Habitat Loss and Other Threats to the Survival of Parnassius apollo (Linnaeus, 1758) in Serbia
Simple Summary
Abstract
1. Introduction
1.1. Taxonomic Background and Regional Status
1.2. Host Plant: Heavy Metals, Habitat, and Climatic Conditions
1.3. Ecological Pressures: Habitat Loss, Succession, and Land-Use Change
- Vegetation succession leads to the encroachment of tall grasses, shrubs, and invasive species, which block sunlight at ground level and displace sun-loving succulents like S. album.
- Loss of habitat heterogeneity erodes the mosaic of rocky surfaces, bare patches, and low grasses, disrupting the availability of microhabitats required for larval and adult stages.
- Decline in spatial connectivity restricts dispersal and gene flow, isolating populations and increasing vulnerability to local extinction.
1.4. Conservation Challenges and Genetic Perspectives
1.5. Aims and Study
2. Materials and Methods
2.1. Study Sites
- Site 1. Serbia, Kopaonik National Park, Raska (Djorov Most), 870 m
- Located approximately 90 km west of the city of Niš and around 40 km south of the city of Kraljevo, in the Serbian Alps (Kopaonik range). In the Jošanica valley, a narrow ecotonal belt of sessile oak forests (Quercetum montanum) appears at the transition between thermophilous oak woodlands and mesophilic montane beech (Fagus) forests.
- Site 2. Serbia, Kopaonik National Park, Brzece (Bele Stene), 1650 m
- Situated approximately 80 km west of Niš and 48 km south of Kraljevo, in the Serbian Alps. This part of Kopaonik is characterized by high-altitude grasslands and subalpine to alpine pastures. On limestone and serpentine substrates, vegetation is more diverse and classified within the class Festuco-Seslerietea, dominated by species such as Sesleria latifolia, Festuca adamovicii, and Festuca panciciana. Intrazonal or azonal plant communities are represented by cliff vegetation, tall-herb communities, and bogs. Specific cliff associations include Edraiantho-Festucetum pancicianae and Silenetum serbicae on serpentinites, and Edraiantho-Saxifragetum sempervivi on limestone substrates.
- Site 3. Serbia, Tara National Park, Rastiste, 1520 m
- Located approximately 15 km northeast of Višegrad (Bosnia and Herzegovina), 45 km west of Užice, and 25 km from the town of Bajina Bašta. The site is composed of subalpine meadows and pastures with low vegetation and shrub cover on limestone bedrock. Sedum album occurs in plant communities alongside thyme (Thymus spp.), St. John’s wort (Hypericum spp.), and savory (Satureja spp.). The surveyed Parnassius apollo habitat consists of a broad mountain meadow and pasture located directly on the national border between Bosnia and Herzegovina and Serbia, both below and above the subalpine beech (Fagus) forest belt.
- Sites 4, 5, and 6. Serbia, Mount Stol—Bucje, Luka, and Bucje 2
- Mount Stol is located between the limestone massifs of Veliki Krs, Mali Krs, and Mount Deli Jovan. The summit of Stol reaches an elevation of 1156 m. The mountain lies approximately 15 km north of the town of Bor and is accessible from several nearby settlements. The research was conducted at multiple sites along the limestone slopes of Mount Stol, which are covered with shrub vegetation, mountain flora, and steppe grasslands. These habitats represent a mosaic of ecologically significant communities, characteristic of eastern Serbia’s karst regions. Mount Stol lies roughly 30 km south of the confluence of the Porecka River with the Danube and 45 km west of the confluence of the Timok River with the Danube. The geographical position of the Stol massif, in combination with its proximity to the Black Sea and the Danube Delta, has played a decisive role in shaping the mountain’s unique vegetation and exceptional biodiversity.
- Site 3 (Rastiste): 3 specimens, collected on 17 July 2024.
- Site 1 (Raska): 3 specimens, collected on 17 July 2023.
2.2. Vegetation Indices and Remote Sensing
2.3. Climate and Thermal Data Analysis
3. Results
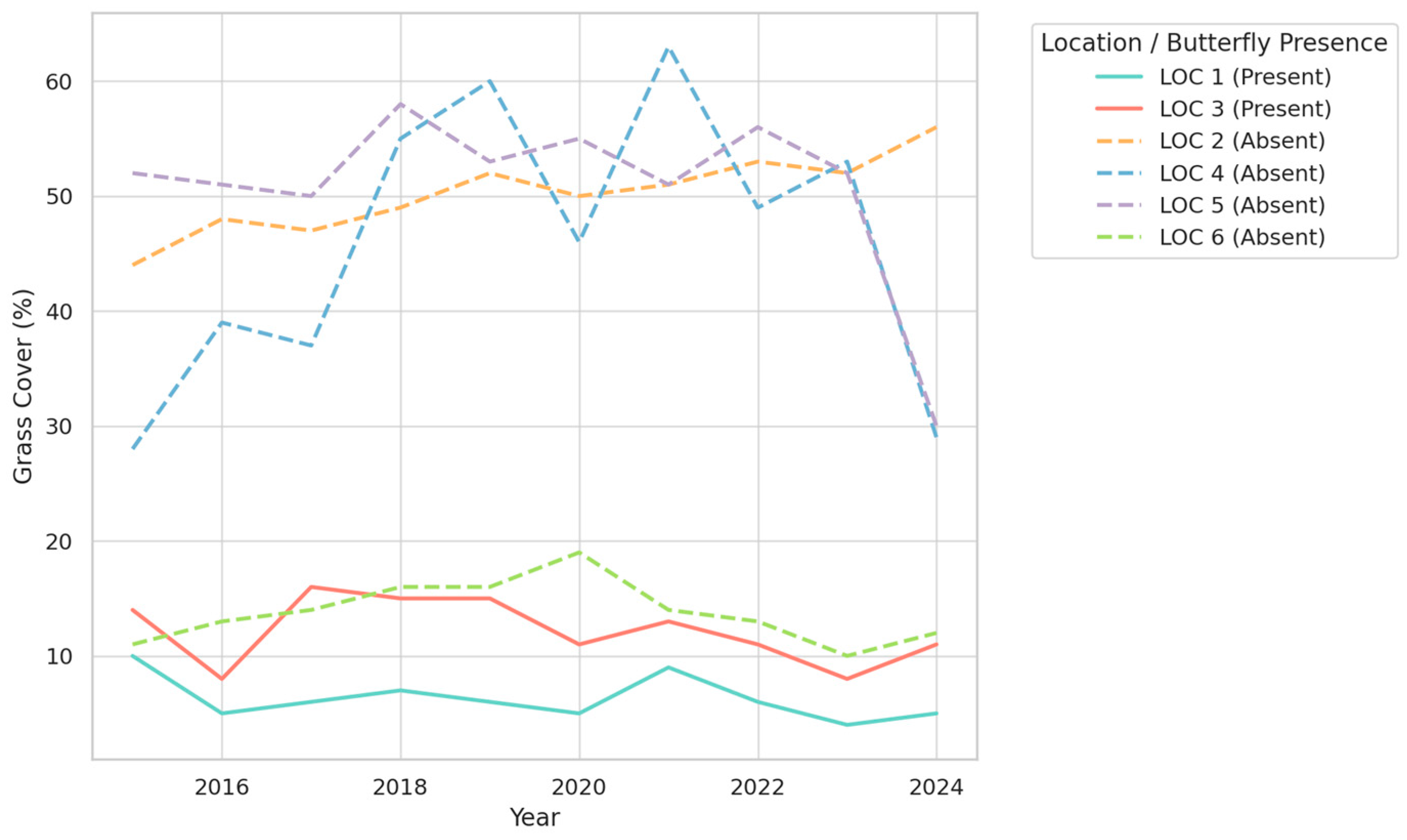
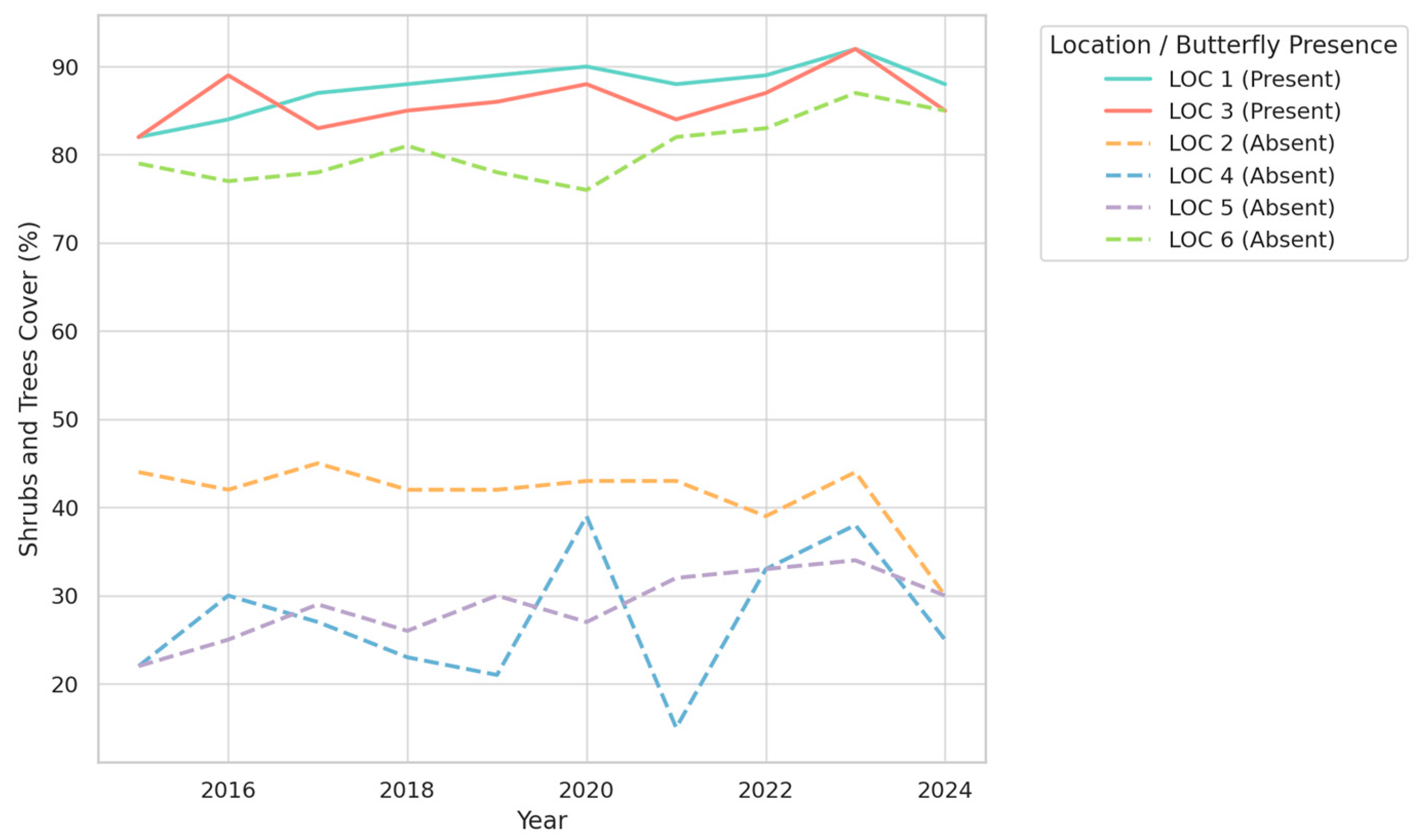
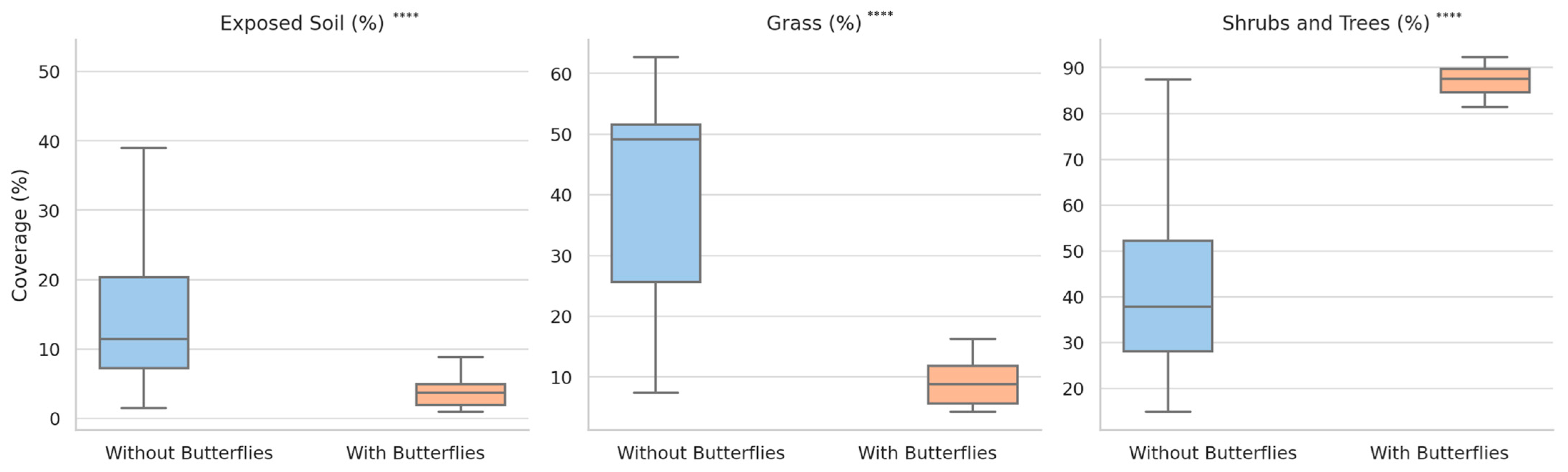
3.1. Temporal Trends in Vegetation Types and Their Association with Butterfly Presence
- −
- Exposed soil was substantially lower at butterfly sites (mean: 3.72%) compared to non-butterfly sites (mean: 16.34%) (U = 58, z = −5.36, p < 0.000001, r = −0.69);
- −
- Grass coverage was also significantly lower in butterfly habitats (mean: 9.00%) than in non-butterfly locations (mean: 39.89%) (U = 42.5, z = −5.61, p < 0.000001, r = −0.72);
- −
- Shrub and tree cover was markedly higher in butterfly-inhabited sites (mean: 87.29%) relative to non-butterfly sites (mean: 44.03%) (U = 779, z = +5.94, p < 0.000001, r = +0.77).
3.2. Vegetation Index-Based Burn Scar Detection on Stol Mountain (Luka, Bucje and Bucje 2 Site Location)
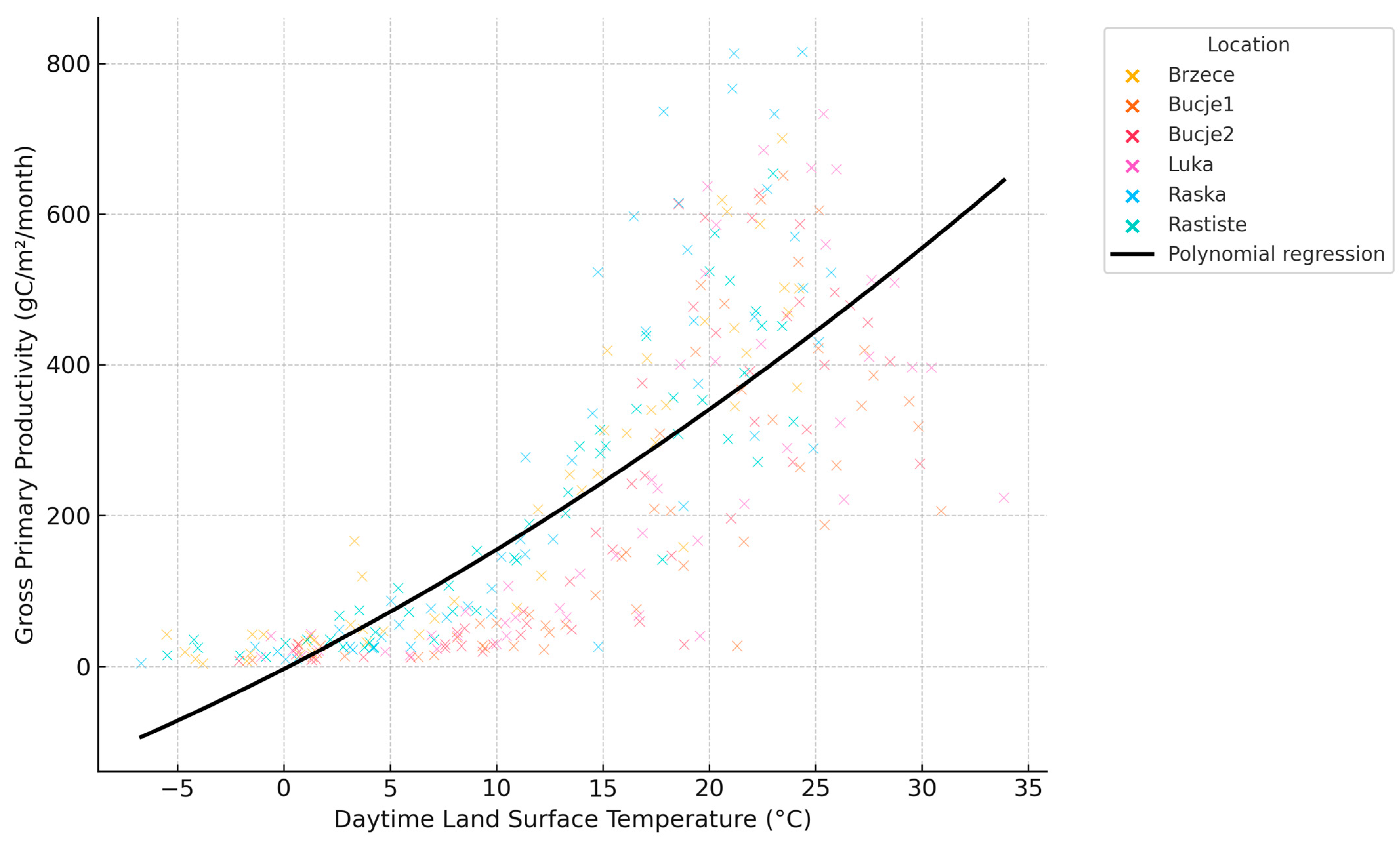
3.3. Ecophysiological Drivers of Gross Primary Productivity: A MODIS-Based Analysis
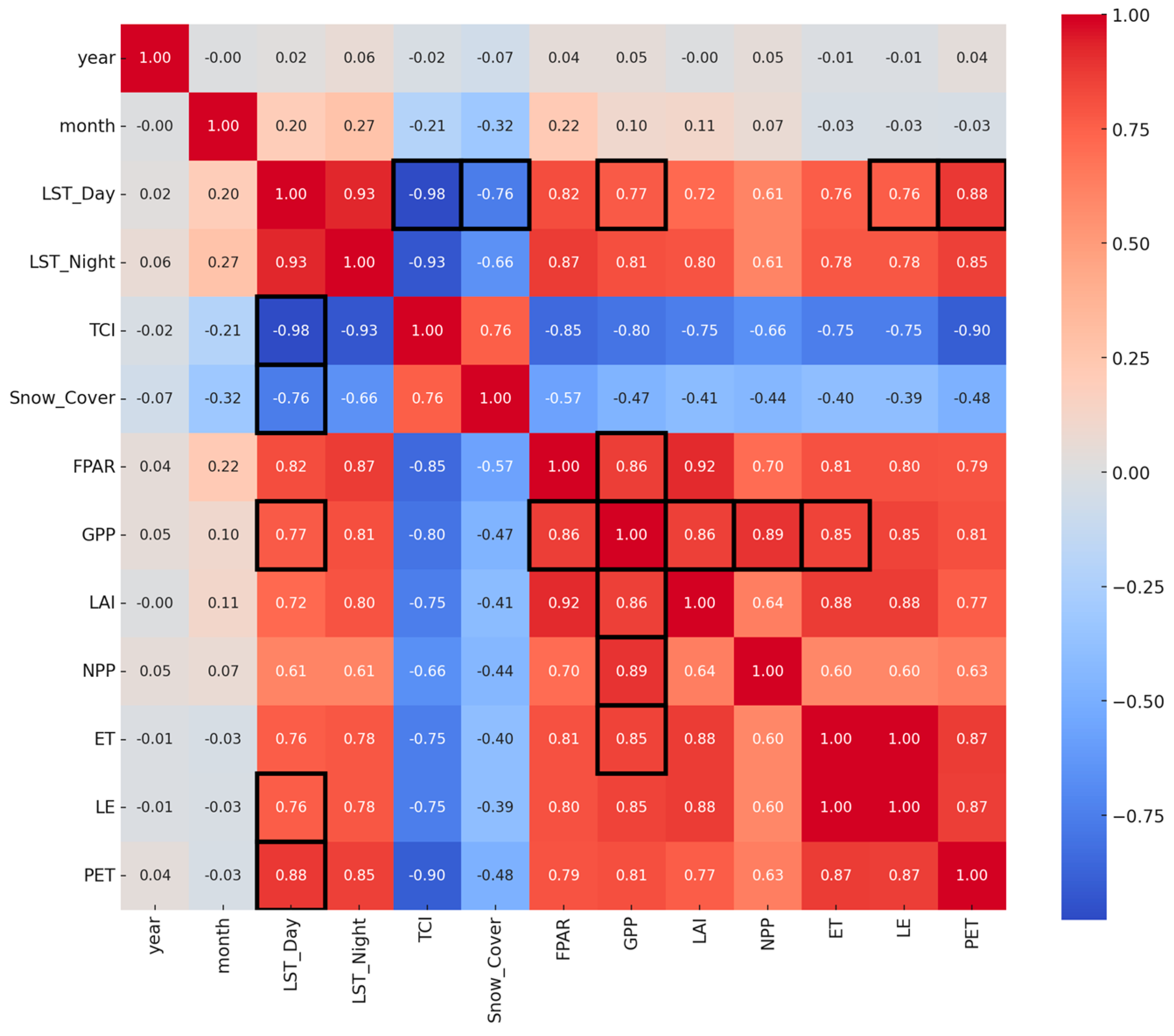
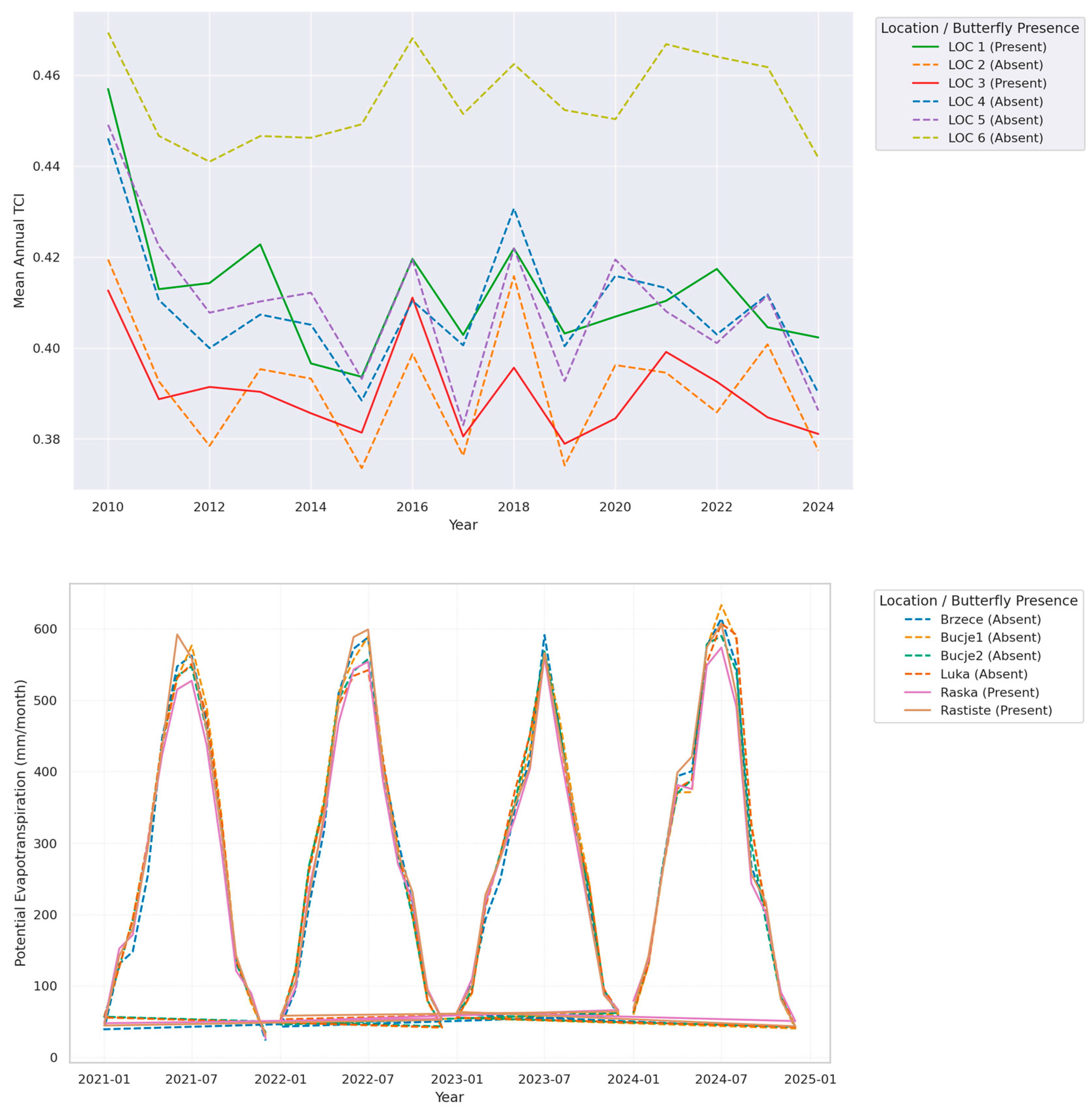
3.4. Interdependence of Climatic Stress Indicators and Vegetation Productivity
3.5. Vegetation Structural and Functional Dynamics: LAI and FPAR Trends (2010–2024)
4. Discussion
5. Conclusions
- Reintroduction of extensive grazing to restore and maintain open habitat mosaics;
- Active suppression of successional overgrowth to support the persistence of Sedum album;
- Continuous remote monitoring of thermal and hydrological stress using satellite-derived indices;
- Strategic fire prevention and management in ecologically sensitive areas.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, A. Der Apollofalter im Kleinziegenfelder Tal—Erhaltung und Sicherung der letzten Population in der Fränkischen Schweiz. ANLiegen. Natur. 2019, 41, 113–122. [Google Scholar] [CrossRef]
- Habel, J.C.; Eibensteiner, P.; Schmitt, T.; Eberle, J. Population ecology of the apollo butterfly Parnassius Apollo in the Austrian Alps—A mark-release recapture study. J. Insect. Conserv. 2025, 29, 39. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Grishutkin, G.F. Biology and Distribution of Parnassius apollo (Linnaeus, 1758), a Rare Species in Mordovia Republic, Russia. Biodiversitas 2018, 19, 431–436. [Google Scholar] [CrossRef]
- Nadler, J.; Bonelli, S.; Dapporto, L.; Karaçetin, E.; Lukhtanov, V.; López Munguira, M.; Micevski, N.; Settele, J.; Tzortzakaki, O.; Verovnik, R.; et al. Parnassius apollo. The IUCN Red List of Threatened Species 2021. E.T16249A122600528. 2021. Available online: https://www.iucnredlist.org/species/16249/122600528 (accessed on 16 July 2025).
- Todisco, V.; Gratton, P.; Cesaroni, D.; Sbordoni, V. Phylogeography of Parnassius apollo: Hints on Taxonomy and Conservation of a Vulnerable Glacial Butterfly Invader. Biol. J. Linn. Soc. 2010, 101, 169–183. [Google Scholar] [CrossRef]
- Nieminen, M.; Nuorteva, P.; Tulisalo, E. The Effect of Metals on the Mortality of Parnassius apollo Larvae (Lepidoptera: Papilionidae). J. Insect Conserv. 2001, 5, 1–7. [Google Scholar] [CrossRef]
- Zečević, M. Pregled Faune Leptira SRBIJE (Macrolepidoptera); Institut za Istraživanja u Poljoprivredi Srbije: Nauka, Beograd, 1996; pp. 1–160. [Google Scholar]
- Zečević, M.; Radovanović, S. Leptiri Timočke Krajine; Zavod za Poljoprivredu, Zaječar i Novinska Ustanova: Timok, Zaječar, 1974. [Google Scholar]
- Petkov, P.; Todorova, V. Beitrag zur Macrolepidopteren-Fauna der Umgebung von Tzaribrod und Tern; Arbeiten der Bulgarischen Naturforschenden Gesellschaft: Sofia, Bulgaria, 1915; Volume VIII, pp. 128–147. [Google Scholar]
- Jakšić, P. Dnevni leptiri (Lepidoptera: Hesperioidea i Papilionoidea) srpskog dela Stare planine. Zaštita Prirode 1999, 51, 69–84. [Google Scholar]
- Langourov, M. New Data on the Butterflies of Western Stara Planina Mts (Bulgaria & Serbia) (Lepidoptera, Papilionoidea). Ecol. Montenegrina 2019, 20, 119–162. [Google Scholar] [CrossRef]
- Chris van, S.; Annabelle, C.; Sue, C.; Dirk, M.; Miguel, L.M.; Martina, Š.; Josef, S.; Rudi, V.; Theo, V.; Martin, W.; et al. European Red List of Butterflies; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Jakšić, P.; Đurić, M. Serbian names for butterflies [Lepidoptera: Hesperioidea and Papilionoidea]. In Symposium of Flora of Southeastern Serbia and Neighbouring Regions, 9th ed.; Ranđelović, V., Ed.; Prirodno-Matematički Fakultet: Niš, Serbia, 2008; pp. 231–237. [Google Scholar]
- Jakšić, P.; Nahirnić, A. Vodič Kroz Faunu Dnevnih Leptira Kopaonika; JP Nacionalni Park Kopaonik: Kopaonik, Serbia, 2014; pp. 1–183. [Google Scholar]
- Stojanović, D.V.; Lelo, S. Prvi nalaz vrste Parnassius apollo (Linnaeus, 1758) (Lepidoptera: Papilionoidea: Papilionidae) na lokalitetu Veliki Stolac. Prilozi Fauni Bosne i Hercegovine 2024, 20, 17–22. [Google Scholar]
- Rákosy, L.; Goia, M. Lepidopterele din România: Lista sistematică și distribuție/The Lepidoptera of Romania: A Distributional Checklist; Presa Universitară Clujeană: Cluj-Napoca, Romania, 2021; p. 369. ISBN 978 606 37 1200 5. [Google Scholar]
- Preston, S.D.; Liao, J.D.; Toombs, T.P.; Tan, S.; Withers, J.; Rohr, J.R. A case study of a conservation flagship species: The monarch butterfly. Biodivers. Conserv. 2021, 30, 2057–2077. [Google Scholar] [CrossRef]
- Moser, H.; Oertli, J. Evidence of a Biochemical Interaction Between Insect and Specific Foodplant in the System Parnassius apollo–Sedum album. Rev. Suisse Zool. 1980, 87, 341–357. [Google Scholar] [CrossRef]
- Nakonieczny, M.; Kędziorski, A.; Michalczyk, K. Apollo Butterfly (Parnassius apollo L.) in Europe—Its History, Decline and Perspectives of Conservation. Funct. Ecosyst. Communities 2007, 1, 56–79. [Google Scholar]
- Mifsud, S.; Stephenson, R.; Thiede, J. Sedum album subsp. rupimelitense (Crassulaceae), a New Vegetatively Reproducing Subspecies from Malta (Maltese Islands, Central Mediterranean). Phytotaxa 2015, 227, 135–146. [Google Scholar] [CrossRef]
- Zlatković, B.; Jovanović, M.; Stojiljković, B.; Krstić, J.; Stojanović, M.; Stojanović, S.; Zdravković, J.; Lakušić, D. Morphological traits of Sedum album sensu stricto (Crassulaceae): Variability patterns across the Balkan Peninsula. Biologia Serbica 2019, 41, 3–12. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Wu, L.; Liu, A.; Zhao, F.-J.; Xu, W. Heavy Metal ATPase 3 (HMA3) Confers Cadmium Hypertolerance on the Cadmium/Zinc Hyperaccumulator Sedum plumbizincicola. New Phytol. 2017, 215, 687–698. [Google Scholar] [CrossRef]
- Tian, S.K.; Xie, R.H.; Wang, H.X.; Hu, Y.; Hou, D.D.; Liao, X.C.; Brown, P.H.; Yang, H.; Lin, X.; Labavitch, J.M.; et al. Uptake, sequestration and tolerance of cadmium at cellular levels in the hyperaccumulator plant species Sedum alfredii. J. Exp. Bot. 2017, 68, 2387–2398. [Google Scholar] [CrossRef]
- Guo, J.-M.; Lei, M.; Yang, J.-X.; Wan, X.-M.; Chen, T.-B.; Zhou, X.-Y.; Gu, S.-P.; Guo, G.-H. Effect of fertilizers on the Cd uptake of two sedum species (Sedum spectabile Boreau and Sedum aizoon L.) as potential Cd accumulators. Ecol. Eng. 2017, 106, 409–414. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Bech, J.; Llugany, M.; Pace, A.; Fenés, E.; Barceló, J. Copper in Plant Species in a Copper Gradient in Catalonia (North East Spain) and Their Potential for Phytoremediation. Plant Soil 2001, 230, 247–256. [Google Scholar] [CrossRef]
- Munkherjee, A.B. Fluxes of Lead, Cadmium and Mercury in the Finnish Environment and the Use of Biomonitors in Checking Trace Metals. Environ. Fenn. 1994, 18, 1–37. [Google Scholar]
- Vesely, J. Effects of Acidification on Trace Metal Transport in Fresh Waters. In Acidification of Freshwater Ecosystems: Implications for the Future; Steinberg, C.E.W., Wright, R.W., Eds.; John Wiley & Sons: London, UK, 1994; pp. 141–151. [Google Scholar]
- Fangmeier, A.; Steubing, L. Cadmium and Lead in the Food Web of a Forest Ecosystem. In Atmospheric Pollutants in Forest Areas; Georgii, H.-W., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1986; pp. 223–234. [Google Scholar]
- Vogel, W.R.; Nopp, H.; Führer, E. Stoffwechsel, Entwicklung und Fortpflanzung von Insekten und Antagonisten unter Schwermetalleinfluss. In FIW-Symposium: Waldsterben in Österreich; Führer, E., Neuhuber, F., Eds.; Universität für Bodenkultur: Wien, Austria, 1988; pp. 228–238. [Google Scholar]
- Hopkin, S.P. Ecophysiology of Metals in Terrestrial Invertebrates; Elsevier: London, UK, 1989. [Google Scholar]
- Tyler, G.; Balsberg-Påhlsson, A.-M.; Bengtsson, G.; Bååth, E.; Tranvik, L. Heavy-Metal Ecology of Terrestrial Plants, Micro-Organisms and Invertebrates: A Review. Water Air Soil Pollut. 1989, 47, 189–215. [Google Scholar] [CrossRef]
- Beeby, A. Toxic Metal Uptake and Essential Metal Regulation in Terrestrial Invertebrates: A Review. In Metal Ecotoxicology: Concepts and Applications; Newman, M.C., McIntosh, A.W., Eds.; Lewis Publishers: Chelsea, MI, USA, 1991; pp. 65–89. [Google Scholar]
- Janssen, M.P.M.; Bruins, A.; de Vries, T.H.; van Straalen, N.M. Comparison of Cadmium Kinetics in Four Soil Arthropod Species. Arch. Environ. Contam. Toxicol. 1991, 20, 305–312. [Google Scholar] [CrossRef]
- van Straalen, N.M.; Donker, M.H. Heavy Metal Adaptation in Terrestrial Arthropods—Physiological and Genetic Aspects. Proc. Exp. Appl. Entomol. NEV Amst. 1994, 5, 3–17. [Google Scholar]
- Migula, P.; Glowacka, E.; Nuorteva, S.-L.; Nuorteva, P.; Tulisalo, E. Time-Related Effects of Intoxication with Cadmium and Mercury in the Red Wood Ant. Ecotoxicology 1997, 6, 307–320. [Google Scholar] [CrossRef]
- Martin, A.-J. Influence of Cadmium Pollution on Social Homeostasis of Red Wood Ants and Using Ants in Environment Bioindication. Ph.D. Dissertation, University of Agricultural Sciences, Tartu, Estonia, 2000. [Google Scholar]
- Bengtsson, G.; Rundgren, S. The Gusum Case: A Brass Mill and the Distribution of Soil Collembola. Can. J. Zool. 1987, 66, 1518–1526. [Google Scholar] [CrossRef]
- Nuorteva, P. Metal Distribution Patterns and Forest Decline; Publications of the Department of Environmental Conservation at the University of Helsinki: Helsinki, Finland, 1990; No. 11. [Google Scholar]
- Nuorteva, P. (1999). Levels of Cadmium and Some Other Metals in Insects. In Proceedings of the XXIV Nordic Congress of Entomology, Tartu, Estonia, 8–11 August 1997; pp. 125–137. [Google Scholar]
- Nuorteva, P.; Li, T.; Tulisalo, E.; Hong, P. Metal Pollution in Relation to Mass Outbreaks of Dendrolimus punctatus Walker on Pinus massoniana Lamb. in China. J. Environ. Sci. 1999, 11, 498–503. [Google Scholar]
- Nuorteva, P.; Soltanpour, A. Relationship of Pemphigid Cecidogenesis and Metal Distribution in Poplar Leaves. Ecol. Chem. 1997, 6, 136–143. [Google Scholar]
- Walther, H.; Klausnitzer, B.; Richter, K. Beeinflussung der Natalität von Aphis fabae Scopoli (Insecta: Homoptera) durch Anthropogene Noxen, insbesondere Schwermetalle. Zool. Anz. Jena 1984, 212, 26–34. [Google Scholar]
- Braun, S.; Flückinger, W. Increased Population of the Aphid Aphis pomi at a Motorway: Part 3. The Effect of Exhaust Gases. Environ. Pollut. Ser. A 1985, 39, 183–192. [Google Scholar] [CrossRef]
- Heliövaara, K. Occurrence of Petrova resinella (Lepidoptera: Tortricidae) in a Gradient of Industrial Air Pollutants. Silva Fenn. 1986, 20, 83–90. [Google Scholar] [CrossRef]
- Heliövaara, K.; Väisänen, R. Parasitization in Petrova resinella Galls in Relation to Industrial Air Pollution. Silva Fennica 1986, 20, 233–236. [Google Scholar][Green Version]
- Heliövaara, K.; Väisänen, R. Air Pollution Levels and Abundance of Forest Insects. In Acidification in Finland; Kauppi, P., Anttila, P., Kenttämies, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 445–467. [Google Scholar]
- Bolsinger, M.; Flückinger, W. Enhanced Aphid Infestation at Motorways: The Role of Ambient Air Pollution. Entomol. Exp. Appl. 1987, 45, 237–243. [Google Scholar][Green Version]
- Koricheva, J.; Haukioja, E. Effects of Air Pollution on Host Plant Quality, Individual Performance, and Population Density of Eriocrania Miners (Lepidoptera: Eriocraniidae). Environ. Entomol. 1992, 21, 1386–1392. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V.; Haukioja, E. Population Dynamics of a Herbivore in an Industrially Modified Landscape: Case Study with Melasoma lapponica (Coleoptera: Chrysomelidae). Acta Phytopathol. Entomol. Hung. 1997, 32, 251–258. [Google Scholar]
- Kozlov, M.V.; Haukioja, E. Pollution-Related Environmental Gradients Around the “Severonikel” Smelter Complex on Kola Peninsula, Northwestern Russia. In The Contaminants in the Nordic Ecosystem; Munawar, M., Luotola, M., Eds.; S.P.B. Academic Publishing: Amsterdam, The Netherlands, 1995; pp. 59–69. [Google Scholar]
- Witkowski, Z.J.; Adamski, P.; Kosior, A.; Plonka, P. Extinction and Reintroduction of Parnassius apollo in the Pieniny National Park (Polish Carpathians). Biologia 1997, 52, 199–208. [Google Scholar]
- Sbaraglia, C.; Samraoui, K.R.; Massolo, A.; Sucháčková Bartoňová, A.; Konvička, M.; Faltýnek Fric, Z. Back to the Future: Climate Change Effects on Habitat Suitability of Parnassius apollo Throughout the Quaternary Glacial Cycles. Insect Conserv. Divers. 2022, 16, 231–242. [Google Scholar] [CrossRef]
- Stojanović, D.V.; Konjević, A. General plan of lepidopterological research for achieving defined goals in the Republic of Serbia with reference to climate changes, alien and migratory species. Topola 2023, 211, 29–44. [Google Scholar] [CrossRef]
- Stojanović, D.V. Erebia aethiops (Esper, 1777) (Lepidoptera: Nymphalidae): A new member of the fauna of higher elevations of the Kopaonik mountain. Topola 2021, 207, 41–45. [Google Scholar] [CrossRef]
- Kukkonen, J.M.; Mussaari, M.; Fred, M.S.; von Numers, M.; Brommer, J.E. A Strong Decline of the Endangered Apollo Butterfly Over 20 Years in the Archipelago of Southern Finland. J. Insect Conserv. 2022, 26, 673–681. [Google Scholar] [CrossRef]
- Kukkonen, J.M.; von Numers, M.; Brommer, J.E. Conserving Apollo Butterflies: Habitat Characteristics and Conservation Implications in Southwest Finland. J. Insect Conserv. 2024, 28, 1199–1210. [Google Scholar] [CrossRef]
- Hatton, L.; Warr, G. The Architecture of the Genome Integrates Scale Independence with Inverse Symmetry. Acad. Mol. Biol. Genom. 2025, 2, 1–20. [Google Scholar] [CrossRef]
- Bussan, S.K. Can Cattle Grazing Benefit Grassland Butterflies? J. Insect Conserv. 2022, 26, 359–374. [Google Scholar] [CrossRef]
- Martínez, J.G.; Ayerbe, E.; Tinaut, A. Landscape Changes, Habitat Characteristics and Use by Parnassius apollo in Sierra Nevada (Spain). Ecosistemas 2023, 32, 2525. [Google Scholar] [CrossRef]
- Adamski, P. Catch Effectiveness Revealed by Site-Related Differences in Capture–Mark–Recapture Methods: A Butterfly Metapopulation Study. Environ. Entomol. 2022, 51, 1234–1240. [Google Scholar] [CrossRef]
- Takáts, K.; Csősz, S.; Szövényi, G.; Katona, G.; Domagała, P.J.; Herczeg, G. From 20 to 2? Landmark-Based Geometric Morphometrics Reveal Negligible Wing-Shape Divergence Between 20 Subspecies of the Apollo Butterfly, Parnassius apollo (Lepidoptera: Papilionidae), in the Carpatho-Pannonian Region. J. Insect Conserv. 2024, 28, 1107–1119. [Google Scholar] [CrossRef]
- Adamski, P.; Ćmiel, A.M. The Long-Term Effect of Over-Supplementation on Recovered Populations: Why Restraint Is a Virtue. Oryx 2022, 56, 564–571. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Skowron Volponi, M.; Węgrzyn, G. Multiple Factors Correlating with Wing Malformations in the Population of Parnassius apollo (Lepidoptera: Papilionidae) Restituted from a Low Number of Individuals: A Mini Review. Insect Sci. 2019, 26, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Condamine, F.L. Limited by the Roof of the World: Mountain Radiations of Apollo Swallowtails Controlled by Diversity-Dependence Processes. Biol. Lett. 2018, 14, 20170622. [Google Scholar] [CrossRef]
- Podsiadlowski, L.; Tunström, K.; Espeland, M.; Wheat, C.W. The Genome Assembly and Annotation of the Apollo Butterfly Parnassius apollo, a Flagship Species for Conservation Biology. Genome Biol. Evol. 2021, 13, evab122. [Google Scholar] [CrossRef]
- Štefánik, M.; Fedor, P. Environmental Stress in Parnassius apollo Reflected Through Wing Geometric Morphometrics in a Historical Collection with a Possible Connection to Habitat Degradation. Nat. Conserv. 2020, 38, 79–99. [Google Scholar] [CrossRef]
- Franzén, M.; Johansson, H.; Askling, J.; Kindvall, O.; Johansson, V.; Forsman, A.; Sunde, J. Long-Distance Movements, Large Population Sizes and Density-Dependent Dispersal in Three Threatened Butterfly Species. Insect Conserv. Divers. 2024, 17, 1033–1045. [Google Scholar] [CrossRef]
- Dequidt-Kebaili, C.; Sherpa, S.; Rioux, D.; Després, L. Demographic inferences and climatic niche modelling shed light on the evolutionary history of the emblematic cold-adapted Apollo butterfly at regional scale. Mol. Ecol. 2021, 31, 448–466. [Google Scholar] [CrossRef]
- Sunde, J.; Askling, J.; Kindvall, O.; Johansson, V.; Franzén, M. Negative Impacts of Future Forest Succession on Three Threatened Butterfly Species. Biodivers. Conserv. 2024, 33, 2885–2910. [Google Scholar] [CrossRef]
- Khunrattanasiri, W. Application of Remote Sensing Vegetation Indices for Forest Cover Assessments. In Concepts and Applications of Remote Sensing in Forestry; Suratman, M.N., Ed.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Gao, S.; Zhong, R.; Yan, K.; Ma, X.; Chen, X.; Pu, J.; Gao, S.; Qi, J.; Yin, G.; Myneni, R.B. Evaluating the Saturation Effect of Vegetation Indices in Forests Using 3D Radiative Transfer Simulations and Satellite Observations. Remote Sens. Environ. 2023, 295, 113665. [Google Scholar] [CrossRef]
- Qian, B.; Ye, H.; Huang, W.; Xie, Q.; Pan, Y.; Xing, N.; Ren, Y.; Guo, A.; Jiao, Q.; Lan, Y. A Sentinel-2-Based Triangular Vegetation Index for Chlorophyll Content Estimation. Agric. For. Meteorol. 2022, 322, 109000. [Google Scholar] [CrossRef]
- Aklilu Tesfaye, A.; Gessesse Awoke, B. Evaluation of the Saturation Property of Vegetation Indices Derived from Sentinel-2 in Mixed Crop-Forest Ecosystem. Spat. Inf. Res. 2020, 29, 109–121. [Google Scholar] [CrossRef]
- Wang, Z.; Sui, L.; Zhang, S. Generating Daily Land Surface Temperature Downscaling Data Based on Sentinel-3 Images. Remote. Sens. 2022, 14, 5752. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, J.; Göttsche, F.-M.; Long, Z.; Ma, J.; Luo, R. Investigation and Validation of Algorithms for Estimating Land Surface Temperature from Sentinel-3 SLSTR Data. Int. J. Appl. Earth Obs. Geoinf. 2020, 91, 102136. [Google Scholar] [CrossRef]
- Hidalgo García, D. Analysis and Precision of the Terrestrial Surface Temperature Using Landsat 8 and Sentinel-3 Images: Study Applied to the City of Granada (Spain). Sustain. Cities Soc. 2021, 71, 102980. [Google Scholar] [CrossRef]
- Cristille, P.-L.; Bernhard, E.; Cox, N.L.J.; Bernard-Salas, J.; Mangin, A. Earth Observation Multi-Spectral Image Fusion with Transformers for Sentinel-2 and Sentinel-3 Using Synthetic Training Data. Remote Sens. 2024, 16, 3107. [Google Scholar] [CrossRef]
- Sobrino, J.A.; Irakulis, I. A Methodology for Comparing the Surface Urban Heat Island in Selected Urban Agglomerations Around the World from Sentinel-3 SLSTR Data. Remote Sens. 2020, 12, 2052. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Wan, Z.; Hook, S.; Hulley, G. MOD11A2 MODIS/Terra Land Surface Temperature/Emissivity 8-Day L3 Global 1km SIN Grid V006. NASA EOSDIS Land Processes DAAC. 2015. Available online: https://www.earthdata.nasa.gov/data/catalog/lpcloud-mod11a2-006 (accessed on 1 June 2025).
- Hall, D.K.; Riggs, G.A. MODIS/Terra Snow Cover 8-Day L3 Global 500m SIN Grid, Version 6. NASA National Snow and Ice Data Center Distributed Active Archive Center. 2016. Available online: https://nsidc.org/data/mod10a2/versions/6 (accessed on 1 June 2025).
- Myneni, R.; Knyazikhin, Y.; Park, T. MOD15A2H MODIS Leaf Area Index/FPAR 8-Day L4 Global 500m SIN Grid V006. NASA EOSDIS Land Processes DAAC. 2015. Available online: https://www.earthdata.nasa.gov/data/catalog/lpcloud-mod15a2h-006 (accessed on 1 June 2025).
- Running, S.W.; Zhao, M. The MODIS Global Terrestrial Primary Productivity Product (MOD17). NASA MODIS Land Products. 2015. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0034425705000106?via%3Dihub (accessed on 1 June 2025).
- Mu, Q.; Zhao, M.; Running, S.W. MODIS Global Terrestrial Evapotranspiration (ET) Products for Science and Applications. NASA MODIS Land Algorithms. 2011. Available online: https://modis.gsfc.nasa.gov/data/dataprod/mod16.php (accessed on 1 June 2025).
- Zhao, M.; Running, S.W. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Running, S.W.; Nemani, R.R.; Heinsch, F.A.; Zhao, M.S.; Reeves, M.; Hashimoto, H. A continuous satellite-derived measure of global terrestrial primary production. BioScience 2004, 54, 547–560. [Google Scholar] [CrossRef]
- Tang, H.; Dubayah, R. Light-driven growth in Amazon evergreen forests explained by seasonal variations of vertical canopy structure. Proc. Natl. Acad. Sci. USA 2017, 114, 2640–2644. [Google Scholar] [CrossRef]
- Berry, J.; Björkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Nemani, R.R.; Keeling, C.D.; Hashimoto, H.; Jolly, W.M.; Piper, S.C.; Tucker, C.J.; Myneni, R.B.; Running, S.W. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 2003, 300, 1560–1563. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Peng, Y.; Ren, P.; Chen, Y.; Zhou, X.; Yang, Z.; Zou, Z.; Peng, C. The Widely Increasing Sensitivity of Vegetation Productivity to Spring Temperature Across Biomes. Geophys. Res. Lett. 2025, 52, e2024GL113892. [Google Scholar] [CrossRef]
- Reitz, O.; Bogena, H.; Neuwirth, B.; Sanchez-Azofeifa, G.; Graf, A.; Bates, J.; Leuchner, M. Environmental Drivers of Gross Primary Productivity and Light Use Efficiency of a Temperate Spruce Forest. J. Geophys. Res. Biogeosciences 2023, 128, 1–18. [Google Scholar] [CrossRef]
- Balde, H.; Hmimina, G.; Goulas, Y.; Latouche, G.; Soudani, K. Synergy between TROPOMI sun-induced chlorophyll fluorescence and MODIS spectral reflectance for understanding the dynamics of gross primary productivity at Integrated Carbon Observatory System (ICOS) ecosystem flux sites. Biogeosciences 2023, 20, 1473–1490. [Google Scholar] [CrossRef]
- Yao, W.; Bie, Q. Global-scale improvement of terrestrial gross primary productivity estimation by integrating optical remote sensing with meteorological data. Ecol. Indic. 2025, 173, 113429. [Google Scholar] [CrossRef]
- Turner, D.P.; Ritts, W.D.; Cohen, W.B.; Gower, S.T.; Running, S.W. Evaluation of MODIS NPP and GPP products across multiple biomes. Remote Sens. Environ. 2006, 102, 282–292. [Google Scholar] [CrossRef]
- Running, S.W.; Mu, Q.; Zhao, M. MOD17A2H MODIS/Terra Gross Primary Productivity 8-Day L4 Global 500m SIN Grid V006. NASA EOSDIS Land Processes DAAC. 2015. Available online: https://www.earthdata.nasa.gov/data/catalog/lpcloud-mod17a2h-006 (accessed on 1 June 2025).
- Chen, X.; Wang, Y.; Li, J. Assessment of agricultural drought severity using multi-temporal remote sensing indices. Sci. Rep. 2024, 14, 3087. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Zhao, M. Quantifying impacts of vegetation greenness change on drought and potential evapotranspiration. Geophys. Res. Lett. 2024, 51, e2024GL111634. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liu, Q. Clarifying the effects of potential evapotranspiration and soil moisture on vegetation productivity. Environ. Sci. Policy 2024, 147, 2389048. [Google Scholar] [CrossRef]
- Imtiaz, F.; Farooque, A.; Randhawa, G.; Hashemi, G.S.E.; Wang, X.; Esau, T.; Acharya, B.; Sadiq, R. Remote sensing-based spatiotemporal dynamics of agricultural drought on Prince Edward Island using Google Earth engine. Ecol. Inform. 2025, 86, 103073. [Google Scholar] [CrossRef]
- Susila, D.; Trigunasih, N.L.; Saifulloh, M. Monitoring agricultural drought in savanna ecosystems using the vegetation health index. Environ. Monit. Assess. 2024, 196, 633907. [Google Scholar] [CrossRef]
- Pu, R.; Yang, C.; Zhang, Y.; Gao, F. A harmonized long-term global climate data record for leaf area index and FPAR from multiple sensors. Earth Syst. Sci. Data 2024, 16, 15–34. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wen, Y.; Zhao, M. HiQ-FPAR: A high-quality global product of fraction of photosynthetically active radiation absorbed by vegetation derived from MODIS. Sci. Data 2025, 12, 93. [Google Scholar] [CrossRef]
- Fric, Z.; Suchackova, B.A.; Massolo, A.; Sbaraglia, C. Climate Change Effects on Habitat Suitability of a Butterfly in the Past, Present, and Future: Biotic Interaction Between Parnassius apollo and Its Host Plants. Master’s Thesis, University of Pisa, Pisa, Italy, 2022. [Google Scholar]
- Kindvall, O.; Franzén, M.; Askling, J.; Forsman, A.; Johansson, V. Subsidized Common Agricultural Policy grazing jeopardizes the protection of biodiversity and Natura 2000 target species. Anim. Conserv. 2022, 25, 597–607. [Google Scholar] [CrossRef]
- Dunskis, A.; Stigenberg, J.; Ågren, J.; Sletvold, N. Determining Oviposition Preferences to Inform Population Reinforcement of the Specialist Chequered Blue Butterfly (Scolitantides orion). Ecol. Solut. Evid. 2024, 5, e12401. [Google Scholar] [CrossRef]
- Johansson, V.; Säwenfalk, D.S.; Bergman, K.O.; Kindvall, O.; Askling, J.; Franzén, M. Oviposition Preferences and Larval Survival of the Marsh Fritillary Butterfly: The Adverse Impact of Grazing. Insect Conserv. Divers. 2024, 17, 1–9. [Google Scholar] [CrossRef]
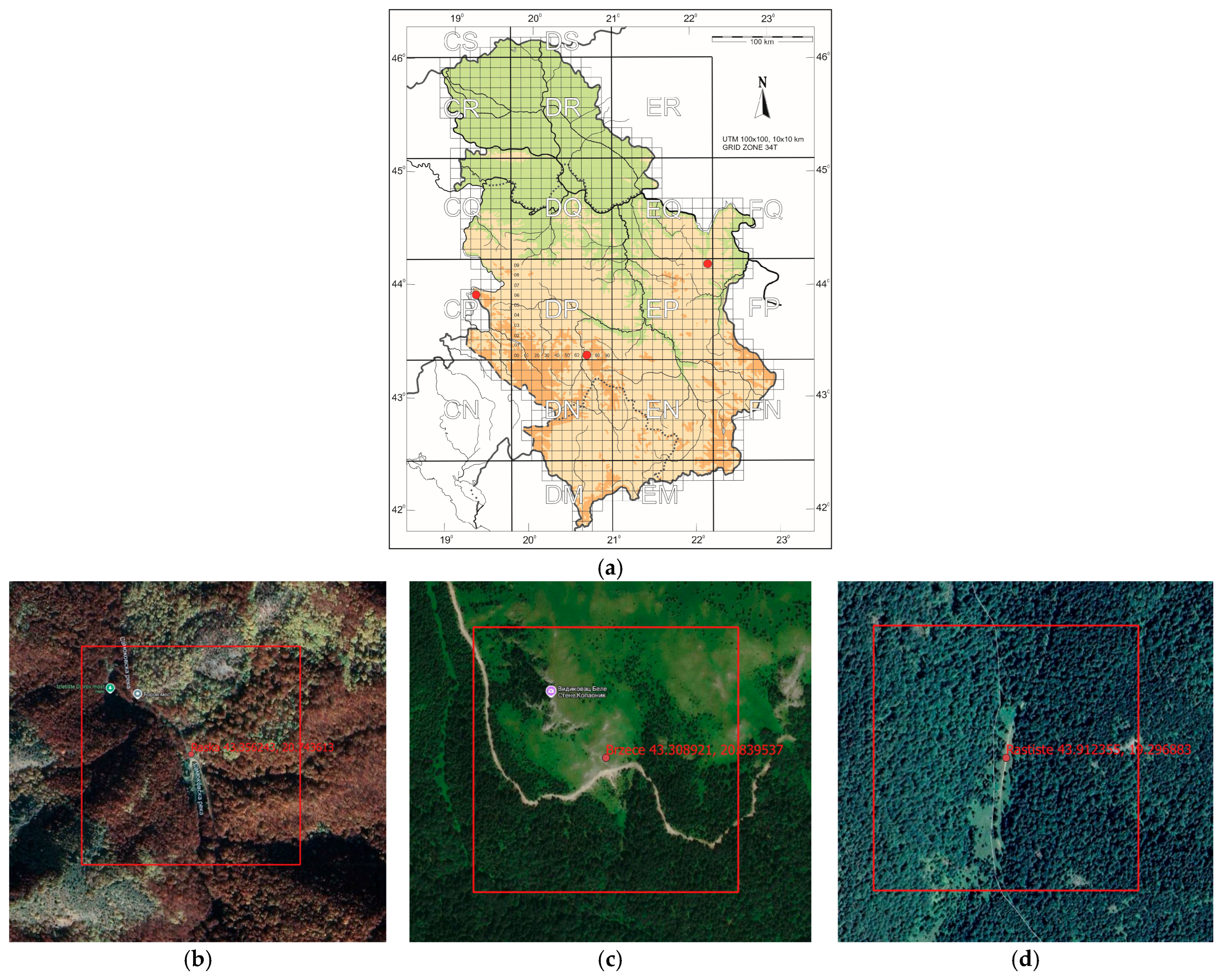
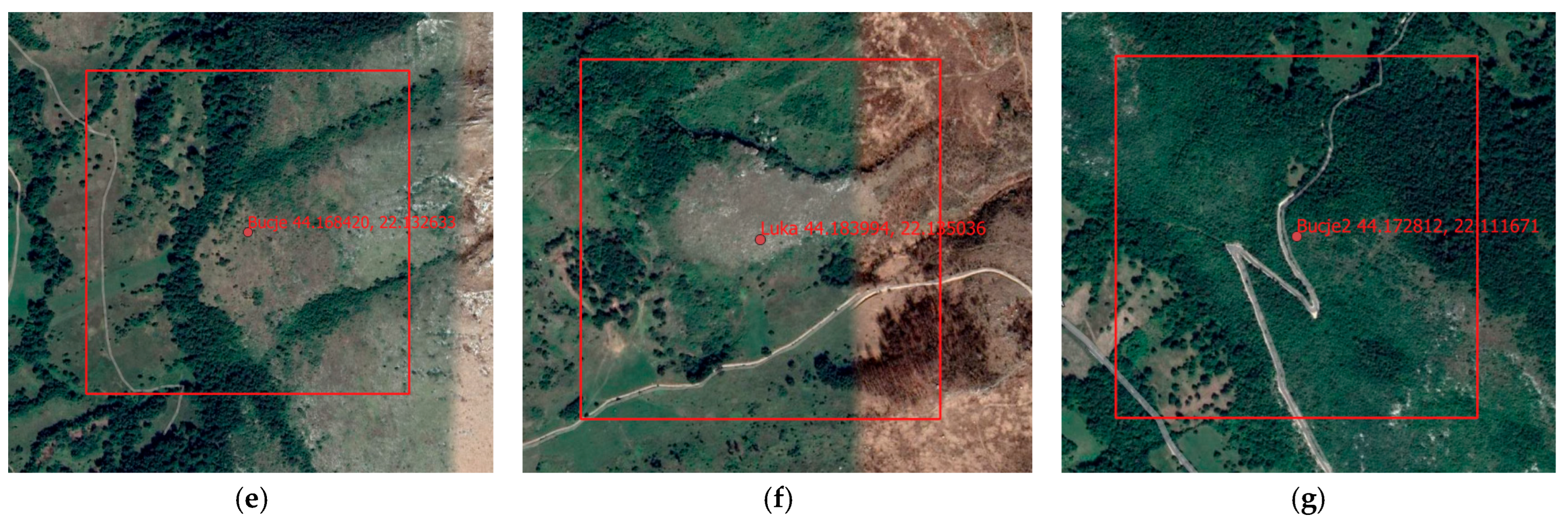






| Site No. | Location | Name | Lat./Lon. (°) |
|---|---|---|---|
| Site 1, Figure 1b | National Park Kopaonik | Raska | 43.356243 20.743613 |
| Site 2, Figure 1c | National Park Kopaonik | Brzece | 43.308921 20.839537 |
| Site 3, Figure 1d | National Park Tara | Rastiste | 43.912355 19.296883 |
| Site 4, Figure 1e | Stol Mountain | Bucje | 44.168420 22.132633 |
| Site 5, Figure 1f | Stol Mountain | Luka | 44.183994 22.135036 |
| Site 6, Figure 1g | Stol Mountain | Bucje 2 | 44.172812 22.111671 |
| Year 2024 | Date | 29.7 | 8.8 | 13.8 | 23.8 | 25.8 | 12.9 | 27.9 | 14.10 |
|---|---|---|---|---|---|---|---|---|---|
| BAI | Minimum value | 2.97 | 3.24 | 2.47 | 2.22 | 1.67 | 3.42 | 3.47 | 2.15 |
| Maximum value | 25.09 | 73.53 | 30.62 | 34.77 | 24.33 | 120.54 | 91.83 | 85.82 | |
| Range | 22.12 | 70.29 | 28.15 | 32.55 | 22.66 | 117.12 | 88.35 | 83.66 | |
| Mean value | 9.39 a | 10.32 a | 10.09 a | 10.93 a | 9.30 a | 27.90 b | 27.95 b | 21.55 b | |
| Standard deviation | 1.72 | 6.42 | 2.01 | 2.42 | 2.02 | 15.31 | 13.62 | 9.78 | |
| NBR | Minimum value | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 |
| Maximum value | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Range | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
| Mean value | 0.17 a | 0.15 a | 0.14 a | 0.11 | 0.09 a | −0.10 b | −0.11 b | −0.10 b | |
| Standard deviation | 0.18 | 0.18 | 0.17 | 0.16 | 0.17 | 0.21 | 0.19 | 0.17 | |
| NBR2 | Minimum value | 0.11 | 0.10 | 0.11 | 0.12 | 0.11 | −0.05 | −0.04 | −0.01 |
| Maximum value | 0.23 | 0.23 | 0.23 | 0.22 | 0.21 | 0.20 | 0.18 | 0.17 | |
| Range | 0.12 | 0.13 | 0.12 | 0.09 | 0.10 | 0.24 | 0.23 | 0.19 | |
| Mean value | 0.17 a | 0.17 a | 0.18 a | 0.18 a | 0.17 a | 0.07 b | 0.05 b | 0.06 b | |
| Standard deviation | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.05 | 0.05 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, D.V.; Višacki, V.; Ranđelović, D.; Ivetić, J.; Orlović, S. Habitat Loss and Other Threats to the Survival of Parnassius apollo (Linnaeus, 1758) in Serbia. Insects 2025, 16, 805. https://doi.org/10.3390/insects16080805
Stojanović DV, Višacki V, Ranđelović D, Ivetić J, Orlović S. Habitat Loss and Other Threats to the Survival of Parnassius apollo (Linnaeus, 1758) in Serbia. Insects. 2025; 16(8):805. https://doi.org/10.3390/insects16080805
Chicago/Turabian StyleStojanović, Dejan V., Vladimir Višacki, Dragana Ranđelović, Jelena Ivetić, and Saša Orlović. 2025. "Habitat Loss and Other Threats to the Survival of Parnassius apollo (Linnaeus, 1758) in Serbia" Insects 16, no. 8: 805. https://doi.org/10.3390/insects16080805
APA StyleStojanović, D. V., Višacki, V., Ranđelović, D., Ivetić, J., & Orlović, S. (2025). Habitat Loss and Other Threats to the Survival of Parnassius apollo (Linnaeus, 1758) in Serbia. Insects, 16(8), 805. https://doi.org/10.3390/insects16080805








