The parasitoids of Parlatoria ziziphi (Lucas) (Hemiptera: Diaspididae): with descriptions of two new species of Aphytis (Hymenoptera: Aphelinidae) from China, and a note on Aphytis species with black-tipped mid tibiae in the male
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Parasitoids
2.2. Photographs and Slides of Parasitoids
2.3. DNA Sequencing and Phylogenetic Analysis
2.4. Terminology and Abbreviations
| FAFU | College of Plant Protection, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China |
| MNCN | Instituto Espanol de Entomologia, Museo Nacional de Ciencias Naturales, Madrid, Spain |
| USNM | United States National Museum of Natural History, Washington, DC, USA |
3. Results
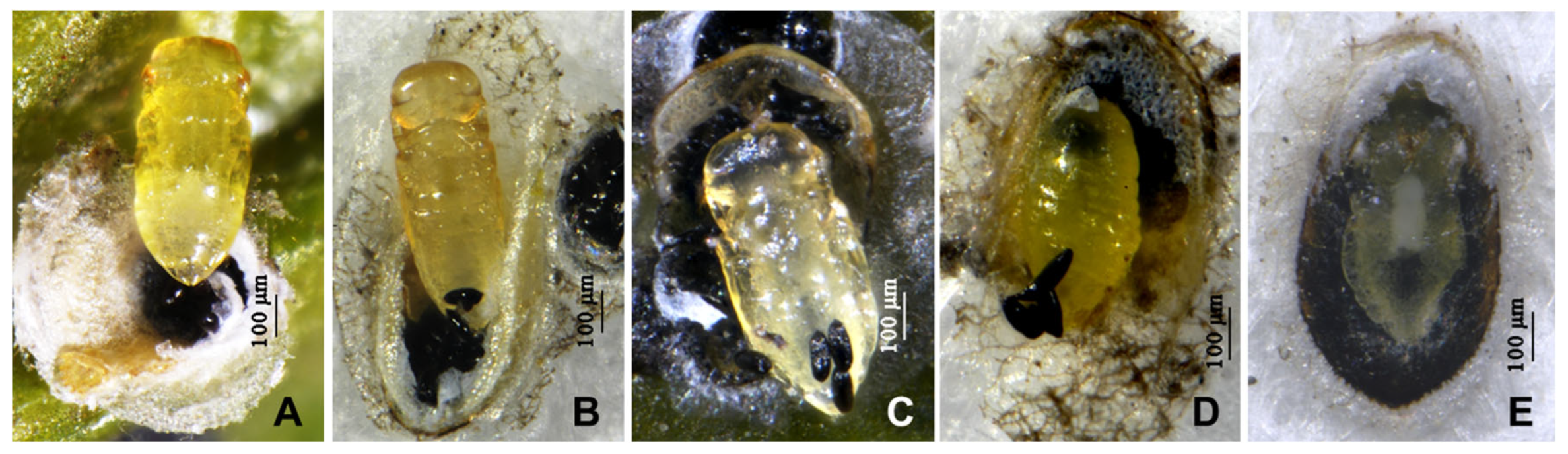
3.1. Parlatoria ziziphi and Pupae or Mature Larvae of Parasitoids (Figure 1 and Figure 2)
3.2. Species Accounts
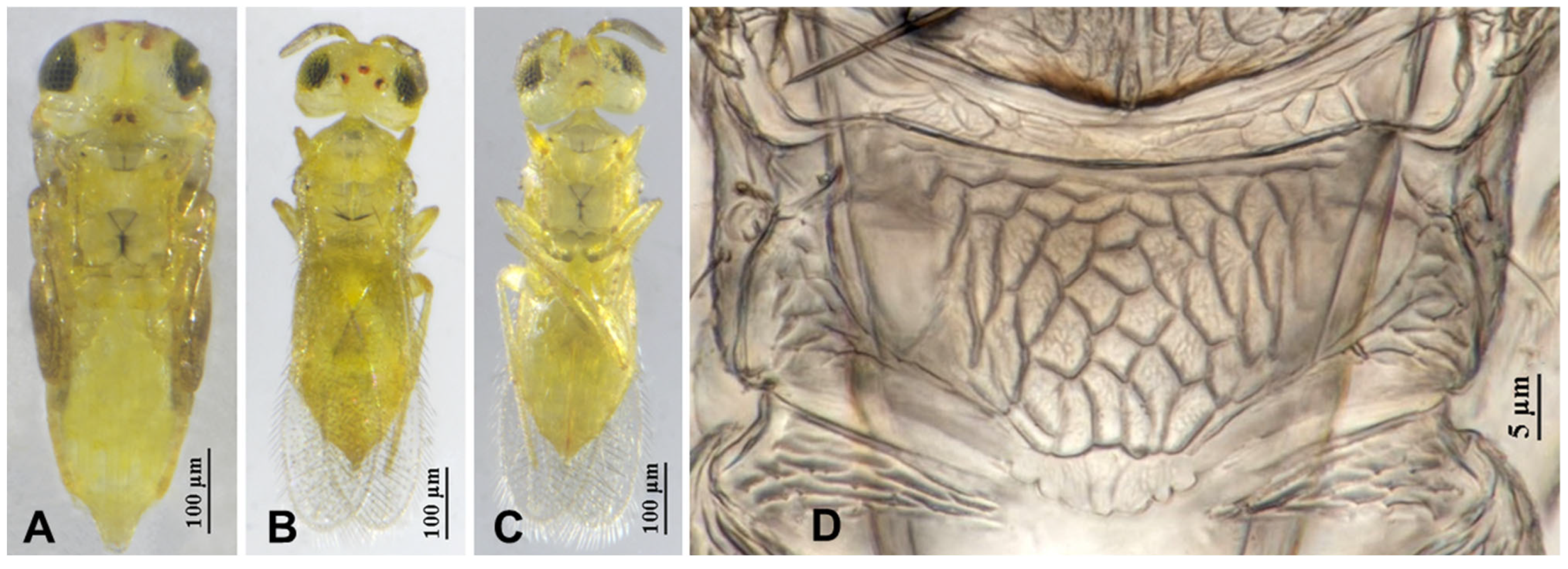
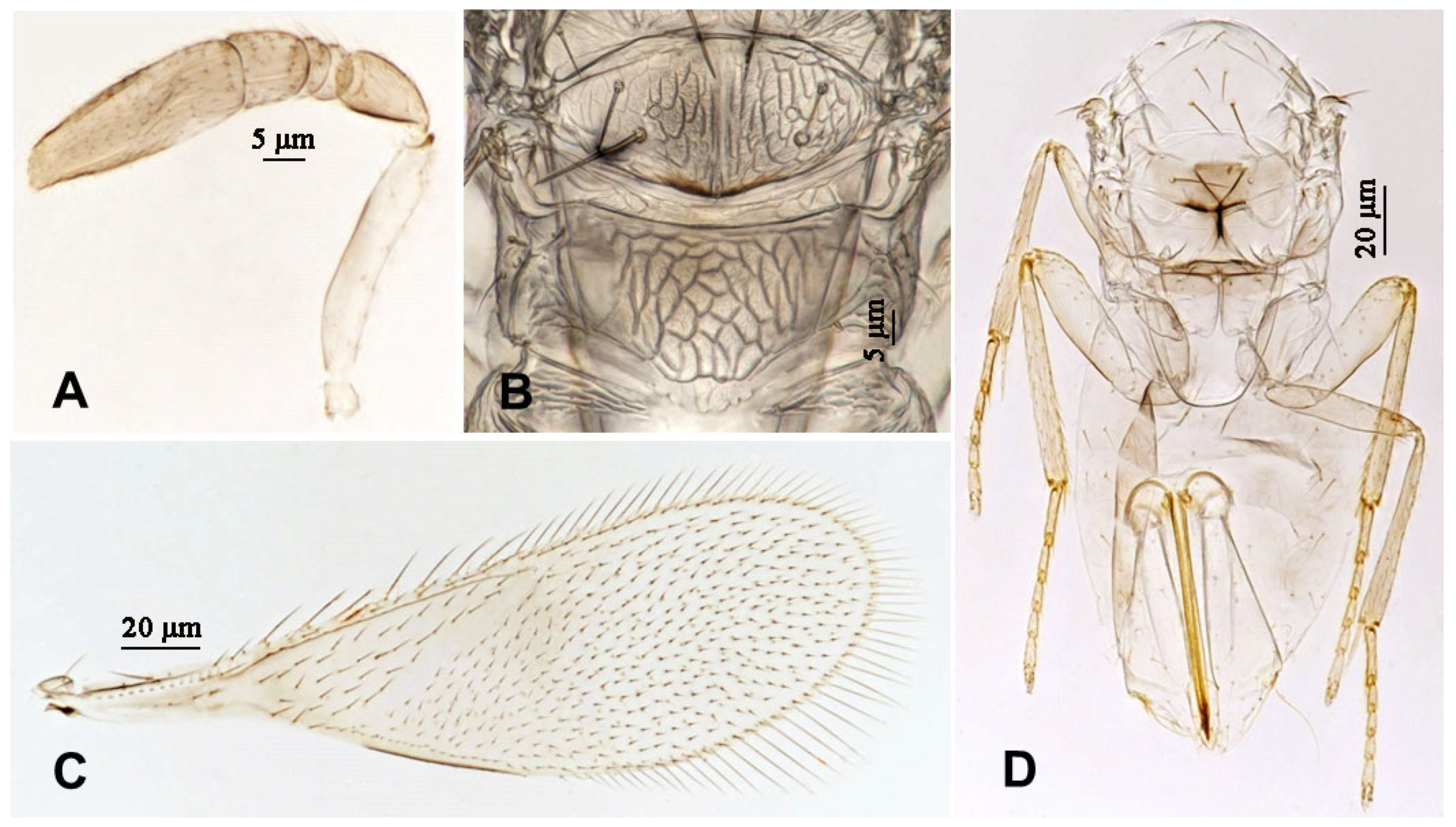
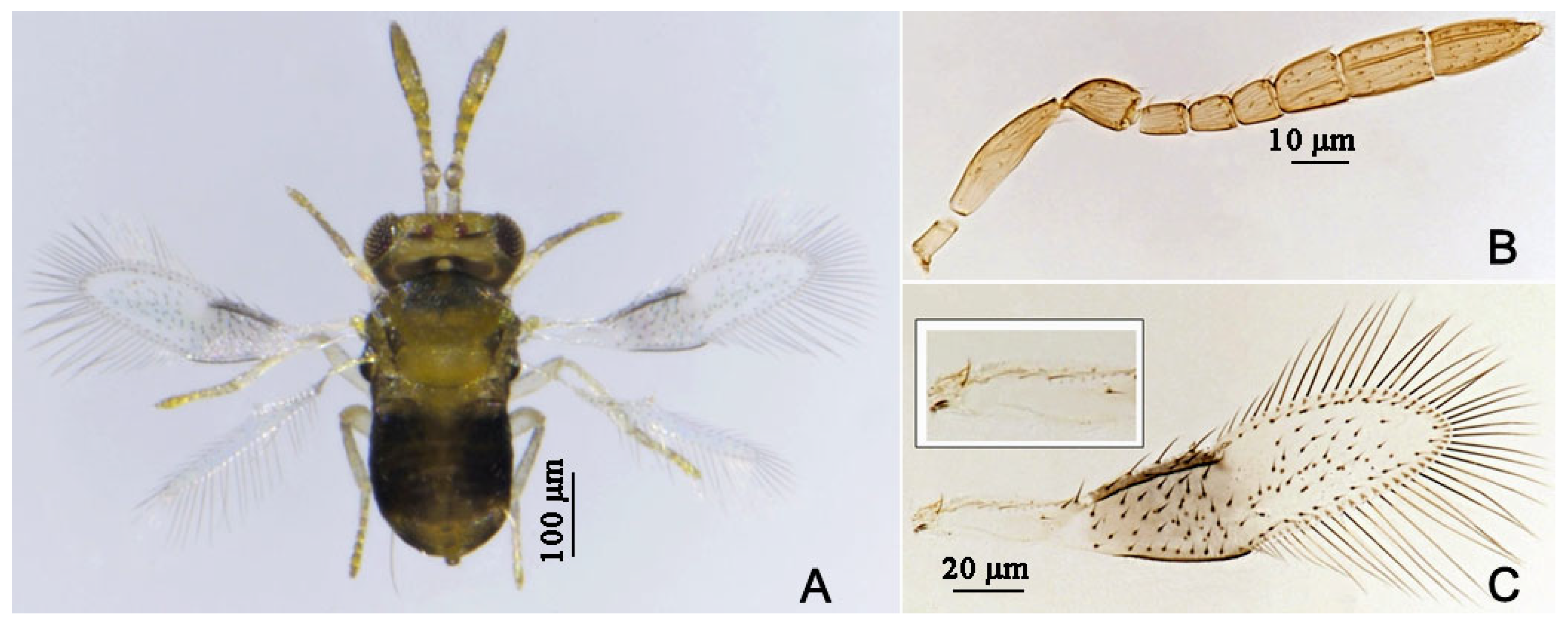
3.2.1. Aphytis nigromaculata Wang & Huang, sp.n. (Figure 3, Figure 4, Figure 5 and Figure 6)


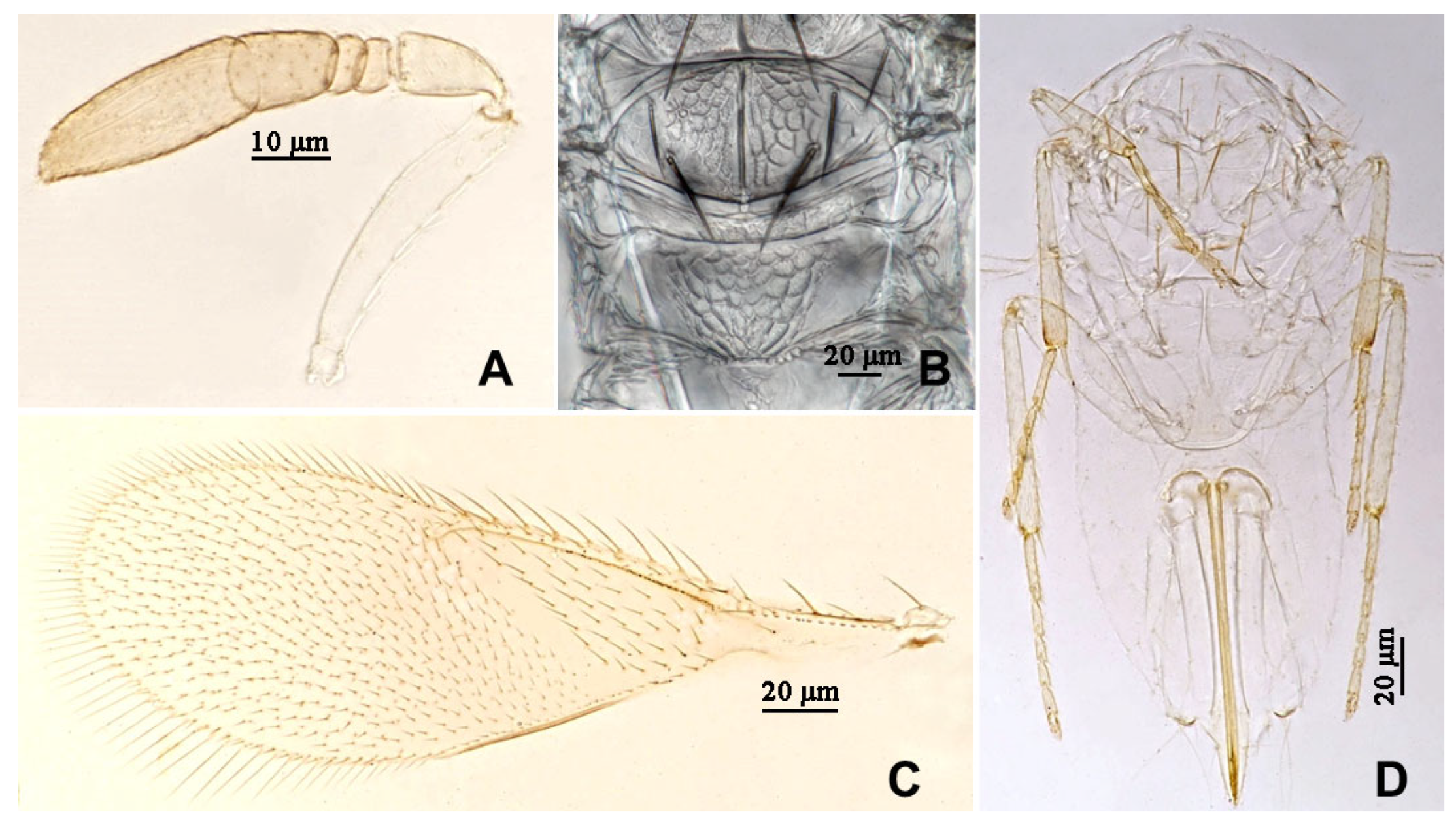
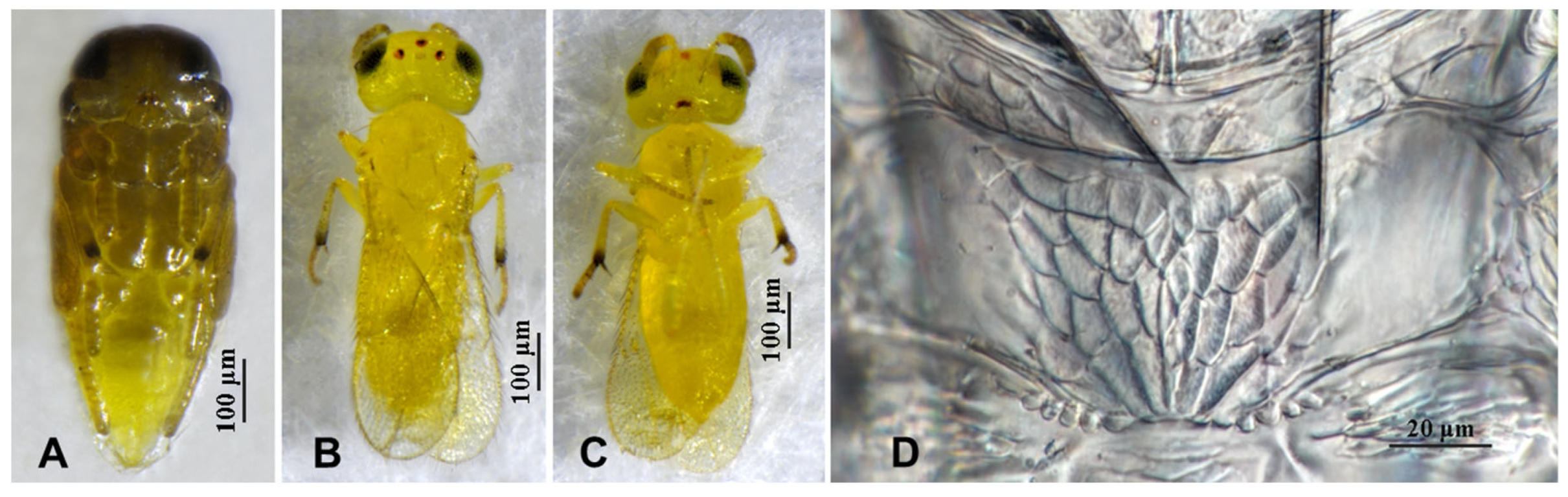
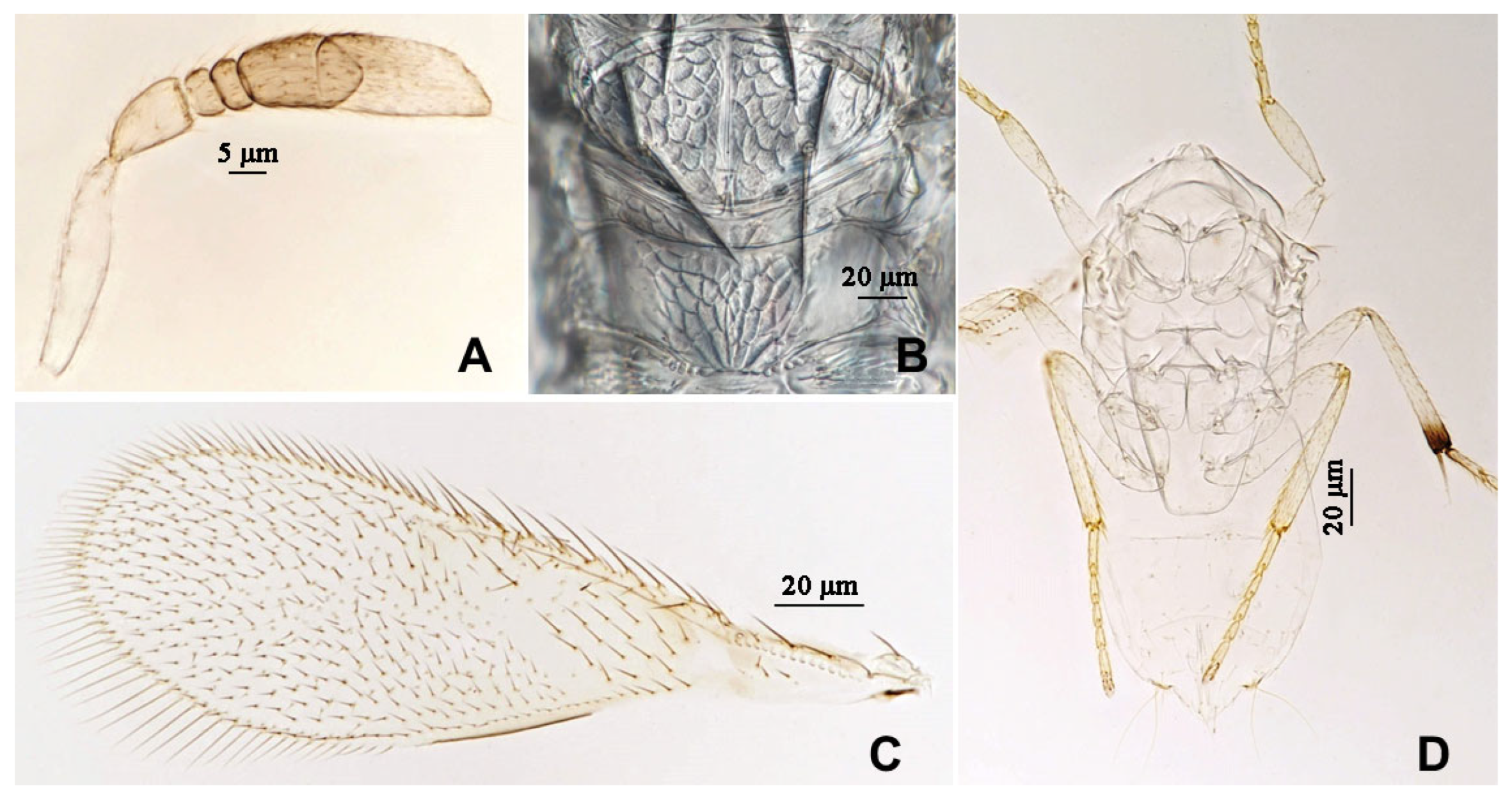
3.2.2. Aphytis jinshanensis Wang & Huang, sp.n. (Figure 7, Figure 8, Figure 9 and Figure 10)
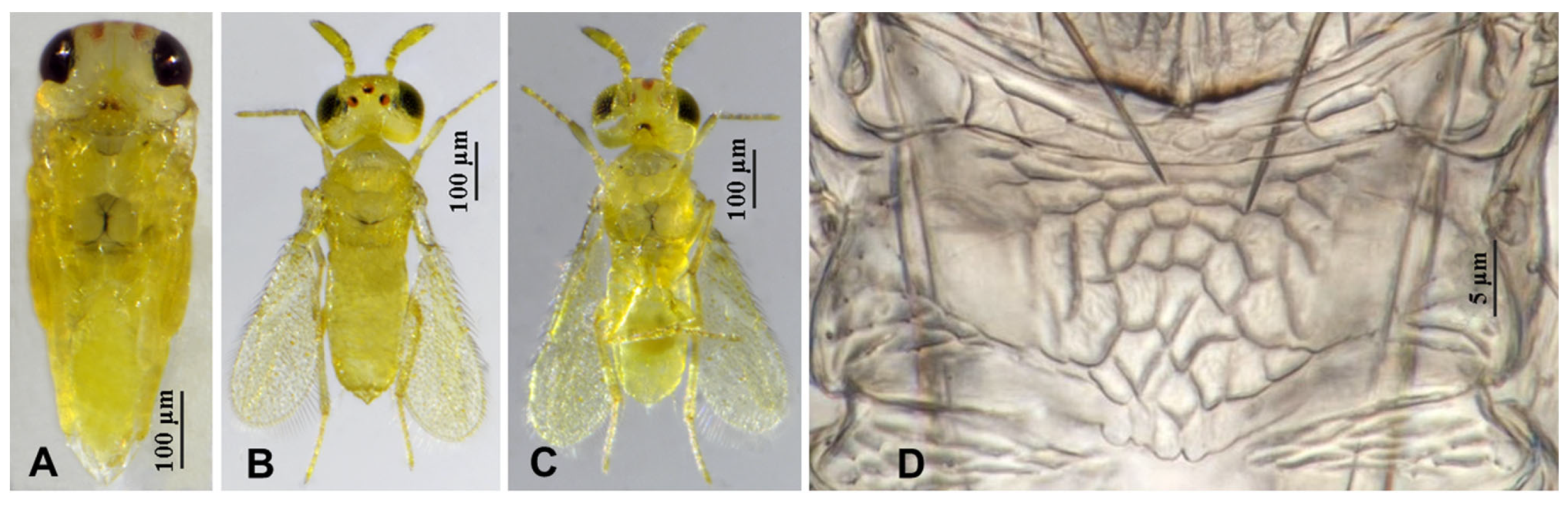
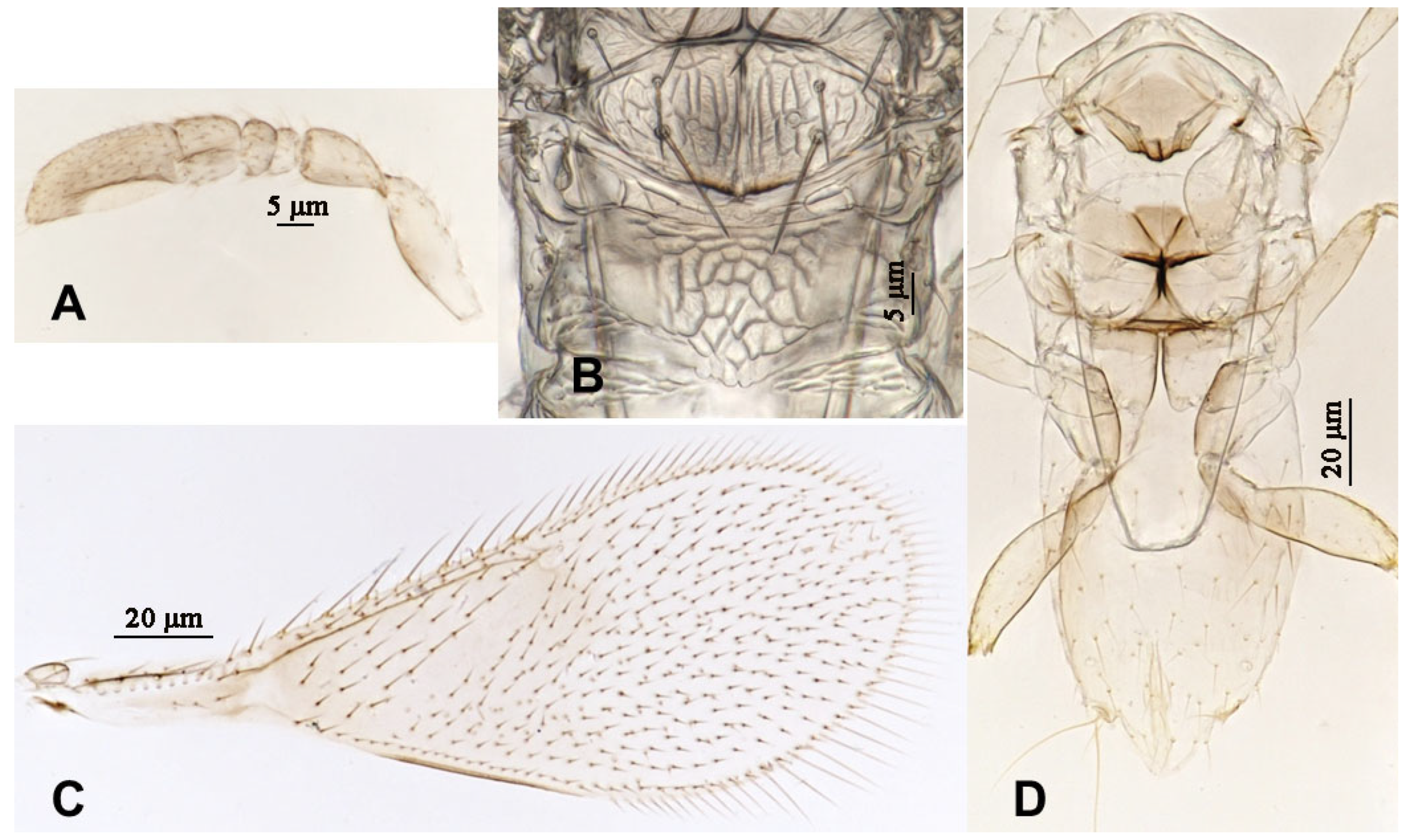
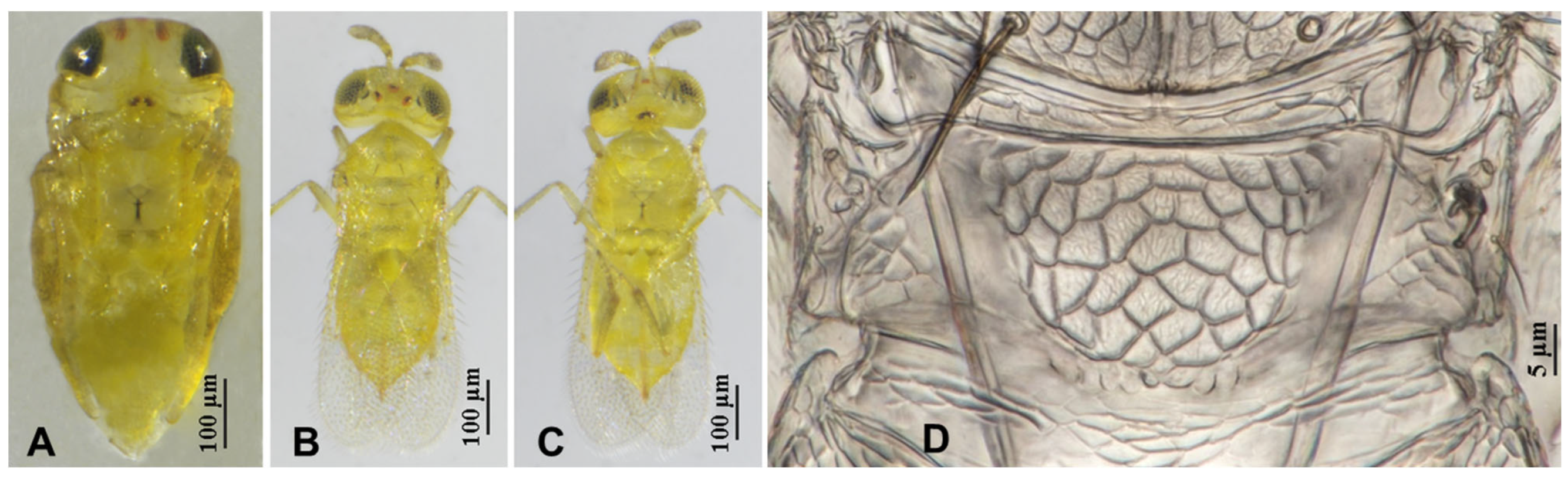
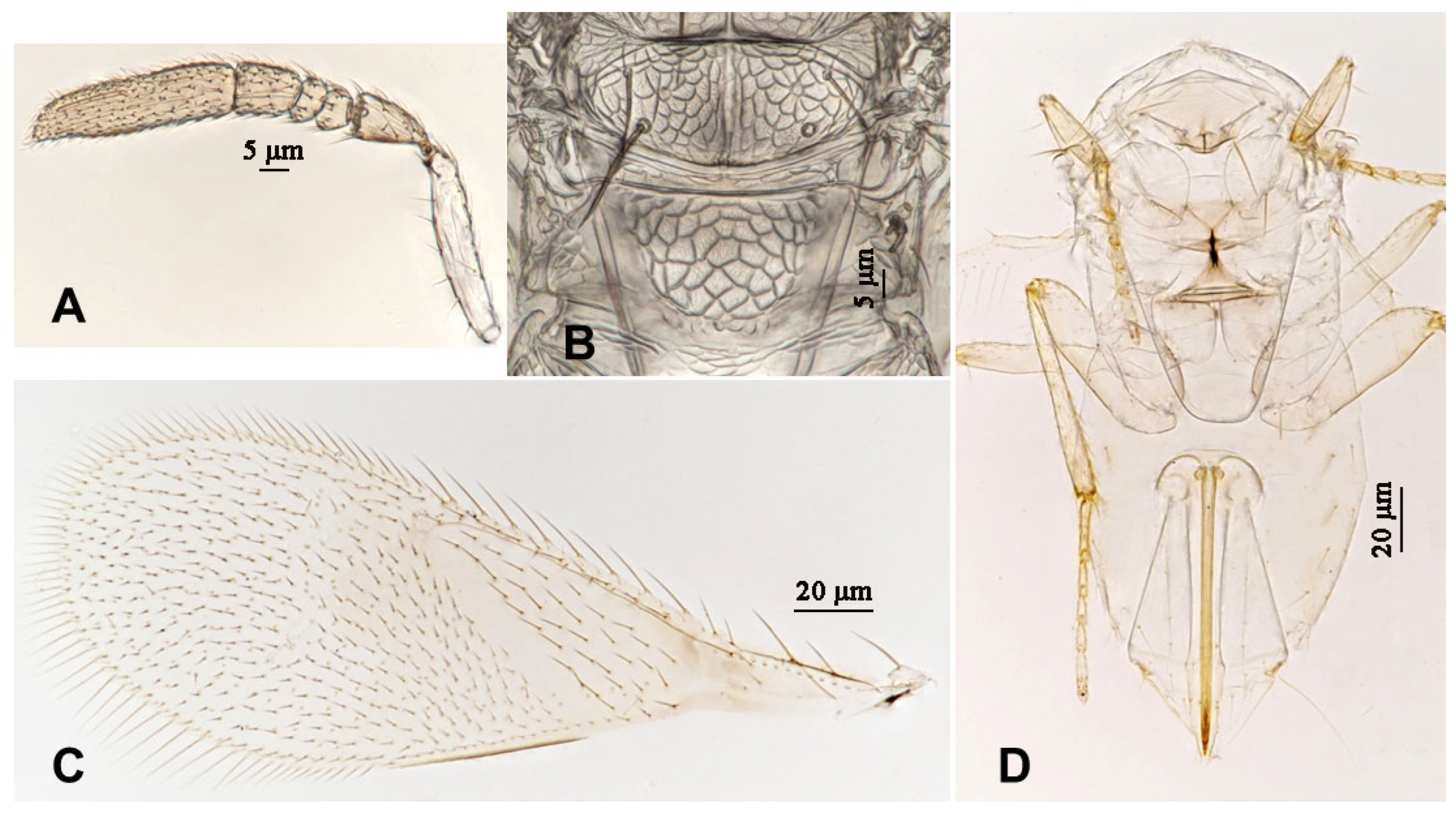
3.2.3. Aphytis chrysomphali (Mercet) [30,31] (Figure 11 and Figure 12)
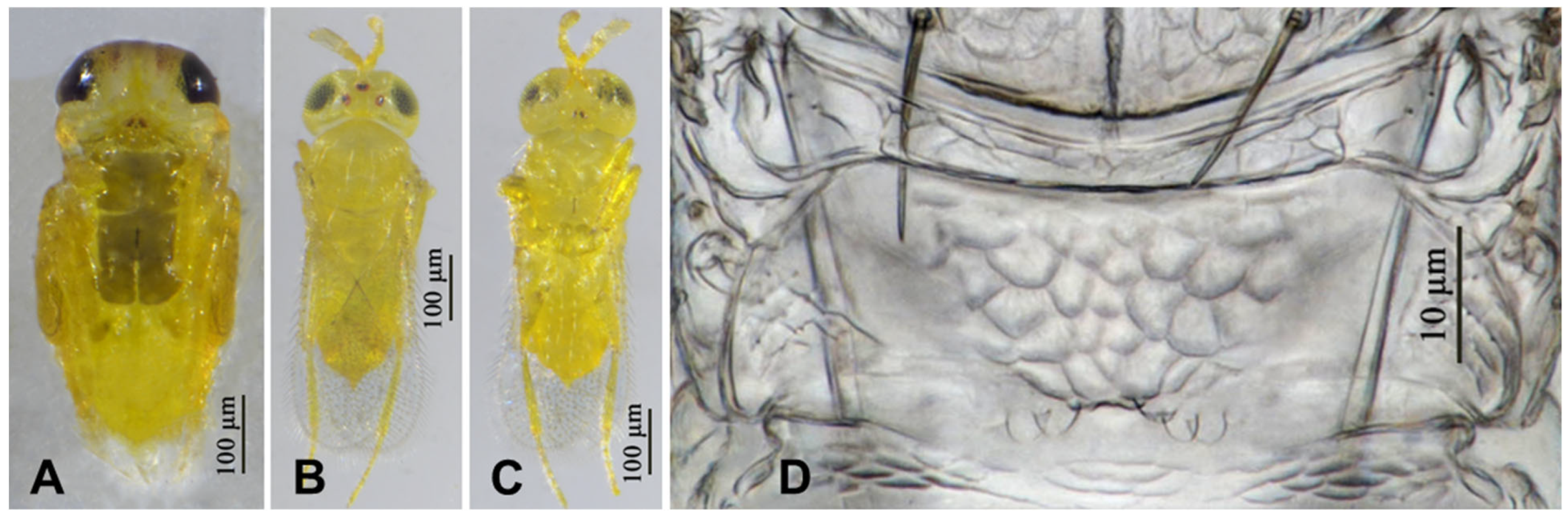
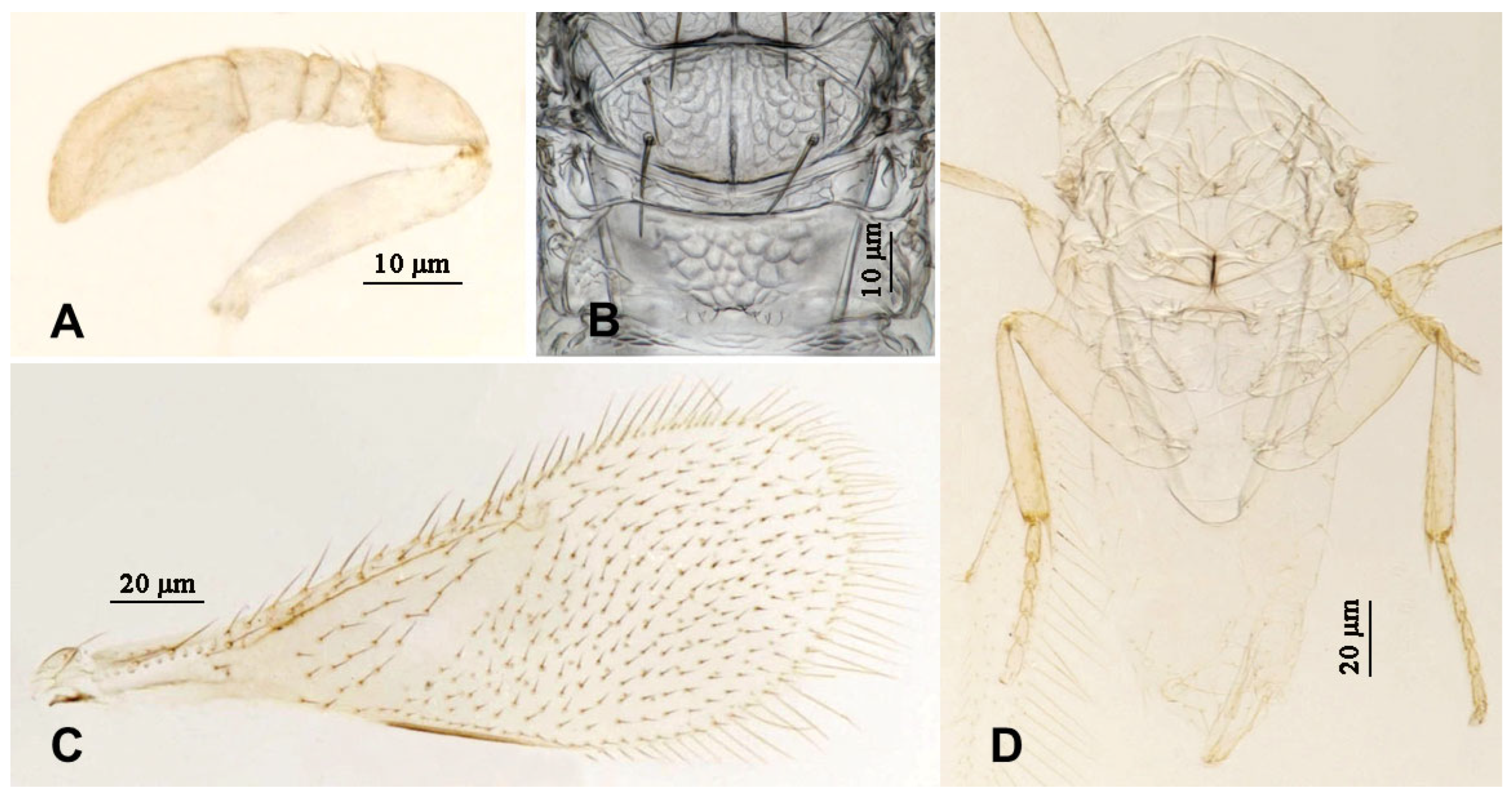
3.2.4. Aphytis nr. melinus DeBach (Figure 13 and Figure 14)
3.2.5. Encarsia citrina (Craw) [32,33] (Figure 15)
3.3. Aphytis Species Having the Mid-Tibia Tipped with Black in the Male
- Propodeum 1.11–1.13x as long as scutellum………………………………………………………………………………………………………………..A. cornuaspis
- --
- Propodeum 0.75–0.90x as long as scutellum………………………………………………………………………………………………………………………………2
- 2.
- Fore wing with unequal setae along the marginal vein; antennal scape with specialized sensilla in the male………………………………..A. salvadorensis
- --
- Without characteristics as above……………………………………………………………………………………………………………………………………………3
- 3.
- Mandibles bidentate…………………………………………………………………………………………………………………………………………..A. erythraeus
- --
- Mandibles tridentate or with 2 denticles and a dorsal truncation……………………………………………………………………………………………………..4
- 4.
- Mid-tibia faintly tipped with brownish in the female; mid-basitarsus black on basal half in the male; antennal F3 in the male obliquely truncate from the dorsal to the ventral, ventral aspect considerably longer than the dorsal; the pupa entirely yellow…………………………………………………..A. mazalae
- --
- Mid-tibia yellow in the female; mid-basitarsus yellow in the male; antennal F3 in the male obliquely truncate from both the dorsal and ventral to the central, forming a point; the pupa with head and thoracic sterna blown to dark brown...……………………………………………….A. nigromaculata, sp.n.
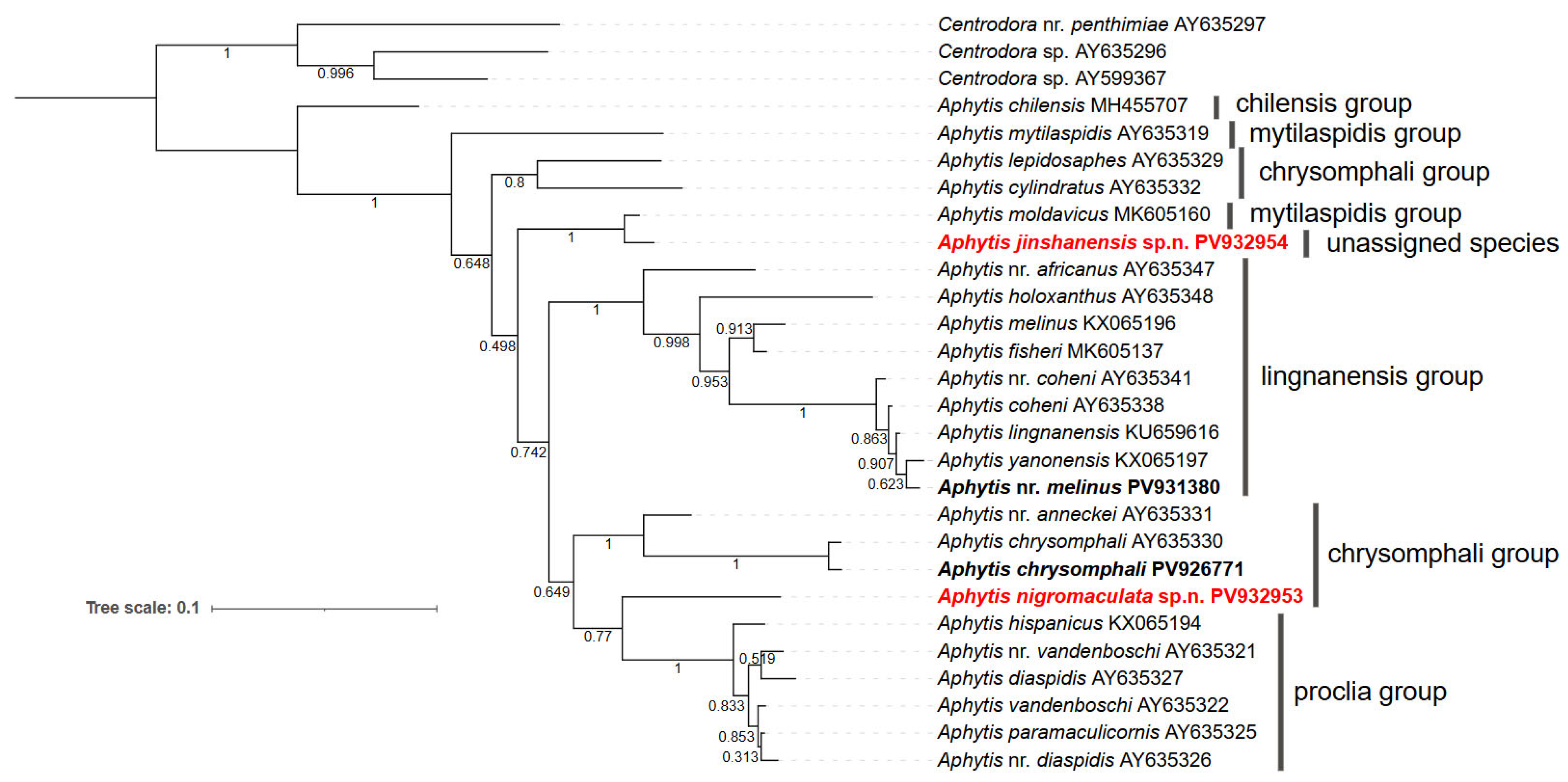
3.4. Phylogenetic Analysis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPPO Global Database. Available online: https://gd.eppo.int (accessed on 13 April 2025).
- Homrahud, D.; Uraichuen, S.; Attathom, T. Cultivation of Aschersonia placenta Berkeley and Broom and its efficacy for controlling Parlatoria ziziphi (Lucas) (Hemiptera: Diaspididae). Agric. Nat. Res. 2016, 50, 179–185. [Google Scholar] [CrossRef]
- Smith, E.S.C.; Bellis, G.A.; Gillespie, P.S. Absence of black parlatoria scale, Parlatoria ziziphi (Lucas) (Hemiptera: Diaspididae) from Australia. Aust. J. Entomol. 2013, 52, 242–245. [Google Scholar] [CrossRef]
- Mansour, R.; Grissa-Lebdi, K.; Suma, P.; Mazzeo, G.; Russo, A. Key scale insects (Hemiptera: Coccoidea) of high economic importance in a Mediterranean area: Host plants, bio-ecological characteristics, natural enemies and pest management strategies—A review. Plant Prot. Sci. 2017, 53, 1–14. [Google Scholar] [CrossRef]
- Coll, M.; Abd-Rabou, S. Effect of oil emulsion sprays on parasitoids of the black parlatoria, Parlatoria ziziphi, in grapefruit. BioControl 1998, 43, 29–37. [Google Scholar] [CrossRef]
- Taibi, A.; Gacemi, A.; Medjdoub, Y.; Medjdoub, I.; Doumandji, S. Citrus infestation by the black scale, Parlatoria ziziphi Lucas (Homoptera: Diaspididae) in Tlemcen, Algeria. J. Entomol. Res. 2016, 40, 217–221. [Google Scholar] [CrossRef]
- Assouguem, A.; Kara, M.; Mechchate, H.; AlZain, M.N.; Noman, O.M.; Imtara, H.; Hano, C.; Ibrahim, M.N.; Benmessaoud, S.; Farah, A.; et al. Evaluation of the effect of different concentrations of spirotetramat on the diaspine scale Parlatoria ziziphi in citrus orchards. Agronomy 2021, 11, 1840. [Google Scholar] [CrossRef]
- Huang, L.L.; Wang, D.W.; Zhang, Q.B.; Lei, H.D.; Yue, B.S. An investigation on the bionomics and control of citrus black scale. J. Plant Prot. 1988, 15, 15–21. [Google Scholar]
- Hu, X.S. Study on the biological characteristics and control of Parlatoria zizyphus and Parlatoria perandii in Southern Hunan. J. Anhui Agric. Sci. 2009, 37, 5034–5036. [Google Scholar]
- Abd-Rabou, S.; Ahmed, N.; Evans, G.A. Encarsia Forester (Hymenoptera: Aphelinidae)–effective parasitoids of armored scale insects (Hemiptera: Diaspididae) in Egypt. Acta Zool. Bulg. 2014, 66, 7–12. [Google Scholar]
- Abd-Rabou, S. Evaluation of Aphytis melinus DeBach (Hymenoptera: Chalcidoidea: Aphelinidae) in citrus orchards as a biocontrol agent of black scale, Parlatoria ziziphi (Lucas) (Hemiptera: Coccoidea: Diaspididae) in Egypt. J. Biol. Control 2009, 23, 37–41. [Google Scholar]
- Shih, Y.T.; Ko, C.C. Fauna Taiwan: Insecta Hymenoptera Aphelinidae; National Taiwan University: Taipei, China, 2020. [Google Scholar]
- Faskha, S.M.; El-Zemaity, M.E.S.; Dahroug, S.M.A. Natural enemies associated with black parlatoria scale, Parlatoria ziziphi (Lucas) in citrus orchards at El-Qualubia Governorate, Egypt. Entomol. Hell. 2021, 30, 33–42. [Google Scholar] [CrossRef]
- Moustafa, M. Scale insects (Coccoidae: Hemiptera) infested citrus trees and thier natural enemies, with a key of these pests in Egypt. Egypt. Acad. J. Biol. Sci. A Entomol. 2012, 5, 1–20. [Google Scholar] [CrossRef]
- Lei, H.D.; Huang, L.L.; Yue, B.S. Investigation on the parasitism of black parlatoria by Encarsia citrina. S. China Fruits 1988, 17, 40. [Google Scholar]
- Ren, Y.S.; Zhang, X.M.; Xu, S.E.; Xu, Z.H.; Li, X.L. Occurrence dynamics of parasitoids of citrus scale in Huangyan District, orange planting area. Zhejiang Ganju 1988, 1, 1–3. [Google Scholar]
- Huang, J. Systematic Studies on Aphelinidae of China (Hymenoptera: Chalcidoidea); Chongqing Publishing House: Chongqing, China, 1994. [Google Scholar]
- Abd-Rabou, S.; Ghahari, H. A revision of Encarsia (Hymenoptera: Aphelinidae) species from Iran. Egypt. J. Agric. Res. 2004, 82, 647–683. [Google Scholar]
- Yang, Z.X. Primary study on the resources of parasitic wasp of citrus armored scale and their natural ability in pest control in Zhejiang province. S. China Fruits 2004, 33, 1–5. [Google Scholar]
- Abd-Rabou, S.; Badary, H. Host range and incidence of Habrolepis diaspidi (Hymenoptera: Encyrtidea) as a parasitoid of armored scale insects (Hemiptera: Diaspididiae). Egypt. J. Agric. Res. 2012, 90, 619–663. [Google Scholar] [CrossRef]
- Noyes, J.S.; Prinsloo, G.L. A review of the Afrotropical and Malagasy taxa of Encyrtidae (Hymenoptera: Chalcidoidea) described by J. Risbec (1949–1959). Ann. Soc. Entomol. Fr. 1998, 34, 71–97. [Google Scholar] [CrossRef]
- Rosen, D.; DeBach, P. Species of Aphytis of the World; Springer: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Noyes, J.S. Collecting and preserving chalcid wasps (Hymenoptera: Chalcidoidea). J. Nat. Hist. 1982, 16, 315–334. [Google Scholar] [CrossRef]
- Polaszek, A.; Ayshford, T.; Yahya, B.E.; Fusu, L. Wallaceaphytis: An unusual new genus of parasitoid wasp (Hymenoptera: Aphelinidae) from Borneo. J. Nat. Hist. 2013, 48, 1111–1123. [Google Scholar] [CrossRef]
- Wang, L.; Hu, H.Y.; Kang, N.; Xi, O.Y. Description of a newly recorded genus Anteris with two newly recorded species in China (Hymenoptera: Scelionidae). Southwest China J. Agric. Sci. 2024, 37, 1635–1644. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.L.; Jakovlic, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Res. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Mercet, R.G. Un parasito dell ‘pollroig’. Bol. R. Soc. Esp. Hist. Nat. 1912, 12, 135–140. [Google Scholar]
- Timberlake, P.H. New species of Hawaiian chalcid flies (Hymenoptera). Proc. Hawaii. Entomol. Soc. 1926, 6, 305–320. [Google Scholar]
- Craw, A. Internal parasites discovered in the San Gabriel Valley: Recommendations and notes. Bull. Calif. State Board Hortic. 1891, 57, 3–7. [Google Scholar]
- Riley, C.V.; Howard, L.O. Mr. Craw on the destructive insects of California. Insect Life 1891, 4, 167–168. [Google Scholar]
- Huang, J.; Polaszek, A. A revision of the Chinese species of Encarsia Förster (Hymenoptera: Aphelinidae): Parasitoids of whiteflies, scale insects and aphids (Hemiptera: Aleyrodidae, Diaspididae, Aphidoidea). J. Nat. Hist. 1998, 32, 1825–1966. [Google Scholar] [CrossRef]
- Wang, Z.H.; Si, Y.; Zhang, H.; Zhang, Z.L.; Polaszek, A.; Huang, J. The pupal pigmentation pattern and pupal development in the species of Aphytis Howard (Hymenoptera: Aphelinidae). Insects 2021, 12, 399. [Google Scholar] [CrossRef]
| Parasitoids (Family/Species) | Distribution | References |
|---|---|---|
| Aphelinidae | ||
| Aphytis chrysomphali | China | [12], Note in this paper |
| A. hispanicus | Egypt | [13] |
| A. lingnanensis | Egypt | [14] |
| A. melinus | Egypt | [11] |
| Encarsia citrina | China, Egypt | [5,10,12,14,15,16,17] |
| E. lounsburyi | Iran | [18] |
| ** Prospaltella inquirenda | Egypt | [13] |
| * Marietta leopardina | Egypt | [5] |
| Encyrtidae | ||
| Comperiella bifasciata | China | [19] |
| ** Habrolepis aspidioti | Egypt | [5] |
| H. diaspidi | Egypt | [20] |
| Species | Host | Distribution | References |
|---|---|---|---|
| Aphytis nigromaculata sp.n. | Parlatoria ziziphi on citrus | China (Fujian) | Described in this paper |
| A. cornuaspis | Lepidosaphes gloverii on citrus | China (Fujian) | [17] |
| A. erythraeus | Aspidiotus elaeidis on olive | Africa | [22] |
| A. mazalae | Aulacaspis murrayae on Murraya funiculara, Aonidiella citrina on Citrus sinensi, Pinnaspis strachani on Ficus palmata | China (Taiwan), Japan, Pakistan | [22] |
| A. salvadorensis | an undetermined scale insect | El Salvador | [22] |
| Primer | Sequence | Cycling Conditions | |||
|---|---|---|---|---|---|
| D2-3551F | 5′-CGTGTTGCTTGATAGTGCAGC-3′ | Denaturation | Annealing | Extension | Cycles |
| D2-4068R | 5′-TTGGTCCGTGTTTCAAGACGGG-3 | 94 °C (30 s) | 58 °C (30 s) | 72 °C (60 s) | 35 |
| Genus/Species | GenBank Accession | Source | Genus/Species | GenBank Accession | Source |
|---|---|---|---|---|---|
| Genus Aphytis | A. lepidosaphes | AY635329 | GenBank | ||
| A. nr. anneckei | AY635331 | GenBank | A. lingnanensis | KU659616 | GenBank |
| A. nr. africanus | AY635347 | GenBank | A. melinus | KX065196 | GenBank |
| A. chrysomphali | PV926771 | This paper | A. nr. melinus | PV931380 | This paper |
| A. chrysomphali | AY635330 | GenBank | A. moldavicus | MK605160 | GenBank |
| A. chilensis | MH455707 | GenBank | A. mytilaspidis | AY635319 | GenBank |
| A. cylindratus | AY635332 | GenBank | A. nigromaculata, sp.n. | PV932953 | This paper |
| A. coheni | AY635338 | GenBank | A. paramaculicornis | AY635325 | GenBank |
| A. nr. coheni | AY635341 | GenBank | A. vandenboschi | AY635322 | GenBank |
| A. diaspidis | AY635327 | GenBank | A. nr. vandenboschi | AY635321 | GenBank |
| A. nr. diaspidis | AY635326 | GenBank | A. yanonensis | KX065197 | GenBank |
| A. fisheri | MK605137 | GenBank | Genus Centrodora | ||
| A. hispanicus | KX065194 | GenBank | Centrodora sp. | AY635296 | GenBank |
| A. holoxanthus | AY635348 | GenBank | C. nr. penthimiae | AY635297 | GenBank |
| A. jinshanensis, sp.n. | PV932954 | This paper | Centrodora sp. | AY599367 | GenBank |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, J.; Ge, J.; Huang, J.; Polaszek, A.; Wang, Z. The parasitoids of Parlatoria ziziphi (Lucas) (Hemiptera: Diaspididae): with descriptions of two new species of Aphytis (Hymenoptera: Aphelinidae) from China, and a note on Aphytis species with black-tipped mid tibiae in the male. Insects 2025, 16, 1070. https://doi.org/10.3390/insects16101070
Xi J, Ge J, Huang J, Polaszek A, Wang Z. The parasitoids of Parlatoria ziziphi (Lucas) (Hemiptera: Diaspididae): with descriptions of two new species of Aphytis (Hymenoptera: Aphelinidae) from China, and a note on Aphytis species with black-tipped mid tibiae in the male. Insects. 2025; 16(10):1070. https://doi.org/10.3390/insects16101070
Chicago/Turabian StyleXi, Jingtao, Junqing Ge, Jian Huang, Andrew Polaszek, and Zhuhong Wang. 2025. "The parasitoids of Parlatoria ziziphi (Lucas) (Hemiptera: Diaspididae): with descriptions of two new species of Aphytis (Hymenoptera: Aphelinidae) from China, and a note on Aphytis species with black-tipped mid tibiae in the male" Insects 16, no. 10: 1070. https://doi.org/10.3390/insects16101070
APA StyleXi, J., Ge, J., Huang, J., Polaszek, A., & Wang, Z. (2025). The parasitoids of Parlatoria ziziphi (Lucas) (Hemiptera: Diaspididae): with descriptions of two new species of Aphytis (Hymenoptera: Aphelinidae) from China, and a note on Aphytis species with black-tipped mid tibiae in the male. Insects, 16(10), 1070. https://doi.org/10.3390/insects16101070







