A New Species of Aprostocetus (Hymenoptera: Eulophidae), a Parasitoid from China of the Invasive Gall Wasp Ophelimus bipolaris (Hymenoptera: Eulophidae) on Eucalyptus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Sampling
2.2. Morphology
2.3. Measurements
2.4. Images
2.5. DNA Extraction, Amplification, and Sequencing
2.6. Sequence Alignments and Phylogenetic Analysis
3. Results
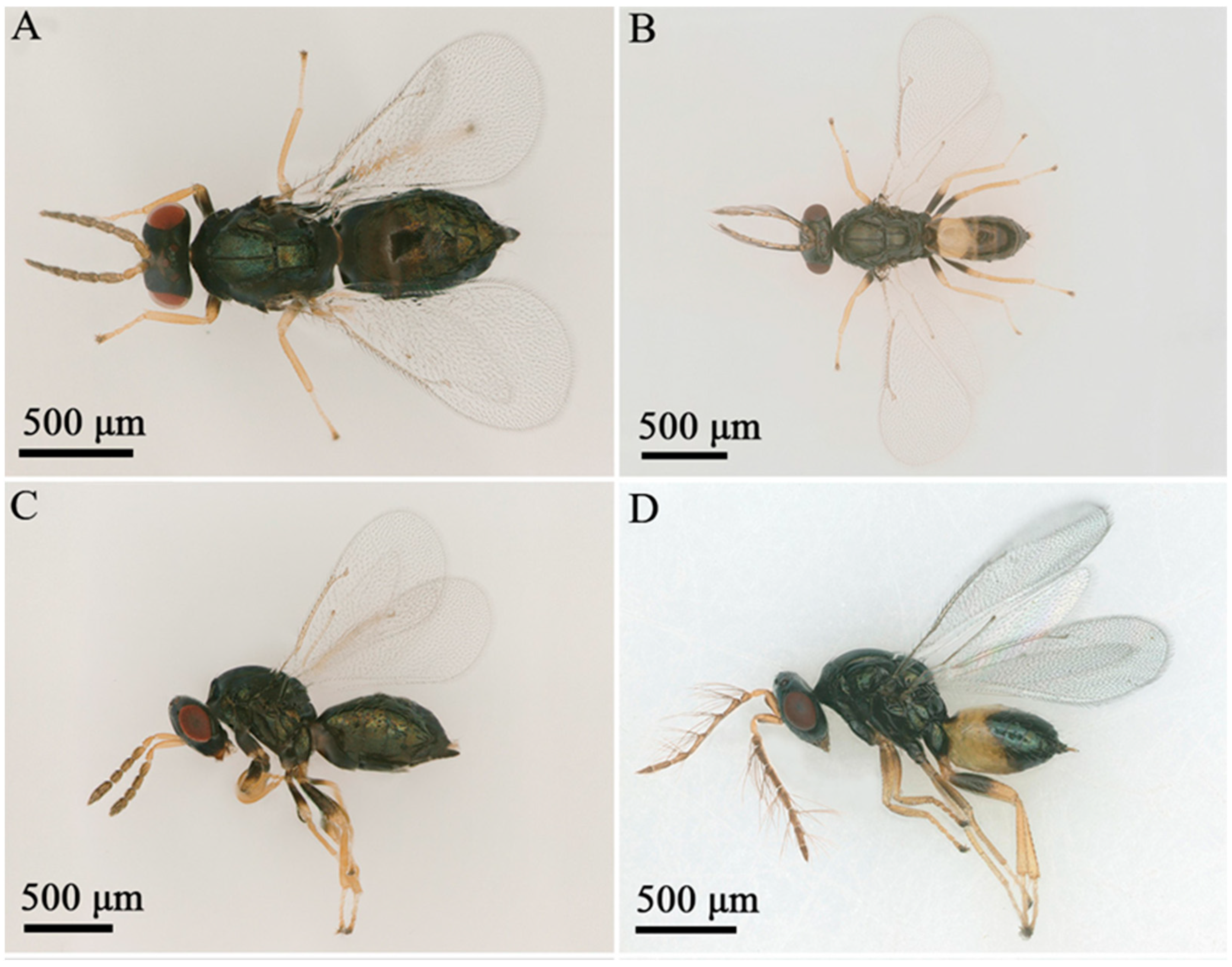
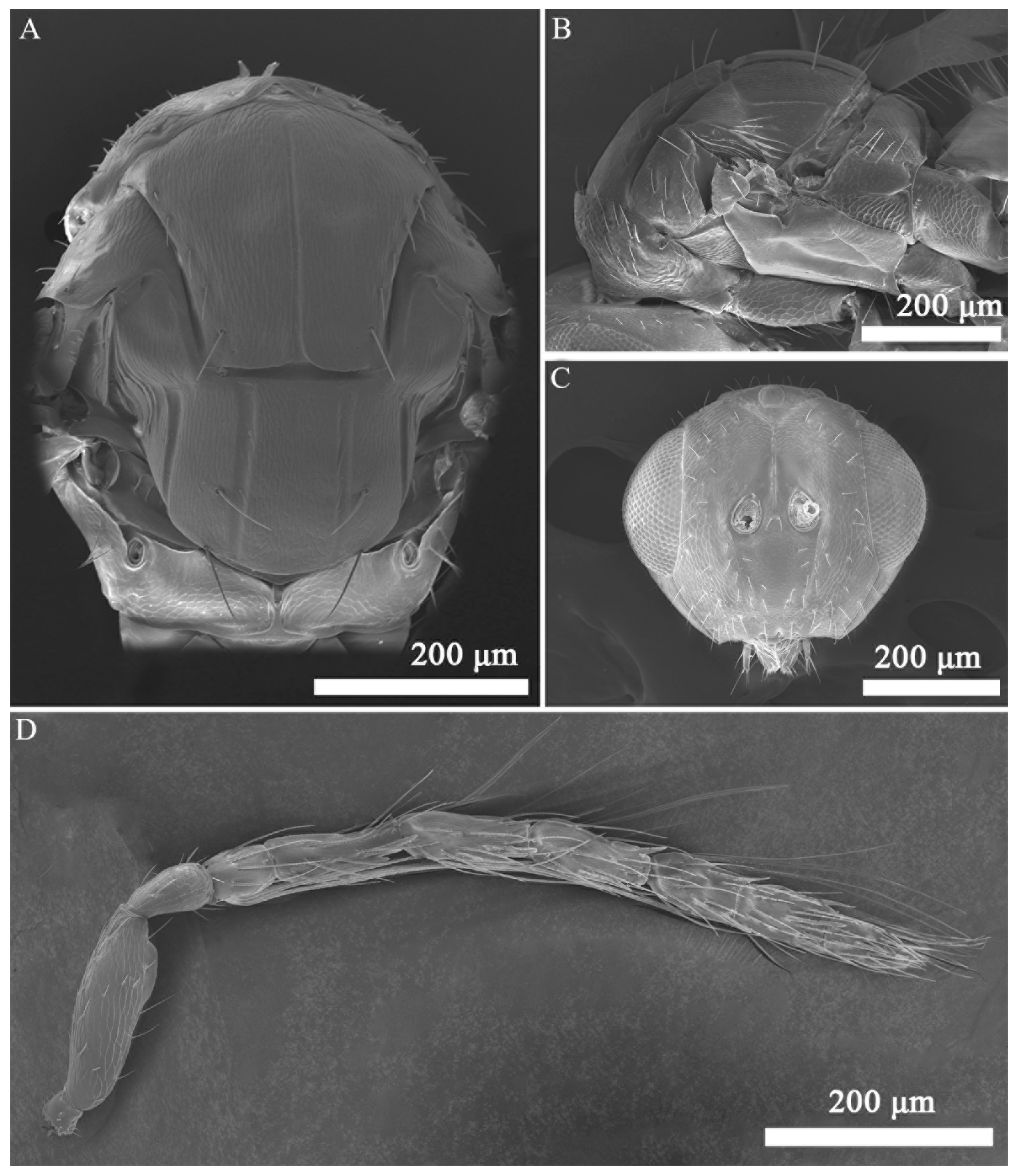
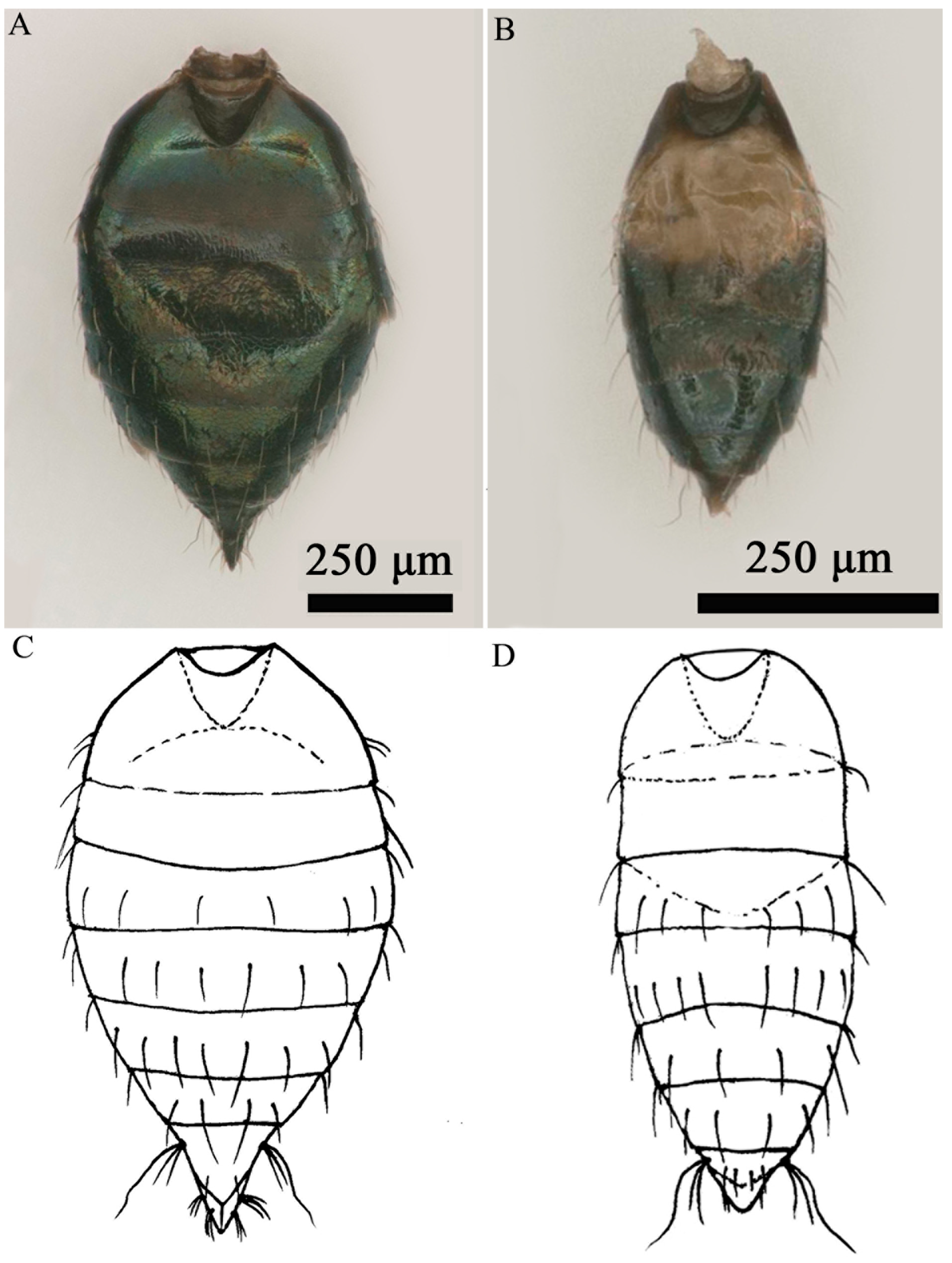
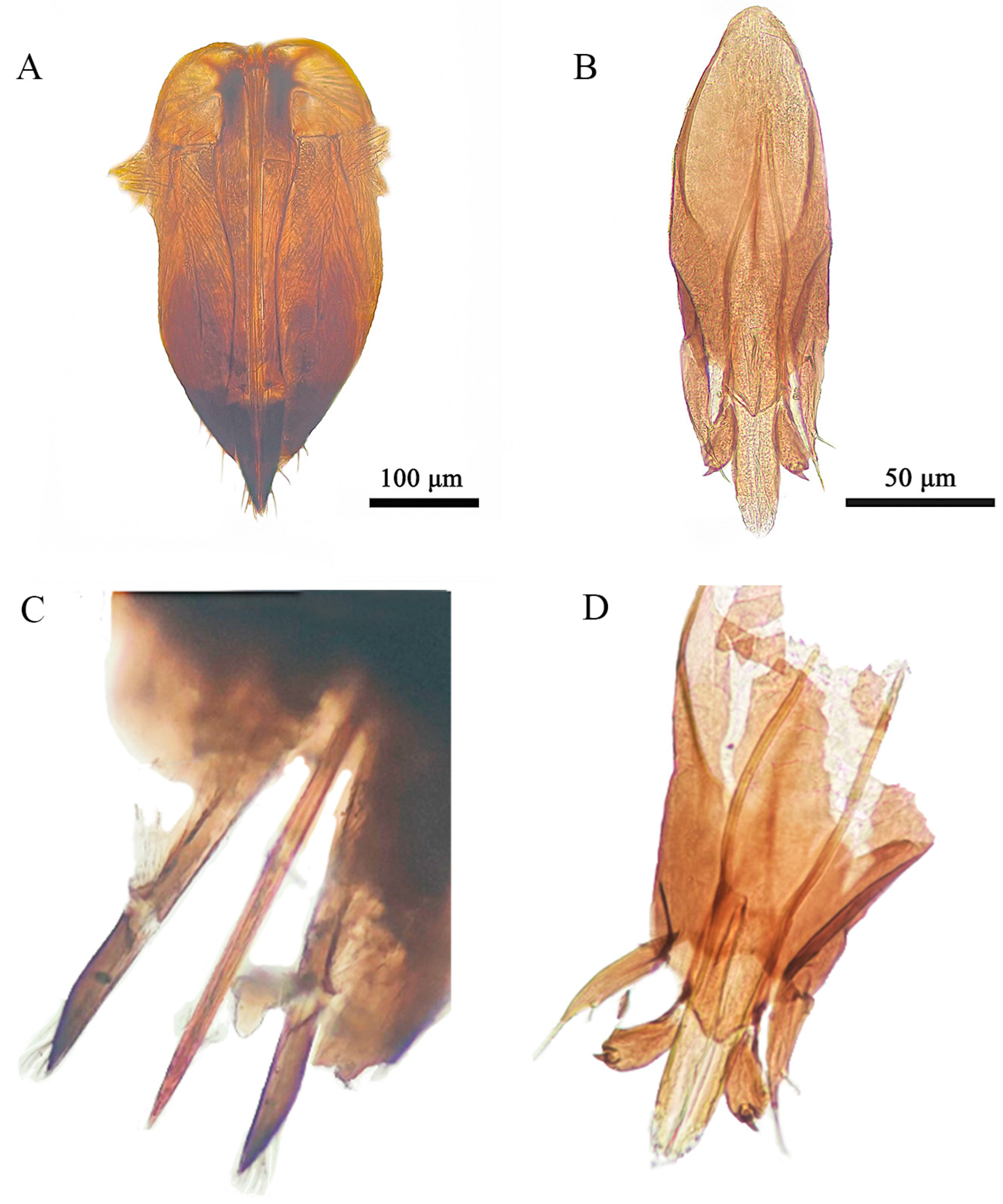
3.1. Taxonomy
- 174. Antenna with funicle slightly more slender … first funicular segment 1.5–2.0× as long as broad……………………………………….…………….A. micantulus (Thomson)
- 174a. Antenna. F1 = F2. F3 0.81× as long as F2.Clava 2.5× as long as F3 and 2.2× as long as broad. Gaster 1.2× as long as broad……….……….……….A. micantulus (Thomson)
- - (a). Antenna. F1 = F2. F3 0.88× as long as F2. Clava 2.1× as long as F3 and 2.0× as long as broad. Gaster 1.6× as long as broad……………………Aprostocetus bipolaris sp. nov.
- 110. Spur of mid tibia fully as long as, or even very slightly longer than basitarsus. Antenna. Species associated with Pinus spp………………………………A. micantulus
- -. Spur of mid tibia 0.87–0.90× length of basitarsus in A. aethiops, virtually as long as basitarsus in A. lycidas mostly on deciduous trees or shrubs………….……..A. aethiops
- 110a. Antenna with ventral plaque 0.29 of length of scape. F1 0.54× as long as F2. F2 0.91× as long as F3. F2 = F3. Whorls of F1 reaching level with tip of F3. Ratio between apodemae of aedeagus and aedeagus 2.2: 1. Digitus with 1 spine. Parasitoid of Dasyneura abietiperda Henschel [46] (Domenichini 1966: 39)….A. micantulus (Thomson)
- -. (a) Antenna with ventral plaque 0.33 of length of scape. F1 0.5× as long as F2. F2 0.91× as long as F3. F3 = F4. Whorls of F1 reaching 1/3 basal part of F2. Ratio between apodemae of aedeagus and aedeagus 2.25: 1. Digitus with 1 spine. Parasitoid of Ophelimus bipolaris (Hymenoptera, Eulophidae)…………..….Aprostocetus bipolaris sp. nov.
- 8. Gaster 4.3–5.3× as long as broad………………….……….…A. (A.) aquaticus Erdoes
- -. Gaster 1.7–4.0× as long as broad…………………….………….……….…….……….9
- 9. The longest cercal seta 1.5× longer than next cercal seta……A. (A.) escherichi Szelyni
- -. The longest cercal seta 2.0× longer than next cercal seta; gaster 1.5–2.7× as long as broad; scutellum 1.15–1.3× as long as broad….……..….………A. (A.) zosimus Walker
- 9a. Antenna female F1 3.0× as long as broad, F2 2.0× as long as broad. Antenna male with ventral plaque 0.6 of length of scape. F1 0.5× as long as F2. F2 = F3 = F4. Whorls of F1 reaching 2/3 basal part of F3. Digitus with 3 spines. Parasitoid of Dasyneura leguminicola Lint (Cecidomyidae)………………………….….…..A. (A.) zosimus Walker
- (a). Antenna female F1 1.9× as long as broad, F2 1.9× as long as broad. Antenna male with ventral plaque 0.33 of length of scape. F1 0.5× as long as F2. F2 0.91× as long as F3. F3 = F4. Whorls of F1 reaching 1/3 basal part of F2. Digitus with 1 spine. Parasitoid of Ophelimus bipolaris (Hymenoptera, Eulophidae)…...….Aprostocetus bipolaris sp. nov.
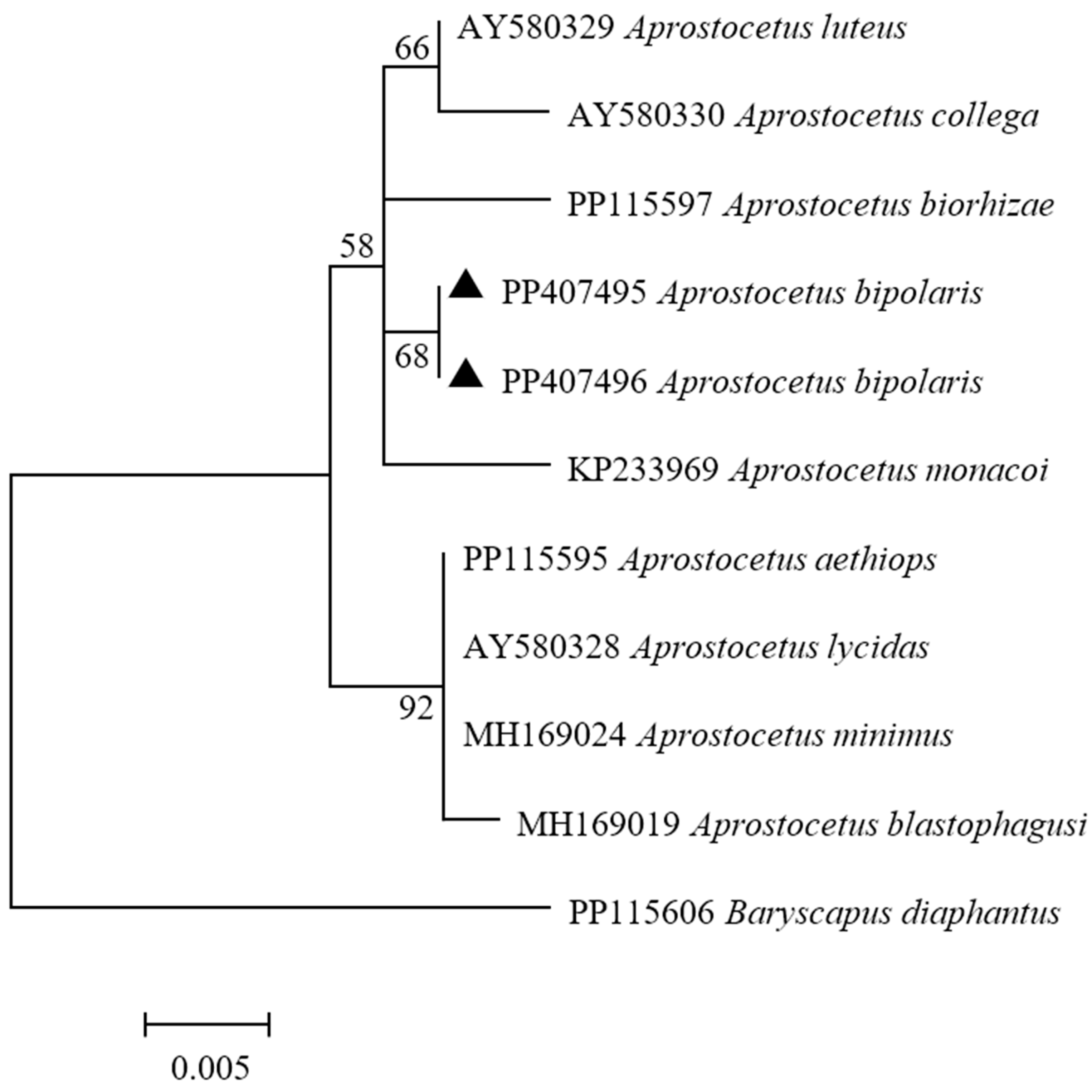
3.2. Phylogenetic Analyses
3.3. Biology
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouček, Z. Australasian Chalcidoidea (Hymenoptera). A Biosystematic Revision of Genera of Fourteen Families, with a Reclassification of Species; CAB International: Wallingford, UK, 1988. [Google Scholar]
- Borowiec, N.; La Salle, J.; Brancaccio, L.; Thaon, M.; Warot, S.; Branco, M.C.; Ris, N.; Malausa, J.C.; Burks, R. Ophelimus mediterraneus sp. n. (Hymenoptera, Eulophidae): A new Eucalyptus gall wasp in the Mediterranean region. Bull. Entomol. Res. 2019, 109, 678–694. [Google Scholar] [CrossRef] [PubMed]
- Withers, T.M.; Raman, A.; Berry, J.A. Host range and biology of Ophelimus eucalyptii (Gahan) (Hym Eulophidae) a pest of New Zealand eucalypts. N. Z. Plant Prot. 2000, 53, 339–344. [Google Scholar] [CrossRef]
- Protasov, A.; Blumberg, D.; Brand, D.; Mendel, Z. Biological control of the eucalyptus gall wasp Ophelimus maskelli (Ashmead): Taxonomy and biology of the parasitoid species Closterocerus chamaeleon (Girault), with information on its establishment in Israel. Biol. Control 2007, 42, 196–206. [Google Scholar] [CrossRef]
- Garcia, A.; Gonçalves, H.; Borowiec, N.; Carlos Franco, J.; Branco, M. Ophelimus sp., a new invasive gall wasp of Eucalyptus globulus in Europe, escapes the parasitism by Closterocerus chamaeleon due to an asynchronous life cycle. Biol. Control 2019, 131, 1–7. [Google Scholar] [CrossRef]
- Molina-Mercader, G.; Angulo, A.O.; Olivares, T.S.; Sanfuentes, E.; Castillo-Salazar, M.; Hasbún, R.; Rojas, E.; Toro-Núñez, Ó.; Benítez, H.A. Ophelimus migdanorum Molina-Mercader sp. nov. (Hymenoptera: Eulophidae): Application of integrative taxonomy for disentangling a polyphenism case in Eucalyptus globulus Labill forest in Chile. Forests 2019, 10, 720. [Google Scholar] [CrossRef]
- Pudjianto, L.J.; Harahap, I.S. Current infestation status and damage severity of Eucalyptus gall wasps, Leptocybe invasa (Fisher & La Salle), and Ophelimus maskelli Ashmead (Hymenoptera: Eulophidae), infesting Eucalyptus germplasms in Tanzania. IOP Conf. Ser. Earth Environ. Sci. 2023, 1208, 12010. [Google Scholar] [CrossRef]
- UCD Community. Universal Chalcidoidea Database Website. 2023. Available online: https://ucd.chalcid.org (accessed on 5 March 2025).
- Gahan, A.B. A list of the phytophagous Chalcidoidea, with descriptions of two new species. Proc. Entomol. Soc. Wash. 1922, 24, 33–58. Available online: https://biostor.org/reference/99253 (accessed on 5 March 2025).
- Burks, R.A.; Mottern, J.L.; Pownall, N.G.; Waterworth, R.; Paine, T.D. First record of Closterocerus chamaeleon, parasitoid of the Eucalyptus gall wasp Ophelimus maskelli (Hymenoptera, Chalcidoidea, Eulophidae), in the New World. ZooKeys 2015, 504, 149–152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mendel, Z.; Protasov, A.; La Salle, J.; Blumberg, D.; Brand, D.; Branco, M. Classical biological control of two Eucalyptus gall wasps; main outcome and conclusions. Biol. Control 2017, 105, 66–78. [Google Scholar] [CrossRef]
- Malumphy, C. First record of Eucalyptus leaf gall wasp, Ophelimus maskelli (Ashmead) (Hymenoptera: Eulophidae), an invasive pest of Eucalyptus spp., in the Canary Islands. Entomol. Mon. Mag. 2018, 154, 213–216. [Google Scholar] [CrossRef]
- Dittrich-Schröder, G.; Hurley, B.P.; Wingfield, M.J.; Nahrung, H.F.; Slippers, B. Invasive gall-forming wasps that threaten non-native plantation-grown Eucalyptus: Diversity and invasion patterns. Agric. For. Entomol. 2020, 22, 285297. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yao, J.M.; Huang, S.B.; Pang, H. Ophelimus bipolaris sp. n. (Hymenoptera, Eulophidae), a new invasive Eucalyptus pest and its host plants in China. Insects 2021, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Clercq, P.D.; Mason, P.G.; Babendreier, D. Benefits and risks of exotic biological control agents. BioControl 2011, 56, 681–698. [Google Scholar] [CrossRef]
- Huber, J.T.; Mendel, Z.; Protasov, A.; La Salle, J. Two new Australian species of Stethynium (Hymenoptera: Mymaridae), larval parasitoids of Ophelimus maskelli (Ashmead) (Hymenoptera: Eulophidae) on Eucalyptus. J. Nat. Hist. 2006, 40, 1909–1921. [Google Scholar] [CrossRef]
- Anisa, R.P.; Hidayat, P.; Buchori, D.; Pratyadhiraksana, G.; Abad, J.I.M.; de S Tavares, W.; Tarigan, M. Parasitoids associated to Ophelimus eucalypti (gahan) (Hymenoptera: Eulophidae) on Eucalyptus (Myrtaceae) plantations in North Sumatra, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2023, 1133, 12040. [Google Scholar] [CrossRef]
- Mendel, Z.; Protasov, A.; Blumberg, D.; Brand, D.; Saphir, N.; Madar, Z.; Salle, J.L. Note: Release and recovery of parasitoids of the eucalyptus gall wasp Ophelimus maskelli in Israel. Phytoparasitica 2007, 35, 330–332. [Google Scholar] [CrossRef]
- Caleca, V.; Verde, G.L.; Rizzo, M.C.; Rizzo, R. Dispersal rate and parasitism by Closterocerus chamaeleon (Girault) after its release in Sicily to control Ophelimus maskelli (Ashmead) (Hymenoptera, Eulophidae). Biol. Control 2011, 57, 66–73. [Google Scholar] [CrossRef]
- Caleca, V. First record in Algeria of two eulophid wasps: Closterocerus chamaeleon (Girault) and its host, the eucalyptus gall wasp Ophelimus maskelli (Asmead) (Hymenoptera Eulophidae). Nat. Sicil. 2010, 34, 201–206. [Google Scholar]
- Sinulingga, N.G.; Tarigan, M.; Tavares, W.D.; Ansor, K.; Pasaribu, I.; Kkadan, S.K.; Panjaitan, R.; Puspita, K.; Abad, J.I.; Durán, Á. The parasitoid Closterocerus chamaeleon has a greater development and survival rate than of its hosts, the Eucalyptus gall wasps Ophelimus eucalypti and Ophelimus maskelli in Sumatra, Indonesia. Ann. Appl. Biol. 2021, 179, 354–367. [Google Scholar] [CrossRef]
- Westwood, J.O. Descriptions of several new British forms amongst the parasitic hymenopterous insects. Lond. Edinb. Phil. Mag. 1833, 2, 443–445. [Google Scholar] [CrossRef]
- Graham, M. A reclassification of the European Tetrastichinae (Hymenoptera: Eulophidae), with a revision of certain genera. Bull. Br. Mus. (Nat. Hist.) Entomol. 1987, 55, 392. [Google Scholar]
- LaSalle, J. North American genera of Tetrastichinae (Hymenoptera: Eulophidae). J. Nat. Hist. 1994, 28, 109–236. [Google Scholar] [CrossRef]
- Kumar, V.; Pant, P.C.; Bisht, V.; Bhat, S.; Kumar, S. Two reared species of the genus Aprostocetus (Hymenoptera: Eulophidae), parasitic on gall forming psylloid Pauropsylla ficicola Kieffer infesting Ficus auriculata Lour. in Uttarakhand, India. J. Entomol. Zool. Stud. 2018, 6, 906–910. [Google Scholar]
- Triapitsyn, S.V. A new species of the genus Aprostocetus Westwood, 1833 (Hymenoptera, Eulophidae: Tetrastichinae) collected by E.S. Sugonyaev in galls on saxauls in Uzbekistan. Entomol. Rev. 2015, 95, 643–646. [Google Scholar] [CrossRef]
- Singh, S. A new species of Aprostocetus Westwood (Hymenoptera: Eulophidae), parasitizing mango leaf gall midge (Diptera: Cecidomyiidae), from India. J. Asia-Pac. Entomol. 2018, 21, 553–559. [Google Scholar] [CrossRef]
- Bae, J.; Jung, S. First record of the genus Aprostocetus (Hymenoptera: Eulophidae) from Korea with the description of a new species: An inquiline of Rhopalomyia giraldii (Diptera: Cecidomyiidae) inducing galls on Artemisia princeps (Asterales: Asteraceae). J. Asia-Pac. Entomol. 2020, 23, 923–929. [Google Scholar] [CrossRef]
- Smith, C.M.; Griffin, M.P.; Fadamiro, H.Y.; Appel, A.G. Toxicity of cockroach gel baits to the oothecal parasitoid Aprostocetus hagenowii (Hymenoptera: Eulophidae) and implications for cockroach integrated pest management. J. Econ. Entomol. 2024, 117, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.G.; Demard, E.P.; Diepenbrock, L.M. Evaluation of potential natural enemies of hibiscus mealybug, Nipaecoccus viridis (Hemiptera: Pseudococcidae) in Florida citrus. Fla. Entomol. 2024, 107, 20240059. [Google Scholar] [CrossRef]
- Yang, M.M.; Lin, Y.C.; Wu, Y.; Fisher, N.; Saimanee, T.; Sangtongpraow, B.; Zhu, C.; Chiu, W.C.; La Salle, J. Two new Aprostocetus species (Hymenoptera: Eulophidae: Tetrastichinae), fortuitous parasitoids of invasive eulophid gall inducers (Tetrastichinae) on Eucalyptus and Erythrina. Zootaxa 2014, 3846, 261–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perkins, R.C.L. Parasites of Insects Attacking Sugar Cane; Bulletin (Hawaiian Sugar Planters’ Association. Experiment Station): Entomological series; Hawaiian Sugar Planters’ Association: Honolulu, HI, USA, 1912; Volume 10, pp. 1–27. [Google Scholar]
- Jacas, J.A.; Peña, J.; Duncan, R.E. Successful oviposition and reproductive biology of Aprostocetus vaquitarum (Hymenoptera: Eulophidae): A predator of Diaprepes abbreviatus (Coleoptera: Curculionidae). Biol. Control 2005, 33, 352–359. [Google Scholar] [CrossRef]
- Tee, H.S.; Saad, A.R.; Lee, C.Y. Suitability of heat- and freeze-killed oothecae of the American cockroach (Dictyoptera: Blattidae) as hosts for an oothecal parasitoid, Aprostocetus hagenowii (Hymenoptera: Eulophidae). J. Econ. Entomol. 2010, 103, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Vongpa, V.; Amornsak, W.; Gordh, G. Development, reproduction and longevity of Aprostocetus sp. (Hymenoptera: Eulophidae), an egg parasitoid of the Brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Agric. Nat. Res. 2016, 50, 291–294. [Google Scholar] [CrossRef]
- Wang, X.; Ramualde, N.; Desurmont, G.A.; Smith, L.; Gundersen-Rindal, D.E.; Grodowitz, M.J. Reproductive traits of the egg parasitoid Aprostocetus fukutai, a promising biological control agent for invasive citrus longhorned beetle Anoplophora chinensis. BioControl 2021, 67, 15–26. [Google Scholar] [CrossRef]
- Ramadan, M.M.; Yalemar, J.A.; Rubinoff, D.; Wright, M.G.; Bokonon-Ganta, A.H.; Wang, X. Aprostocetus nitens (Hymenoptera: Eulophidae), an ectoparasitoid proposed for biological control of the destructive erythrina gall wasp, Quadrastichus erythrinae, in Hawaiʻi. Insects 2025, 16, 519. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Limonta, L. Anoplophora malasiaca Thomson (Coleoptera Cerambycidae Lamiinae Lamiini) in Europe. Boll. Zool. Agrar. Bachic. 2001, 33, 65–68. [Google Scholar]
- Burks, R.A.; Gibson, G.A.P.; Heraty, J.M. External morphology of adults Chalcidoidea. In Chalcidoidea of the World; Heraty, J.M., Woolly, J.B., Eds.; CABI: Wallingford, UK, 2025. [Google Scholar]
- Gillespie, J.J.; Munro, J.B.; Heraty, J.M.; Yoder, M.J.; Owen, A.K.; Carmichael, A.E. A secondary structural model of the 28S rRNA expansion segments D2 and D3 for Chalcidoid wasps (Hymenoptera: Chalcidoidea). Mol. Biol. Evol. 2005, 22, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Cruaud, A.; Jabbour-Zahab, R.; Genson, G.; Cruaud, C.; Couloux, A.; Kjellberg, F.; Van Noort, S.; Rasplus, J.Y. Laying the foundations for a new classification of Agaonidae (Hymenoptera: Chalcidoidea), a multilocus phylogenetic approach. Cladistics 2010, 26, 359–387. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Domenichini, G. Hym. Eulophidae, Palearctic Tetrastichinae. In Index of Entomohpagous Insects; Delucchi, V., Remaudiere, G., Eds.; Le François: Paris, France, 1966. [Google Scholar]
- Kostjukov, V.V. Family Eulophidae Subfamily Tetrastichinae. In Key to the Insects of Russian Far East; Lehr, P.A., Ed.; Dal’nauka: Vladivostok, Russia, 1995. [Google Scholar]
- Khan, M.A.; Agnihotri, M.; Sushil, S.N. Taxonomic studies of eulophid parasitoids (Hymenoptera: Chalcidoidea) of India. Pantnagar J. Res. 2005, 2, 1–203. [Google Scholar]
- Narendran, T.C. Indian Chalcidoid Parasitoids Of The Tetrastichinae (Hymenoptera: Eulophidae); Zoological Survey of India: Kolkata, India, 2007. [Google Scholar]
- Pujade-Villar, J. Sobre las especies de Aprostocetus Westwood, 1833, recolectadas en Cataluña en agallas de cinípidos producidas sobre especies del género Rosa y Quercus (Hym., Chalcidoidea, Eulophidae). Orsis 1992, 7, 79–85. [Google Scholar]
- Wang, J.; Chen, Y.M.; Yang, X.B.; Lv, R.E.; Desneux, N.; Zang, L.S. Parasitism and suitability of Aprostocetus brevipedicellus on Chinese oak silkworm, Antheraea pernyi, a dominant factitious host. Insects 2021, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Queffelec, J.; Wooding, A.L.; Greeff, J.M.; Garnas, J.R.; Hurley, B.P.; Wingfield, M.J.; Slippers, B. Mechanisms that influence sex ratio variation in the invasive hymenopteran Sirex noctilio in South Africa. Ecol. Evol. 2019, 9, 7966–7973. [Google Scholar] [CrossRef] [PubMed]




| Sex | Accession Number | |
|---|---|---|
| 28S | COI | |
| Female | PP407495 | PV555247 |
| Male | PP407496 | PV555248 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.-H.; Li, Y.-H.; Hu, J.; Qin, Y.; Li, J.; Yefremova, Z.; Zheng, X.-L. A New Species of Aprostocetus (Hymenoptera: Eulophidae), a Parasitoid from China of the Invasive Gall Wasp Ophelimus bipolaris (Hymenoptera: Eulophidae) on Eucalyptus. Insects 2025, 16, 755. https://doi.org/10.3390/insects16080755
Su J-H, Li Y-H, Hu J, Qin Y, Li J, Yefremova Z, Zheng X-L. A New Species of Aprostocetus (Hymenoptera: Eulophidae), a Parasitoid from China of the Invasive Gall Wasp Ophelimus bipolaris (Hymenoptera: Eulophidae) on Eucalyptus. Insects. 2025; 16(8):755. https://doi.org/10.3390/insects16080755
Chicago/Turabian StyleSu, Jing-Hui, Yuan-Hao Li, Jin Hu, Yan Qin, Jun Li, Zoya Yefremova, and Xia-Lin Zheng. 2025. "A New Species of Aprostocetus (Hymenoptera: Eulophidae), a Parasitoid from China of the Invasive Gall Wasp Ophelimus bipolaris (Hymenoptera: Eulophidae) on Eucalyptus" Insects 16, no. 8: 755. https://doi.org/10.3390/insects16080755
APA StyleSu, J.-H., Li, Y.-H., Hu, J., Qin, Y., Li, J., Yefremova, Z., & Zheng, X.-L. (2025). A New Species of Aprostocetus (Hymenoptera: Eulophidae), a Parasitoid from China of the Invasive Gall Wasp Ophelimus bipolaris (Hymenoptera: Eulophidae) on Eucalyptus. Insects, 16(8), 755. https://doi.org/10.3390/insects16080755






