Expression of Heat Shock Protein 90 Genes Induced by High Temperature Mediated Sensitivity of Aphis glycines Matsumura (Hemiptera: Aphididae) to Insecticides
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphid and Host Source
2.2. Total RNA Extraction, cDNA Synthesis, and Gene Cloning
2.3. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.4. Gene Expression Responds to Thermal Treatments
2.5. Gene Expression Responds to Insecticide Treatment
2.6. RNA Interference
2.7. Bioassays
2.8. Data Analysis
3. Results
3.1. AgHsp90 Genes Profiling at Different Developmental Stages
3.2. AgHsp90 Genes Profiling in Response to Different Thermal Stresses
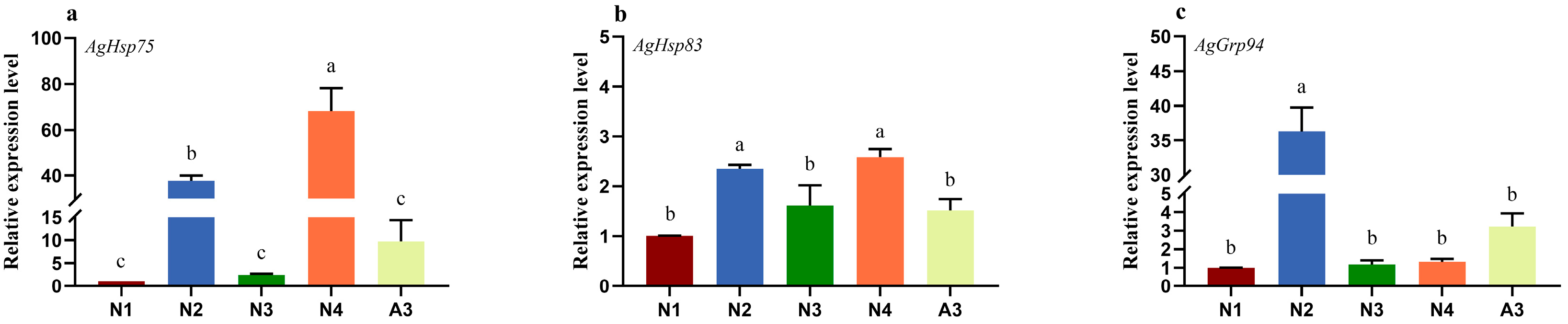
3.3. Expression Levels of AgHsp90 Genes Following Insecticide Exposure
3.4. Effect of RNAi on AgHsp90 Gene Expression and Bioassays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Global Warming of 1.5 °C; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Eds.; Cambridge University Press: Cambridge, UK, 2018; 616p. [Google Scholar] [CrossRef]
- Heilongjiang Meteorological Bureau. Ambient Temperature Data of Harbin (2010–2020); Heilongjiang Meteorological Bureau: Harbin, China, 2020. [Google Scholar]
- Gao, G.; Feng, L.; Perkins, L.E.; Sharma, S.; Lu, Z. Effect of the frequency and magnitude of extreme temperature on the life history traits of the large cotton aphid, Acyrthosiphon gossypii (Hemiptera: Aphididae): Implications for their population dynamics under global warming. Entomol. Gen. 2018, 37, 103–113. [Google Scholar] [CrossRef]
- Abdel-Hady, A.A.A.; Ramadan, M.M.; Lü, J.; Hashem, A.S. High-temperature shock consequences on the red flour beetle (Tribolium castaneum) and the rice weevil (Sitophilus oryzae). J. Therm. Biol. 2021, 100, 103062. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Desneux, N.; Lu, Y. Impact of temperature on survival rate, fecundity, and feeding behavior of two aphids, Aphis gossypii and Acyrthosiphon gossypii, when reared on cotton. Insects 2021, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Bowler, K. Heat death in poikilotherms: Is there a common cause? J. Therm. Biol. 2018, 76, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Dampc, J.; Kula-Maximenko, M.; Molon, M.; Durak, R. Enzymatic defense response of apple aphid Aphis pomi to increased temperature. Insects 2020, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Durak, R.; Dampc, J.; Kula-Maximenko, M.; Mołoń, M.; Durak, T. Changes in antioxidative, oxidoreductive and detoxification enzymes during development of aphids and temperature increase. Antioxidants 2021, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, F.; Mikani, A.; Moharramipour, S. Thermal tolerance variations and physiological adjustments in a winter active and a summer active aphid species. J. Therm. Biol. 2021, 98, 102950. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Ma, F.; Wei, J.; Li, H.; Ma, H.; Sun, P. Physiological functions of heat shock proteins. Curr. Protein Pept. Sci. 2020, 21, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Guo, M.; Wang, D.; Li, T.; Li, R.; Li, D.; Cheng, S.; Zhen, C.; Zhang, L. Molecular and functional characterization of heat-shock protein 70 in Aphis gossypii under thermal and xenobiotic stresses. Pestic. Biochem. Physiol. 2024, 199, 105774. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Liu, P.; Wang, W.; Liang, G.M.; Lu, Y.H. Characterizing three heat shock protein 70 genes of Aphis gossypii and their expression in response to temperature and insecticide stress. J. Agric. Food Chem. 2025, 73, 2842–2852. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, R.; Kim, S.H. Crystal structure of small heat-shock protein. Nature 1998, 394, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, D.W.; Landis, D.A.; Brodeur, J.; Heimpel, G.E.; Desneux, N. Ecology and management of the soybean aphid in North America. Annu. Rev. Entomol. 2011, 56, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Schenk-Hamlin, D.; Zhan, W.; Ragsdale, D.; Heimpel, G. The soybean aphid in China: A historical review. Ann. Entomol. Soc. Am. 2004, 97, 209–218. [Google Scholar] [CrossRef]
- Wang, R.-Y.; Ghabrial, S.A. Effect of aphid behavior on efficiency of transmission of soybean mosaic virus by the soybean-colonizing aphid, Aphis glycines. Plant Dis. 2002, 86, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.H.; Alleman, R.; Hogg, D.B.; Grau, C.R. First report of transmission of soybean mosaic virus and alfalfa mosaic virus by Aphis glycines in the new world. Plant Dis. 2001, 85, 561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davis, J.A.; Radcliffe, E.B.; Ragsdale, D.W. Soybean aphid, Aphis glycines Matsumura, a new vector of potato virus Y in potato. Am. J. Potato Res. 2005, 82, 197–201. [Google Scholar] [CrossRef]
- Chen, B.; Feder, M.E.; Kang, L. Evolution of heat-shock protein expression underlying adaptive responses to environmental stress. Mol. Ecol. 2018, 27, 3040–3054. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Liu, X.-Y.; Li, B.; Li, M.-Y.; Li, S.-G.; Liu, S. A heat shock protein protects against oxidative stress induced by lambda-cyhalothrin in the green peach aphid Myzus persicae. Pestic. Biochem. Physiol. 2022, 181, 104995. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Y.; Wang, Y.; Chang, Y.; Wu, Q.; Gong, W.; Du, Y. Effect of high temperature on abamectin and thiamethoxam tolerance in Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae). Insects 2024, 15, 399. [Google Scholar] [CrossRef] [PubMed]
- Han, L.L.; Zhu, M.H.; Dong, T.Y.; Zhao, K.J.; Qu, Z.C.; Yang, L.; Han, X.X. Effects of heat shock and imidacloprid on the expressions of Hsp70 and Hsc70 mRNA in the Aphis glycines (Hemiptera: Aphididae). Acta Entomol. Sin. 2014, 57, 387–394. [Google Scholar]
- Lu, K.; Chen, X.; Liu, W.; Zhang, Z.; Wang, Y.; You, K.; Li, Y.; Zhang, R.; Zhou, Q. Characterization of heat shock protein 70 transcript from Nilaparvata lugens (Stål): Its response to temperature and insecticide stresses. Pestic. Biochem. Physiol. 2017, 142, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Li, S.-Y.; Yao, L.; Liu, Y.; Chen, Y.-R.; Yang, H.-J.; Fan, D. Stress expression of AgHsp75 gene in soybean aphid (Aphis glycines) under imidacloprid and temperature stress. Chin. J. Biol. Control 2020, 36, 913–919. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Zhang, Z.; Zhao, J.; Ma, F.; Zheng, M.; Yang, M.; Sang, X.; Ma, K.; Li, L. Selection of reference genes for normalization of qRT–PCR analysis in the soybean aphid Aphis glycines Matsumura (Hemiptera: Aphididae). J. Econ. Entomo. 2022, 115, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, J.; Cui, L.; Wang, Q.; Huang, W.; Ji, X.; Yang, Q.; Rui, C. Overexpression of ATP-binding cassette transporters associated with sulfoxaflor resistance in Aphis gossypii Glover. Pest Manag. Sci. 2021, 77, 4064–4072. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-S.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Tan, J.-Y.; Wang, Y.; Qi, M.-H.; Peng, J.-N.; Chen, D.-X.; Liu, S.; Li, M.-Y. A heat shock protein 70 protects the green peach aphid (Myzus persicae) against high-temperature stress. J. Asia-Pac. Entomol. 2022, 25, 101992. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Q.; Duan, X.; Song, C.; Chen, M. Transcription of four Rhopalosiphum padi (L.) heat shock protein genes and their responses to heat stress and insecticide exposure. Comp. Biochem. Physiol. A 2017, 205, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Shim, J.-K.; Lee, K.-Y. Identification of glucose-regulated protein 78 (grp78) cDNA from Aphis gossypii and its expression during development, heat shock and nutritional ingestion. Physiol. Entomol. 2016, 41, 193–201. [Google Scholar] [CrossRef]
- Li, H.; Du, Y. Molecular cloning and characterization of an Hsp90/70 organizing protein gene from Frankliniella occidentalis (Insecta: Thysanoptera, Thripidae). Gene 2013, 520, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, Y.; Zhao, X.; Chen, J.; Li, S.; Ye, J.; Hao, D. Accumulation of heat shock protein 20s in the ovary and testis of Monochamus alternatus protects reproduction against high temperatures. Entomol. Gen. 2023, 43, 1021–1030. [Google Scholar] [CrossRef]
- Shu, Y.; Du, Y.; Wang, J. Molecular characterization and expression patterns of Spodoptera litura heat shock protein 70/90, and their response to zinc stress. Comp. Biochem. Physiol. A 2010, 158, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.-Y.; Jin, X.; He, L.-C.; Zhang, X.-X.; Yang, C.-L.; Zhang, C.-Y. Cloning, Expression profiles of Arma chinensis heat shock protein genes AcHsp83a and AcHsp83b, and their response to high and low temperature and UV-B stress. Acta Entomol. Sin. 2023, 66, 1425–1434. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, H.; Wang, Z.; Long, G.-Y.; Jin, D.-C. Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci. Rep. 2018, 8, 8773. [Google Scholar] [CrossRef] [PubMed]
- Dumas, P.; Morin, M.D.; Boquel, S.; Moffat, C.E.; Morin, P.J. Expression status of heat shock proteins in response to cold, heat, or insecticide exposure in the Colorado potato beetle Leptinotarsa decemlineata. Cell Stress Chaperones 2019, 24, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Q.; Zhang, Y.-L.; Wang, X.-Q.; Dong, H.; Gao, P.; Jia, L.-Y. Characterization of multiple heat-shock protein transcripts from Cydia pomonella: Their response to extreme temperature and insecticide exposure. J. Agric. Food Chem. 2016, 64, 4288–4298. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.-Y.; Chen, Z.-D.; Jiang, S.-D.; Miao, Z.-Q.; Wang, J.-J.; Wei, D.-D. Characterization and expression of heat shock protein 70s in Liposcelis bostrychophila: Insights into their roles in insecticidal stress response. J. Stored Prod. Res. 2024, 106, 102289. [Google Scholar] [CrossRef]
- Oliver, S.V.; Brooke, B.D. The effect of elevated temperatures on the life history and insecticide resistance phenotype of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Malar. J. 2017, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Li, S.; Shi, C.; Zhang, J.; Zhang, G.; Jin, Z.; Li, C.; Wang, W.; Zhang, Y. Implication of heat-shock protein 70 and UDP-glucuronosyltransferase in thiamethoxam-induced whitefly Bemisia tabaci thermotolerance. J. Pest Sci. 2018, 91, 469–478. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Hranitz, J.M.; McGonigle, M.B.; Manweiler, R.E.; Smith, D.R.; Barthell, J.F. Acute exposure to sublethal doses of neonicotinoid insecticides increases heat tolerance in honey bees. PLoS ONE 2022, 17, e0240950. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, L.; Liu, Y.; He, L.; Li, M.; Lu, W.; Xue, C. Molecular characterization and expression of a heat shock protein gene (Hsp90) from the carmine spider mite, Tetranychus cinnabarinus (Boisduval). J. Insect Sci. 2010, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sheng, Y.; Bai, L.; Zhang, Y.; Xiao, Y.; Xiao, L.; Tan, Y.; Shen, Y. Characterizing heat shock protein 90 gene of Apolygus lucorum (Meyer-Dür) and its expression in response to different temperature and insecticide stresses. Cell Stress Chaperones 2014, 19, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.M.; Liu, Q.X. Study on Insecticide-Induced Resurgence of Cotton Aphid. Acta Ecol. Sin. 1992, 4, 341–347. [Google Scholar]
- Koo, H.-N.; An, J.-J.; Park, S.-E.; Kim, J.-I.; Kim, G.-H. Regional susceptibilities to 12 insecticides of melon and cotton aphid, Aphis gossypii (Hemiptera: Aphididae) and a point mutation associated with imidacloprid resistance. Crop Prot. 2014, 55, 91–97. [Google Scholar] [CrossRef]
- Silva, A.X.; Bacigalupe, L.D.; Luna-Rudloff, M.; Figueroa, C.C. Insecticide Resistance Mechanisms in the Green Peach Aphid Myzus persicae (Hemiptera: Aphididae) II: Costs and Benefits. PLoS ONE 2012, 7, 36810. [Google Scholar] [CrossRef] [PubMed]
- Sangster, T.A.; Lindquist, S.; Queitsch, C. Under cover: Causes, effects and implications of Hsp90-mediated genetic capacitance. Bioessays 2004, 26, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.Q.; Chen, Y.; Wang, X.Y.; Liu, P.B.; Xu, L.; Zhao, T.H. Adaptation of the dwarf forms of soybean aphid, Aphis glycines to environment and impact on soybean yield. Chin. J. Appl. Entomol. 2011, 48, 1638–1645. [Google Scholar]
- De Beeck, L.O.; Verheyen, J.; Olsen, K.; Stoks, R. Negative effects of pesticides under global warming can be counteracted by a higher degradation rate and thermal adaptation. J. Appl. Ecol. 2017, 54, 1847–1855. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chang, Y.W.; Du, Y.Z. Temperature affects the tolerance of Liriomyza trifolii to insecticide abamectin. Ecotox. Environ. Safe 2021, 218, 112307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Chang, Y.W.; Du, Y.Z. Transcriptome analysis reveals gene expression differences in Liriomyza trifolii exposed to combined heat and abamectin exposure. PeerJ 2021, 9, e12064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Chang, Y.W.; Gong, W.R.; Hu, J.; Du, Y.Z. The development of abamectin resistance in Liriomyza trifolii and its contribution to thermotolerance. Pest Manag. Sci. 2024, 80, 2053–2060. [Google Scholar] [CrossRef] [PubMed]




| Insecticides | Slope (±SE) | LC30 (95% CL)(mg/L) | LC50 (95% CL)(mg/L) | χ2 | df | p |
|---|---|---|---|---|---|---|
| Imidacloprid | 1.59 ± 0.33 | 2.30 (0.90–4.02) | 4.91 (2.60–8.54) | 2.93 | 5 | 0.72 |
| Lambda-cyhalothrin | 1.51 ± 0.42 | 0.79 (0.11–1.62) | 1.75 (0.55–3.18) | 0.45 | 5 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Jia, Y.; Dai, C.; Wang, X.; Liu, J.; Tian, Z. Expression of Heat Shock Protein 90 Genes Induced by High Temperature Mediated Sensitivity of Aphis glycines Matsumura (Hemiptera: Aphididae) to Insecticides. Insects 2025, 16, 772. https://doi.org/10.3390/insects16080772
Han X, Jia Y, Dai C, Wang X, Liu J, Tian Z. Expression of Heat Shock Protein 90 Genes Induced by High Temperature Mediated Sensitivity of Aphis glycines Matsumura (Hemiptera: Aphididae) to Insecticides. Insects. 2025; 16(8):772. https://doi.org/10.3390/insects16080772
Chicago/Turabian StyleHan, Xue, Yulong Jia, Changchun Dai, Xiaoyun Wang, Jian Liu, and Zhenqi Tian. 2025. "Expression of Heat Shock Protein 90 Genes Induced by High Temperature Mediated Sensitivity of Aphis glycines Matsumura (Hemiptera: Aphididae) to Insecticides" Insects 16, no. 8: 772. https://doi.org/10.3390/insects16080772
APA StyleHan, X., Jia, Y., Dai, C., Wang, X., Liu, J., & Tian, Z. (2025). Expression of Heat Shock Protein 90 Genes Induced by High Temperature Mediated Sensitivity of Aphis glycines Matsumura (Hemiptera: Aphididae) to Insecticides. Insects, 16(8), 772. https://doi.org/10.3390/insects16080772






