842
APA StyleKrzyżaniak, M., Kosewska, O., Białoskórski, P., Warmiński, K., Stolarski, M. J., Graban, Ł., Lajszner, W., Sikorski, Ł., Wilke, A., & Eisele, T. (2025). Impact of Enzymatically Treated Substrate on Yellow Mealworm Development and Composition. Insects, 16(8), 842. https://doi.org/10.3390/insects16080842








