Proteomic Responses of the Springtail Folsomia candida to Drought
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Animals
2.2. Test Soil
2.3. Drought Exposure Experiment
2.4. Body Mass and Body Water Content of Springtails
2.5. Sample Preparation for Proteomics
2.6. Data Acquisition
2.7. Data Analysis
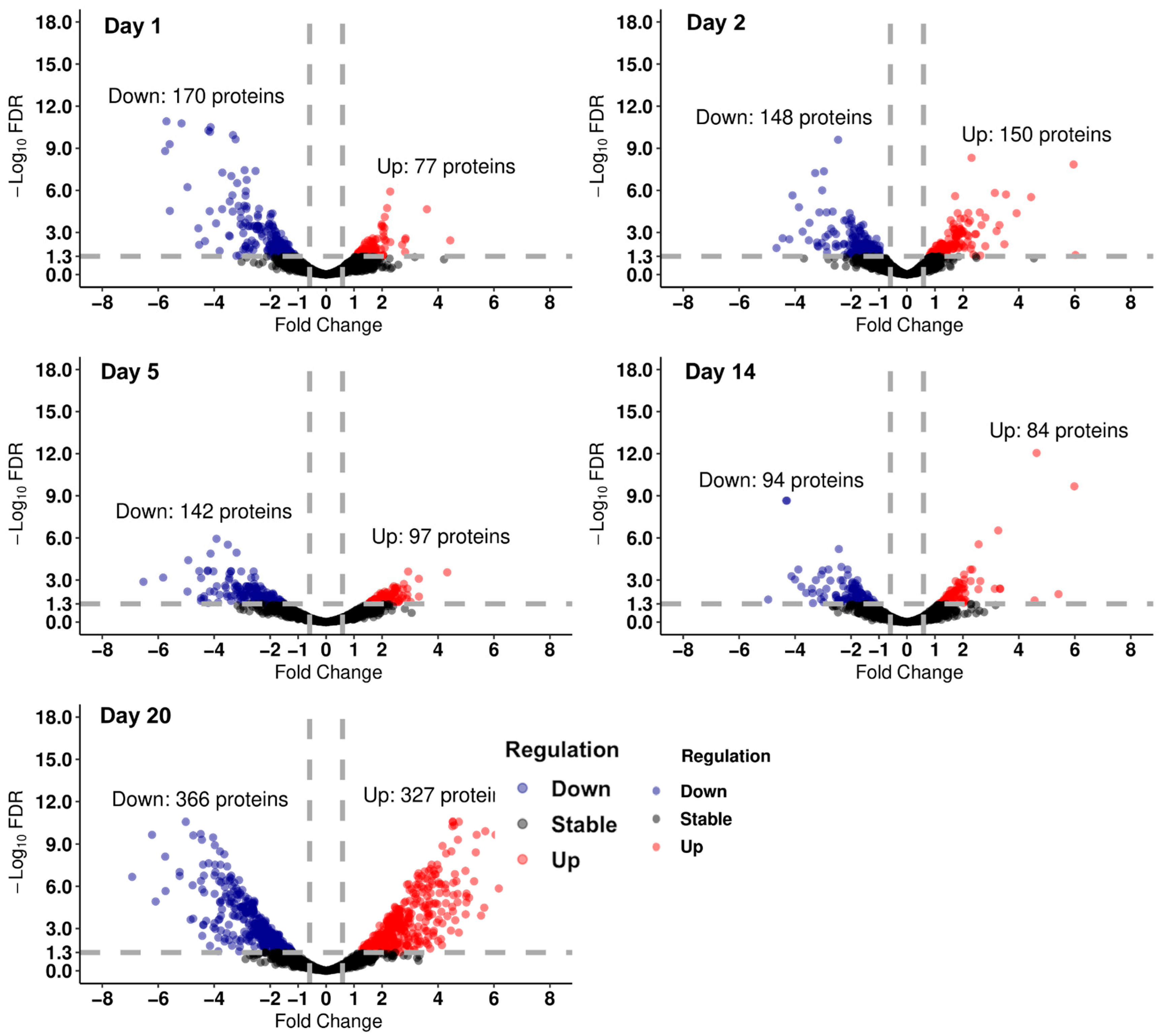
2.8. Differential Abundance Analysis and Pathway Enrichment of Proteomics Data
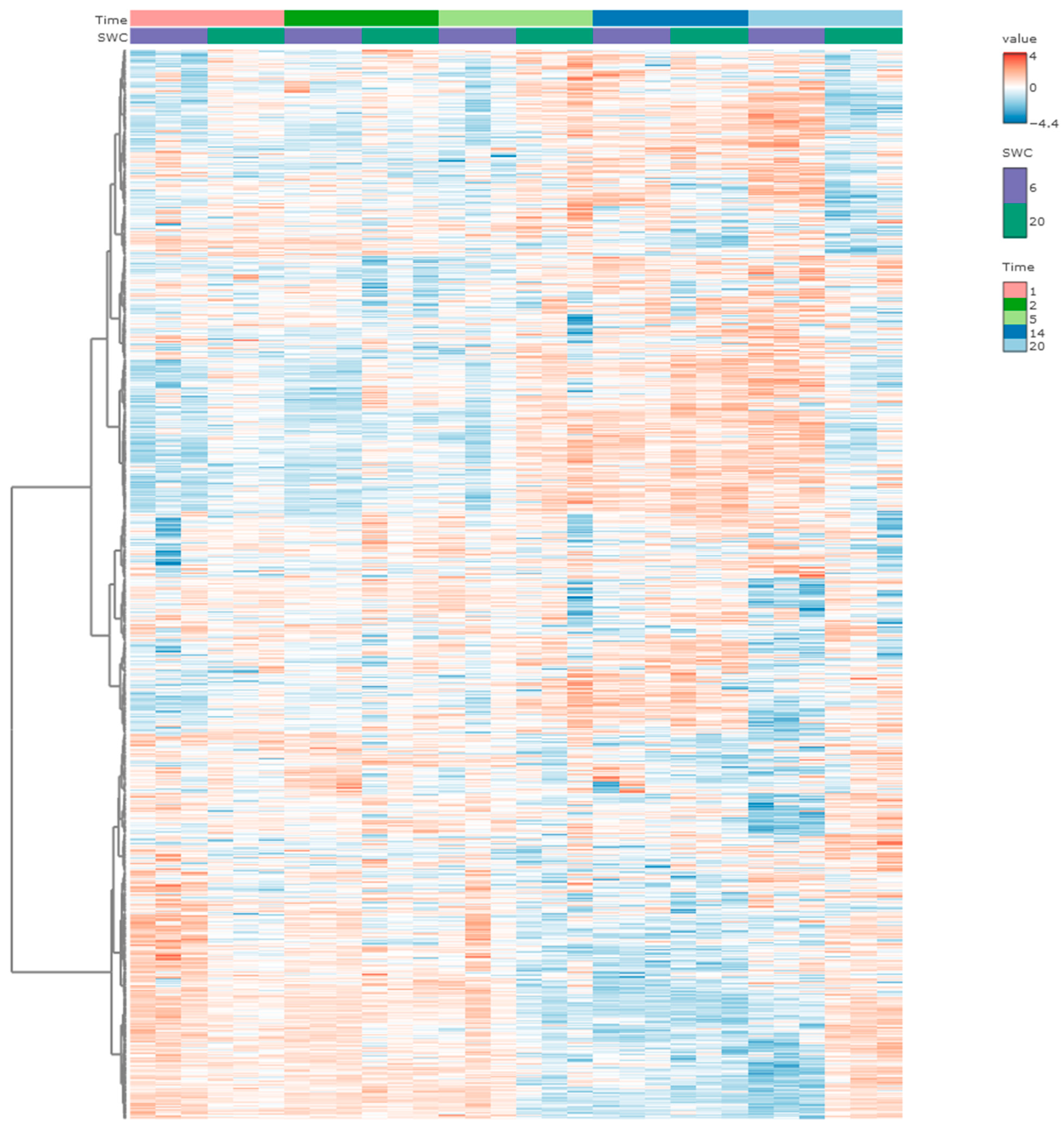
2.9. Heatmap Analysis and Two-Way ANOVA Analysis
3. Results
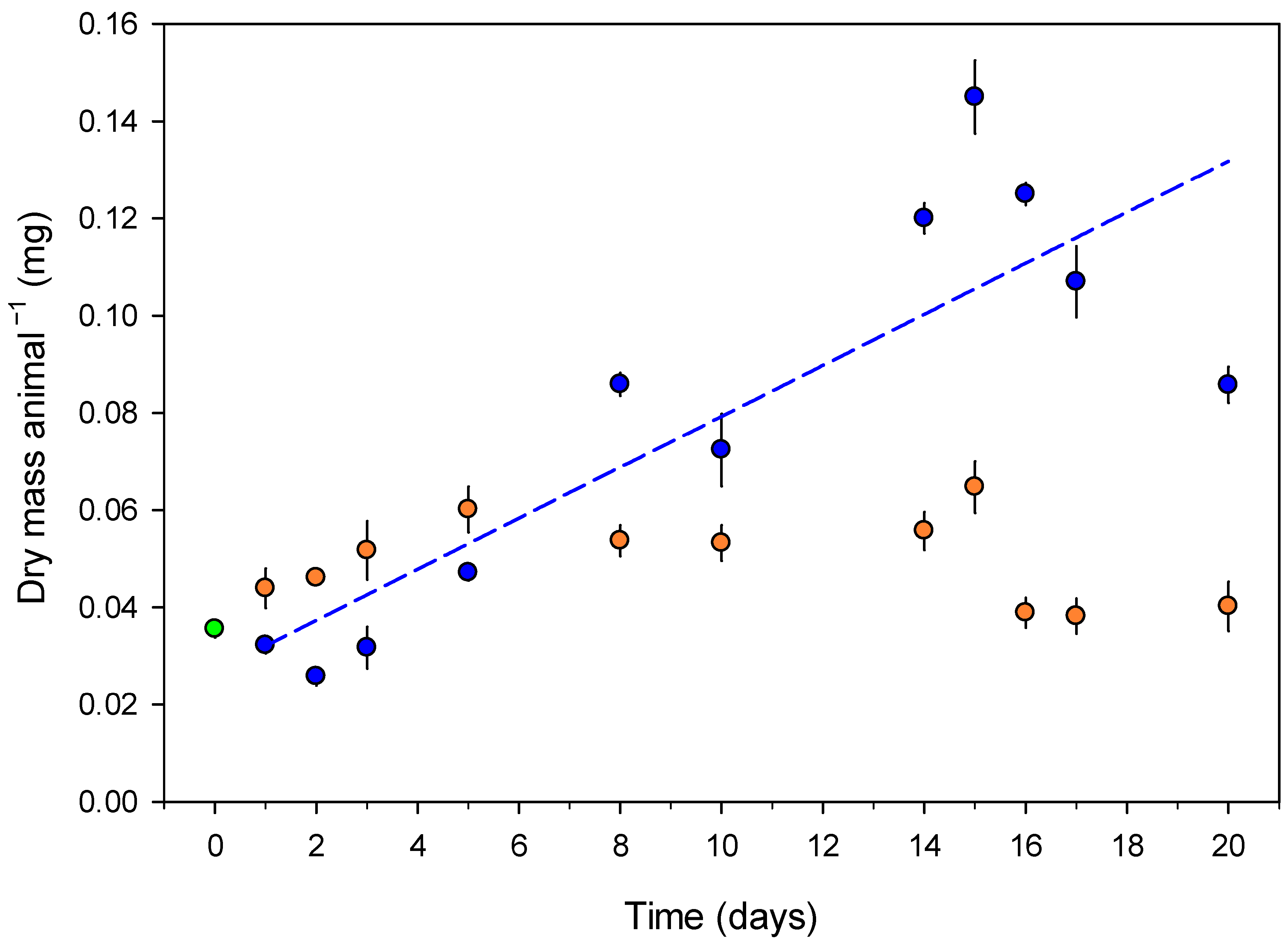
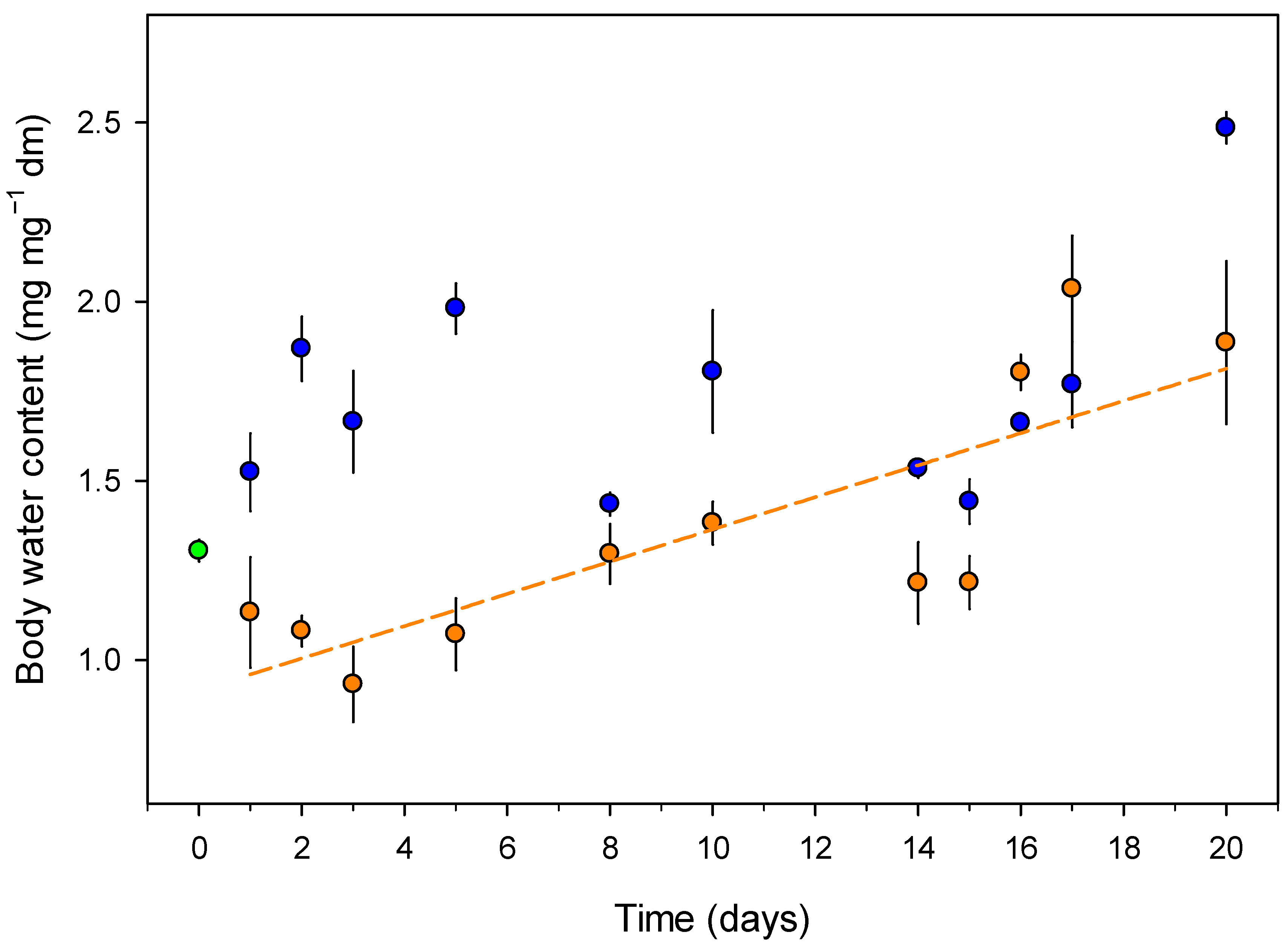
3.1. Growth and Body Water Content
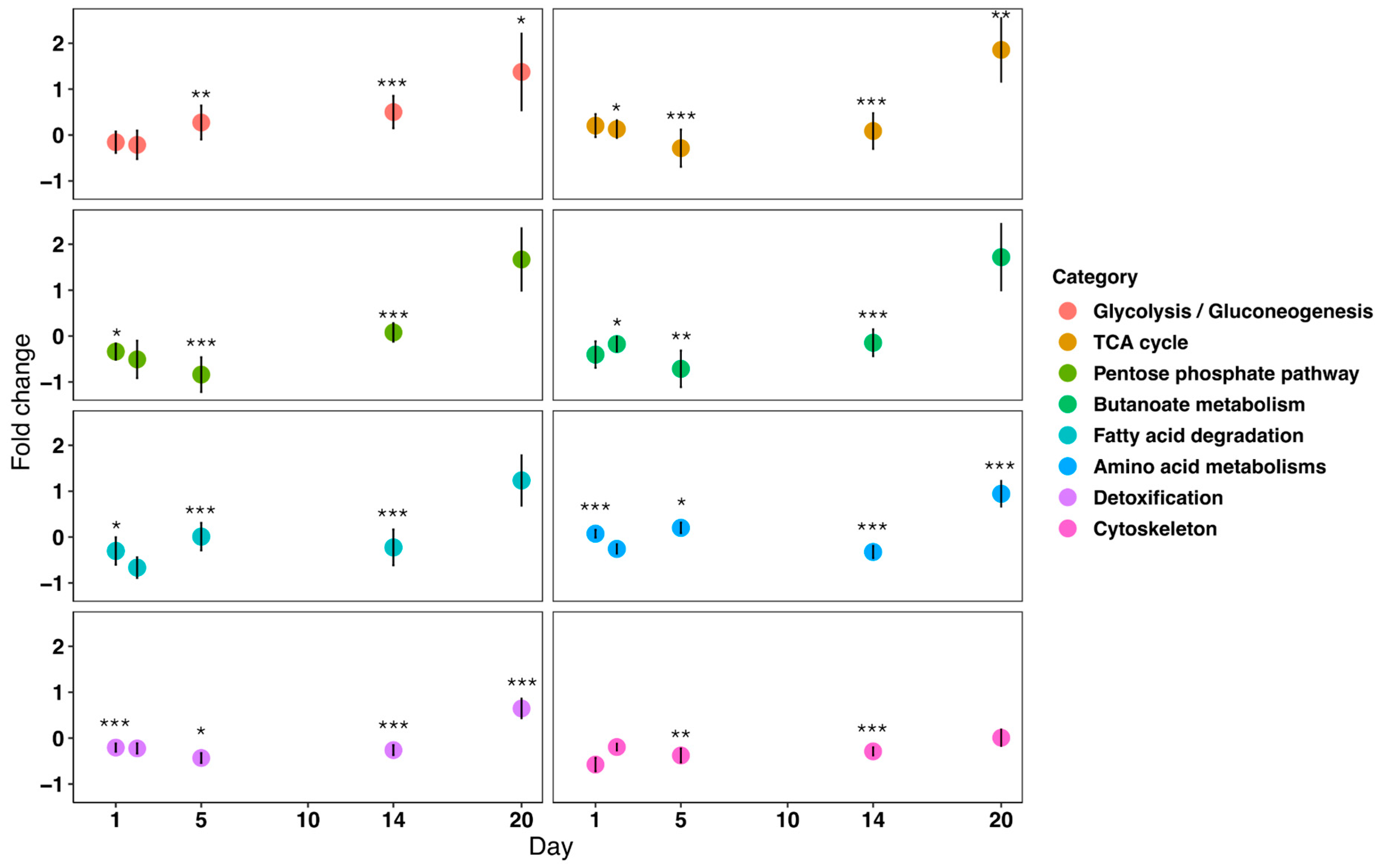
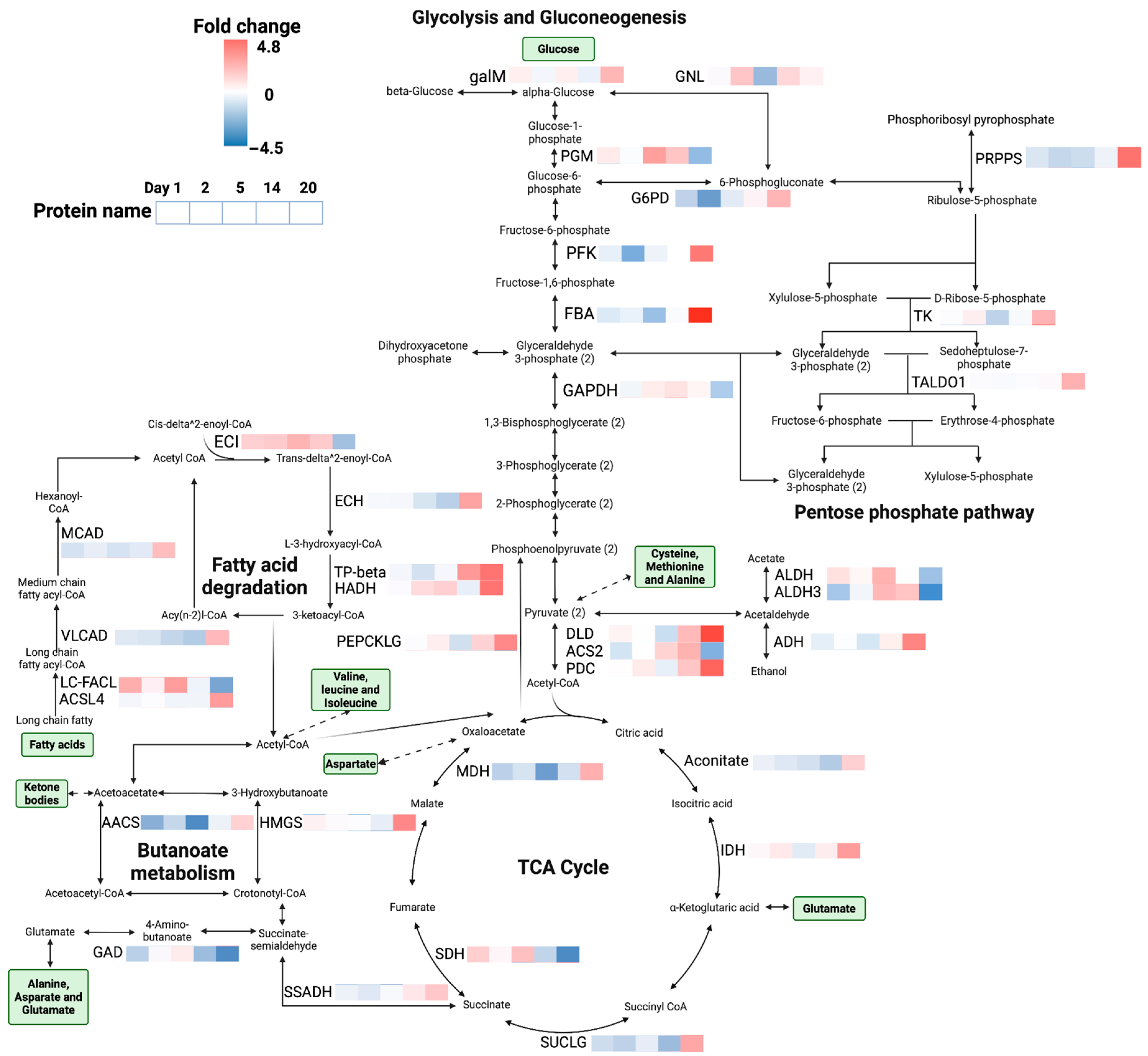
3.2. Proteomics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Potapov, A.M.; Guerra, C.A.; van den Hoogen, J.; Babenko, A.; Bellini, B.C.; Berg, M.P.; Chown, S.L.; Deharveng, L.; Kováč, Ľ.; Kuznetsova, N.A.; et al. Globally invariant metabolism but density-diversity mismatch in springtails. Nat. Commun. 2023, 14, 674. [Google Scholar] [CrossRef]
- Bødvarsson, H. Alimentary studies of seven common soil-inhabiting Collembola of southern Sweden. Entomol. Scand. 1970, 1, 74–80. [Google Scholar]
- Ngosong, C.; Raupp, J.; Richnow, H.H.; Ruess, L. Tracking Collembola feeding strategies by the natural C-13 signal of fatty acids in an arable soil with different fertilizer regimes. Pedobiologia 2011, 54, 225–233. [Google Scholar] [CrossRef]
- Potapov, A.A.; Semenina, E.E.; Korotkevich, A.Y.; Kuznetsova, N.A.; Tiunov, A.V. Connecting taxonomy and ecology: Trophic niches of collembolans as related to taxonomic identity and life forms. Soil Biol. Biochem. 2016, 101, 20–31. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Chan, K.F. Experimental evidence that soil fauna enhance nutrient mineralization and plant nutrient uptake in montane grassland ecosystems. Soil Biol. Biochem. 1999, 31, 1007–1014. [Google Scholar]
- Filser, J.; Faber, J.H.; Tiunov, A.V.; Brussaard, L.; Frouz, J.; De Deyn, G.; Uvarov, A.V.; Berg, M.P.; Lavelle, P.; Loreau, M.; et al. Soil fauna: Key to new carbon models. Soil 2016, 2, 565–582. [Google Scholar] [CrossRef]
- Peguero, G.; Sol, D.; Arnedo, M.; Petersen, H.; Salmon, S.; Ponge, J.F.; Maspons, J.; Emmett, B.; Beier, C.; Schmidt, I.K.; et al. Fast attrition of springtail communities by experimental drought and richness-decomposition relationships across Europe. Glob. Change Biol. 2019, 25, 2727–2738. [Google Scholar] [CrossRef]
- Vannier, G. The porosphere as an ecological medium emphasized in Professor Ghilarov’s work on soil animal adaptations. Biol. Fertil. Soils 1987, 3, 39–44. [Google Scholar]
- Blankinship, J.C.; Niklaus, P.A.; Hungate, B.A. A meta-analysis of responses of soil biota to global change. Oecologia 2011, 165, 553–565. [Google Scholar] [CrossRef]
- Peng, Y.; Penuelas, J.; Vesterdal, L.; Yue, K.; Peguero, G.; Fornara, D.A.; Hedenec, P.; Steffens, C.; Wu, F.Z. Responses of soil fauna communities to the individual and combined effects of multiple global change factors. Ecol. Lett. 2022, 25, 1961–1973. [Google Scholar] [CrossRef]
- EEA. Climate Change, Impacts and Vulnerability in Europe 2016; An Indicator-Based Report; European Environment Agency: Copenhagen, Denmark, 2017. [Google Scholar]
- IPCC. Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2022; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Aupic-Samain, A.; Baldy, V.; Delcourt, N.; Krogh, P.H.; Gauquelin, T.; Fernandez, C.; Santonja, M. Water availability rather than temperature control soil fauna community structure and prey-predator interactions. Funct. Ecol. 2021, 35, 1550–1559. [Google Scholar] [CrossRef]
- Holmstrup, M. Water relations and drought sensitivity of Folsomia candida eggs (Collembola: Isotomidae). Eur. J. Entomol. 2019, 116, 229–234. [Google Scholar] [CrossRef]
- Wang, Y.; Slotsbo, S.; Holmstrup, M. Soil dwelling springtails are resilient to extreme drought in soil, but their reproduction is highly sensitive to small decreases in soil water potential. Geoderma 2022, 421, 115913. [Google Scholar]
- Bayley, M.; Holmstrup, M. Water vapor absorption in arthropods by accumulation of myoinositol and glucose. Science 1999, 285, 1909–1911. [Google Scholar]
- Holmstrup, M.; Sjursen, H.; Ravn, H.; Bayley, M. Dehydration tolerance and water vapour absorption in two species of soil-dwelling Collembola by accumulation of sugars and polyols. Funct. Ecol. 2001, 15, 647–653. [Google Scholar]
- Sjursen, H.; Bayley, M.; Holmstrup, M. Enhanced drought tolerance of a soil-dwelling springtail by pre-acclimation to a mild drought stress. J. Insect Physiol. 2001, 47, 1021–1027. [Google Scholar]
- Holmstrup, M. The springtail Megaphorura arctica survives extremely high osmolality of body fluids during drought. J. Comp. Physiol. B 2018, 188, 939–945. [Google Scholar] [CrossRef]
- Holmstrup, M.; Bayley, M. Protaphorura tricampata, a euedaphic and highly permeable springtail that can sustain activity by osmoregulation during extreme drought. J. Insect Physiol. 2013, 59, 1104–1110. [Google Scholar] [CrossRef]
- Holmstrup, M.; Slotsbo, S.; Rozsypal, J.; Henriksen, P.G.; Bayley, M. Accumulation of free amino acids during exposure to drought in three springtail species. J. Insect Physiol. 2015, 82, 114–121. [Google Scholar] [CrossRef]
- Clark, M.S.; Thorne, M.A.S.; Purac, J.; Burns, G.; Hillyard, G.; Popovic, Z.D.; Grubor-Lajsic, G.; Worland, M.R. Surviving the cold: Molecular analyses of insect cryoprotective dehydration in the Arctic springtail Megaphorura arctica (Tullberg). BMC Genom. 2009, 10, 328. [Google Scholar] [CrossRef]
- Timmermans, M.J.; Roelofs, D.; Nota, B.; Ylstra, B.; Holmstrup, M. Sugar sweet springtails: On the transcriptional response of Folsomia candida (Collembola) to desiccation stress. Insect Mol. Biol. 2009, 18, 737–746. [Google Scholar] [CrossRef]
- Thorne, M.A.S.; Worland, M.R.; Feret, R.; Deery, M.J.; Lilley, K.S.; Clark, M.S. Proteomics of cryoprotective dehydration in Megaphorura arctica Tullberg 1876 (Onychiuridae: Collembola). Insect Mol. Biol. 2011, 20, 303–310. [Google Scholar] [CrossRef]
- Faddeeva-Vakhrusheva, A.; Kraaijeveld, K.; Derks, M.F.L.; Anvar, S.Y.; Agamennone, V.; Suring, W.; Kampfraath, A.A.; Ellers, J.; Le Ngoc, G.; van Gestel, C.A.M.; et al. Coping with living in the soil: The genome of the parthenogenetic springtail Folsomia candida. BMC Genom. 2017, 18, 14. [Google Scholar] [CrossRef]
- Bahrndorff, S.; Marien, J.; Loeschcke, V.; Ellers, J. Dynamics of heat-induced thermal stress resistance and Hsp70 expression in the springtail, Orchesella cincta. Funct. Ecol. 2009, 23, 233–239. [Google Scholar] [CrossRef]
- Zougman, A.; Selby, P.J.; Banks, R.E. Suspension trapping (STrap) sample preparation method for bottom- up proteomics analysis. Proteomics 2014, 14, 1006–1010. [Google Scholar] [CrossRef]
- Kumar, R.; Kane, H.; Wang, Q.; Hibberd, A.; Jensen, H.M.; Kim, H.S.; Bak, S.Y.; Auzanneau, I.; Bry, S.; Christensen, N.; et al. Identification and characterization of a novel species of genus Akkermansia with metabolic health effects in a diet-induced obesity mouse model. Cells 2022, 11, 2084. [Google Scholar] [CrossRef]
- Kall, L.; Canterbury, J.D.; Weston, J.; Noble, W.S.; MacCoss, M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 2007, 4, 923–925. [Google Scholar] [CrossRef]
- Silva, J.C.; Gorenstein, M.V.; Li, G.Z.; Vissers, J.P.C.; Geromanos, S.J. Absolute quantification of proteins by LCMSE: A virtue of parallel MS acquisition. Mol. Cell Proteom. 2006, 5, 144–156. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Levin, Y. The role of statistical power analysis in quantitative proteomics. Proteomics 2011, 11, 2565–2567. [Google Scholar] [CrossRef]
- Yu, G.C.; Wang, L.G.; Han, Y.Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics-A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Barrett, J. Metabolic responses to anabiosis in the 4th stage juveniles of Ditylenchus dipsaci (nematoda). Proc. R. Soc. Ser. B-Biol. Sci. 1982, 216, 159–177. [Google Scholar] [CrossRef]
- Bayley, M.; Overgaard, J.; Høj, A.S.; Malmendal, A.; Nielsen, N.C.; Holmstrup, M.; Wang, T. Metabolic changes during estivation in the common earthworm Aporrectodea caliginosa. Physiol. Biochem. Zool. 2010, 83, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Madin, K.A.C.; Loomis, S.H. Anhydrobiosis in nematodes: Metabolism during resumption of activity. J. Exp. Zool. 1977, 201, 57–64. [Google Scholar] [CrossRef]
- Gleason, L.U.; Fekete, F.J.; Tanner, R.L.; Dowd, W.W. Multi-omics reveals largely distinct transcript- and protein-level responses to the environment in an intertidal mussel. J. Exp. Biol. 2023, 226, jeb245962. [Google Scholar] [CrossRef]
- Wasteneys, G.O.; Galway, M.E. Remodeling the cytoskeleton for growth and form: An overview with some new views. Annu. Rev. Plant Biol. 2003, 54, 691–722. [Google Scholar] [CrossRef]
- Holmstrup, M.; Hedlund, K.; Boriss, H. Drought acclimation and lipid composition in Folsomia candida: Implications for cold shock, heat shock and acute desiccation stress. J. Insect Physiol. 2002, 48, 961–970. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Slotsbo, S.; Bak, S.Y.; Martyniuk, C.J.; Holmstrup, M. Proteomic Responses of the Springtail Folsomia candida to Drought. Insects 2025, 16, 707. https://doi.org/10.3390/insects16070707
Wang Y, Slotsbo S, Bak SY, Martyniuk CJ, Holmstrup M. Proteomic Responses of the Springtail Folsomia candida to Drought. Insects. 2025; 16(7):707. https://doi.org/10.3390/insects16070707
Chicago/Turabian StyleWang, Yang, Stine Slotsbo, Steffen Y. Bak, Christopher J. Martyniuk, and Martin Holmstrup. 2025. "Proteomic Responses of the Springtail Folsomia candida to Drought" Insects 16, no. 7: 707. https://doi.org/10.3390/insects16070707
APA StyleWang, Y., Slotsbo, S., Bak, S. Y., Martyniuk, C. J., & Holmstrup, M. (2025). Proteomic Responses of the Springtail Folsomia candida to Drought. Insects, 16(7), 707. https://doi.org/10.3390/insects16070707






