Modeling the Historical and Future Potential Global Distribution of the Pepper Weevil Anthonomus eugenii Using the Ensemble Approach
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overall Modeling Workflow
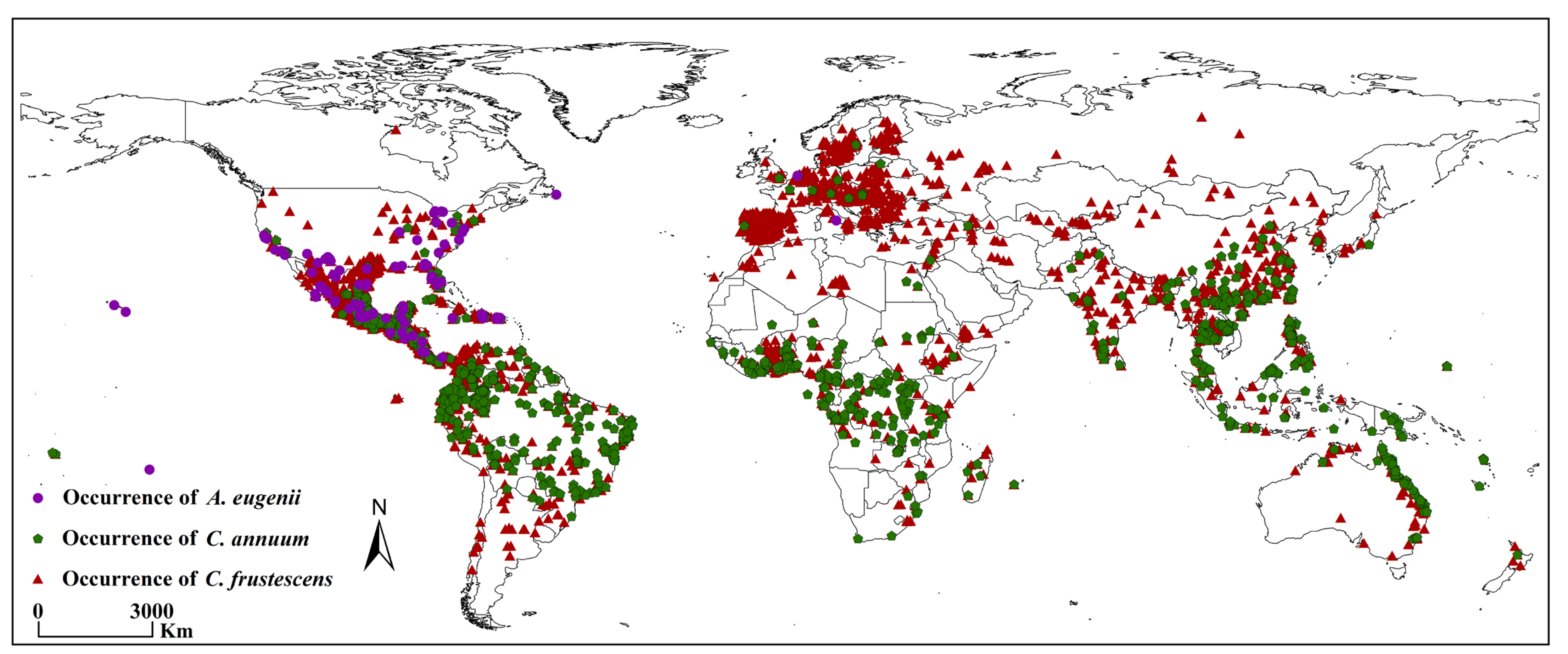
2.2. Acquisition of Global Presence Records of A. eugenii, C. annuum, and C. frutescens
2.3. Selection of Climate Data in Different Climate Scenarios
2.4. Using RF to Project the Potential Global Distribution of A. eugenii, C. annuum, and C. frutescens
2.4.1. Modeling Process
2.4.2. Evaluation of Model Accuracy
2.4.3. Classification of Suitability
2.5. Using CLIMEX to Project the Potential Global Distribution of A. eugenii
2.5.1. CLIMEX Model
2.5.2. Parameter Fitting
2.5.3. Classification of EI Values
2.6. Potential Global Distribution of A. eugenii Hosts
2.7. Potential Global Distribution of A. eugenii Considering Its Hosts
2.8. Potential Global Distribution of A. eugenii Predicted Using the Ensemble Model
3. Results
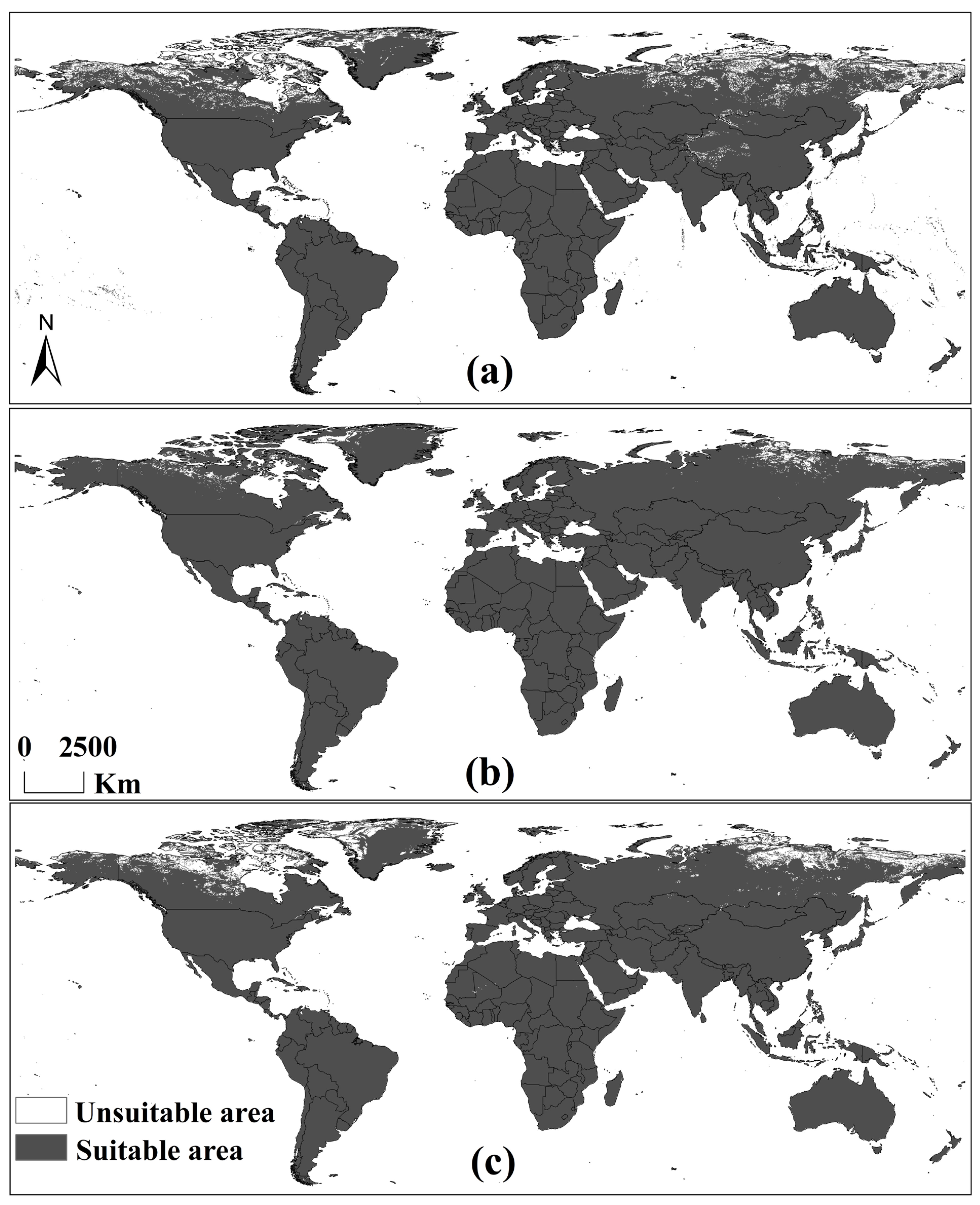
3.1. Global Hosts’ Potential Distribution for A. eugenii Projected Using RF
3.2. Potential Global Distribution of A. eugenii Projected Using RF Considering the Hosts
3.2.1. Model Performance and Key Environmental Factors
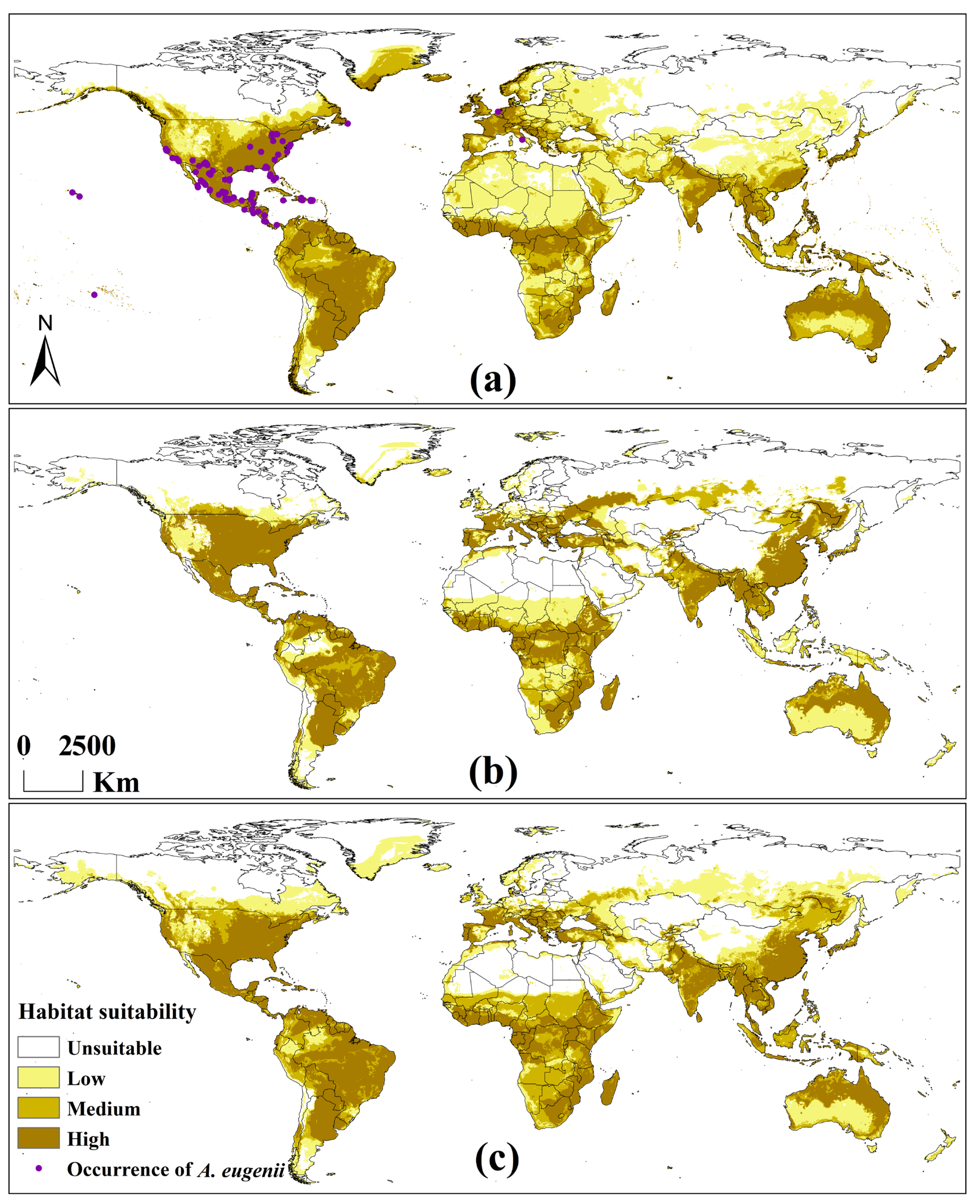
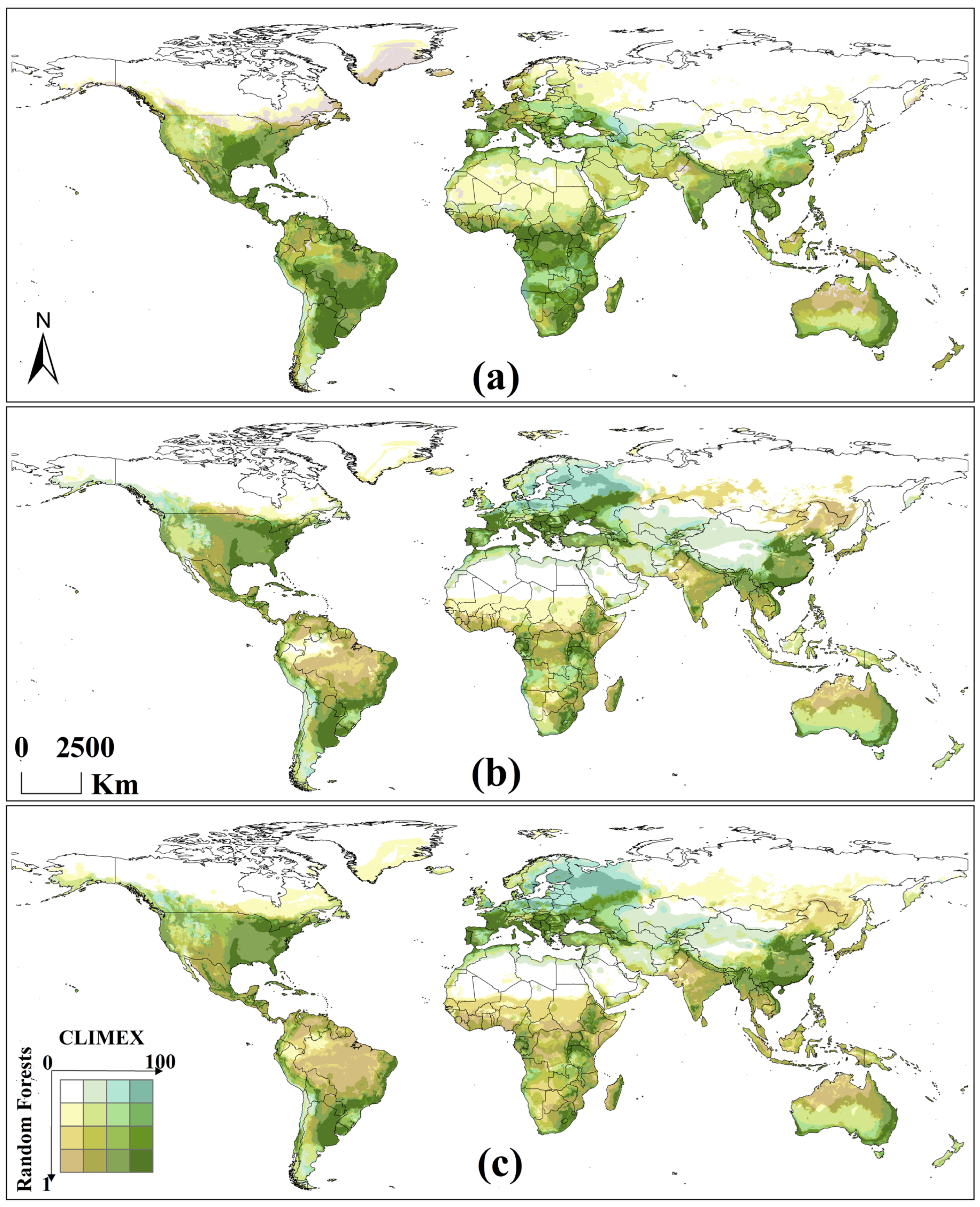
3.2.2. Potential Global Distribution Under Historical Climate Scenario
3.2.3. Potential Global Distribution Under Future Climate Scenarios
3.3. Potential Global Distribution of A. eugenii Projected Using CLIMEX Considering the Hosts
3.3.1. Model Performance
3.3.2. Potential Global Distribution Under Historical Climate Scenario
3.3.3. Potential Global Distribution Under Future Climate Scenarios
3.4. Potential Global Distribution of A. eugenii Projected Using Ensemble Model
3.4.1. Potential Global Distribution Under Historical Climate Scenario
3.4.2. Potential Global Distribution Under Future Climate Scenarios
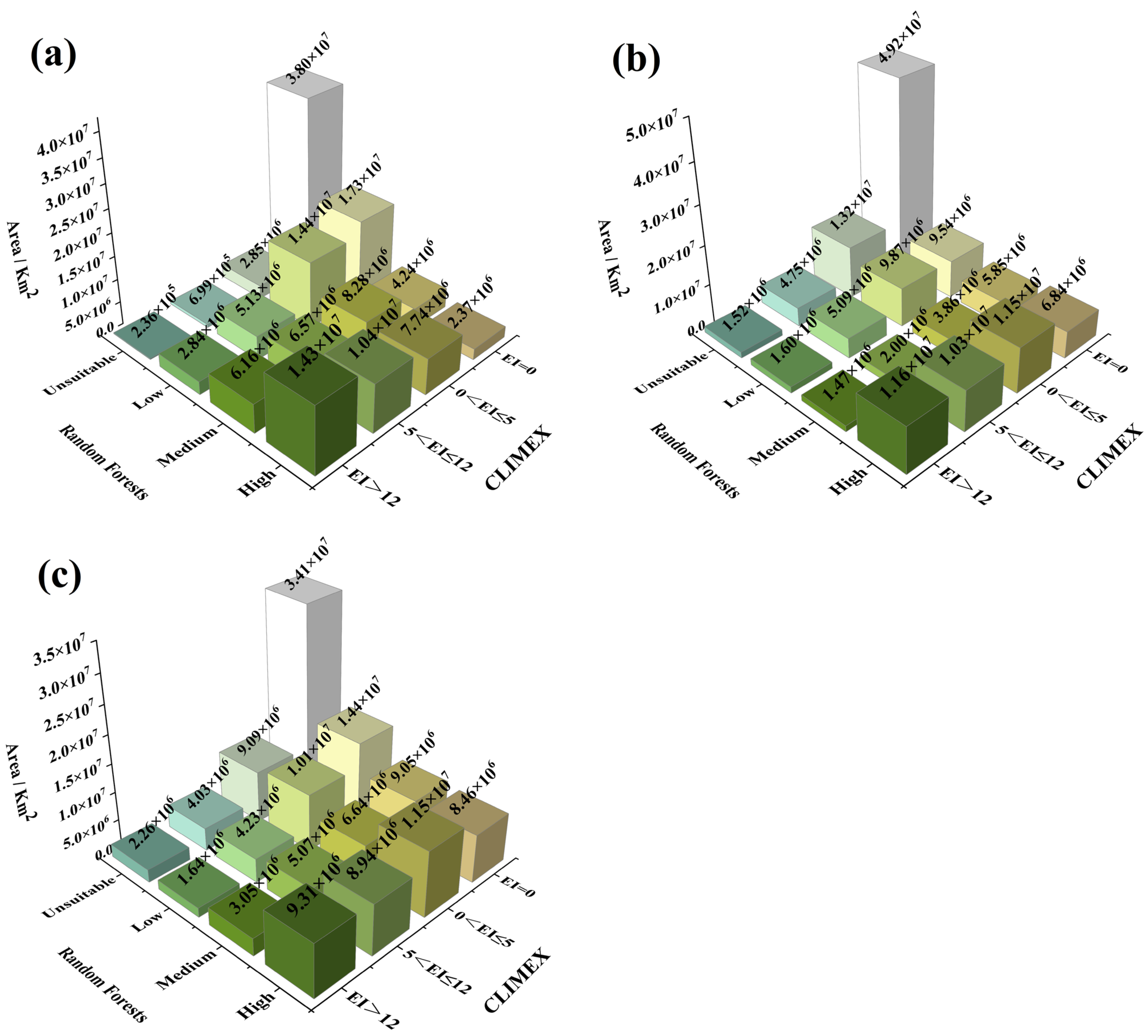
3.4.3. Distribution Area of Different Suitability Levels
4. Discussion
4.1. Potential Global Distribution Range Changes of A. eugenii Under Historical and Future Climate Conditions
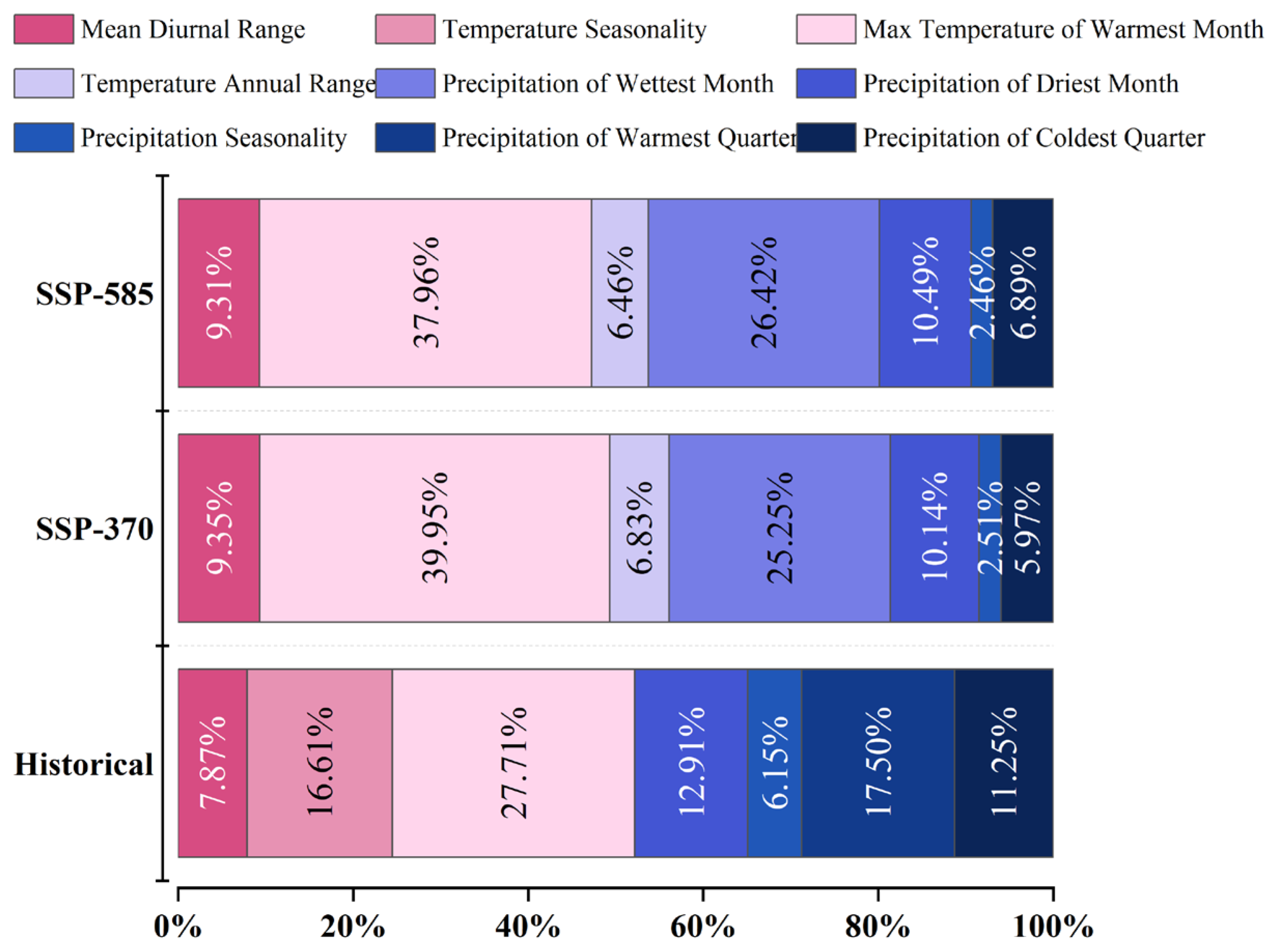
4.2. Major Climatic Factors Affecting the Potential Global Distribution of A. eugenii
4.3. Quarantine Measures and Management Plan for A. eugenii
4.4. Limitations and Future Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A. eugenii | Anthonomus eugenii |
| C. annuum | Capsicum annuum |
| C. frutescens | Capsicum frutescens |
| RF | Random Forests |
References
- Huang, H.; Zhou, L.; Lin, Q.; Wang, Y.; Wu, P. Be alert to the invasion of devastating pest pepper weevil Anthonomus eugenii into China. J. Plant Prot. 2023, 50, 1237–1243. [Google Scholar] [CrossRef]
- Clausen, C.P. Curculionidae. In Introduced Parasites and Predators of Arthropod Pests and Weeds: A World Review; Clausen, C.P., Ed.; United States Department of Agriculture: Washington, DC, USA, 1978; Volume 480, pp. 259–276. [Google Scholar]
- Speranza, S.; Colonnelli, E.; Garonna, A.P.; Laudonia, S. First Record of Anthonomus eugenii (Coleoptera: Curculionidae) in Italy. Fl. Entomol. 2014, 97, 844–845. [Google Scholar] [CrossRef]
- Labbé, R.M.; Hilker, R.; Gagnier, D.; McCreary, C.; Gibson, G.A.P.; Fernández-Triana, J.; Mason, P.G.; Gariepy, T.D. Natural enemies of Anthonomus eugenii (Coleoptera: Curculionidae) in Canada. Can. Entomol. 2018, 150, 404–411. [Google Scholar] [CrossRef]
- Fernández, D.C. An Integrated Approach to Understanding Anthonomus eugenii Cano (Coleoptera: Curculionidae): An Exotic Pest of Greenhouse and Field Pepper Crops. Ph.D. Thesis, University of Windsor, Windsor, ON, Canada, 2021. [Google Scholar]
- Seal, D.R.; Martin, C.G. Pepper Weevil (Coleoptera: Curculionidae) Preferences for Specific Pepper Cultivars, Plant Parts, Fruit Colors, Fruit Sizes, and Timing. Insects 2016, 7, 9. [Google Scholar] [CrossRef]
- Rodríguez-Leyva, E.; Stansly, P.A.; Schuster, D.J.; Bravo-Mosqueda, E. Diversity and distribution of parasitoids of Anthonomus eugenii (Coleoptera: Curculionidae) from Mexico and prospects for biological control. Fl. Entomol. 2007, 90, 693–702. [Google Scholar] [CrossRef]
- Rodriguez-Del-Bosque, L.A.; Reyes-Rosas, M.A. Damage, survival, and parasitism of Anthonomus eugenii (Coleoptera: Curculionidae) on piquin pepper in northern Mexico. Southwest. Entomol. 2003, 28, 293–294. [Google Scholar]
- Fernández, D.C.; VanLaerhoven, S.L.; McCreary, C.; Labbé, R.M. An Overview of the Pepper Weevil (Coleoptera: Curculionidae) as a Pest of Greenhouse Peppers. J. Integr. Pest. Manag. 2020, 11, 26. [Google Scholar] [CrossRef]
- Addesso, K.M.; Stansly, P.A.; Kostyk, B.C.; McAuslane, H.J. Organic Treatments for Control of Pepper Weevil (Coleoptera: Curculionidae). Fl. Entomol. 2014, 97, 1148–1156. [Google Scholar] [CrossRef]
- Toapanta, M.A. Population ecology, life history, and biological control of the pepper weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae). Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2001. [Google Scholar]
- Wu, P.; Haseeb, M.; Diedrick, W.; Ouyang, H.; Zhang, R.; Kanga, L.H.B.; Legaspi, J.C. Influence of plant direction, layer, and spacing on the infestation levels of Anthonomus eugenii (Coleoptera: Curculionidae) in open Jalapeño pepper fields in North Florida. Fl. Entomol. 2019, 102, 501–508. [Google Scholar] [CrossRef]
- IPCC Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 10.
- Saddam, B.; Wei, C. Impact of climate change on the potential distributions of two cicada species, Platypleura octoguttata and Lemuriana apicalis (Hemiptera: Cicadidae), in India and their conservation implications. Eur. J. Entomol. 2025, 122, 99–110. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Warren, M.S.; Hill, J.K.; Thomas, J.A.; Asher, J.; Fox, R.; Huntley, B.; Roy, D.B.; Telfer, M.G.; Jeffcoate, S.; Harding, P.; et al. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 2001, 414, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate Change and Bark Beetles of the Western United States and Canada: Direct and Indirect Effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef]
- Ning, H.; Dai, L.; Fu, D.; Liu, B.; Wang, H.; Chen, H. Factors Influencing the Geographical Distribution of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytidae) in China. Forests 2019, 10, 425. [Google Scholar] [CrossRef]
- Azrag, A.G.; Mohamed, S.A.; Ndlela, S.; Ekesi, S. Predicting the habitat suitability of the invasive white mango scale, Aulacaspis tubercularis; Newstead, 1906 (Hemiptera: Diaspididae) using bioclimatic variables. Pest. Manag. Sci. 2022, 78, 4114–4126. [Google Scholar] [CrossRef]
- Xie, L.; Wu, X.; Li, X.; Chen, M.; Zhang, N.; Zong, S.; Yan, Y. Impacts of climate change and host plant availability on the potential distribution of Bradysia odoriphaga (Diptera: Sciaridae) in China. Pest. Manag. Sci. 2024, 80, 2724–2737. [Google Scholar] [CrossRef]
- Tourinho, L.; Vale, M.M. Choosing among correlative, mechanistic, and hybrid models of species’ niche and distribution. Integr. Zool. 2023, 18, 93–109. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Zhang, G.; Sun, X.; Chen, S.; Huang, L. Assessing the quality ecology of endemic tree species in China based on machine learning models and UPLC methods: The example of Eucommia ulmoides Oliv. J. Clean. Prod. 2024, 452, 142021. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Maywald, G.F.; Yonow, T.; Zurcher, E.J.; Herrmann, N.I.; Sutherst, R.W. CLIMEX Version 4: Exploring the Effects of Climate on Plants, Animals and Diseases; CSIRO: Canberra, Australia, 2015. [Google Scholar]
- Xiao, K.; Ling, L.; Deng, R.; Huang, B.; Cao, Y.; Wu, Q.; Ning, H.; Chen, H. Projecting the Potential Global Distribution of Sweetgum Inscriber, Acanthotomicus suncei (Coleoptera: Curculionidae: Scolytinae) Concerning the Host Liquidambar styraciflua Under Climate Change Scenarios. Insects 2024, 15, 897. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, W.H. Assessing potential European areas of Pierce’s disease mediated by insect vectors by using spatial ensemble model. Front. Plant Sci. 2023, 14, 1209694. [Google Scholar] [CrossRef]
- Ullah, F.; Zhang, Y.; Gul, H.; Hafeez, M.; Desneux, N.; Qin, Y. Potential economic impact of Bactrocera dorsalis on Chinese citrus based on simulated geographical distribution with MaxEnt and CLIMEX models. Entomol. Gen. 2023, 43, 821–830. [Google Scholar] [CrossRef]
- GBIF. GBIF.org GBIF Occurrence Download. 2024. Available online: https://doi.org/10.15468/dl.687jgz (accessed on 20 May 2024).
- GBIF. GBIF.org GBIF Occurrence Download. 2024. Available online: https://doi.org/10.15468/dl.myg4qe (accessed on 29 May 2024).
- GBIF. GBIF.org GBIF Occurrence Download. 2024. Available online: https://doi.org/10.15468/dl.hbhrye (accessed on 29 May 2024).
- Kriticos, D.J.; Webber, B.L.; Leriche, A.; Ota, N.; Macadam, I.; Bathols, J.; Scott, J.K. CliMond: Global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Nakicenovic, N.; Alcamo, J.; Davis, G.; Vries, B.d.; Fenhann, J.; Gaffin, S.; Gregory, K.; Grubler, A.; Jung, T.Y.; Kram, T.; et al. Special Report on Emissions Scenarios; WHO: Gevena, Switzerland, 2000. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Ebi, K.L.; Kemp-Benedict, E.; Riahi, K.; Rothman, D.S.; van Ruijven, B.J.; van Vuuren, D.P.; Birkmann, J.; Kok, K.; et al. The roads ahead: Narratives for shared socioeconomic pathways describing world futures in the 21st century. Glob. Environ. Change 2017, 42, 169–180. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Gueguen, M.; Engler, R.; Breiner, F.; Lafourcade, B. Biomod2: Ensemble Platform for Species Distribution Modeling. Available online: https://CRAN.R-project.org/package=biomod2 (accessed on 2 May 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2025. [Google Scholar]
- Chen, X.; Xiao, K.; Deng, R.; Wu, L.; Cui, L.; Ning, H.; Ai, X.; Chen, H. Projecting the future redistribution of Pinus koraiensis (Pinaceae: Pinoideae: Pinus) in China using machine learning. Front. For. Glob. Chang. 2024, 7. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Hou, C.; Xie, Y.; Zhang, Z. An improved convolutional neural network based indoor localization by using Jenks natural breaks algorithm. China Commun. 2022, 19, 291–301. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Maywald, G.F. A computerised system for matching climates in ecology. Agric. Ecosyst. Environ. 1985, 13, 281–299. [Google Scholar] [CrossRef]
- Ge, X.; He, S.; Zhu, C.; Wang, T.; Xu, Z.; Zong, S. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest. Manag. Sci. 2019, 75, 160–169. [Google Scholar] [CrossRef]
- Toapanta, M.A.; Schuster, D.J.; Stansly, P.A. Development and life history of Anthonomus eugenii (Coleoptera:Curculionidae) at constant temperatures. Environ. Entomol. 2005, 34, 999–1008. [Google Scholar] [CrossRef]
- Rossini, L.; Contarini, M.; Severini, M.; Talano, D.; Speranza, S. A modelling approach to describe the Anthonomus eugenii (Coleoptera: Curculionidae) life cycle in plant protection: A priori and a posteriori analysis. Fl. Entomol. 2020, 103, 259–263. [Google Scholar] [CrossRef]
- Zhu, J.j.; Peng, Q.; Liang, Y.l.; Wu, X.; Hao, W.l. Leaf Gas Exchange, Chlorophyll Fluorescence, and Fruit Yield in Hot Pepper (Capsicum anmuum L.) Grown Under Different Shade and Soil Moisture During the Fruit Growth Stage. J. Integr. Agric. 2012, 11, 927–937. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Tan, X.M.; Qi, F.J.; Teng, Z.W.; Fan, Y.J.; Shang, M.Q.; Lu, Z.Z.; Wan, F.H.; Zhou, H.X. The host shift of Bactrocera dorsalis: Early warning of the risk of damage to the fruit industry in northern China. Entomol. Gen. 2022, 42, 691–699. [Google Scholar] [CrossRef]
- Xian, X.; Zhao, H.; Wang, R.; Huang, H.; Chen, B.; Zhang, G.; Liu, W.; Wan, F. Climate change has increased the global threats posed by three ragweeds (Ambrosia L.) in the Anthropocene. Sci. Total Environ. 2023, 859, 160252. [Google Scholar] [CrossRef]
- Hulme, P.E. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simoes, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R package for comparative ecological biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Trenberth, K.E. Recent Observed Interdecadal Climate Changes in the Northern Hemisphere. Bull. Amer. Meteorol. Soc. 1990, 71, 988–993. [Google Scholar] [CrossRef]
- Pysek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Follett, P.A.; Neven, L.G. Current trends in quarantine entomology. Annu. Rev. Entomol. 2006, 51, 359–385. [Google Scholar] [CrossRef]
- Haack, R.A.; Herard, F.; Sun, J.; Turgeon, J.J. Managing Invasive Populations of Asian Longhorned Beetle and Citrus Longhorned Beetle: A Worldwide Perspective. Annu. Rev. Entomol. 2010, 55, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Labbe, R.M.; Gagnier, D.; Rizzato, R.; Tracey, A.; McCreary, C. Assessing New Tools for Management of the Pepper Weevil (Coleoptera: Curculionidae) in Greenhouse and Field Pepper Crops. J. Econ. Entomol. 2020, 113, 1903–1912. [Google Scholar] [CrossRef]
- Wiens, J.J.; Graham, C.H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, S.; Wang, T.; Zong, S.; Ge, X. Modeling the pest-pathogen threats in a warming world for the red turpentine beetle (Dendroctonus valens) and its symbiotic fungus (Leptographium procerum). Pest. Manag. Sci. 2024, 80, 3423–3435. [Google Scholar] [CrossRef]
- Newbold, T. Applications and limitations of museum data for conservation and ecology, with particular attention to species distribution models. Prog. Phys. Geogr. 2010, 34, 3–22. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef]
- Liu, C.; Wolter, C.; Xian, W.; Jeschke, J.M. Species distribution models have limited spatial transferability for invasive species. Ecol. Lett. 2020, 23, 1682–1692. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J. Cross-validation of species distribution models: Removing spatial sorting bias and calibration with a null model. Ecology 2012, 93, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Helmstetter, N.A.; Conway, C.J.; Stevens, B.S.; Goldberg, A.R. Balancing transferability and complexity of species distribution models for rare species conservation. Divers. Distrib. 2021, 27, 95–108. [Google Scholar] [CrossRef]
- Qiao, H.; Feng, X.; Escobar, L.E.; Peterson, A.T.; Soberon, J.; Zhu, G.; Papes, M. An evaluation of transferability of ecological niche models. Ecography 2019, 42, 521–534. [Google Scholar] [CrossRef]
- Rousseau, J.S.; Betts, M.G. Factors influencing transferability in species distribution models. Ecography 2022, 2022, e06060. [Google Scholar] [CrossRef]

| CLIMEX Parameter | Temperate | Semi-Arid | Final Parameter Value |
|---|---|---|---|
| Temperature requirements | |||
| DV0—Lower temperature threshold (°C) | 8 | 10 | 9.6 |
| DV1—Lower optimum temperature (°C) | 18 | 20 | 28 |
| DV2—Upper optimum temperature (°C) | 24 | 32 | 31 |
| DV3—Upper temperature threshold (°C) | 28 | 38 | 33 |
| PDD—Degree-days per generation (°C days) | 600 | 0 | 256.4 |
| Soil moisture | |||
| SM0—Lower soil moisture threshold | 0.25 | 0.1 | 0.1 |
| SM1—Lower optimal soil moisture | 0.8 | 0.2 | 0.7 |
| SM2—Upper optimal soil moisture | 1.5 | 0.25 | 0.85 |
| SM3—Upper soil moisture threshold | 2.5 | 0.3 | 1.5 |
| Cold stress | |||
| TTCS—Cold stress temperature threshold (°C) | 0 | 0 | −10 |
| THCS—Cold stress temperature rate (week−1) | 0 | 0 | −0.01 |
| Heat stress | |||
| TTHS—Heat stress temperature threshold (°C) | 30 | 39 | 41.7 |
| THHS—Heat stress temperature rate (week−1) | 0.005 | 0.002 | 0.005 |
| Dry stress | |||
| SMDS—Dry stress threshold | 0.2 | 0.05 | 0.1 |
| HDS—Dry stress rate (week−1) | −0.005 | −0.005 | −0.005 |
| Wet stress | |||
| SMWS—Wet stress threshold | 2.5 | 0.4 | 2 |
| HWS—Wet stress rate (week−1) | 0.002 | 0.01 | 0.001 |
| Target Species | Climate Scenario | Period | AUC * | Kappa * | TSS * |
|---|---|---|---|---|---|
| A. eugenii | History | 1970–2000 | 0.9259 | 0.8519 | 0.8710 |
| SSP370 | 2081–2100 | 0.8113 | 0.623 | 0.6257 | |
| SSP585 | 2081–2100 | 0.9057 | 0.8117 | 0.8204 | |
| C. annuum | History | 1970–2000 | 0.8521 | 0.7042 | 0.7189 |
| SSP370 | 2081–2100 | 0.8575 | 0.7150 | 0.7367 | |
| SSP585 | 2081–2100 | 0.8654 | 0.7308 | 0.7512 | |
| C. frutescens | History | 1970–2000 | 0.8566 | 0.7133 | 0.7282 |
| SSP370 | 2081–2100 | 0.8689 | 0.7380 | 0.7575 | |
| SSP585 | 2081–2100 | 0.8743 | 0.7487 | 0.7695 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, K.; Ling, L.; Deng, R.; Huang, B.; Wu, Q.; Cao, Y.; Ning, H.; Chen, H. Modeling the Historical and Future Potential Global Distribution of the Pepper Weevil Anthonomus eugenii Using the Ensemble Approach. Insects 2025, 16, 803. https://doi.org/10.3390/insects16080803
Xiao K, Ling L, Deng R, Huang B, Wu Q, Cao Y, Ning H, Chen H. Modeling the Historical and Future Potential Global Distribution of the Pepper Weevil Anthonomus eugenii Using the Ensemble Approach. Insects. 2025; 16(8):803. https://doi.org/10.3390/insects16080803
Chicago/Turabian StyleXiao, Kaitong, Lei Ling, Ruixiong Deng, Beibei Huang, Qiang Wu, Yu Cao, Hang Ning, and Hui Chen. 2025. "Modeling the Historical and Future Potential Global Distribution of the Pepper Weevil Anthonomus eugenii Using the Ensemble Approach" Insects 16, no. 8: 803. https://doi.org/10.3390/insects16080803
APA StyleXiao, K., Ling, L., Deng, R., Huang, B., Wu, Q., Cao, Y., Ning, H., & Chen, H. (2025). Modeling the Historical and Future Potential Global Distribution of the Pepper Weevil Anthonomus eugenii Using the Ensemble Approach. Insects, 16(8), 803. https://doi.org/10.3390/insects16080803






