Harnessing Insects as Novel Food Ingredients: Nutritional, Functional, and Processing Perspectives
Simple Summary
Abstract
1. Introduction
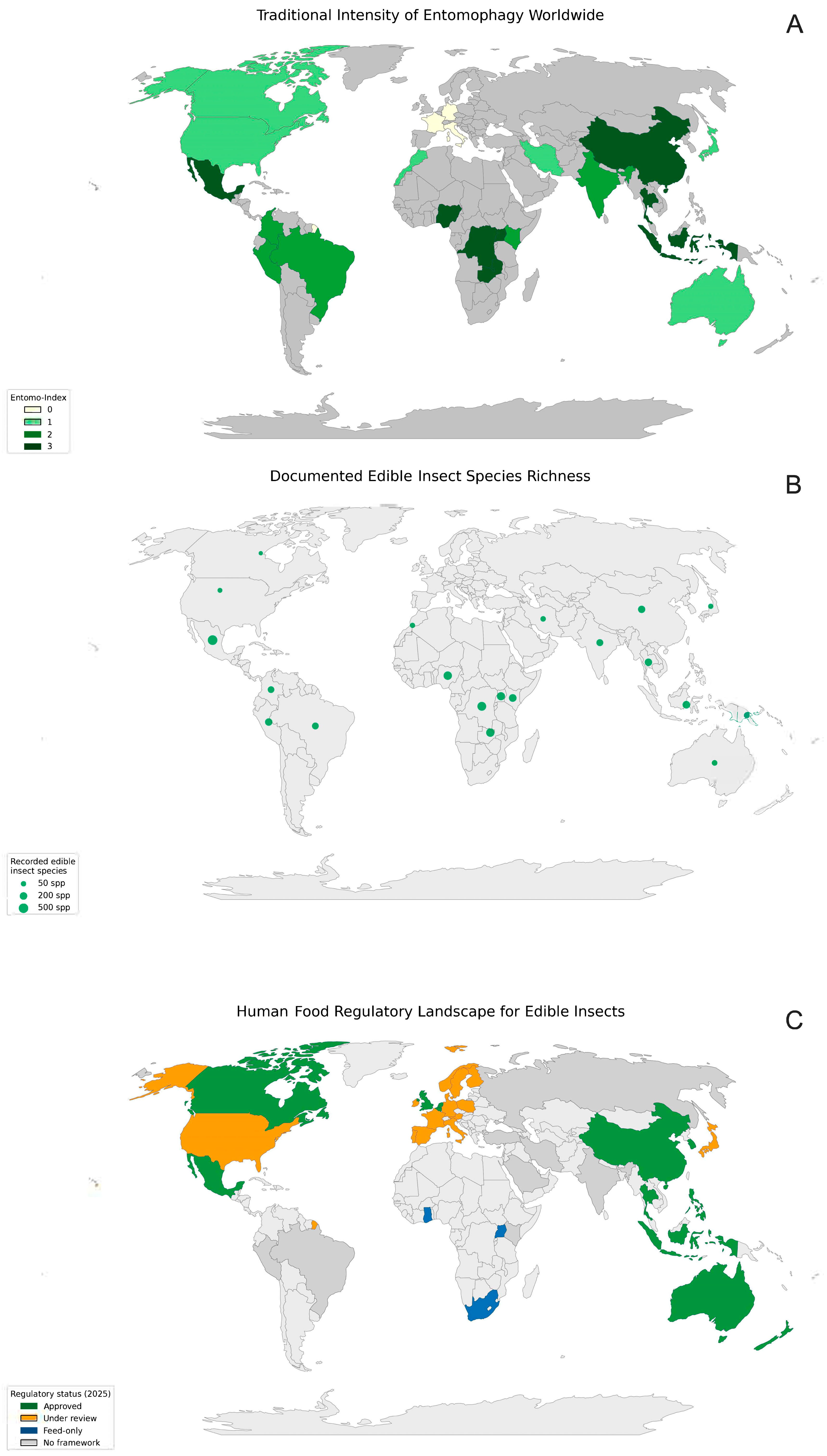
1.1. Global Drivers of Entomophagy
1.2. Overview of Key Edible Insect Species Used in Food Systems
1.3. The Regulatory Status of Edible Insects in Europe and the United States
1.4. Veterinary Oversight of Insect Farms
2. Nutritional Composition and Ingredient Forms of Edible Insects
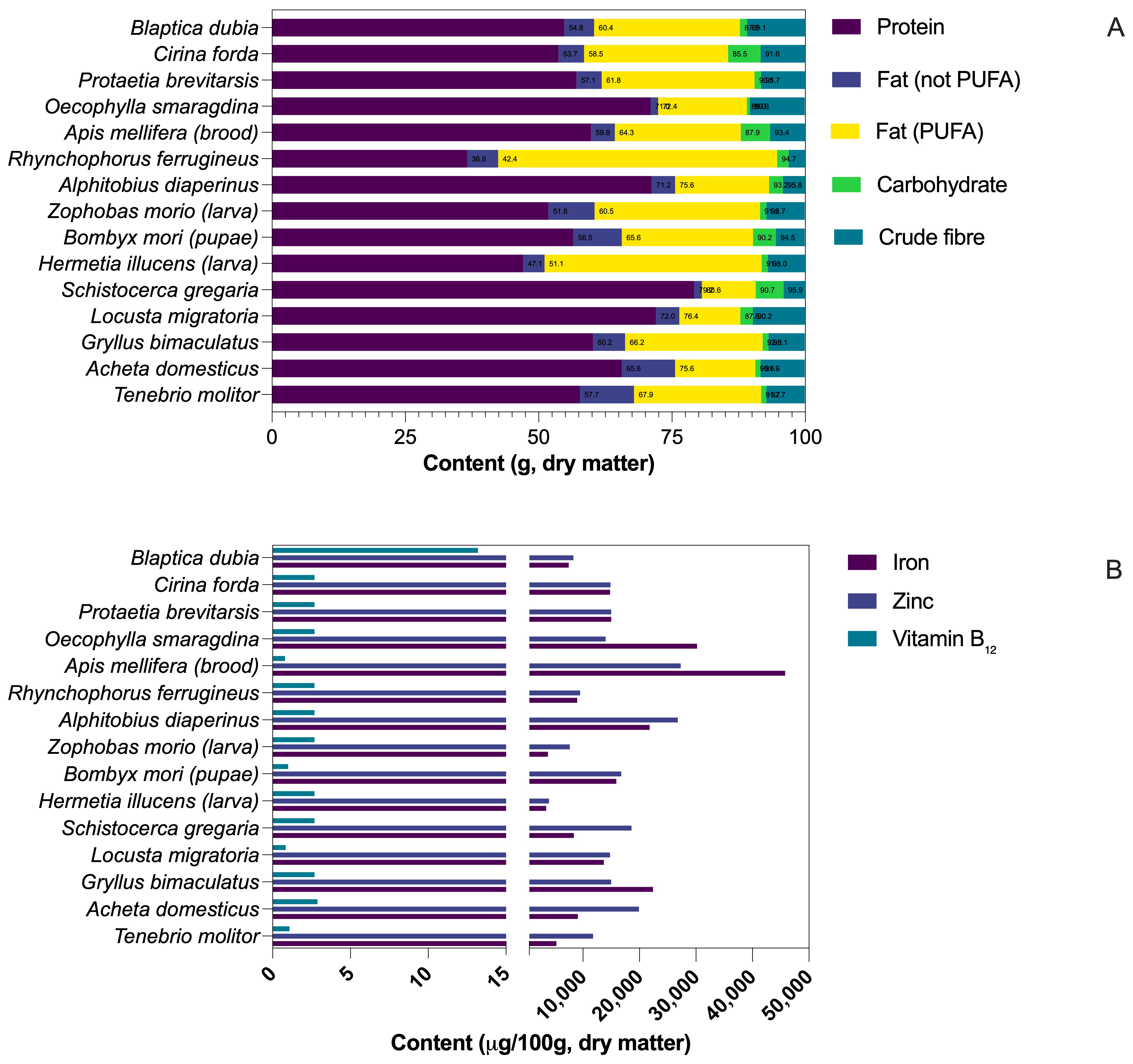
2.1. Protein Content, Quality, and Derived Ingredients
2.2. Lipid Fractions and Extracted Oils
2.3. Carbohydrates and Chitinous Fiber
2.4. Micronutrients, Pigments, and Bioactive Compounds
2.5. Safety and Quality Considerations
3. Processing Technologies to Convert Edible Insects into Food Ingredients
3.1. Raw Material Preparation
3.2. Protein Extraction and Fractionation
3.3. Lipid Extraction and Defatting Methods
3.4. Emerging Non-Thermal and Advanced Thermal Technologies
4. Functional Properties and Roles of Insect-Derived Ingredients in Foods
4.1. Functional Properties
4.1.1. Water- and Oil-Holding Capacity
4.1.2. Emulsification and Foaming Behavior
4.1.3. Gelation, Viscosity, and Texturizing Potential
4.1.4. Antioxidants and Antimicrobial
4.2. Functional Roles
4.2.1. Flavor and Aroma
4.2.2. Texture and Structure
4.2.3. Appearance and Color
5. Applications in Food Formulation and Product Development
5.1. Bakery Products (Breads, Biscuits, and Snacks)
5.2. Pasta and Noodles
5.3. Meat Products and Extenders
5.4. Dairy Analogs and Beverages
6. Sustainability and Life-Cycle Considerations
7. Future Directions and Research Gaps
7.1. Nutritional Quality and Techno-Functional Optimization
7.2. Flavor and Consumer Palatability
7.3. Safety, Technology, and Market Translation
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| AA | amino acid |
| BSF | black soldier fly |
| CFU | colony-forming unit |
| CP | crude protein |
| DM | dry matter |
| EAA | essential amino acid |
| FAO | Food and Agriculture Organization of the United Nations |
| GHG | greenhouse gas |
| GRAS | generally recognized as safe |
| HPLC | high-performance liquid chromatography |
| IC50 | half-maximal inhibitory concentration |
| LCA | life-cycle assessment |
| OHC | oil-holding capacity |
| PBM | population balance modeling |
| PUFA | polyunsaturated fatty acid |
| RSM | response surface methodology |
| SD | standard deviation |
| TEA | techno-economic analysis |
| WHC | water-holding capacity |
References
- Lisboa, H.M.; Pasquali, M.B.; dos Anjos, A.I.; Sarinho, A.M.; de Melo, E.D.; Andrade, R.; Batista, L.; Lima, J.; Diniz, Y.; Barros, A. Innovative and Sustainable Food Preservation Techniques: Enhancing Food Quality, Safety, and Environmental Sustainability. Sustainability 2024, 16, 8223. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Nascimento, A.; Arruda, A.; Sarinho, A.; Lima, J.; Batista, L.; Dantas, M.F.; Andrade, R. Unlocking the Potential of Insect-Based Proteins: Sustainable Solutions for Global Food Security and Nutrition. Foods 2024, 13, 1846. [Google Scholar] [CrossRef]
- Department of Economic and Social Affairs, United Nations. World Population Prospects 2022: Summary of Results; United Nations: New York, NY, USA, 2022; pp. 1–24. ISBN 978-92-1-148373-4. [Google Scholar]
- Lange, K.W.; Nakamura, Y. Edible insects as future food: Chances and challenges. J. Future Foods 2021, 1, 38–46. [Google Scholar] [CrossRef]
- Skrivervik, E. Insects’ contribution to the bioeconomy and the reduction of food waste. Heliyon 2020, 6, e03934. [Google Scholar] [CrossRef]
- Doi, H.; Gałęcki, R.; Mulia, R.N. The merits of entomophagy in the post COVID-19 world. Trends Food Sci. Technol. 2021, 110, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Suleria, H.A.R.; Rauf, A. Edible insects as innovative foods: Nutritional and functional assessments. Trends Food Sci. Technol. 2019, 86, 352–359. [Google Scholar] [CrossRef]
- Puzari, M. Prospects of entomophagy. Int. J. Trop. Insect Sci. 2021, 41, 1989–1992. [Google Scholar] [CrossRef]
- Olivadese, M.; Dindo, M.L. Edible Insects: A Historical and Cultural Perspective on Entomophagy with a Focus on Western Societies. Insects 2023, 14, 690. [Google Scholar] [CrossRef]
- Huis, A.V.; Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Stull, V.J.; Wamulume, M.; Mwalukanga, M.I.; Banda, A.; Bergmans, R.S.; Bell, M.M. “We like insects here”: Entomophagy and society in a Zambian village. Agric. Hum. Values 2018, 35, 867–883. [Google Scholar] [CrossRef]
- Jagadeesan, P.; Siddiqui, A.; Muzahem, S.; Al Shareef, A.J.M. Would edible insects become part of our gastronomic quest? Edelweiss Appl. Sci. Technol. 2024, 8, 737–745. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EU) 2015/2283 of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union 2015, L 327, 1–22.
- Sun-Waterhouse, D.; Waterhouse, G.I.N.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Gao, J.; Dong, Y. Transforming insect biomass into consumer wellness foods: A review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef]
- Omuse, E.R.; Tonnang, H.E.Z.; Yusuf, A.A.; Machekano, H.; Egonyu, J.P.; Kimathi, E.; Mohamed, S.F.; Kassie, M.; Subramanian, S.; Onditi, J.; et al. The global atlas of edible insects: Analysis of diversity and commonality contributing to food systems and sustainability. Sci. Rep. 2024, 14, 5045. [Google Scholar] [CrossRef]
- Siddiqui, S.; Aidoo, O.; Ghisletta, M.; Osei-Owusu, J.; Saraswati, Y.; Bhardwaj, K.; Khalid, W.; Fernando, I.; Golik, A.; Nagdalian, A. African edible insects as human food–a comprehensive review. J. Insects Food Feed. 2023, 10, 51–78. [Google Scholar] [CrossRef]
- Baiano, A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Conway, A.; Jaiswal, S.; Jaiswal, A.K. The Potential of Edible Insects as a Safe, Palatable, and Sustainable Food Source in the European Union. Foods 2024, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef]
- Xie, B.; Zhu, Y.; Chu, X.; Pokharel, S.S.; Qian, L.; Chen, F. Research Progress and Production Status of Edible Insects as Food in China. Foods 2024, 13, 1986. [Google Scholar] [CrossRef] [PubMed]
- Imathiu, S. Benefits and food safety concerns associated with consumption of edible insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Malla, N.; Roos, N. Are insects a good source of protein for humans? J. Insects Food Feed 2023, 9, 841–844. [Google Scholar] [CrossRef]
- Kozlu, A.; Ngasakul, N.; Klojdová, I.; Baigts-Allende, D.K. Edible insect-processing techniques: A strategy to develop nutritional food products and novelty food analogs. Eur. Food Res. Technol. 2024, 250, 1253–1267. [Google Scholar] [CrossRef]
- Halpern, B.S.; Frazier, M.; Verstaen, J.; Rayner, P.-E.; Clawson, G.; Blanchard, J.L.; Cottrell, R.S.; Froehlich, H.E.; Gephart, J.A.; Jacobsen, N.S. The environmental footprint of global food production. Nat. Sustain. 2022, 5, 1027–1039. [Google Scholar] [CrossRef]
- Oonincx, D.G.; De Boer, I.J. Environmental impact of the production of mealworms as a protein source for humans–a life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed]
- Diener, S.; Studt Solano, N.M.; Roa Gutiérrez, F.; Zurbrügg, C.; Tockner, K. Biological Treatment of Municipal Organic Waste using Black Soldier Fly Larvae. Waste Biomass Valorization 2011, 2, 357–363. [Google Scholar] [CrossRef]
- Parodi, A.; Gerrits, W.J.J.; Van Loon, J.J.A.; De Boer, I.J.M.; Aarnink, A.J.A.; Van Zanten, H.H.E. Black soldier fly reared on pig manure: Bioconversion efficiencies, nutrients in the residual material, greenhouse gas and ammonia emissions. Waste Manag. 2021, 126, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Hlongwane, Z.T.; Slotow, R.; Munyai, T.C. The Role of Edible Insects in Rural Livelihoods, and Identified Challenges in Vhembe District, Limpopo, South Africa. Resources 2021, 10, 123. [Google Scholar] [CrossRef]
- Van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Marimuthu, S.B.; Meijer, N. Regulations on insects as food and feed: A global comparison. J. Insects Food Feed 2021, 7, 849–856. [Google Scholar] [CrossRef]
- Commission, E. Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). Off. J. Eur. Union 2016, 59, 1–208. [Google Scholar]
- Commision, E. Regulation (EU) 2019/4 of the European Parliament and of the Council of 11 December 2018 on the Manufacture, Placing on the Market and Use of Medicated Feed, Amending Regulation (EC) No 183/2005 of the European Parliament and of the Council and Repealing. Off. J. Eur. Union 2018, 62. [Google Scholar]
- Union, E. Commission Implementing Regulation (EU) 2023/5 of 3 January 2023 Authorising the Placing on the Market of Acheta Domesticus (House Cricket) Partially Defatted Powder as a Novel Food and Amending Implementing Regulation (EU) 2017/2470 (Text with EEA Relevance), Document 32023R0005. Off. J. Eur. Union 2023, 50, 9–14. [Google Scholar]
- EFSA Panel on Animal Health and Welfare (AHAW); More, S.; Bicout, D.; Bøtner, A.; Butterworth, A.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Schmidt, C.G.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Bluetongue. Efsa J. 2017, 15, e04957. [Google Scholar] [PubMed]
- Elissen, H.; van der Weide, R.; Gollenbeek, L.; Wevers, K. Legislative Issues Surrounding the Application of Insect Larvae and Compost Worms for Biobased Valorization of Manure, Digestate and Other Organics; Stichting Wageningen Research, Wageningen Plant Research: Wageningen, The Netherlands, 2023. [Google Scholar]
- Oliveira, L.A.; Pereira, S.M.S.; Dias, K.A.; Paes, S.d.S.; Grancieri, M.; Jimenez, L.G.S.; de Carvalho, C.W.P.; de Oliveira, E.E.; Martino, H.S.D.; Della Lucia, C.M. Nutritional content, amino acid profile, and protein properties of edible insects (Tenebrio molitor and Gryllus assimilis) powders at different stages of development. J. Food Compos. Anal. 2024, 125, 105804. [Google Scholar] [CrossRef]
- Kröncke, N.; Benning, R. Influence of Dietary Protein Content on the Nutritional Composition of Mealworm Larvae (Tenebrio molitor L.). Insects 2023, 14, 261. [Google Scholar] [CrossRef]
- Perez-Santaescolastica, C.; de Pril, I.; van de Voorde, I.; Fraeye, I. Fatty Acid and Amino Acid Profiles of Seven Edible Insects: Focus on Lipid Class Composition and Protein Conversion Factors. Foods 2023, 12, 4090. [Google Scholar] [CrossRef]
- Köhler, R.; Kariuki, L.; Lambert, C.; Biesalski, H.K. Protein, amino acid and mineral composition of some edible insects from Thailand. J. Asia-Pac. Entomol. 2019, 22, 372–378. [Google Scholar] [CrossRef]
- Oonincx, D.; Finke, M. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed 2021, 7, 639–660. [Google Scholar] [CrossRef]
- Nsevolo Miankeba, P.; Taofic, A.; Kiatoko, N.; Mutiaka, K.; Francis, F.; Caparros Megido, R. Protein Content and Amino Acid Profiles of Selected Edible Insect Species from the Democratic Republic of Congo Relevant for Transboundary Trade across Africa. Insects 2022, 13, 994. [Google Scholar] [CrossRef]
- Hammer, L.; Moretti, D.; Abbühl-Eng, L.; Kandiah, P.; Hilaj, N.; Portmann, R.; Egger, L. Mealworm larvae (Tenebrio molitor) and crickets (Acheta domesticus) show high total protein in vitro digestibility and can provide good-to-excellent protein quality as determined by in vitro DIAAS. Front. Nutr. 2023, 10, 1150581. [Google Scholar] [CrossRef]
- Malla, N.; Nørgaard, J.V.; Roos, N. Protein quality of edible insects in the view of current assessment methods. Anim. Front. 2023, 13, 50–63. [Google Scholar] [CrossRef]
- Lampová, B.; Kopecká, A.; Šmíd, P.; Kulma, M.; Kurečka, M.; Ogrinc, N.; Heath, D.; Kouřimská, L.; Doskočil, I. Evaluating protein quality in edible insects: A comparative analysis of house cricket, yellow mealworm, and migratory locust using DIAAS methodologies. LWT 2024, 213, 117062. [Google Scholar] [CrossRef]
- Wu, X.; He, K.; Velickovic, T.C.; Liu, Z. Nutritional, functional, and allergenic properties of silkworm pupae. Food Sci. Nutr. 2021, 9, 4655–4665. [Google Scholar] [CrossRef]
- Nachtigall, L.; Grune, T.; Weber, D. Proteins and Amino Acids from Edible Insects for the Human Diet—A Narrative Review Considering Environmental Sustainability and Regulatory Challenges. Nutrients 2025, 17, 1245. [Google Scholar] [CrossRef]
- Oibiokpa, F.I.; Akanya, H.O.; Jigam, A.A.; Saidu, A.N.; Egwim, E.C. Protein quality of four indigenous edible insect species in Nigeria. Food Sci. Hum. Wellness 2018, 7, 175–183. [Google Scholar] [CrossRef]
- Oriolowo, O.B.; Abubakar, D.; Bidda, R.D.; Masa’udu, S. Nutritional comparison of the pallid emperor moth, Cirina Forda and the atlantic mackerel, scombrus scomber. Acta Entomol. Zool. 2021, 2, 24–31. [Google Scholar] [CrossRef]
- Martins da Silva, R.; Köhler, A.; de Cássia de Souza Schneider, R.; Prado de Vargas, D.; Lúcia Köhler, A.; da Costa e Silva, D.; Soares, J. Proximate and fatty acid profile analysis of Tenebrio molitor and Zophobas morio using different killing methods. Food Chem. 2024, 445, 138719. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Gutierrez-Docio, A.; Navarro del Hierro, J.; Reglero, G.; Martin, D. Extracts from the edible insects Acheta domesticus and Tenebrio molitor with improved fatty acid profile due to ultrasound assisted or pressurized liquid extraction. Food Chem. 2020, 314, 126200. [Google Scholar] [CrossRef]
- Mancini, S.; Mattioli, S.; Paolucci, S.; Fratini, F.; Dal Bosco, A.; Tuccinardi, T.; Paci, G. Effect of Cooking Techniques on the in vitro Protein Digestibility, Fatty Acid Profile, and Oxidative Status of Mealworms (Tenebrio molitor). Front. Vet. Sci. 2021, 8, 675572. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, J.; Ghosh, S.; Megu, K.; Jung, C.; Meyer-Rochow, V.B. Nutritional and anti-nutritional composition of Oecophylla smaragdina (Hymenoptera: Formicidae) and Odontotermes sp. (Isoptera: Termitidae): Two preferred edible insects of Arunachal Pradesh, India. J. Asia-Pac. Entomol. 2016, 19, 711–720. [Google Scholar] [CrossRef]
- Devi, M.R.; Ummalyma, S.B.; Brockmann, A.; Raina, V.; Rajashekar, Y. Nutritional properties of giant water bug, Lethocerus indicus a traditional edible insect species of North-East India. Bioengineered 2023, 14, 2252669. [Google Scholar] [CrossRef]
- Kavle, R.R.; Carne, A.; Bekhit, A.E.-D.A.; Kebede, B.; Agyei, D. Proximate composition and lipid nutritional indices of larvae and pupae of the edible Huhu beetle (Prionoplus reticularis) endemic to New Zealand. J. Food Compos. Anal. 2022, 110, 104578. [Google Scholar] [CrossRef]
- Kibet, S.; Mudalungu, C.M.; Ochieng, B.O.; Mokaya, H.O.; Kimani, N.M.; Tanga, C.M. Nutritional composition of edible wood borer beetle larvae in Kenya. PLoS ONE 2024, 19, e0304944. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-F.; Siriamornpun, S.; Li, D. Polyunsaturated Fatty Acid Content of Edible Insects in Thailand. J. Food Lipids 2006, 13, 277–285. [Google Scholar] [CrossRef]
- Hasnan, F.F.B.; Feng, Y.; Sun, T.; Parraga, K.; Schwarz, M.; Zarei, M. Insects as Valuable Sources of Protein and Peptides: Production, Functional Properties, and Challenges. Foods 2023, 12, 4243. [Google Scholar] [CrossRef]
- Zaalberg, R.M.; Nielsen, H.M.; Noer, N.K.; Schou, T.M.; Jensen, K.; Thormose, S.; Kargo, M.; Slagboom, M. A bio-economic model for estimating economic values of important production traits in the black soldier fly (Hermetia illucens). J. Insects Food Feed 2024, 10, 1411–1421. [Google Scholar] [CrossRef]
- Nawoya, S.; Geissmann, Q.; Karstoft, H.; Bjerge, K.; Akol, R.; Katumba, A.; Mwikirize, C.; Gebreyesus, G. Prediction of black soldier fly larval sex and morphological traits using computer vision and deep learning. Smart Agric. Technol. 2025, 11, 100953. [Google Scholar] [CrossRef]
- Suryati, T.; Julaeha, E.; Farabi, K.; Ambarsari, H.; Hidayat, A.T. Lauric Acid from the Black Soldier Fly (Hermetia illucens) and Its Potential Applications. Sustainability 2023, 15, 10383. [Google Scholar] [CrossRef]
- Matthäus, B.; Piofczyk, T.; Katz, H.; Pudel, F. Renewable Resources from Insects: Exploitation, Properties, and Refining of Fat Obtained by Cold-Pressing from Hermetia illucens (Black Soldier Fly) Larvae. Eur. J. Lipid Sci. Technol. 2019, 121, 1800376. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Son, Y.J.; Kim, S.H.; Yun, E.Y.; Kang, H.J.; Hwang, I.K. Physicochemical properties and oxidative stabilities of mealworm (Tenebrio molitor) oils under different roasting conditions. Food Sci. Biotechnol. 2016, 25, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, R.; Nowacka, M.; Bryś, J.; Rybak, K.; Szulc, K. Quality assessment of yellow mealworm (Tenebrio molitor L.) powders processed by pulsed electric field and convective drying. Sci. Rep. 2024, 14, 27792. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zaini, N.S.; Lim, E.J.; Ahmad, N.H.; Gengatharan, A.; Wan-Mohtar, W.; Abd Rahim, M.H. The Review of Cooking, Drying, and Green Extraction Methods on General Nutritional Properties of Mealworms and Locusts. Food Bioproc. Tech. 2023, 16, 1904–1918. [Google Scholar] [CrossRef]
- Silva, H.A.; Paiva, E.G.; Lisboa, H.M.; Duarte, E.; Cavalcanti-Mata, M.; Gusmão, T.; de Gusmão, R. Role of chitosan and transglutaminase on the elaboration of gluten-free bread. J. Food Sci. Technol. 2020, 57, 1877–1886. [Google Scholar] [CrossRef]
- Ojha, S.; Bekhit, A.E.-D.; Grune, T.; Schlüter, O.K. Bioavailability of nutrients from edible insects. Curr. Opin. Food Sci. 2021, 41, 240–248. [Google Scholar] [CrossRef]
- Marei, N.H.; El-Samie, E.A.; Salah, T.; Saad, G.R.; Elwahy, A.H. Isolation and characterization of chitosan from different local insects in Egypt. Int. J. Biol. Macromol. 2016, 82, 871–877. [Google Scholar] [CrossRef]
- Lima, D.B.; Almeida, R.D.; Pasquali, M.; Borges, S.P.; Fook, M.L.; Lisboa, H.M. Physical characterization and modeling of chitosan/peg blends for injectable scaffolds. Carbohydr. Polym. 2018, 189, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.B.; de Souza, M.A.A.; de Lima, G.G.; Ferreira Souto, E.P.; Oliveira, H.M.L.; Fook, M.V.L.; de Sá, M.J.C. Injectable bone substitute based on chitosan with polyethylene glycol polymeric solution and biphasic calcium phosphate microspheres. Carbohydr. Polym. 2020, 245, 116575. [Google Scholar] [CrossRef] [PubMed]
- Psarianos, M.; Aghababaei, F.; Schlüter, O.K. Bioactive compounds in edible insects: Aspects of cultivation, processing and nutrition. Food Res. Int. 2025, 203, 115802. [Google Scholar] [CrossRef]
- Ordoñez-Araque, R.; Quishpillo-Miranda, N.; Ramos-Guerrero, L. Edible Insects for Humans and Animals: Nutritional Composition and an Option for Mitigating Environmental Damage. Insects 2022, 13, 944. [Google Scholar] [CrossRef] [PubMed]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Borges, M.; Tejera, R.; Díaz, L.; Esparza, P.; Ibáñez, E. Natural dyes extraction from cochineal (Dactylopius coccus). New extraction methods. Food Chem. 2012, 132, 1855–1860. [Google Scholar] [CrossRef]
- Ferreyra-Suarez, D.; Paredes-Vargas, L.; Jafari, S.M.; García-Depraect, O.; Castro-Muñoz, R. Extraction pathways and purification strategies towards carminic acid as natural-based food colorant: A comprehensive review. Adv. Colloid Interface Sci. 2024, 323, 103052. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ortiz, L.M.; Hincapié, C.A.; Hincapié-Llanos, G.A.; Osorio, M. Potential uses of silkworm pupae (Bombyx mori L.) in food, feed, and other industries: A systematic review. Front. Insect Sci. 2024, 4, 1445636. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Ma, Z.; Mirón-Mérida, V.; Mondor, M.; Hernández-Álvarez, A.J. Chapter 5—Insect processing technologies. In Insects as Food and Food Ingredients; García-Vaquero, M., Álvarez García, C., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 67–92. [Google Scholar]
- Cavalcanti, A.; Furtado, G.T.; Lisboa, H.M.; Fook, M.V.L. Morphological characterization of chitin extraction. J. Chitin Chitosan Sci. 2013, 1, 157–160. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Wynants, E.; Frooninckx, L.; Van Miert, S.; Geeraerd, A.; Claes, J.; Van Campenhout, L. Risks related to the presence of Salmonella sp. during rearing of mealworms (Tenebrio molitor) for food or feed: Survival in the substrate and transmission to the larvae. Food Control 2019, 100, 227–234. [Google Scholar] [CrossRef]
- Martins, G.M.V.; de Sousa, S.; Duarte, M.E.M.; Cavalcanti-Mata, M.E.R.M.; Oliveira, H.M.L. Modeling the combinatory effects of parboiling and cooking on red paddy rice (Oryza sativa L.) properties. LWT 2021, 147, 111607. [Google Scholar] [CrossRef]
- Liang, Z.; Zhu, Y.; Leonard, W.; Fang, Z. Recent advances in edible insect processing technologies. Food Res. Int. 2024, 182, 114137. [Google Scholar] [CrossRef]
- Nascimento, A.; Lúcio, A.; Nery, A.; Andrade, R.; Sarinho, A.M.; Lima, J.; Batista, L.; Lisboa, H.M. Engineering effects of hydrocolloids on drying kinetics, powder characteristics, and antioxidant preservation in grape powder. J. Food Eng. 2025, 387, 112319. [Google Scholar] [CrossRef]
- Kröncke, N.; Grebenteuch, S.; Keil, C.; Demtröder, S.; Kroh, L.; Thünemann, A.F.; Benning, R.; Haase, H. Effect of Different Drying Methods on Nutrient Quality of the Yellow Mealworm (Tenebrio molitor L.). Insects 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.-J.; Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Lenaerts, S.; Callens, A.; Van Campenhout, L. Effect of blanching followed by refrigerated storage or industrial microwave drying on the microbial load of yellow mealworm larvae (Tenebrio molitor). Food Control 2017, 71, 311–314. [Google Scholar] [CrossRef]
- Keil, C.; Grebenteuch, S.; Kröncke, N.; Kulow, F.; Pfeif, S.; Kanzler, C.; Rohn, S.; Boeck, G.; Benning, R.; Haase, H. Systematic studies on the antioxidant capacity and volatile compound profile of yellow mealworm larvae (T. molitor L.) under different drying regimes. Insects 2022, 13, 166. [Google Scholar] [CrossRef]
- Kröncke, N.; Böschen, V.; Woyzichovski, J.; Demtröder, S.; Benning, R. Comparison of suitable drying processes for mealworms (Tenebrio molitor). Innov. Food Sci. Emerg. Technol. 2018, 50, 20–25. [Google Scholar] [CrossRef]
- Fombong, F.T.; Van Der Borght, M.; Vanden Broeck, J. Influence of freeze-drying and oven-drying post blanching on the nutrient composition of the edible insect Ruspolia differens. Insects 2017, 8, 102. [Google Scholar] [CrossRef]
- Lenaerts, S.; Van Der Borght, M.; Callens, A.; Van Campenhout, L. Suitability of microwave drying for mealworms (Tenebrio molitor) as alternative to freeze drying: Impact on nutritional quality and colour. Food Chem. 2018, 254, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Liceaga, A.M. Processing insects for use in the food and feed industry. Curr. Opin. Insect Sci. 2021, 48, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Sindermann, D.; Heidhues, J.; Kirchner, S.; Stadermann, N.; Kühl, A. Industrial processing technologies for insect larvae. J. Insects Food Feed 2021, 7, 857–876. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhang, L.; Zhou, S.; You, L.; Song, J. Application of edible insects to food products: A review on the functionality, bioactivity and digestibility of insect proteins under high-pressure/ultrasound processing. Food Chem. 2025, 468, 142469. [Google Scholar] [CrossRef]
- Mishyna, M.; Martinez, J.-J.I.; Chen, J.; Benjamin, O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera). Food Res. Int. 2019, 116, 697–706. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.-K.; Jeong, C.H.; Yong, H.I.; Cha, J.Y.; Kim, B.-K.; Choi, Y.-S. Biological activity and processing technologies of edible insects: A review. Food Sci. Biotechnol. 2021, 30, 1003–1023. [Google Scholar] [CrossRef]
- Kee, P.E.; Cheng, Y.-S.; Chang, J.-S.; Yim, H.S.; Tan, J.C.Y.; Lam, S.S.; Lan, J.C.-W.; Ng, H.S.; Khoo, K.S. Insect biorefinery: A circular economy concept for biowaste conversion to value-added products. Environ. Res. 2023, 221, 115284. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; He, Q.; Wang, D. Dynamic Analysis of Major Components in the Different Developmental Stages of Tenebrio molitor. Front. Nutr. 2021, 8, 689746. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of partially defatted house cricket (Acheta domesticus) powder as a novel food pursuant to Regulation (EU) 2015/2283. Efsa J. 2022, 20, e07258. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Sun, Q.; Tan, X.; You, C.; Huang, Y.; Zhou, M. Growth and Fatty Acid Composition of Black Soldier Fly Hermetia illucens (Diptera: Stratiomyidae) Larvae Are Influenced by Dietary Fat Sources and Levels. Animals 2022, 12, 486. [Google Scholar] [CrossRef]
- Clarkson, C.; Mirosa, M.; Birch, J. Potential of Extracted Locusta Migratoria Protein Fractions as Value-Added Ingredients. Insects 2018, 9, 20. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Coscueta, E.; Cunha, L.; Pintado, M. Evaluation of the ultrafiltration method to obtain a high-value protein concentrate from the edible insect Tenebrio molitor. J. Insects Food Feed 2023, 10, 491–504. [Google Scholar] [CrossRef]
- Purschke, B.; Meinlschmidt, P.; Horn, C.; Rieder, O.; Jäger, H. Improvement of techno-functional properties of edible insect protein from migratory locust by enzymatic hydrolysis. Eur. Food Res. Technol. 2018, 244, 999–1013. [Google Scholar] [CrossRef]
- Laroche, M.; Perreault, V.; Marciniak, A.; Gravel, A.; Chamberland, J.; Doyen, A. Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal. Foods 2019, 8, 572. [Google Scholar] [CrossRef]
- Pokorski, P.; Michałowska, D.; Moczkowska-Wyrwisz, M.; Strojny-Cieślak, B.; Custodio-Mendoza, J.A.; Aktaş, H.; Kurek, M.A. Edible insect protein concentrates: Optimized salt-assisted extraction methods evaluation. Food Chem. 2025, 466, 142225. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bibek, B.; and Lamsal, B.P. Protein, lipid, and chitin fractions from insects: Method of extraction, functional properties, and potential applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 6415–6431. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Fröhling, A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Cold atmospheric pressure plasma processing of insect flour from Tenebrio molitor: Impact on microbial load and quality attributes in comparison to dry heat treatment. Innov. Food Sci. Emerg. Technol. 2016, 36, 277–286. [Google Scholar] [CrossRef]
- Sweers, L.J.H.; Lakemond, C.M.M.; Fogliano, V.; Boom, R.M.; Mishyna, M.; Keppler, J.K. Biorefining of liquid insect fractions by microfiltration to increase functionality. J. Food Eng. 2024, 364, 111821. [Google Scholar] [CrossRef]
- Rose, A.; Jaczynski, J.; Matak, K. Extraction of lipids from insect powders using a one-step organic solvent extraction process. Future Foods 2021, 4, 100073. [Google Scholar] [CrossRef]
- Ravi, H.K.; Vian, M.A.; Tao, Y.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F. Alternative solvents for lipid extraction and their effect on protein quality in black soldier fly (Hermetia illucens) larvae. J. Clean. Prod. 2019, 238, 117861. [Google Scholar] [CrossRef]
- Cantero-Bahillo, E.; Del Hierro, J.N.; Hernández, D.M.; Fernández-Felipe, M.T.; Fornari, T.; Martín, D. Supercritical-CO2 for defatting and production of bioactive extracts from black soldier fly (Hermetia illucens) larvae. J. Insects Food Feed 2022, 8, 1441–1454. [Google Scholar] [CrossRef]
- Hurtado-Ribeira, R.; Hernández, D.M.; Villanueva-Bermejo, D.; García-Risco, M.R.; Hernández, M.D.; Vázquez, L.; Fornari, T.; Martin, D. The Interaction of Slaughtering, Drying, and Defatting Methods Differently Affects Oxidative Quality of the Fat from Black Soldier Fly (Hermetia illucens) Larvae. Insects 2023, 14, 368. [Google Scholar] [CrossRef]
- Feng, W.; Xiong, H.; Wang, W.; Duan, X.; Yang, T.; Wu, C.; Yang, F.; Wang, T.; Wang, C. A facile and mild one-pot process for direct extraction of lipids from wet energy insects of black soldier fly larvae. Renew. Energy 2020, 147, 584–593. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.F.; van Boekel, M.A.J.S.; Lakemond, C.M.M. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Feng, W.; Xiong, H.; Wang, W.; Duan, X.; Yang, T.; Wu, C.; Yang, F.; Xiong, J.; Wang, T.; Wang, C. Energy consumption analysis of lipid extraction from black soldier fly biomass. Energy 2019, 185, 1076–1085. [Google Scholar] [CrossRef]
- Khampakool, A.; Soisungwan, S.; You, S.; Park, S.H. Infrared assisted freeze-drying (IRAFD) to produce shelf-stable insect food from Protaetia brevitarsis (white-spotted flower chafer) larva. Food Sci. Anim. Resour. 2020, 40, 813. [Google Scholar] [CrossRef]
- Barutçu Mazı, I.; Mazı, B.G.; Sevgili, H. Infrared drying characteristics of locust (L. migratoria): Physical and techno-functional characterization of locust powder. J. Food Sci. Technol. 2025, 1–13. [Google Scholar] [CrossRef]
- Fonseca, M.T.; Vital, A.C.; Silva, M.B.; Monteiro, S.S.; Nascimento, A.; Trindade, A.P.; Lisboa, H.M.; Pasquali, M.B. Improving the stability of spray-dried probiotic acerola juice: A study on hydrocolloids’ efficacy and process variables. Food Bioprod. Process. 2024, 147, 209–218. [Google Scholar] [CrossRef]
- Ojha, S.; Rossi, G.; Pathak, N.; Durek, J.; Mahajan, P.; Schlüter, O.K. Microbial growth and physicochemical quality changes during modified atmosphere storage of high hydrostatic pressure processed Tenebrio molitor paste. Food Bioprocess Technol. 2024, 17, 1914–1925. [Google Scholar] [CrossRef]
- Kingwascharapong, P.; Chaijan, M.; Karnjanapratum, S. Ultrasound-assisted extraction of protein from Bombay locusts and its impact on functional and antioxidative properties. Sci. Rep. 2021, 11, 17320. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-H.; Xiao, S.; Wang, J.-H.; Wang, B.; Cai, Y.-X.; Hu, W.-F. Comparative study of the effects of ultrasound-assisted alkaline extraction on black soldier fly (Hermetia illucens) larvae protein: Nutritional, structural, and functional properties. Ultrason. Sonochemistry 2023, 101, 106662. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.A.; Queiroz, L.S.; Tavares, G.M.; Feyissa, A.H.; Silva, N.F.N.; Casanova, F. Edible insect proteins: How can they be a driver for food innovation? Curr. Opin. Food Sci. 2024, 58, 101195. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of functional properties of edible insects and protein preparations thereof. LWT 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef]
- Ma, Z.; Mondor, M.; Valencia, F.G.; Hernández-Álvarez, A.J. Current state of insect proteins: Extraction technologies, bioactive peptides and allergenicity of edible insect proteins. Food Funct. 2023, 14, 8129–8156. [Google Scholar] [CrossRef] [PubMed]
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta domesticus) and Field Cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Cortazar-Moya, S.; Mejía-Garibay, B.; López-Malo, A.; Morales-Camacho, J.I. Nutritional composition and techno-functionality of non-defatted and defatted flour of edible insect Arsenura armida. Food Res. Int. 2023, 173, 113445. [Google Scholar] [CrossRef]
- Zielińska, E. Evaluating the Functional Characteristics of Certain Insect Flours (Non-Defatted/Defatted Flour) and Their Protein Preparations. Molecules 2022, 27, 6339. [Google Scholar] [CrossRef]
- Jeong, M.-S.; Lee, S.-D.; Cho, S.-J. Effect of Three Defatting Solvents on the Techno-Functional Properties of an Edible Insect (Gryllus bimaculatus) Protein Concentrate. Molecules 2021, 26, 5307. [Google Scholar] [CrossRef]
- López-Gámez, G.; del Pino-García, R.; López-Bascón, M.A.; Verardo, V. From feed to functionality: Unravelling the nutritional composition and techno-functional properties of insect-based ingredients. Food Res. Int. 2024, 178, 113985. [Google Scholar] [CrossRef]
- Villaseñor, V.M.; Enriquez-Vara, J.N.; Urías-Silva, J.E.; Mojica, L. Edible Insects: Techno-functional Properties Food and Feed Applications and Biological Potential. Food Rev. Int. 2022, 38, 866–892. [Google Scholar] [CrossRef]
- Mannozzi, C.; Foligni, R.; Mozzon, M.; Aquilanti, L.; Cesaro, C.; Isidoro, N.; Osimani, A. Nonthermal technologies affecting techno-functional properties of edible insect-derived proteins, lipids, and chitin: A literature review. Innov. Food Sci. Emerg. Technol. 2023, 88, 103453. [Google Scholar] [CrossRef]
- da Silva, E.S.; Xiong, J.; Medeiros, F.G.M.d.; Grace, M.; Moncada, M.; Lila, M.A.; Hoskin, R.T. Spray dried insect protein-polyphenol particles deliver health-relevant value-added food ingredients. Future Foods 2024, 9, 100315. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Lúcio, A.; Andrade, R.; Sarinho, A.M.; Lima, J.; Batista, L.; Costa, M.E.; Nascimento, A.; Pasquali, M.B. Leveraging Quantitative Structure-Activity Relationships to Design and Optimize Wall Material Formulations for Antioxidant Encapsulation via Spray Drying. Food Chem. Adv. 2025, 7, 100948. [Google Scholar] [CrossRef]
- Gravel, A.; Marciniak, A.; Couture, M.; Doyen, A. Effects of Hexane on Protein Profile, Solubility and Foaming Properties of Defatted Proteins Extracted from Tenebrio molitor Larvae. Molecules 2021, 26, 351. [Google Scholar] [CrossRef]
- Mintah, B.K.; He, R.; Dabbour, M.; Xiang, J.; Agyekum, A.A.; Ma, H. Techno-functional attribute and antioxidative capacity of edible insect protein preparations and hydrolysates thereof: Effect of multiple mode sonochemical action. Ultrason. Sonochemistry 2019, 58, 104676. [Google Scholar] [CrossRef]
- Leni, G.; Soetemans, L.; Caligiani, A.; Sforza, S.; Bastiaens, L. Degree of Hydrolysis Affects the Techno-Functional Properties of Lesser Mealworm Protein Hydrolysates. Foods 2020, 9, 381. [Google Scholar] [CrossRef]
- Ribeiro, S.; Almeida, R.; Batista, L.; Lima, J.; Sarinho, A.; Nascimento, A.; Lisboa, H. Investigation of Guar Gum and Xanthan Gum Influence on Essential Thyme Oil Emulsion Properties and Encapsulation Release Using Modeling Tools. Foods 2024, 13, 816. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, Y.; Li, X.; Huang, M.; Qiao, C.; Sun, J. Properties of co-gel between Tenebrio Molitor larvae protein and myofibrillar protein induced by transglutaminase. Food Chem. 2024, 443, 138609. [Google Scholar] [CrossRef] [PubMed]
- Sweers, L.J.H.; Keppler, J.K.; Feng, S.; Aponte Zea, J.; van Bokhorst-van de Veen, H.; Timmermans, R.A.H.; Boom, R.M.; Fogliano, V.; Lakemond, C.M.M.; Mishyna, M. High hydrostatic pressure for decontamination of soluble insect proteins prevents protein denaturation better than blanching. Innov. Food Sci. Emerg. Technol. 2024, 96, 103743. [Google Scholar] [CrossRef]
- Dion-Poulin, A.; Laroche, M.; Doyen, A.; Turgeon, S.L. Functionality of Cricket and Mealworm Hydrolysates Generated after Pretreatment of Meals with High Hydrostatic Pressures. Molecules 2020, 25, 5366. [Google Scholar] [CrossRef]
- Tarahi, M.; Aghababaei, F.; McClements, D.J.; Pignitter, M.; Hadidi, M. Bioactive peptides derived from insect proteins: Preparation, biological activities, potential applications, and safety issues. Food Chem. 2025, 465, 142113. [Google Scholar] [CrossRef]
- García-Gutiérrez, N.; Salvador, A.; Sanz, T.; Ferrando, M.; Güell, C.; Méndez, C.; de Lamo-Castellví, S. Rheological and Textural Characterisation of Chickpea Dough and Baked 3D-Printed Snacks Enriched with Alphitobius diaperinus and Locusta migratoria Powders. Food Bioprocess Technol. 2024, 17, 5199–5207. [Google Scholar] [CrossRef]
- Aguilera, Y.; Pastrana, I.; Rebollo-Hernanz, M.; Benitez, V.; Álvarez-Rivera, G.; Viejo, J.L.; Martín-Cabrejas, M.A. Investigating edible insects as a sustainable food source: Nutritional value and techno-functional and physiological properties. Food Funct. 2021, 12, 6309–6322. [Google Scholar] [CrossRef]
- Gantner, M.; Król, K.; Piotrowska, A.; Sionek, B.; Sadowska, A.; Kulik, K.; Wiącek, M. Adding Mealworm (Tenebrio molitor L.) Powder to Wheat Bread: Effects on Physicochemical, Sensory and Microbiological Qualities of the End-Product. Molecules 2022, 27, 6155. [Google Scholar] [CrossRef]
- Tanga, C.M.; Nzomo, A.M.; Ndegwa, P.N.; Ekesi, S.; Khamis, F.M.; Akutse, K.S.; Ong’amo, G.; Ochieng, B.O.; Kababu, M.; Beesigamukama, D.; et al. Desert locust (Schistocerca gregaria) flour as an emerging functional ingredient for baking flavorful and nutritious whole wheat bread. Appl. Food Res. 2025, 5, 100802. [Google Scholar] [CrossRef]
- Chao, C.; Lee, J.H.; Kim, H.W.; Kim, I.W.; Park, H.J.; Lee, S.H. Effect of mealworm component and extrusion temperature on fibrous structure formation and physicochemical properties of meat analog extrudates. Food Chem. 2025, 483, 144206. [Google Scholar] [CrossRef]
- Bolat, B.; Ugur, A.E.; Oztop, M.H.; Alpas, H. Effects of High Hydrostatic Pressure assisted degreasing on the technological properties of insect powders obtained from Acheta domesticus & Tenebrio molitor. J. Food Eng. 2021, 292, 110359. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Nogueira Silva, N.F.; Jessen, F.; Mohammadifar, M.A.; Stephani, R.; Fernandes de Carvalho, A.; Perrone Í, T.; Casanova, F. Edible insect as an alternative protein source: A review on the chemistry and functionalities of proteins under different processing methods. Heliyon 2023, 9, e14831. [Google Scholar] [CrossRef]
- Sathe, S.K.; Zaffran, V.D.; Gupta, S.; Li, T. Protein solubilization. J. Am. Oil Chem. Soc. 2018, 95, 883–901. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Senphan, T.; Sigh, A.; Hutamekalin, P.; Nuthong, P.; Benjakul, S. Characteristics and Bioactivities of Protein Hydrolysate from Cricket (Acheta domesticus) Powder Defatted Using Ethanol with Aid of Vacuum Impregnation. Foods 2024, 13, 3250. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhao, X.; Kuang, Z.; Ye, M.; Luo, G.; Xiao, G.; Liao, S.; Li, L.; Xiong, Z. Optimization of antioxidant peptide production in the hydrolysis of silkworm (Bombyx mori L.) pupa protein using response surface methodology. J. Food Agric. Environ. 2013, 11, 952–956. [Google Scholar]
- Vercruysse, L.; Smagghe, G.; Beckers, T.; Van Camp, J. Antioxidative and ACE inhibitory activities in enzymatic hydrolysates of the cotton leafworm, Spodoptera littoralis. Food Chem. 2009, 114, 38–43. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Delgado-Osorio, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; del Carmen Almécija-Rodríguez, M.; Rufián-Henares, J.Á.; Fernandez-Bayo, J.D. Improved nutritional and antioxidant properties of black soldier fly larvae reared on spent coffee grounds and blood meal by-products. Food Res. Int. 2024, 196, 115151. [Google Scholar] [CrossRef]
- Yeerong, K.; Chantawannakul, P.; Anuchapreeda, S.; Wangtueai, S.; Chaiyana, W. Optimization of Hydrolysis Conditions, Isolation, and Identification of Biologically Active Peptides Derived from Acheta domesticus for Antioxidant and Collagenase Inhibition. Antioxidants 2024, 13, 367. [Google Scholar] [CrossRef]
- Hara, S.; Yamakawa, M. A novel antibacterial peptide family isolated from the silkworm, Bombyx mori. Biochem. J. 1995, 310, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Axen, A.; Carlsson, A.; Engström, Å.; Bennich, H. Gloverin, an antibacterial protein from the immune hemolymph of Hyalophora pupae. Eur. J. Biochem. 1997, 247, 614–619. [Google Scholar] [CrossRef]
- Brattsten, L.B.; Wilkinson, C.F. A microsomal enzyme inhibitor in the gut contents of the house cricket (Acheta domesticus). Comp. Biochem. Physiol. Part B Comp. Biochem. 1973, 45, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Zahedinezhad, S.; Loh, J.Y. A review on insect meals in aquaculture: The immunomodulatory and physiological effects. Int. Aquat. Res. 2020, 12, 100–115. [Google Scholar]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and antimicrobial activity of chitosan and its derivatives: A concise review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef]
- Chen, S.; Wei, X.; Sui, Z.; Guo, M.; Geng, J.; Xiao, J.; Huang, D. Preparation of Antioxidant and Antibacterial Chitosan Film from Periplaneta americana. Insects 2021, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Cermeño, M.; Bascón, C.; Amigo-Benavent, M.; Felix, M.; FitzGerald, R.J. Identification of peptides from edible silkworm pupae (Bombyx mori) protein hydrolysates with antioxidant activity. J. Funct. Foods 2022, 92, 105052. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhu, Z.; Li, X.; Sun, S.; Wang, W.; Sadiq, F.A. Identification and characterization of two novel antioxidant peptides from silkworm pupae protein hydrolysates. Eur. Food Res. Technol. 2021, 247, 343–352. [Google Scholar] [CrossRef]
- Yi, H.-Y.; Chowdhury, M.; Huang, Y.-D.; Yu, X.-Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef]
- Wojda, I.; Cytryńska, M.; Zdybicka-Barabas, A.; Kordaczuk, J. Insect defense proteins and peptides. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins; Springer: Berlin/Heidelberg, Germany, 2020; pp. 81–121. [Google Scholar]
- Luo, Y.; Ye, B.; Gao, S.; Tang, G.; Li, D.; Huang, Y.; Huang, Z.; Yang, X.; Naman, A.; Qian, Y. Evaluation of antibacterial activities of hemolymph and methanol extracts from black soldier fly (Hermetia illucens). J. Insects Food Feed 2024, 10, 1197–1209. [Google Scholar] [CrossRef]
- Mendoza-Salazar, A.; Santiago-López, L.; Torres-Llanez, M.J.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. In vitro antioxidant and antihypertensive activity of edible insects flours (mealworm and grasshopper) fermented with Lactococcus lactis strains. Fermentation 2021, 7, 153. [Google Scholar] [CrossRef]
- Malila, Y.; Owolabi, I.O.; Chotanaphuti, T.; Sakdibhornssup, N.; Elliott, C.T.; Visessanguan, W.; Karoonuthaisiri, N.; Petchkongkaew, A. Current challenges of alternative proteins as future foods. NPJ Sci. Food 2024, 8, 53. [Google Scholar] [CrossRef]
- Pontes, E.; Viera, V.; Silva, G.; Silva Neto, M.d.; Mendes, B.; Tome, A.; Almeida, R.; Santos, N.C.; Gusmão, R.d.; Lisboa, H.; et al. Effect of Malvaviscus arboreus Flower and Leaf Extract on the Functional, Antioxidant, Rheological, Textural, and Sensory Properties of Goat Yogurt. Foods 2024, 13, 3942. [Google Scholar] [CrossRef] [PubMed]
- Bless, I.; Bastian, S.E.P.; Gould, J.; Yang, Q.; Wilkinson, K.L. Development of a lexicon for the sensory description of edible insects commercially available in Australia. Food Res. Int. 2024, 190, 114574. [Google Scholar] [CrossRef]
- Perez-Santaescolastica, C.; De Winne, A.; Devaere, J.; Fraeye, I. The flavour of edible insects: A comprehensive review on volatile compounds and their analytical assessment. Trends Food Sci. Technol. 2022, 127, 352–367. [Google Scholar] [CrossRef]
- Park, M.K.; Shin, D.-M.; Choi, Y.-S. Comparison of volatile compound profiles derived from various livestock protein alternatives including edible-insect, and plant-based proteins. Food Chem. X 2024, 23, 101570. [Google Scholar] [CrossRef]
- Szlachciuk, J.; Żakowska-Biemans, S. Breaking the Taboo: Understanding the Relationship between Perception, Beliefs, Willingness to Eat Insects, and Food Neophobia among Polish Adults. Foods 2024, 13, 944. [Google Scholar] [CrossRef]
- Tan, H.S.G.; Fischer, A.R.; van Trijp, H.C.; Stieger, M. Tasty but nasty? Exploring the role of sensory-liking and food appropriateness in the willingness to eat unusual novel foods like insects. Food Qual. Prefer. 2016, 48, 293–302. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Santos, C.; Lima, R.C.; Pintado, M.E.; Cunha, L.M. Impact of defatting and drying methods on the overall liking and sensory profile of a cereal bar incorporating edible insect species. Future Foods 2022, 6, 100190. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Lima, R.C.; Maia, M.R.G.; Almeida, A.A.; Fonseca, A.J.M.; Cabrita, A.R.J.; Cunha, L.M. Impact of defatting freeze-dried edible crickets (Acheta domesticus and Gryllodes sigillatus) on the nutritive value, overall liking and sensory profile of cereal bars. LWT 2019, 113, 108335. [Google Scholar] [CrossRef]
- An, J.; Wicaksana, F.; Woo, M.W.; Liu, C.; Tian, J.; Yao, Y. Current food processing methods for obtaining umami peptides from protein-rich foods: A review. Trends Food Sci. Technol. 2024, 153, 104704. [Google Scholar] [CrossRef]
- Cho, J.-H.; Zhao, H.-L.; Kim, J.-S.; Kim, S.-H.; Chung, C.-H. Characteristics of fermented seasoning sauces using Tenebrio molitor larvae. Innov. Food Sci. Emerg. Technol. 2018, 45, 186–195. [Google Scholar] [CrossRef]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Nutritional, sensory, and texture quality of bread and cookie enriched with house cricket (Acheta domesticus) powder. J. Food Process. Preserv. 2020, 44, e14601. [Google Scholar] [CrossRef]
- Ruszkowska, M.; Tańska, M.; Kowalczewski, P.Ł. Extruded Corn Snacks with Cricket Powder: Impact on Physical Parameters and Consumer Acceptance. Sustainability 2022, 14, 16578. [Google Scholar] [CrossRef]
- Żołnierczyk, A.K.; Szumny, A. Sensory and Chemical Characteristic of Two Insect Species: Tenebrio molitor and Zophobas morio Larvae Affected by Roasting Processes. Molecules 2021, 26, 2697. [Google Scholar] [CrossRef]
- Gantner, M.; Sadowska, A.; Piotrowska, A.; Kulik, K.; Sionek, B.; Kostyra, E. Wheat Bread Enriched with House Cricket Powder (Acheta domesticus L.) as an Alternative Protein Source. Molecules 2024, 29, 711. [Google Scholar] [CrossRef] [PubMed]
- El Hosry, L.; Elias, V.; Chamoun, V.; Halawi, M.; Cayot, P.; Nehme, A.; Bou-Maroun, E. Maillard Reaction: Mechanism, Influencing Parameters, Advantages, Disadvantages, and Food Industrial Applications: A Review. Foods 2025, 14, 1881. [Google Scholar] [CrossRef]
- Ribeiro, J.C.R.; Lima, R.C.; Cunha, L.M. Sensory Profile and Consumer Acceptance of Edible Insects and Insect-Based Foods; The Royal Society of Chemistry: London, UK, 2024. [Google Scholar]
- Kavle, R.R.; Pritchard, E.; Bekhit, A.E.-D.A.; Carne, A.; Agyei, D. Nutrient Content and Functionalities of Edible Insects. In Edible Insects Processing for Food and Feed; CRC Press: Boca Raton, FL, USA, 2023; pp. 53–84. [Google Scholar]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Gumienna, M.; Rybicka, I.; Górna, B.; Sarbak, P.; Dziedzic, K.; Kmiecik, D. Nutritional Value and Biological Activity of Gluten-Free Bread Enriched with Cricket Powder. Molecules 2021, 26, 1184. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.; Walkowiak, K.; Masewicz, Ł.; Bartczak, O.; Lewandowicz, J.; Kubiak, P.; Baranowska, H.M. Gluten-Free Bread with Cricket Powder-Mechanical Properties and Molecular Water Dynamics in Dough and Ready Product. Foods 2019, 8, 240. [Google Scholar] [CrossRef]
- da Rosa Machado, C.; Thys, R.C.S. Cricket powder (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innov. Food Sci. Emerg. Technol. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Nissen, L.; Samaei, S.P.; Babini, E.; Gianotti, A. Gluten free sourdough bread enriched with cricket flour for protein fortification: Antioxidant improvement and Volatilome characterization. Food Chem. 2020, 333, 127410. [Google Scholar] [CrossRef]
- Ramos, N.J.d.S.; Rocha, E.B.M.; Gusmão, T.A.S.; Nascimento, A.; Lisboa, H.M.; de Gusmão, R.P. Optimizing gluten-free pasta quality: The impacts of transglutaminase concentration and kneading time on cooking properties, nutritional value, and rheological characteristics. LWT 2023, 189, 115485. [Google Scholar] [CrossRef]
- Mishyna, M.; Keppler, J.K.; Chen, J. Techno-functional properties of edible insect proteins and effects of processing. Curr. Opin. Colloid Interface Sci. 2021, 56, 101508. [Google Scholar] [CrossRef]
- Scholliers, J.; Steen, L.; Fraeye, I. Gelation of a combination of insect and pork proteins as affected by heating temperature and insect:meat ratio. Food Res. Int. 2020, 137, 109703. [Google Scholar] [CrossRef] [PubMed]
- Smetana, S.; Ashtari Larki, N.; Pernutz, C.; Franke, K.; Bindrich, U.; Toepfl, S.; Heinz, V. Structure design of insect-based meat analogs with high-moisture extrusion. J. Food Eng. 2018, 229, 83–85. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Ianniciello, D.; Tedesco, F.; Salvia, R.; Scieuzo, C.; Schmitt, E.; Capece, A.; Falabella, P. Insect-derived chitosan, a biopolymer for the increased shelf life of white and red grapes. Int. J. Biol. Macromol. 2024, 275, 133149. [Google Scholar] [CrossRef]
- Tafi, E.; Triunfo, M.; Guarnieri, A.; Ianniciello, D.; Salvia, R.; Scieuzo, C.; Ranieri, A.; Castagna, A.; Lepuri, S.; Hahn, T.; et al. Preliminary investigation on the effect of insect-based chitosan on preservation of coated fresh cherry tomatoes. Sci. Rep. 2023, 13, 7030. [Google Scholar] [CrossRef]
- Ho, I.; Peterson, A.; Madden, J.; Huang, E.; Amin, S.; Lammert, A. Will it cricket? Product development and evaluation of cricket (Acheta domesticus) powder replacement in sausage, pasta, and brownies. Foods 2022, 11, 3128. [Google Scholar] [CrossRef] [PubMed]
- Puleo, S.; Fiore, A.; Sieghartsleitner, A.; Russo, G.; Grigor, J.; Di Monaco, R. Cricket flour integration in biscuits: A study on formulation and consumer acceptance. J. Insects Food Feed 2025, 1, 1–14. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, N.F.; Allergens, F.; Turck, D.; Bohn, T.; Cámara, M.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Jos, Á.; Maciuk, A.; et al. Safety of frozen and dried forms of whole yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2025, 23, e9155. [Google Scholar] [CrossRef]
- Janssen, R.H.; Canelli, G.; Sanders, M.G.; Bakx, E.J.; Lakemond, C.M.; Fogliano, V.; Vincken, J.-P. Iron-polyphenol complexes cause blackening upon grinding Hermetia illucens (black soldier fly) larvae. Sci. Rep. 2019, 9, 2967. [Google Scholar] [CrossRef]
- Kowalski, S.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Mazurek, A. Wheat bread supplementation with various edible insect flours. Influence of chemical composition on nutritional and technological aspects. LWT 2022, 159, 113220. [Google Scholar] [CrossRef]
- Ramírez-Rivera, E.J.; Hernández-Santos, B.; Juárez-Barrientos, J.M.; Torruco-Uco, J.G.; Ramírez-Figueroa, E.; Rodríguez-Miranda, J. Effects of formulation and process conditions on chemical composition, color parameters, and acceptability of extruded insect-rich snack. J. Food Process. Preserv. 2021, 45, e15499. [Google Scholar] [CrossRef]
- Cooksey, C.J. The red insect dyes: Carminic, kermesic and laccaic acids and their derivatives. Biotech. Histochem. 2019, 94, 100–107. [Google Scholar] [CrossRef]
- Manzo, G.M.O.; Flores, H.E.M.; López, J.O.R.; Portillo, L. Carmine red from cochineal (Dactylopius coccus), a natural dye: A review. Cienc. Nicolaita 2025, 26–34. [Google Scholar] [CrossRef]
- Nisal, A.; Trivedy, K.; Mohammad, H.; Panneri, S.; Sen Gupta, S.; Lele, A.; Manchala, R.; Kumar, N.S.; Gadgil, M.; Khandelwal, H. Uptake of azo dyes into silk glands for production of colored silk cocoons using a green feeding approach. ACS Sustain. Chem. Eng. 2014, 2, 312–317. [Google Scholar] [CrossRef]
- Ide, J.-Y. Effect of color on the feeding preference of a generalist grasshopper, Acrida cinerea (Orthoptera: Acrididae). J. Asia-Pac. Entomol. 2025, 28, 102415. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Z.; Shi, K.; Jiang, Z.; Guan, C.; Zhang, L.; Yang, T.; Xie, F. Shellac-based materials: Structures, properties, and applications. Int. J. Biol. Macromol. 2024, 279, 135102. [Google Scholar] [CrossRef] [PubMed]
- Mafu, A.; Ketnawa, S.; Phongthai, S.; Schönlechner, R.; Rawdkuen, S. Whole Wheat Bread Enriched with Cricket Powder as an Alternative Protein. Foods 2022, 11, 2142. [Google Scholar] [CrossRef] [PubMed]
- Mihaly Cozmuta, A.; Uivarasan, A.; Peter, A.; Nicula, C.; Kovacs, D.E.; Mihaly Cozmuta, L. Yellow Mealworm (Tenebrio molitor) Powder Promotes a High Bioaccessible Protein Fraction and Low Glycaemic Index in Biscuits. Nutrients 2023, 15, 997. [Google Scholar] [CrossRef]
- Ayensu, J.; Lutterodt, H.; Annan, R.A.; Edusei, A.; Loh, S.P. Nutritional composition and acceptability of biscuits fortified with palm weevil larvae (Rhynchophorus phoenicis Fabricius) and orange-fleshed sweet potato among pregnant women. Food Sci. Nutr. 2019, 7, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Carcea, M. Quality and nutritional/textural properties of durum wheat pasta enriched with cricket powder. Foods 2020, 9, 1298. [Google Scholar] [CrossRef]
- Carpentieri, S.; Orkusz, A.; Harasym, J.; Ferrari, G. Exploring the Use of Tenebrio molitor Larvae Proteins to Functionalize Durum Wheat Pasta. Foods 2025, 14, 1194. [Google Scholar] [CrossRef]
- Musika, J.; Kapcum, C.; Itthivadhanapong, P.; Musika, T.; Hanmontree, P.; Piayura, S. Enhancing nutritional and functional properties of gluten-free Riceberry rice pasta supplemented with cricket powder using D-optimal mixture design. Front. Sustain. Food Syst. 2024, 8, 1417045. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Fernández, M.; Martin, D.; Escudero, R.; Navarro del Hierro, J. Use of Mealworm (Tenebrio molitor) Flour as Meat Replacer in Dry Fermented Sausages. Foods 2025, 14, 1019. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Pintado, T.; Cruz, T.d.M.P.; da Silva, M.C.A. Cricket (Acheta domesticus) flour as meat replacer in frankfurters: Nutritional, technological, structural, and sensory characteristics. Innov. Food Sci. Emerg. Technol. 2023, 83, 103245. [Google Scholar] [CrossRef]
- Kiiru, S.M.; Kinyuru, J.N.; Kiage, B.N.; Martin, A.; Marel, A.K.; Osen, R. Extrusion texturization of cricket flour and soy protein isolate: Influence of insect content, extrusion temperature, and moisture-level variation on textural properties. Food Sci. Nutr. 2020, 8, 4112–4120. [Google Scholar] [CrossRef]
- Wójtowicz, A.; Combrzyński, M.; Biernacka, B.; Oniszczuk, T.; Mitrus, M.; Różyło, R.; Gancarz, M.; Oniszczuk, A. Application of edible insect flour as a novel ingredient in fortified snack pellets: Processing aspects and physical characteristics. Processes 2023, 11, 2561. [Google Scholar] [CrossRef]
- Ruszkowska, M.; Tańska, M.; Miedzianka, J.; Kowalczewski, P.Ł. Field Cricket (Gryllus bimaculatus) and Spirulina (Arthrospira platensis) Powders as Environmentally Friendly Protein Enrichment Ingredients in Corn Snacks. Foods 2024, 13, 2390. [Google Scholar] [CrossRef]
- Azzollini, D.; Derossi, A.; Fogliano, V.; Lakemond, C.; Severini, C. Effects of formulation and process conditions on microstructure, texture and digestibility of extruded insect-riched snacks. Innov. Food Sci. Emerg. Technol. 2018, 45, 344–353. [Google Scholar] [CrossRef]
- Severini, C.; Azzollini, D.; Albenzio, M.; Derossi, A. On printability, quality and nutritional properties of 3D printed cereal based snacks enriched with edible insects. Food Res. Int. 2018, 106, 666–676. [Google Scholar] [CrossRef]
- Karwacka, K.; Łobacz, A.; Ziajka, J.; Lis, A.; Małkowska-Kowalczyk, M.; Baranowska, M. Use of House Cricket (Acheta domesticus) Powder in Yoghurt Products. Foods 2024, 13, 2426. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.M.; da Costa, D.V.; Trombete, F.M.; Câmara, A.K.F.I. Edible insects as a sustainable alternative to food products: An insight into quality aspects of reformulated bakery and meat products. Curr. Opin. Food Sci. 2022, 46, 100864. [Google Scholar] [CrossRef]
- Bas, A.; El, S.N. Nutritional evaluation of biscuits enriched with cricket flour (Acheta domesticus). Int. J. Gastron. Food Sci. 2022, 29, 100583. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Burt, K.G.; Takumi, K.; Isimar, L.; Jamie, K.; Avia, G.; Lalitha, S.; and Stopler, M. Acceptance of Using Cricket Flour as a Low Carbohydrate, High Protein, Sustainable Substitute for All-Purpose Flour in Muffins. J. Culin. Sci. Technol. 2020, 18, 201–213. [Google Scholar] [CrossRef]
- Akande, O.A.; Falade, O.O.; Badejo, A.A.; Adekoya, I. Assessment of Mulberry Silkworm Pupae and African Palm Weevil larvae as alternative protein sources in snack fillings. Heliyon 2020, 6, e03754. [Google Scholar] [CrossRef]
- Duda, A.; Adamczak, J.; Chełmińska, P.; Juszkiewicz, J.; Kowalczewski, P. Quality and nutritional/textural properties of durum wheat pasta enriched with cricket powder. Foods 2019, 8, 46. [Google Scholar] [CrossRef]
- Çabuk, B.; Yılmaz, B. Fortification of traditional egg pasta (erişte) with edible insects: Nutritional quality, cooking properties and sensory characteristics evaluation. J. Food Sci. Technol. 2020, 57, 2750–2757. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.I.; Cha, J.Y.; Park, S.-Y.; Jung, S.; Choi, Y.-S. Drying-induced restructured jerky analog developed using a combination of edible insect protein and textured vegetable protein. Food Chem. 2022, 373, 131519. [Google Scholar] [CrossRef]
- Cho, S.Y.; Ryu, G.H. Effects of mealworm larva composition and selected process parameters on the physicochemical properties of extruded meat analog. Food Sci. Nutr. 2021, 9, 4408–4419. [Google Scholar] [CrossRef]
- Scholliers, J.; Steen, L.; Glorieux, S.; Van de Walle, D.; Dewettinck, K.; Fraeye, I. The effect of temperature on structure formation in three insect batters. Food Res. Int. 2019, 122, 411–418. [Google Scholar] [CrossRef]
- Choi, N.; Park, S.; Park, Y.; Park, G.; Oh, S.; Kim, Y.-a.; Lim, Y.; Jang, S.; Kim, Y.; Ahn, K.-S. Effects of edible insect powders as meat partial substitute on physicochemical properties and storage stability of pork patties. Food Sci. Anim. Resour. 2024, 44, 817. [Google Scholar] [CrossRef]
- Rocchetti, G.; Leni, G.; Rebecchi, A.; Dordoni, R.; Giuberti, G.; Lucini, L. The distinctive effect of different insect powders as meat extenders in beef burgers subjected to cooking and in vitro gastrointestinal digestion. Food Chem. 2024, 442, 138422. [Google Scholar] [CrossRef]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Nakagawa, K.; Chantanuson, R.; Boonarsa, P.; Seephua, N.; Siriamornpun, S. Meat analogue preparation from cricket and rice powder mixtures with controlled textural and nutritional quality by freeze alignment technique. Food Chem. X 2024, 22, 101402. [Google Scholar] [CrossRef] [PubMed]
- Combrzyński, M.; Oniszczuk, T.; Wójtowicz, A.; Biernacka, B.; Wojtunik-Kulesza, K.; Bąkowski, M.; Różyło, R.; Szponar, J.; Soja, J.; Oniszczuk, A. Nutritional characteristics of new generation extruded snack pellets with edible cricket flour processed at various extrusion conditions. Antioxidants 2023, 12, 1253. [Google Scholar] [CrossRef] [PubMed]
- Dridi, C.; Millette, M.; Uscanga, B.R.A.; Salmieri, S.; Allahdad, Z.; Lacroix, M. Evaluation of the Nutritional Quality and In Vivo Digestibility of Probiotic Beverages Enriched with Cricket Proteins. Food Bioprocess Technol. 2023, 16, 1992–2000. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Chon, J.-W.; Song, K.-Y.; Kim, D.-H.; Kim, H.; Seo, K.-H. Sensory profiles of protein-fortified kefir prepared using edible insects (silkworm pupae, Bombyx mori): A preliminary study. J. Dairy Sci. Biotechnol. 2017, 35, 262–265. [Google Scholar] [CrossRef]
- Barton, A.; Richardson, C.D.; McSweeney, M.B. Consumer attitudes toward entomophagy before and after evaluating cricket (Acheta domesticus)-based protein powders. J. Food Sci. 2020, 85, 781–788. [Google Scholar] [CrossRef]
- Tang, Q.; Chung, S.-J. Effect of explicit frames on the sensitivity and acceptance of mealworm in protein shake. Food Qual. Prefer. 2023, 109, 104924. [Google Scholar] [CrossRef]
- Neves, V.; Campos, L.; Ribeiro, N.; Costa, R.; Correia, P.; Gonçalves, J.; Henriques, M. Insect flour as milk protein substitute in fermented dairy products. Food Biosci. 2024, 60, 104379. [Google Scholar] [CrossRef]
- Morais, R.; Soares, P.I.; Morais, S.K.; Oriente, S.; Nascimento, A.; Melo, M.O.; Sousa, F.M.; Cavalcanti-Mata, M.; Lisboa, H.M.; Gusmão, R.P.; et al. Development and Characterization of Symbiotic Buffalo Petit Suisse Cheese Utilizing Whey Retention and Inulin Incorporation. Foods 2023, 12, 4343. [Google Scholar] [CrossRef]
- Godfray, H.; Pretty, J.; Thomas, S.; Warham, E.; Beddington, J. Linking policy on climate and food. Science 2011, 331, 1013–1014. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.; de Boer, I.J. Comparing environmental impacts for livestock products: A review of life cycle assessments. Livest. Sci. 2010, 128, 1–11. [Google Scholar] [CrossRef]
- Smetana, S.; Bhatia, A.; Batta, U.; Mouhrim, N.; Tonda, A. Environmental impact potential of insect production chains for food and feed in Europe. Anim. Front. 2023, 13, 112–120. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Dias, C.; Finnigan, J.; Moran, D.; Rounsevell, M.D. Could consumption of insects, cultured meat or imitation meat reduce global agricultural land use? Glob. Food Secur. 2017, 15, 22–32. [Google Scholar] [CrossRef]
- Miglietta, P.P.; De Leo, F.; Ruberti, M.; Massari, S. Mealworms for food: A water footprint perspective. Water 2015, 7, 6190–6203. [Google Scholar] [CrossRef]
- Dagevos, H. A literature review of consumer research on edible insects: Recent evidence and new vistas from 2019 studies. J. Insects Food Feed 2021, 7, 249–260. [Google Scholar] [CrossRef]
- Aivazidou, E.; Tsolakis, N.; Iakovou, E.; Vlachos, D. The emerging role of water footprint in supply chain management: A critical literature synthesis and a hierarchical decision-making framework. J. Clean. Prod. 2016, 137, 1018–1037. [Google Scholar] [CrossRef]
- Suckling, J.; Druckman, A.; Moore, C.D.; Driscoll, D. The environmental impact of rearing crickets for live pet food in the UK, and implications of a transition to a hybrid business model combining production for live pet food with production for human consumption. Int. J. Life Cycle Assess. 2020, 25, 1693–1709. [Google Scholar] [CrossRef]
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Smetana, S.; Palanisamy, M.; Mathys, A.; Heinz, V. Sustainability of insect use for feed and food: Life Cycle Assessment perspective. J. Clean. Prod. 2016, 137, 741–751. [Google Scholar] [CrossRef]
- Bava, L.; Jucker, C.; Gislon, G.; Lupi, D.; Savoldelli, S.; Zucali, M.; Colombini, S. Rearing of Hermetia illucens on different organic by-products: Influence on growth, waste reduction, and environmental impact. Animals 2019, 9, 289. [Google Scholar] [CrossRef]
- Cohen, A.C. Ecology of insect rearing systems: A mini-review of insect rearing papers from 1906–2017. Adv. Entomol. 2018, 6, 86. [Google Scholar] [CrossRef]
- Vesterlund, M.; Borisová, S.; Emilsson, E. Data center excess heat for mealworm farming, an applied analysis for sustainable protein production. Appl. Energy 2024, 353, 121990. [Google Scholar] [CrossRef]
- Corona-Mariscal, A.; Sanjuan, N.; Güell, C.; Clemente, G. Assessing the environmental sustainability of insects as a source of functional proteins: A prospective LCA. Future Foods 2024, 10, 100457. [Google Scholar] [CrossRef]
- Caccialanza, A.; Cerrato, D.; Galli, D. Sustainability practices and challenges in the meat supply chain: A systematic literature review. Br. Food J. 2023, 125, 4470–4497. [Google Scholar] [CrossRef]
- Parniakov, O.; Mikhrovska, M.; Wiktor, A.; Alles, M.; Ristic, D.; Bogusz, R.; Nowacka, M.; Devahastin, S.; Mujumdar, A.; Heinz, V. Insect processing for food and feed: A review of drying methods. Dry. Technol. 2022, 40, 1500–1513. [Google Scholar] [CrossRef]
- Berardy, A.; Costello, C.; Seager, T. Life cycle assessment of soy protein isolate. In Proceedings of the International Symposium on Sustainable Systems and Technologies, Dearborn, MI, USA, 18–20 May 2015. [Google Scholar]
- Orkusz, A. Edible Insects versus Meat-Nutritional Comparison: Knowledge of Their Composition Is the Key to Good Health. Nutrients 2021, 13, 1207. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Cazarolli, L.H.; Pigatto, T.; Trento, E.; Retcheski, M.C.; Quast, L.B.; Romão, S.; Tormen, L.; Pinto, V.Z. Exploring side streams upcycling for crickets farming: Insects biology and chemical composition. Food Biosci. 2025, 68, 106431. [Google Scholar] [CrossRef]
- Ochiai, M.; Suzuki, Y.; Suzuki, R.; Iwata, K.; Murayama, M. Low protein digestibility-corrected amino acid score and net nitrogen-to-protein conversion factor value of edible insects. Food Chem. 2024, 454, 139781. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Tsuda, M.; Wada, Y.; Shibasaki, T.; Nakamura, H.; Miyaji, K. Nutritional evaluation of milk-, plant-, and insect-based protein materials by protein digestibility using the INFOGEST digestion method. J. Agric. Food Chem. 2023, 71, 2503–2513. [Google Scholar] [CrossRef]
- Grossmann, K.K.; Merz, M.; Appel, D.; De Araujo, M.M.; Fischer, L. New insights into the flavoring potential of cricket (Acheta domesticus) and mealworm (Tenebrio molitor) protein hydrolysates and their Maillard products. Food Chem. 2021, 364, 130336. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Whanmek, K.; Santivarangkna, C. Physicochemical, microbiological and nutritional quality of fermented cricket (Acheta domesticus) paste. LWT 2023, 189, 115444. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Dewettinck, K.; Gellynck, X.; Schouteten, J.J. Replacing vegetable oil by insect oil in food products: Effect of deodorization on the sensory evaluation. Food Res. Int. 2021, 141, 110140. [Google Scholar] [CrossRef]
- Fernandes, B.; Oliveira, M.C.; Marques, A.C.; dos Santos, R.G.; Serrano, C. Microencapsulation of Essential Oils and Oleoresins: Applications in Food Products. Foods 2024, 13, 3873. [Google Scholar] [CrossRef]
- de Oliveira Alencar, D.D.; de Souza, E.L.; da Cruz Almeida, E.T.; da Silva, A.L.; Oliveira, H.M.L.; Cavalcanti, M.T. Microencapsulation of Cymbopogon citratus DC Stapf Essential Oil with Spray Drying: Development, Characterization, and Antioxidant and Antibacterial Activities. Foods 2022, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Kolobe, S.D.; Manyelo, T.G.; Sebola, N.A.; Malematja, E.; Monnye, M. Prospects of rearing selected southern African swarming insects for animal feed: A review on insect farming and the economic value of edible insects. Agric. Food Secur. 2024, 13, 6. [Google Scholar] [CrossRef]
- Riekkinen, K.; Väkeväinen, K.; Korhonen, J. The Effect of Substrate on the Nutrient Content and Fatty Acid Composition of Edible Insects. Insects 2022, 13, 590. [Google Scholar] [CrossRef]
- Emilia, M.; Magdalena, C.; Weronika, G.; Julia, W.; Danuta, K.; Jakub, S.; Bożena, C.; Krzysztof, K. IgE-based analysis of sensitization and cross-reactivity to yellow mealworm and edible insect allergens before their widespread dietary introduction. Sci. Rep. 2025, 15, 1466. [Google Scholar] [CrossRef] [PubMed]
- Wangorsch, A.; Jamin, A.; Spiric, J.; Vieths, S.; Scheurer, S.; Mahler, V.; Hofmann, S.C. Allergic Reaction to a Commercially Available Insect Snack Caused by House Cricket (Acheta domesticus) Tropomyosin. Mol. Nutr. Food Res. 2024, 68, 2300420. [Google Scholar] [CrossRef]
- Ni, D. Allergenic potential of insect proteins: Cross-reactivity, detection, degradation, and implications for food safety and labelling. Food Sci. Anim. Prod. 2025, 3, 9240127. [Google Scholar] [CrossRef]
- Cruz-López, S.O.; Escalona-Buendía, H.B.; Martinez-Arellano, I.; Domínguez-Soberanes, J.; Alvarez-Cisneros, Y.M. Physicochemical and techno-functional characterization of soluble proteins extracted by ultrasound from the cricket Acheta domesticus. Heliyon 2024, 10, e40718. [Google Scholar] [CrossRef]
- Sweers, L.J.H.; Politiek, R.G.A.; Lakemond, C.M.M.; Bruins, M.E.; Boom, R.M.; Fogliano, V.; Mishyna, M.; Keppler, J.K.; Schutyser, M.A.I. Dry fractionation for protein enrichment of animal by-products and insects: A review. J. Food Eng. 2022, 313, 110759. [Google Scholar] [CrossRef]
- Wockenfuss, L.; Lammers, V.; Heinz, V.; Sozer, N.; Silventoinen-Veijalainen, P. Two steps of dry fractionation: Comparison and combination of air classification and electrostatic separation for protein enrichment from defatted rapeseed press cake. J. Food Eng. 2023, 357, 111623. [Google Scholar] [CrossRef]
- Abro, Z.; Sibhatu, K.T.; Fetene, G.M.; Alemu, M.H.; Tanga, C.M.; Sevgan, S.; Kassie, M. Global review of consumer preferences and willingness to pay for edible insects and derived products. Glob. Food Secur. 2025, 44, 100834. [Google Scholar] [CrossRef]
- Hansen, L.S.; Laursen, S.F.; Bahrndorff, S.; Sørensen, J.G.; Sahana, G.; Kristensen, T.N.; Nielsen, H.M. The unpaved road towards efficient selective breeding in insects for food and feed—A review. Entomol. Exp. Appl. 2025, 173, 498–521. [Google Scholar] [CrossRef]
- Ashizawa, R.; Rubio, N.; Letcher, S.; Parkinson, A.; Dmitruczyk, V.; Kaplan, D.L. Entomoculture: A Preliminary Techno-Economic Assessment. Foods 2022, 11, 3037. [Google Scholar] [CrossRef] [PubMed]

| Scientific Name (Common Name) | EU Novel Food Status | Typical US Market Status |
|---|---|---|
| Tenebrio molitor (Coleoptera: Tenebrionidae) (yellow mealworm, larva) | Authorized—dried, frozen, paste, and UV-treated powder (implementing Regs 2021/882 and 2022/169) | Widely marketed as whole larva and powder; no FDA objection when produced under CGMP |
| Acheta domesticus (Orthoptera: Gryllidae) (house cricket) | Authorized—frozen, dried, and partially defatted powder (Regs 2022/188 and 2023/5) (EUR-Lex) | Principal species in US retail flours and bars |
| Locusta migratoria (Orthoptera: Acrididae) | Authorized—frozen, dried, ground (Reg 2021/1975) | Sold chiefly as whole-insect snack; emerging powders |
| Alphitobius diaperinus (Coleoptera: Tenebrionidae) (lesser mealworm) | Authorized—frozen, paste, dried, powder (Reg 2023/58) | Commercial powders and savory snacks |
| Hermetia illucens (Diptera: Stratiomyidae) (black soldier fly) | EFSA opinion under review; not yet authorized for food; already authorized for feed | Pilot human food products; self-GRAS dossiers in preparation |
| Gryllodes sigillatus (Orthoptera: Gryllidae) (banded cricket) | Application submitted; no decision (dossier NF 2021/2313) | Niche US start-ups; GRAS self-determination |
| Zophobas morio (Coleoptera: Tenebrionidae) (king-mealworm) | No EU file to date | Limited US online sales |
| Oecophylla smaragdina (Hymenoptera: Formicidae) (Asian weaver ant) | Unregulated; traditional food in S-E Asia only | Not marketed for food |
| Lethocerus indicus (Hemiptera: Belostomatidae) (giant water bug) | Unregulated; traditional Thai/Vietnamese delicacy | Not marketed for food |
| Imbrasia ertli (Lepidoptera: Saturniidae) (saturniid moth caterpillar) | Unregulated; regional African consumption | Not marketed in EU/US |
| Prionoplus reticularis (Coleoptera: Cerambycidae) (Huhu beetle) | Unregulated | Not marketed |
| Odontotermes spp. (Blattodea: Termitidae) (subterranean termites) | Unregulated | Not marketed |
| Ingredient (Species and Preparation) | Protein Solubility | Protein Digestibility | Protein Quality | Experimental Conditions/Notes |
|---|---|---|---|---|
| A. domesticus (House cricket)—Whole/defatted | ~96% at pH 11; drops to ~11–15% near pI. | 79–93% in vitro total protein digestibility (depending on processing) [42]. | PDCAAS ≈ 84% (0.84)—limiting amino acid: Leucine [43]. | PDCAAS measured in rats (ref. pattern 6 mo–3 yr child). Digestible indispensable amino acids (DIAASs) for cricket protein up to 89% for adults. High digestibility relative to plant proteins. |
| T. molitor (Yellow mealworm)—Whole/defatted | ~97% at pH 11 (isolate); ~15% at pH 4 (near pI). | 91–99% in vitro digestibility of protein (high unless over-dried) [42]. | PDCAAS ~76–86% (limiting SAA: Met+Cys) [44]. | PDCAAS from rat assays; higher PDCAAS reported with mild processing: essential amino acids sufficiently high to meet requirements except slightly low in sulfur AAs. |
| B. mori (Silkworm pupae)—Protein concentrate | High protein solubility in extracts (e.g., water-soluble fraction). | ~90% (est.)—silkworm proteins are highly digestible [45]. | PDCAAS ~99–100%—complete amino acid profile (limiting AA effectively none; Leu at 99–100%) [46]. | PDCAAS determined via rat assay; exceptionally high foaming stability noted, possibly due to hydrophobic amino acid content. |
| Cirina forda (Lepidoptera: Saturniidae) (African caterpillar)—Whole flour | ~90% at pH 5.5; solubility improves at extreme pH (55% at pH 11). | ~85–87% in vivo digestibility, but lower net protein utilization due to amino acid imbalance [47]. | PDCAAS ~42%—very low. Poor amino acid balance—deficient in sulfur AAs [48]. | Values from rat feeding tests. Low PDCAAS despite decent digestibility implies one or more essential AAs far below requirements. |
| Ingredient (Species and Form) | Water-Holding Capacity (WHC) (g/g) | Oil-Holding Capacity (OHC) (g/g) | Emulsifying Capacity (EC) (%) | Emulsion Stability (ES) (%) | Foaming Capacity (FC) (%) | Foam Stability (FS) (%) | Gelation (w/v) | Experimental Conditions/Notes |
|---|---|---|---|---|---|---|---|---|
| Mealworm larvae (T. molitor)—Protein isolate [123] | 3.95 ± 0.2 | 2.74 ± 0.06 | 66.6 ± 2.2 | 51.3 ± 0.5 | 32.7 ± 0.9 | 30.3 ± 0.5 | No gel | Solubility ~97% at pH 11; near pI (pH 4) solubility ~15%. |
| Locust (Schistocerca gregaria (Orthoptera: Acrididae) )—Protein isolate [123] | 2.31 ± 0.19 | 3.22 ± 0.16 | 67.8 ± 1.6 | 50.4 ± 2.0 | 32.0 ± 1.9 | 6.2 ± 0.7 | No gel | Solubility ~90% at pH 11. Low foam stability (only ~6%). |
| Cricket (G. sigillatus)—Protein isolate [124] | 3.44 ± 0.13 | 3.33 ± 0.11 | 72.6 ± 1.9 | 62 ± 1.2 | 125 ± 25 | 92.0 ± 1.9 | No gel | Extremely high foaming capacity and stability. Solubility 30% at pH = 3 ~96% and pH= 11. |
| Mealworm larvae (T. molitor)—Whole flour [125] | 0.6 ± 0.19 | 0.71 ± 0.33 | 65.9 ± 1.5 | 27.6 ± 1.2 | 31.0 ± 1.4% | 26.0 ± 0.9% | No gel | Non-defatted flour (~52% protein). Lower WHC/OHC than isolates; emulsions unstable (ES ~28%). |
| Locust (S. gregaria)—Whole flour [117,126] | 2.17 ± 0.09 | 1.64 ± 0.06 | 69.2 ± 0.6 | 48.1 ± 0.6 | 22.3 ± 1.4 | 19.3 ± 0.9 | No gel | High protein content (~76%) in flour. Emulsions quite stable (ES ~48%) even in whole flour form. |
| Cricket (G. sigillatus)—Whole flour [127] | 2.34 ± 0.28 | 2.82 ± 0.08 | 62.0 ± 1.3 | 31.7 ± 0.9 | 41.0 ± 1.4 | 34.7 ± 2.8 | No gel | Protein ~70% in flour. Balanced WHC/OHC ~2–3 g/g; moderate foam and emulsion stability. |
| African caterpillar (C. forda)—Defatted flour [126] | 3.00 ± 0.00 | 3.58 ± 0.00 | 36.7 ± 0.1 | 45.4 ± 0.2 | 7.1 ± 0.2 | 3.0 ± 0.0 | Gels at 6% | High solubility (~55%) achieved at pH 11. Shows exceptionally high water and oil binding (>>200%) after defatting. Minimum gelation concentration 6% (w/v). |
| Ingredient (Preparation) | Cricket (A. domesticus Protein Hydrolysate) [152] | Silkworm (B. mori Pupae Protein Peptides) [153] | Locust (S. gregaria Protein Hydrolysate) [154] | Black Soldier Fly (H. illucens) [155] |
|---|---|---|---|---|
| Antioxidant Activity | 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging IC50 ≈ 455 µg/mL; 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging assay (ABTS) IC50 ≈ 71 µg/mL. Also 6.27 µmol TE/g (DPPH) and 19.5 µmol TE/g Ferric-Reducing Antioxidant Power (FRAP) in defatted-cricket Alcalase hydrolysate. | Moderate antioxidant capacity with hydrolysis yielding ~66% free-radical scavenging. | Relatively low antioxidant activity, peptides from S. littoralis, a related species, showed weak DPPH/FRAP activity. | DPPH: ~110 mmol TE/kg, FRAP: ~100 mmol TE/kg, ABTS: ~600 mmol TE/kg, depending on feed. |
| Antimicrobial Effects | Protein hydrolysates (PHs) show significant inhibition of collagenase and hyaluronidase, enzymes involved in skin aging and degradation of connective tissue [156]. An inhibitor from the gut contents of A. domesticus targets microsomal oxidation, particularly affecting Reduced Nicotinamide Adenine Dinucleotide Phosphate (NADPH) cytochrome c reductase, with greater activity against insect enzymes than mammalian ones. | Lebocin 1–3 (O-glycosylated, 51 aa) completely inhibit Micrococcus luteus at 3–6 µM; partial activity on E. coli (~25 µM) [157]. Gloverins (14 kDa glycine-rich) active vs. Gram-negative strains at 50–200 µM, isoform-dependent [158]. | Orthopteran defensin-like peptides reported for locusts; typical MIC 0.1–5 µM vs. Gram-positive (M. luteus, S. aureus) and low-tens µM vs. Gram-negative bacteria. No direct data yet for protein hydrolysate, but presence of defensin genes suggests comparable potency. | Broad-spectrum antimicrobial: insect-derived chitosan (low MW) is effective against Gram(+) and Gram(−) bacteria. Minimum inhibitory concentrations are in the hundreds of µg/mL range (e.g., MIC > 500 µg/mL for E. coli). |
| Enzyme Inhibition | Protein hydrolysates (PHs) show significant inhibition of collagenase and hyaluronidase, enzymes involved in skin aging and degradation of connective tissue [156]. An inhibitor from the gut contents of A. domesticus targets microsomal oxidation, particularly affecting NADPH cytochrome c reductase, with greater activity against insect enzymes than mammalian ones [159]. | Silkworm albumin fraction showed very potent ACE inhibition (IC50 = 0.047 mg/mL). Ultrasonication increases ACE-I activity ~40–67%. | Locust protein showed inhibition of pro-inflammatory enzymes (lipoxygenase and Cyclo-oxygenase (COX-2)) in vitro. | General literature suggests weak/no known ACE or Lipoxygenase (LOX) inhibition. |
| Immunomodulation [160] | Peptide-rich cricket meals up-regulate innate immune markers in vivo: in African catfish, inclusion of 10–30% cricket meal elevated lysozyme activity, total leukocyte count, and glutathione-based antioxidant enzymes, improving survival after Aeromonas challenge. | Polysaccharides (silkrose/dipterose) and chitin/chitosan fractions act as immunostimulants. Diets containing defatted silkworm pupae (25–50%). Serum activities of the antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) were significantly increased and reduced lipid peroxidation in mirror carp; White Blood Cell (WBC) counts rose in rainbow trout, and peptidoglycan recognition protein BmPGRP-S5-mediated phagocytosis toward E. coli was demonstrated. | Limited but emerging evidence: partial fishmeal replacement (≤25%) with Locusta meal maintains or improves hematological indices and glutathione S-transferase (GST) antioxidant activity in tilapia and catfish, indicating preserved non-specific immunity; high inclusion rates can depress these markers. | Multiple bioactive drivers—chitin, medium-chain fatty acids (lauric acid), and diet-dependent antimicrobial peptides (AMPs). Feeding trials show raised lysozyme, complement activity, leukocyte counts, and up-regulation of IL-1β, IL-17F, and TNF-α genes in crayfish, catfish, and sturgeon; in vitro digestion/fermentation of BSF meal releases high Short-Chain Fatty Acids (SCFAs) levels that can further modulate gut immunity. |
| Experimental Conditions/Notes | Antioxidant activity measured by DPPH and ABTS assays in vitro. Peptides obtained via enzymatic or gastrointestinal digestion. Defatting the cricket powder enhances peptide activity. | Silkworm protein hydrolysates produced by Alcalase, etc., exhibit strong angiotensin-converting enzyme (ACE)-inhibitory activity. Peptide Tyr-Ala-Asn from silkworm reduced blood pressure in hypertensive rats. Antioxidant assays (DPPH, etc.) indicate silkworm peptides can reach ~66% radical scavenging under optimal conditions. | Hydrolysate produced via simulated Gastro Intestinal digestion. The presence of peptides that inhibit COX-2/LOX suggests an anti-inflammatory potential beyond antioxidant activity (even if intrinsic antioxidant power is modest). | Measured using Trolox-Equivalent Antioxidant Capacity (TEAC)-DPPH, TEAC-FRAP, TEAC-ABTS, and Folin–Ciocalteu. In vitro digestion + fermentation simulated small/large intestine. Digestion phase accounted for >75% of activity. Fermentation led to high SCFA release in blood meal-fed larvae. |
| Product Category and Format | Typical Insect Ingredient/Inclusion Level | Reported Benefits (Nutrition/Techno-Function/Sensory) | Representative Studies |
|---|---|---|---|
| Bakery (bread, biscuits, cookies, muffins, breakfast cereals, protein bars) | • Cricket or mealworm whole flour 5–15% (w/w dough) • Palm weevil larvae flour ≤70% in composite biscuits | ↑ protein (+30–60%), improves EAA balance (adds Lys and Trp); ↑ Fe, Zn water-binding softens crumb; ≤10% keeps color and texture acceptable; flavor easily masked with cocoa/spices | [209,210,211] |
| Pasta and noodles | • Cricket/grasshopper flour 10–15% semolina • Mealworm flour 5–10% | ↑ protein and iron; higher DIAASs; minor darkening but texture comparable to durum pasta; acceptance high when sauced | [212,213,214] |
| Meat products and extenders (sausages, patties, meatballs, burgers) | • Defatted cricket/locust/mealworm flour 5–15% of batter • Mealworm paste, 10% in hybrid burgers | Binds water and fat → lower cook loss; ↑ protein, Fe, Zn, PUFA; can lower SFA; texture/juiciness maintained; color slightly darker; allergen labeling needed | [181,215,216] |
| Meat analogs (high-moisture extrusion; jerky-style) | • Cricket flour + soy isolate (15–30% insect solids) • Insect/plant blends for jerky analogs | Forms fibrous “muscle-like” texture (anisotropic index ≤ 2.8); insect proteins supply complete AA profile; very high inclusion can lower tensile strength—blend optimization required | [181,217] |
| Extruded snacks and crisps | • Cricket/mealworm flour 5–20% with corn or rice grits | ↑ protein 2–4 g per 30 g serving; expansion ratio ↓ above ~10% insect; crunch and flavor acceptable with seasoning | [218,219,220,221] |
| Dairy analogs and beverages (protein shakes, yogurt-type, kefir) | • Cricket/buffalo worm protein powder 5–12% (RTD shakes) • Silkworm pupae or buffalo worm flour replacing 5–10% milk solids | Whey-like protein boost; fermentation kinetics retained; ↑ Fe, Zn, vit B12; “nutty/earthy” flavor must be masked; slight beige color | [222,223] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisboa, H.M.; Andrade, R.; Lima, J.; Batista, L.; Costa, M.E.; Sarinho, A.; Pasquali, M.B. Harnessing Insects as Novel Food Ingredients: Nutritional, Functional, and Processing Perspectives. Insects 2025, 16, 783. https://doi.org/10.3390/insects16080783
Lisboa HM, Andrade R, Lima J, Batista L, Costa ME, Sarinho A, Pasquali MB. Harnessing Insects as Novel Food Ingredients: Nutritional, Functional, and Processing Perspectives. Insects. 2025; 16(8):783. https://doi.org/10.3390/insects16080783
Chicago/Turabian StyleLisboa, Hugo M., Rogério Andrade, Janaina Lima, Leonardo Batista, Maria Eduarda Costa, Ana Sarinho, and Matheus Bittencourt Pasquali. 2025. "Harnessing Insects as Novel Food Ingredients: Nutritional, Functional, and Processing Perspectives" Insects 16, no. 8: 783. https://doi.org/10.3390/insects16080783
APA StyleLisboa, H. M., Andrade, R., Lima, J., Batista, L., Costa, M. E., Sarinho, A., & Pasquali, M. B. (2025). Harnessing Insects as Novel Food Ingredients: Nutritional, Functional, and Processing Perspectives. Insects, 16(8), 783. https://doi.org/10.3390/insects16080783








