Fall Webworm Host Plant Preferences Generate a Reduced Predation Enemy-Free Space in Its Interaction with Parasitoids
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects Rearing
2.2. Larvae Performance on Host Plants
2.3. Volatiles Collection
2.4. Gas Chromatography and GC-MS
2.5. Gas Chromatography-Electroantennography
2.6. Chemicals
2.7. Behavioral Assays
2.7.1. Responses of Mated Female H. cunea to Different Plants
2.7.2. Responses of 1-Day-Old Mated C. cunea Females to Various Odor Sources
- (i)
- In total, 5 g of uncontaminated plant leaves were positioned in one arm of the Y-tube olfactometer, while 5 g of leaves from the same plant, consumed by H. cunea, were placed in the other arm. The leaves were separated using 70 mesh absorbent cotton gauze to prevent physical contact. Forty mated female C. cunea (1-day-old) were released at the base of the common arm of the Y-tube and monitored for a maximum of 5 min. Females failing to exhibit orientation behavior within 5 min were excluded from the analysis. Females that traversed 1 cm beyond the Y-junction and remained there for at least 10 s were recorded to have made a selection. Following each assay, the tube was cleansed with soap, water, hexane, and air dried, and its orientation was reversed. Five independent biological tests were performed for each odor source. Each parasitoid was utilized only once. All bioassays were conducted at 25 °C and 60% relative humidity (RH). The female response percentage was calculated as follows: Rs = 100% × (parasitoids that selected the treatment arm)/(parasitoids that selected the treatment arm + parasitoids that selected the control arm).
- (ii)
- The responses of C. cunea to the eight different plant leaves after they were consumed by H. cunea were investigated using a four-arm olfactometer. The experiment was conducted as follows: group 1: the attraction of four plants to the wasps was compared; group 2: attraction to the other four plants was compared; group 3: the two most attractive plants in group 1 were compared with the two most attractive plants in group 2; and group 4: the two least attractive plants in group 1 were compared with group 2 again. Leaves (5 g) consumed by H. cunea were placed inside the chambers of four randomly selected arms of the olfactometer. Forty 1-day-old mated female C. cunea were introduced in the center of the arena, and their selection of the four arms was recorded. A selection was defined as entering one of the four arms and remaining there for at least 10 s. The odor sources in the olfactometer were replaced, and their positions were altered at every assay. Additionally, the entire arena and odor chambers were cleansed with soapy water followed by hexane and then air-dried. Each parasitoid was utilized only once. Nine independent biological tests were conducted, and the bioassays were performed at 25 °C and 60% RH.
- (iii)
- For chemical standard testing, test compounds at various concentrations (10, 100, 1000, and 10,000 ng/μL, 10 μL) were applied to filter papers. After a 20 s period to allow for solvent evaporation, the filter papers were positioned in one arm of the Y-tube olfactometer. A corresponding filter paper with 10 μL of hexane was placed in the opposite arm (solvent control). The bioassay procedures with C. cunea were conducted as previously described.
- (iv)
- The responses of C. cunea to 10 μL of synthetic blends of EAD-active compounds were examined using a four-arm olfactometer. Filter papers containing synthetic blends of EAD-active compounds (10 ng/uL for each component), synthetic blends (excluding tridecane), and hexane were positioned within the chambers of three randomly selected arms of the olfactometer. The fourth arm received 10 μL hexane and functioned as a solvent control. The bioassay procedures with C. cunea were conducted as previously described.
2.7.3. Responses of Female Adult H. cunea to Different Compounds
2.8. Statistical Analysis
3. Results
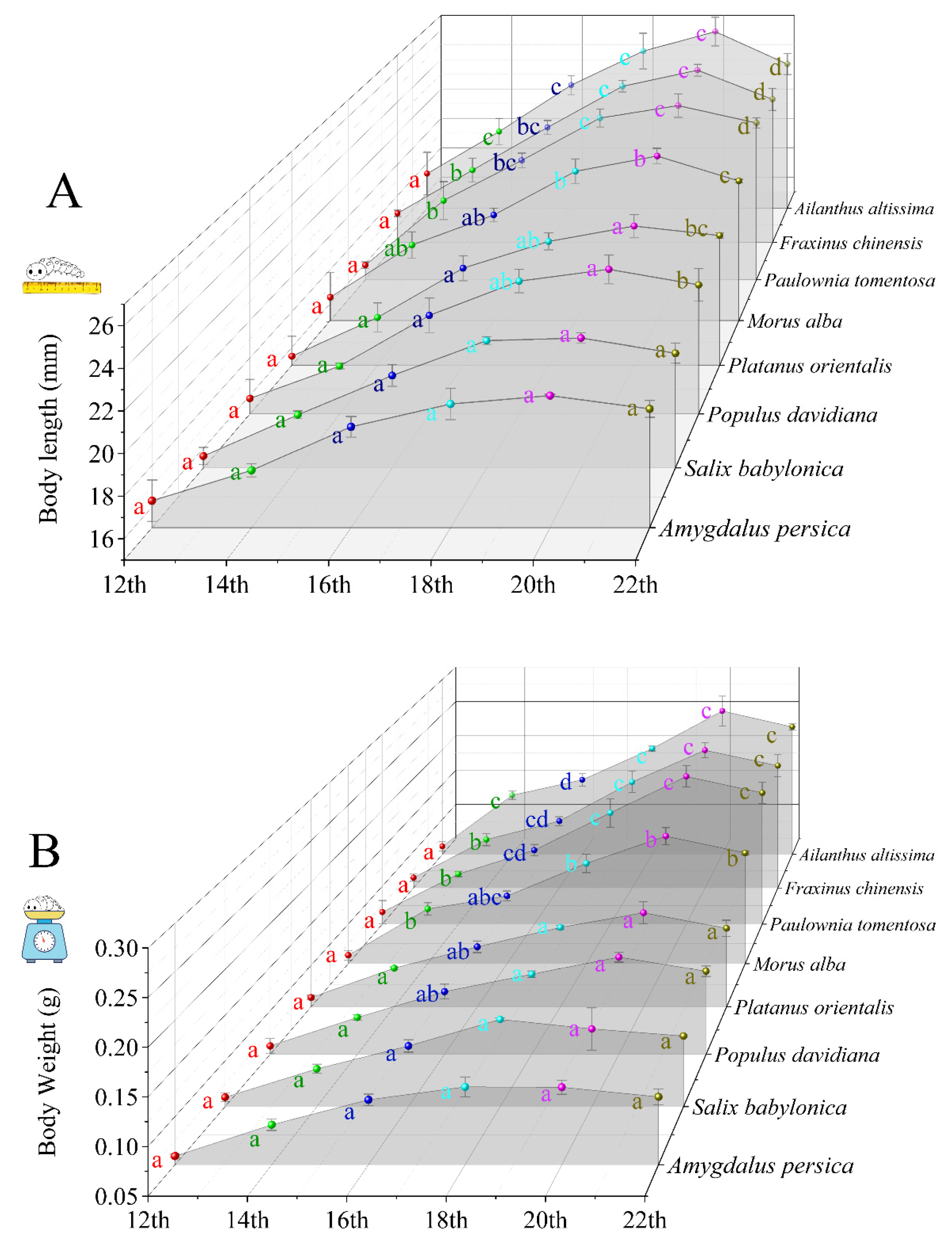
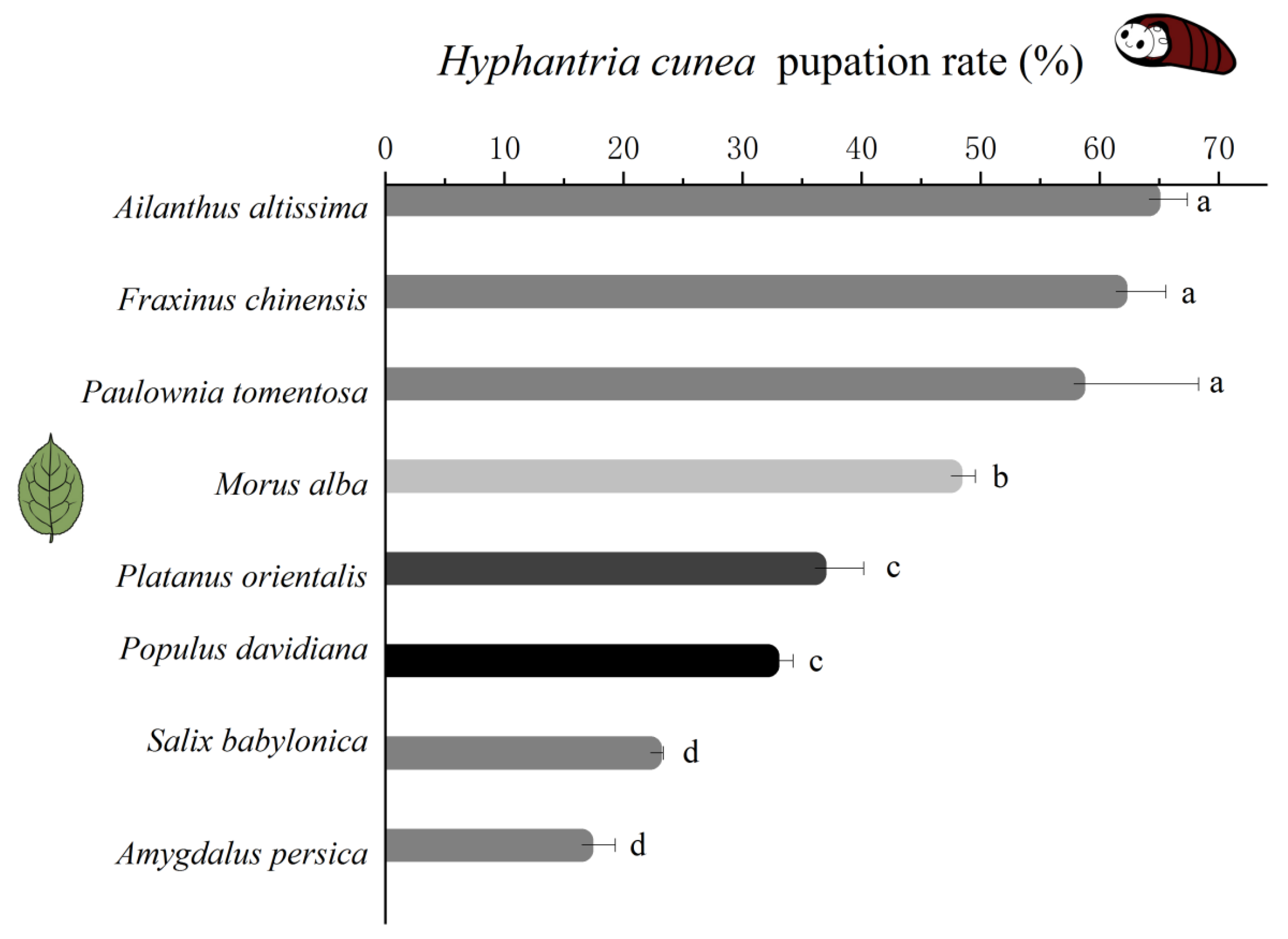
3.1. Growth of H. cunea Fed Different Plant Leaves
3.2. H. cunea Preference of Eight Plant Species
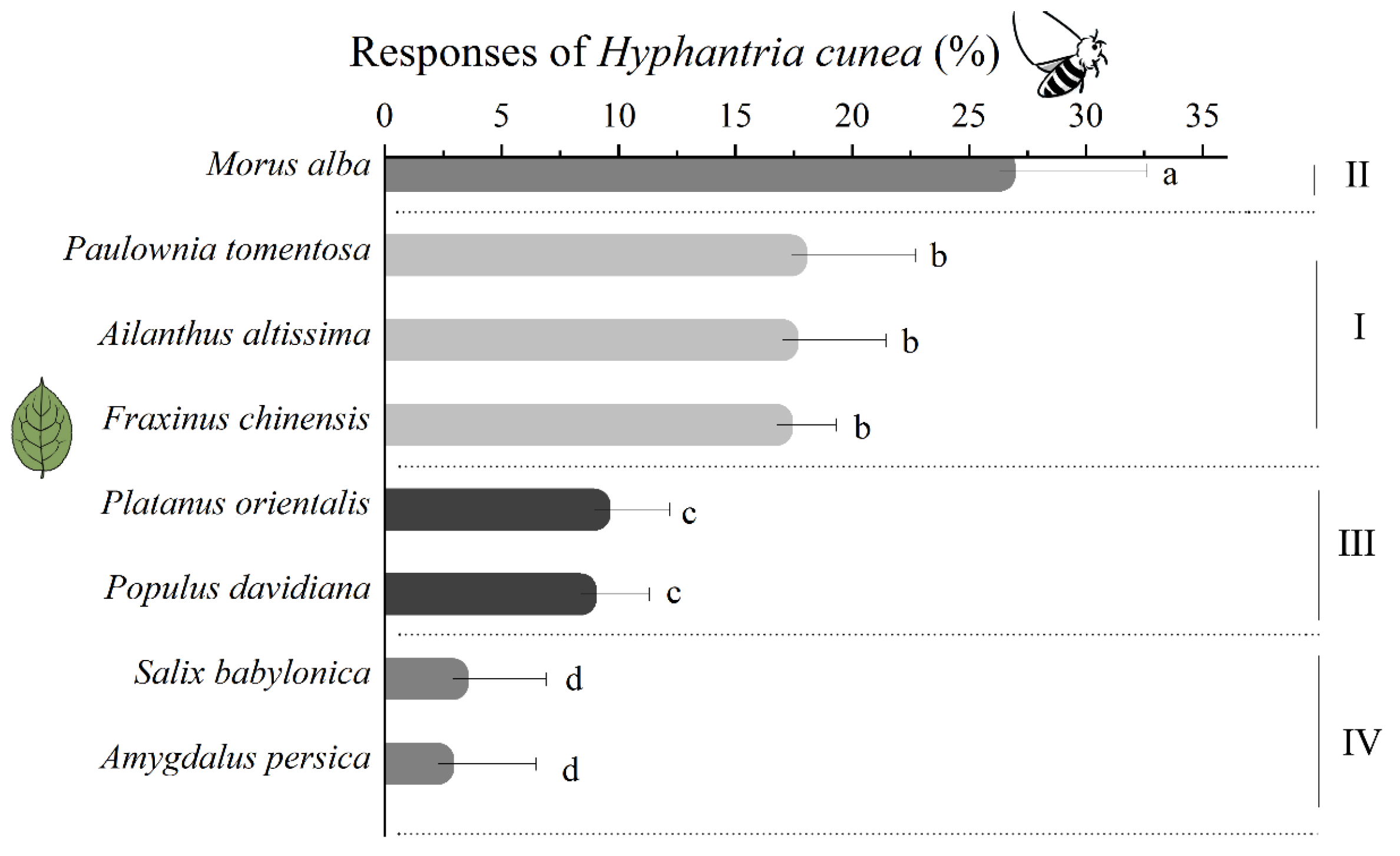
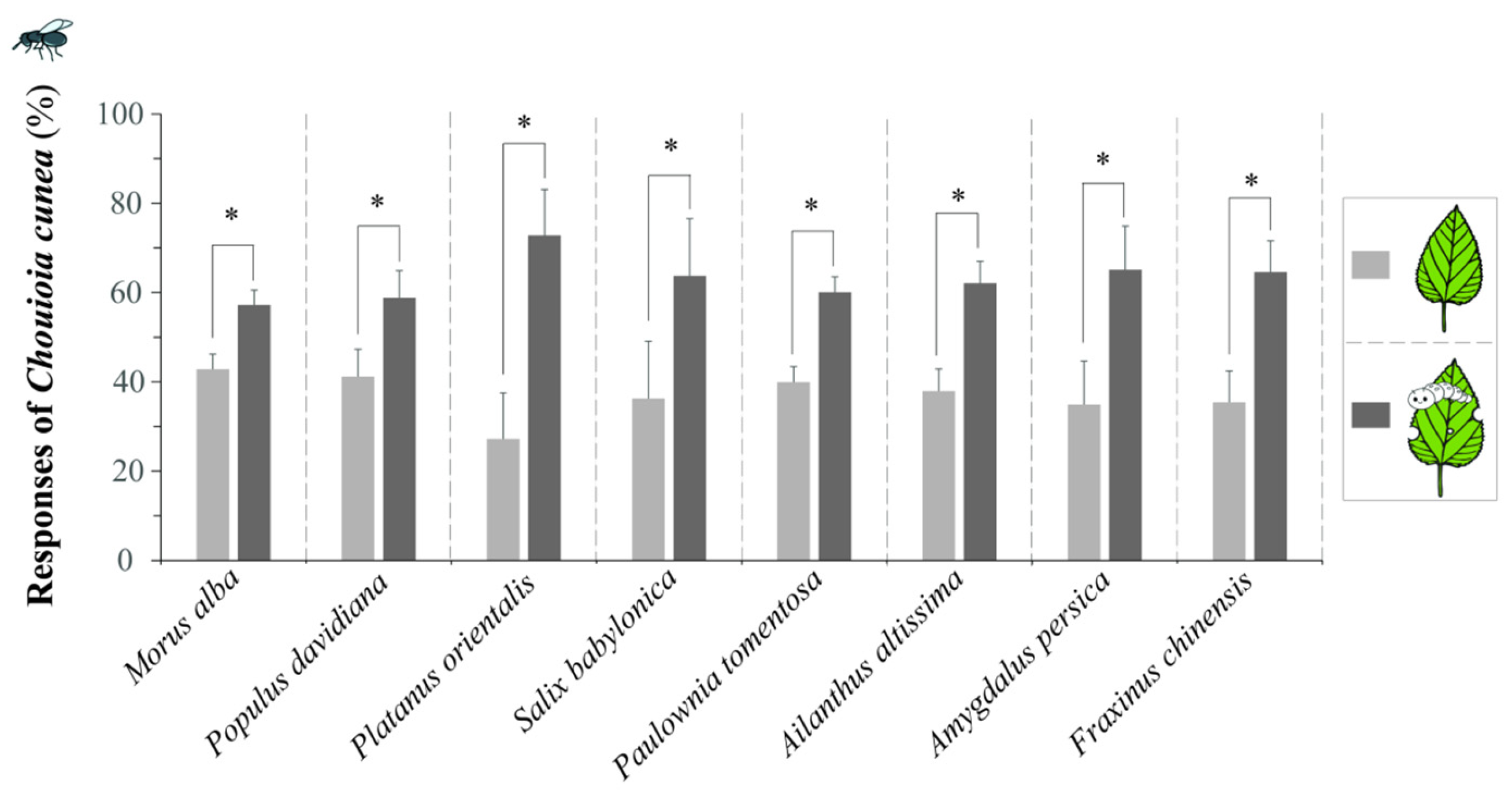
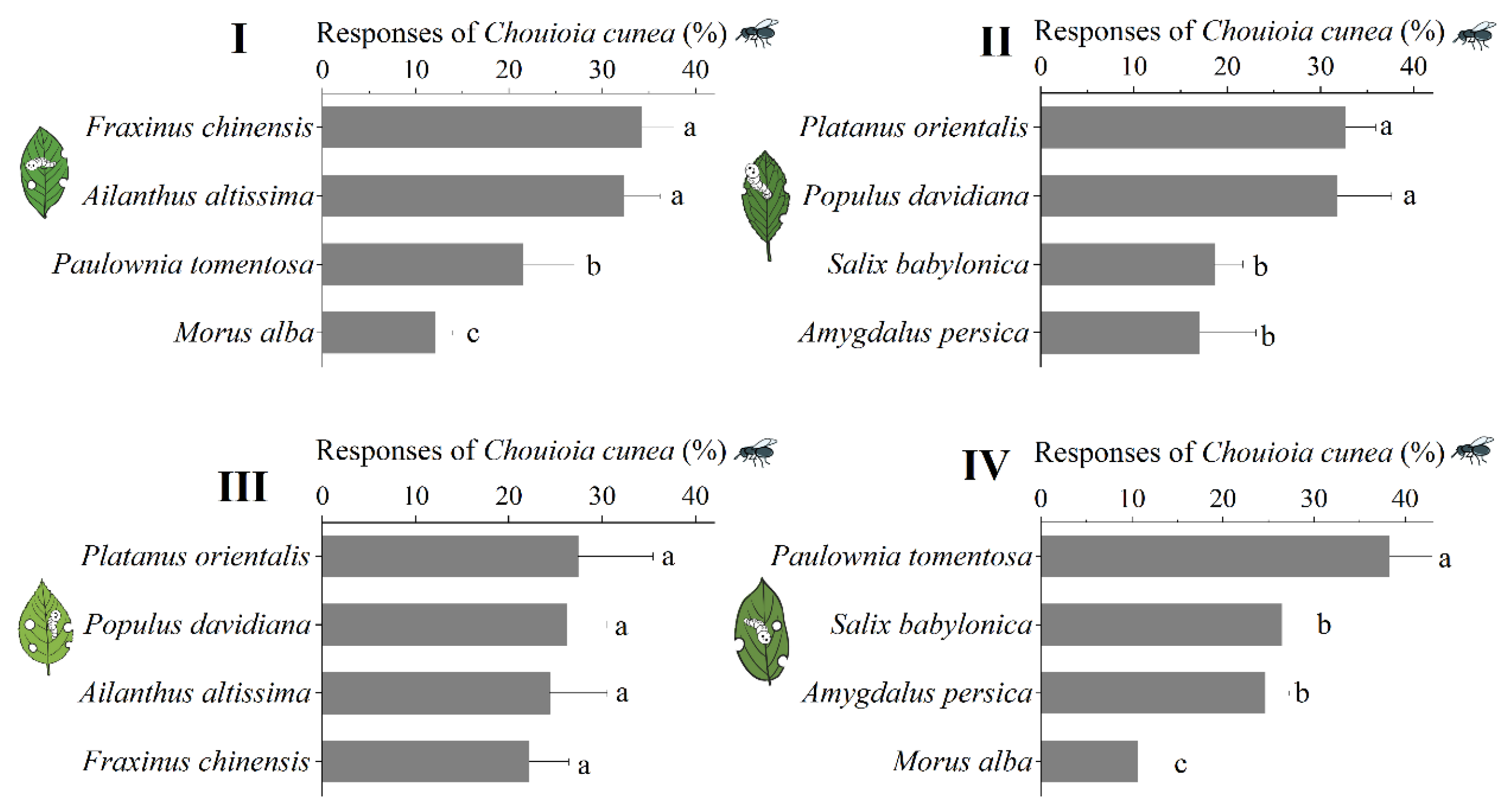
3.3. Responses of C. cunea to Leaves Before and After Feeding by H. cunea
3.4. Comparison of Responses of C. cunea to Different Plant Leaves Fed upon by H. cunea
3.5. Chemical Analysis
3.6. Olfactometer Bioassays
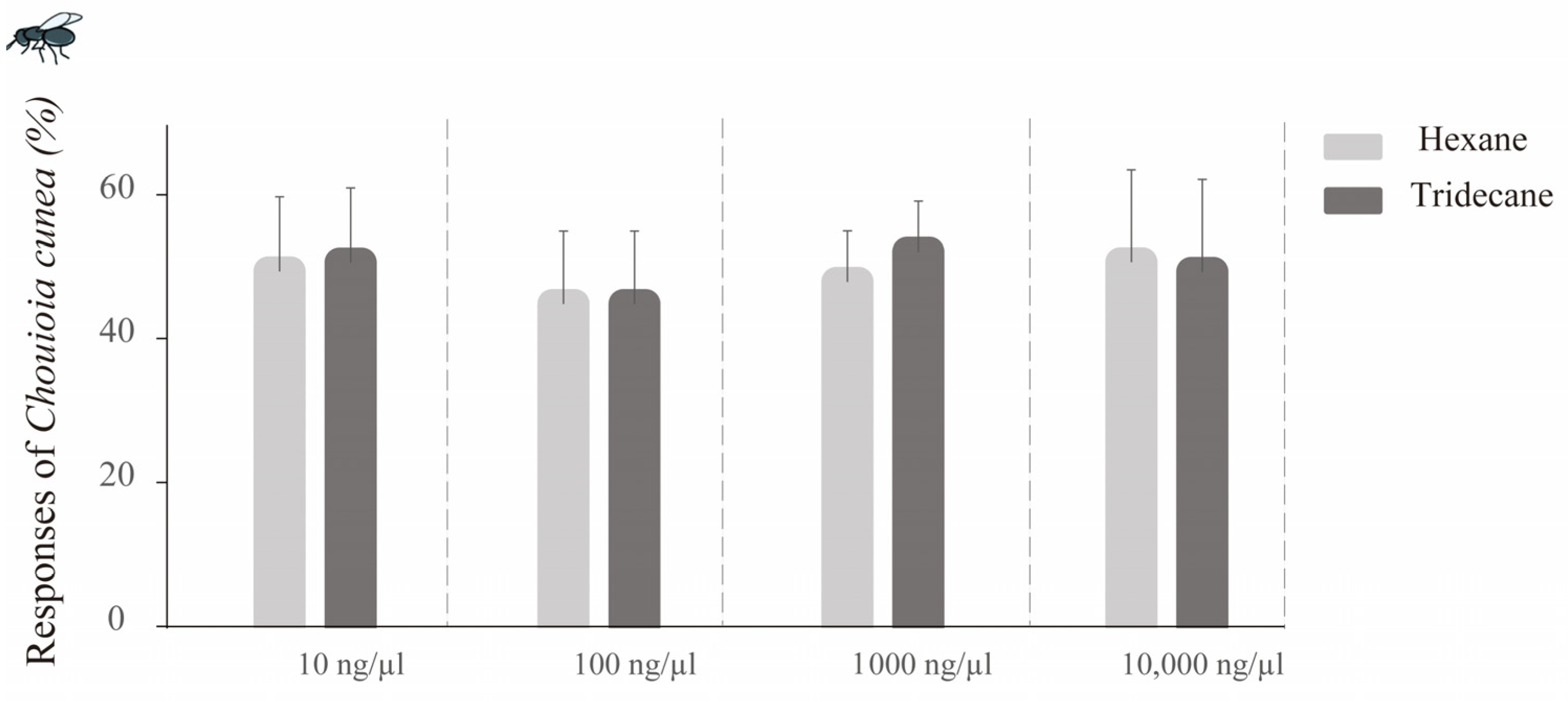
3.6.1. Tridecane vs. Hexane
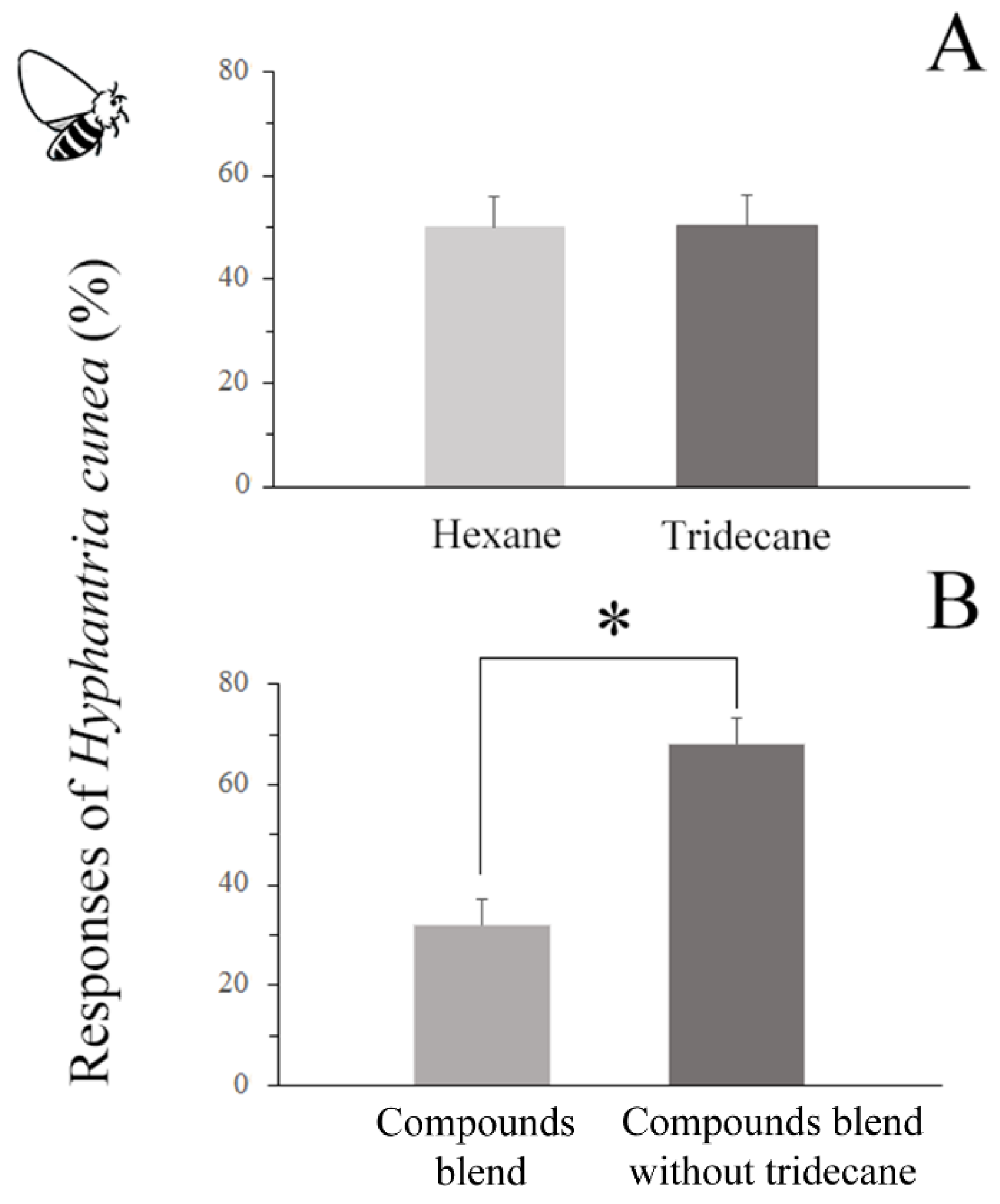
3.6.2. Responses of C. cunea to Synthetic Blends of EAD-Active Compounds
3.6.3. Responses of H. cunea Females to Different Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biere, A.; Elzinga, J.A.; Honders, S.C.; Harvey, J.A. A plant pathogen reduces the enemy-free space of an insect herbivore on a shared host plant. Proc. R. Soc. B-Biol. Sci. 2002, 269, 2197–2204. [Google Scholar] [CrossRef]
- Singer, M.S.; Rodrigues, D.; Stireman, J.O.; Carriere, Y. Roles of food quality and enemy-free space in host use by a generalist insect herbivore. Ecology 2004, 85, 2747–2753. [Google Scholar] [CrossRef]
- Hu, X.Y.; Su, S.L.; Liu, Q.S.; Jiao, Y.Y.; Peng, Y.F.; Li, Y.H.; Turlings, T.C.J. Caterpillar-induced rice volatiles provide enemy-free space for the offspring of the brown planthopper. Elife 2020, 9, e55421. [Google Scholar] [CrossRef]
- Singer, M.S.; Stireman, J.O. Does anti-parasitoid defense explain host-plant selection by a polyphagous caterpillar? Oikos 2003, 100, 554–562. [Google Scholar] [CrossRef]
- Jeffries, M.J.; Lawton, J.H. Enemy free space and the structure of ecological communities. Biol. J. Linn. Soc. 1984, 23, 269–286. [Google Scholar] [CrossRef]
- Price, P.W.; Fernandes, G.W.; Waring, G.L. Adaptive nature of insect galls. Environ. Entomol. 1987, 16, 15–24. [Google Scholar] [CrossRef]
- Lill, J.T.; Marquis, R.J.; Ricklefs, R.E. Host plants influence parasitism of forest caterpillars. Nature 2002, 417, 170–173. [Google Scholar] [CrossRef]
- Mulatu, B.; Applebaum, S.W.; Coll, M. A recently acquired host plant provides an oligophagous insect herbivore with enemy-free space. Oikos 2004, 107, 231–238. [Google Scholar] [CrossRef]
- Alhmedi, A.; Bylemans, D.; Bangels, E.; Belien, T. Cultivar-mediated effects on apple-Dysaphis plantaginea interaction. J. Pest Sci. 2021, 95, 1303–1315. [Google Scholar] [CrossRef]
- Ohsaki, N.; Sato, Y. Food plant choice of pieris butterflies as a trade-off between parasitoid avoidance and quality of plants. Ecology 1994, 75, 59–68. [Google Scholar] [CrossRef]
- Vidal, M.C.; Murphy, S.M. Bottom-up vs. top-down effects on terrestrial insect herbivores: A meta-analysis. Ecol. Lett. 2018, 21, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Englishloeb, G.M.; Brody, A.K.; Karban, R. Host-plant-mediated interactions between a generalist folivore and its tachinid parasitoid. J. Anim. Ecol. 1993, 62, 465–471. [Google Scholar] [CrossRef]
- Williams, I.S.; Jones, T.H.; Hartley, S.E. The role of resources and natural enemies in determining the distribution of an insect herbivore population. Ecol. Entomol. 2001, 26, 204–211. [Google Scholar] [CrossRef]
- Murphy, S.M.; Lill, J.T.; Bowers, M.D.; Singer, M.S. Enemy-Free Space for Parasitoids. Environ. Entomol. 2014, 43, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Xie, B.Y.; Li, X.H.; Gao, Z.X.; Li, D.M. Research progress on the invasive species, Hyphantria cunea. Entomol. Knowl. 2003, 40, 13–18. [Google Scholar]
- Gao, B.J.; Du, J.; Gao, S.H.; Liu, J.X. Genetic diversity and differentiations of fall webworm (Hyphantria cunea) populations. Sci. Silvae Sin. 2010, 8, 120–124. [Google Scholar]
- Zhang, X.X.; Wang, Z.J. Research progress on the Hyphantria cunea (Drury) of alien invasive species. J. Anhui Agric. Sci. 2009, 37, 215–219. [Google Scholar]
- Yang, Z.Q. A new genus and species of Eulophidae (Hymenoptera: Chalcidoidea) parasitizing Hyphantria cunea (Drury) (Lepidoptera: Arctiidae) in China. Entomotaxonomia 1989, 11, 117–123. [Google Scholar]
- Yang, Z.Q. Anatomy of internal reproductive system of Chouioia cunea (Hymenoptera, Chalcidoidea, Eulophidae). Sci. Silvae Sin. 1995, 31, 23–26. [Google Scholar]
- Yang, Z.Q. Advance in bio-control researches of the important forest insect pests with natural enemies in China. Chin. J. Biol. Control 2004, 20, 221–227. [Google Scholar]
- Yang, Z.Q.; Wang, X.Y.; Wei, J.R.; Qu, H.R.; Qiao, X.R. Survey of the native insect natural enemies of Hyphantria cunea (Drury) (Lepidoptera: Arctiidae) in China. Bull. Entomol. Res. 2008, 98, 293–302. [Google Scholar] [CrossRef]
- Zheng, Y.N.; Qi, J.Y.; Sun, S.H.; Yang, C.C. Advance in research of Chouioia cunea Yang (Hymenoptera: Eulophidade) and its biocontrol application in China. Chin. J. Biol. Control 2012, 28, 275–281. [Google Scholar]
- Pan, L.; Gao, W.; Liu, X.; Qin, D.; Zhang, T.; Ren, R.; Zhang, W.; Sun, M.; Gao, C.; Bai, P.; et al. Parasitoids as taxonomists: How does the parasitoid Chouioia cunea distinguish between a host and a non-host? Pest. Manag. Sci. 2023, 79, 4547–4556. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Yuan, Y.F.; Wu, L.; Chen, M. Effects of host plants on the feeding behavior and detoxification enzyme activities in Hyphantria cunea (Lepidoptera: Arctiidae) larvae. Acta Entomol. Sin. 2018, 61, 232–239. [Google Scholar]
- Hu, D.Q.; Li, R.; Song, X.X. Research on feeding habits of Hyphantria cunea larvae in Qingdao area. Heilongjiang Agric. Sci. 2019, 10, 66–69. [Google Scholar]
- Wang, F.; Zhang, L.H.; Han, H.Z.; Wang, X.L.; Zhang, Y.; Li, S.H. Feeding preference of Hyphantria cunea Drury larvae to common garden plants in Suqian area. J. Henan Agric. Univ. 2020, 54, 1002–1008. [Google Scholar]
- Costa, A.; Reeve, J.D. Olfactory Experience Modifies Semiochemical Responses in a Bark Beetle Predator. J. Chem. Ecol. 2011, 37, 1166–1176. [Google Scholar] [CrossRef]
- Lo Giudice, D.; Riedel, M.; Rostas, M.; Peri, E.; Colazza, S. Host Sex Discrimination by an Egg Parasitoid on Brassica Leaves. J. Chem. Ecol. 2011, 37, 622–628. [Google Scholar] [CrossRef]
- Mouratidis, A.; Vacas, S.; Herrero, J.; Navarro-Llopis, V.; Dicke, M.; Tena, A. Parasitic wasps avoid ant-protected hemipteran hosts via the detection of ant cuticular hydrocarbons. Proc. R. Soc. B-Biol. Sci. 2021, 288, 20201684. [Google Scholar] [CrossRef]
- Piesik, D.; Wenda-Piesik, A.; Krasinska, A.; Wrzesinska, D.; Delaney, K.J. Volatile organic compounds released by Rumex confertus following Hypera rumicis herbivory and weevil responses to volatiles. J. Appl. Entomol. 2016, 140, 308–316. [Google Scholar] [CrossRef]
- Gantner, M.; Najda, A.; Piesik, D. Effect of phenolic acid content on acceptance of hazel cultivars by filbert aphid. Plant Prot. Sci. 2019, 55, 116–122. [Google Scholar] [CrossRef]
- Piesik, D.; Wenda-Piesik, A. Sitophilus granarius responses to blends of five groups of cereal kernels and one group of plant volatiles. J. Stored Prod. Res. 2015, 63, 63–66. [Google Scholar] [CrossRef]
- Zhu, G.; Pan, L.; Zhao, Y.; Zhang, X.; Wang, F.; Yu, Y.; Fan, W.; Liu, Q.; Zhang, S.; Li, M. Chemical investigations of volatile kairomones produced by Hyphantria cunea (Drury), a host of the parasitoid Chouioia cunea Yang. Bull. Entomol. Res. 2017, 107, 234–240. [Google Scholar] [CrossRef]
- Awater-Salendo, S.; Hilker, M.; Fürstenau, B. Kairomone-induced changes in foraging activity of the larval ectoparasitoid Holepyris sylvanidis are linked with an increased number of male parasitoid offspring. Front. Ecol. Evol. 2023, 11, 1158081. [Google Scholar] [CrossRef]
- Liu, C.M.; Xu, F.Y.; Jiang, J.H.; Wu, J.Y.; Wang, M.M.; Li, Y.; Wang, Y.H.; Wei, D.X. Effects of main tree species in northern Jiangsu on occurrence and larval growth of Hyphantria cunea. J. Jiangsu For. Sci. Technol. 2013, 40, 6–8. [Google Scholar]
- Qin, X.B.; Li, D.J.; Shao, W.H.; Li, Z.P.; Jiang, C.P.; Qu, H.R.; Wang, C.Z. Problems and suggestions on the control of Hyphantria cunea in Shandong Province. For. Pest Dis. 2000, 19, 41–43. [Google Scholar]
- Yu, S.W. Oviposition habit and host selection of Hyphantria cunea in Langfang. Mod. Rural. Sci. Technol. 2016, 4, 54–55. [Google Scholar]
- Chen, H.; Su, H.H.; Zhang, S.; Jing, T.X.; Liu, Z.; Yang, Y.Z. The Effect of Mirid Density on Volatile-Mediated Foraging Behaviour of Apolygus lucorum and Peristenus spretus. Insects 2021, 12, 870. [Google Scholar] [CrossRef]
- Murphy, S.M.; Loewy, K.J. Trade-offs in host choice of an herbivorous insect based on parasitism and larval performance. Oecologia 2015, 179, 741–751. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Turlings, T.C.J. Advances and challenges in the identification of volatiles that mediate interactions among plants and arthropods. Analyst 2006, 131, 24–32. [Google Scholar] [CrossRef]
- Jayanthi, P.D.K.; Subramoniam, A.; Kumar, P.S.; Jayanthimala, B.R.; Rekha, A. Do conspecific herbivores track resource depletion through host phenology-specific HIPVs? Curr. Sci. 2021, 121, 286–293. [Google Scholar] [CrossRef]
- Cai, P.M.; Song, Y.Z.; Huo, D.; Lin, J.; Zhang, H.M.; Zhang, Z.H.; Huang, F.M.; Xiao, C.M.; Ji, Q.E. Chemical cues mediating behavioral and electrophysiological responses of Fopius Arisanus (Hymenoptera: Braconidae): The Role Of Herbivore-Induced Plant Volatiles. Appl. Ecol. Environ. Res. 2020, 18, 5475–5489. [Google Scholar] [CrossRef]
- Du, Y.W.; Shi, X.B.; Zhao, L.C.; Yuan, G.G.; Zhao, W.W.; Huang, G.H.; Chen, G. Chinese Cabbage Changes Its Release of Volatiles to Defend against Spodoptera litura. Insects 2022, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, B.; Chakraborti, U.; Mandal, A.; Paul, B.; Bhadra, K. Attraction of Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae) to Host Plant Cucurbita maxima Duchesne (Cucurbitaceae) Volatiles. Agronomy 2022, 12, 2640. [Google Scholar] [CrossRef]
- Song, Y.Z.; Guo, Y.Q.; Cai, P.M.; Chen, W.B.; Liu, C.M. Alteration of volatile chemical composition in tobacco plants due to green peach aphid (Myzus persicae sulzer) (Hemiptera: Aphididae) feeding. Appl. Ecol. Environ. Res. 2021, 19, 159–169. [Google Scholar] [CrossRef]
- Malek, R.; Kaser, J.M.; Anfora, G.; Ciolli, M.; Khrimian, A.; Weber, D.C.; Hoelmer, K.A. Trissolcus japonicus foraging behavior: Implications for host preference and classical biological control. Biol. Control 2021, 161, 104700. [Google Scholar] [CrossRef]
- Fraga, D.F.; Parker, J.; Busoli, A.C.; Hamilton, G.C.; Nielsen, A.L.; Rodriguez-Saona, C. Behavioral responses of predaceous minute pirate bugs to tridecane, a volatile emitted by the brown marmorated stink bug. J. Pest Sci. 2017, 90, 1107–1118. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Wu, W.W.; Li, G.K. Study of the Alarming Volatile Characteristics of Tessaratoma papillosa Using SPME-GC-MS. J. Chromatogr. Sci. 2009, 47, 291–296. [Google Scholar] [CrossRef][Green Version]
- Gasch, T.; Vilcinskas, A. The chemical defense in larvae of the earwig Forficula auricularia. J. Insect Physiol. 2014, 67, 1–8. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, A.Q.; Ryu, J.; Del Mármol, J. Structural basis of odor sensing by insect heteromeric odorant receptors. Science 2024, 28, 1460–1467. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Wäckers, F. Recruitment of Predators and Parasitoids by Herbivore-Injured Plants; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Vet, L.E.M.; Dicke, M. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 1992, 37, 141–172. [Google Scholar] [CrossRef]
- van Alphen, J.J.M.; Jervis, M.A. Insect Natural Enemies: Practical Approaches to Their Study and Evolution.; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Li, M.; Yang, Y.X.; Yao, Y.H.; Xiang, W.F.; Han, J.Y.; Wang, Y.H.; Bai, P.H.; Wang, J.; Zhu, G.P.; Man, L.; et al. Isolation and identification of attractants from the pupae of three lepidopteran species for the parasitoid Chouioia cunea Yang. Pest Manag. Sci. 2020, 76, 1920–1928. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; Van Loon, J.J.A.J.J.L.; Dicke, M. Insect–Plant Biology, 2nd ed.; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]

| Compound | Retention Time | Emission Rate (ng/min from 1 g Leaves) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M. alba | P. davidiana | P. orientalis | A. altissima | S. babylonica | A. persica | P. tomentosa | F. chinensis | |||
| 1 | Undecane | 8.85 | 31.48 | 9.02 | 17.87 | 44.36 | 16.52 | 15.70 | 17.51 | 19.07 |
| 2 | Tridecane | 11.43 | N | 30.76 | 30.26 | 30.40 | 4.98 | 9.12 | 21.10 | 27.72 |
| 3 | Tetradecane | 12.60 | 22.85 | 15.11 | 11.41 | 19.25 | 13.70 | 19.91 | 14.11 | 35.97 |
| 4 | Hexadecane | 14.86 | 20.63 | 15.98 | 23.20 | 42.31 | 13.50 | 21.01 | 17.21 | 13.36 |
| 5 | Nonadecane | 17.85 | 17.23 | 8.91 | 21.80 | 24.60 | 13.68 | 15.68 | 13.62 | 14.68 |
| 6 | Heneicosane | 24.81 | 17.17 | 11.47 | 18.57 | 20.24 | 13.42 | 13.95 | 13.56 | 22.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Gao, W.; Song, Z.; Li, X.; Wei, Y.; Qin, G.; Hu, Y.; Sun, Z.; Gao, C.; Bai, P.; et al. Fall Webworm Host Plant Preferences Generate a Reduced Predation Enemy-Free Space in Its Interaction with Parasitoids. Insects 2025, 16, 804. https://doi.org/10.3390/insects16080804
Pan L, Gao W, Song Z, Li X, Wei Y, Qin G, Hu Y, Sun Z, Gao C, Bai P, et al. Fall Webworm Host Plant Preferences Generate a Reduced Predation Enemy-Free Space in Its Interaction with Parasitoids. Insects. 2025; 16(8):804. https://doi.org/10.3390/insects16080804
Chicago/Turabian StylePan, Lina, Wenfang Gao, Zhiqin Song, Xiaoyu Li, Yipeng Wei, Guangyan Qin, Yiping Hu, Zeyang Sun, Cuiqing Gao, Penghua Bai, and et al. 2025. "Fall Webworm Host Plant Preferences Generate a Reduced Predation Enemy-Free Space in Its Interaction with Parasitoids" Insects 16, no. 8: 804. https://doi.org/10.3390/insects16080804
APA StylePan, L., Gao, W., Song, Z., Li, X., Wei, Y., Qin, G., Hu, Y., Sun, Z., Gao, C., Bai, P., Zhu, G., Wang, W., & Li, M. (2025). Fall Webworm Host Plant Preferences Generate a Reduced Predation Enemy-Free Space in Its Interaction with Parasitoids. Insects, 16(8), 804. https://doi.org/10.3390/insects16080804










