The Role of Heat Shock Proteins in Insect Stress Response, Immunity, and Climate Adaptation
Simple Summary
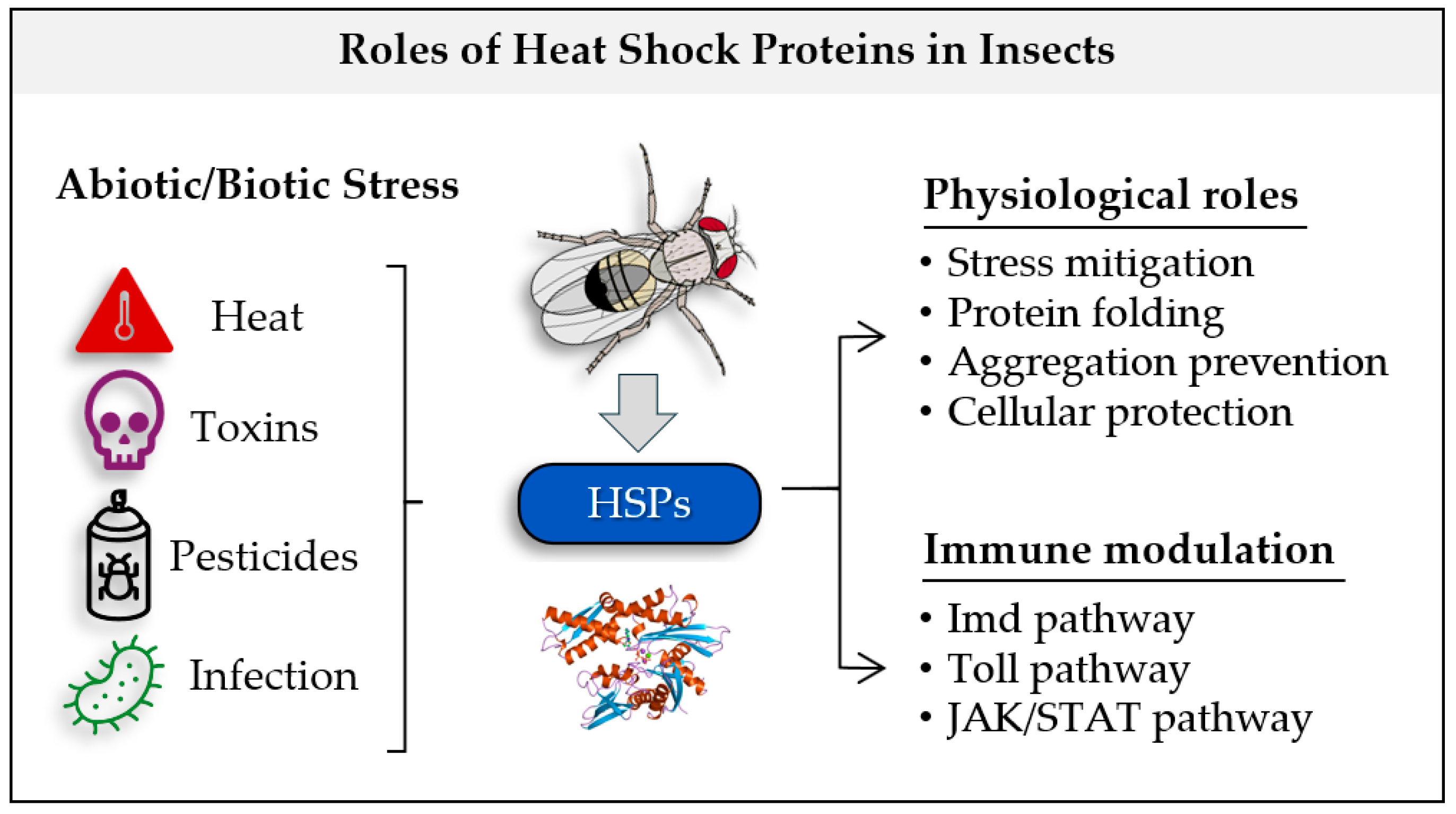
Abstract
1. Introduction
2. Thermal Stress and Heat Shock Proteins
3. Dehydration Stress and Heat Shock Proteins
4. Biotic Stresses and Heat Shock Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grgac, R.; Rozsypal, J.; Des Marteaux, L.; Stetina, T.; Kostal, V. Stabilization of Insect Cell Membranes and Soluble Enzymes by Accumulated Cryoprotectants during Freezing Stress. Proc. Natl. Acad. Sci. USA 2022, 119, e2211744119. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S. Effects of Climate Change in Agricultural Insect Pest. Acta Sci. Agric. 2019, 3, 74–80. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Ritossa, F. A New Puffing Pattern Induced by Temperature Shock and DNP in Drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Ritossa, F. Discovery of the Heat Shock Response. Cell Stress Chaperones 1996, 1, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Tutar, L.; Tutar, Y. Heat Shock Proteins; An Overview. Curr. Pharm. Biotechnol. 2010, 11, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat Shock Proteins: Biological Functions, Pathological Roles, and Therapeutic Opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Alagar Boopathy, L.R.; Jacob-Tomas, S.; Alecki, C.; Vera, M. Mechanisms Tailoring the Expression of Heat Shock Proteins to Proteostasis Challenges. J. Biol. Chem. 2022, 298, 101796. [Google Scholar] [CrossRef] [PubMed]
- Åkerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat Shock Factors: Integrators of Cell Stress, Development and Lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Matsukage, A.; Hirose, F.; Yoo, M.A.; Yamaguchi, M. The DRE/DREF Transcriptional Regulatory System: A Master Key for Cell Proliferation. Biochim. Biophys. Acta Gene Regul. Mech. 2008, 1779, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, R.C.; Lis, J.T. GAGA Factor Binding to DNA via a Single Trinucleotide Sequence Element. Nucleic Acids Res. 1998, 26, 2672–2678. [Google Scholar] [CrossRef] [PubMed]
- Stephanou, A.; Latchman, D.S. Transcriptional Regulation o f the Heat Shock Protein Genes by STAT Family Transcription Factors. Gene Expr. 2018, 7, 311. [Google Scholar]
- Westerheide, S.D.; Anckar, J.; Stevens, S.M.; Sistonen, L.; Morimoto, R.I. Stress-Inducible Regulation of Heat Shock Factor 1 by the Deacetylase SIRT. Science 2009, 323, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.K.; Weirich, C.S.; Guyon, J.R.; Sif, S.; Kingston, R.E. Transcriptional Activation Domains of Human Heat Shock Factor 1 Recruit Human SWI/SNF. Mol. Cell. Biol. 2001, 21, 5826–5837. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Feder, M.E.; Kang, L. Evolution of Heat-Shock Protein Expression Underlying Adaptive Responses to Environmental Stress. Mol. Ecol. 2018, 27, 3040–3054. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.M.; Zhang, Q.; Zhang, Y.L.; Zhang, G.Z.; Zhang, Z.; Yu, Q.Y. Heat Shock Protein 70 Family in Response to Multiple Abiotic Stresses in the Silkworm. Insects 2021, 12, 928. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-R.; Wang, C.; Ban, F.-X.; Zhu, D.-T.; Liu, S.-S.; Wang, X.-W. Genome-Wide Identification and Characterization of HSP Gene Superfamily in Whitefly (Bemisia tabaci) and Expression Profiling Analysis under Temperature Stress. Insect Sci. 2019, 26, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Pauli, D.; Arrigo, A.-P.; Tissières, A. Heat Shock Response in Drosophila. Experientia 1992, 48, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Jagla, T.; Dubińska-Magiera, M.; Poovathumkadavil, P.; Daczewska, M.; Jagla, K. Developmental Expression and Functions of the Small Heat Shock Proteins in Drosophila. Int. J. Mol. Sci. 2018, 19, 3441. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yin, Z.; Li, H.; Guo, J. HSP Gene Superfamily in Aspongopus chinensis Dallas: Unravelling Identification, Characterisation and Expression Patterns during Diapause and Non-Diapause Stages. Bull. Entomol. Res. 2024, 114, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Moghaddam, S.H.H.; Du, X.; Zhong, B.X.; Chen, Y.Y. Comparative Analysis on the Expression of Inducible HSPs in the Silkworm, Bombyx mori. Mol. Biol. Rep. 2012, 39, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tettamanti, G.; Bassal, T.; Heryanto, C.; Eleftherianos, I.; Mohamed, A. Regulators and Signalling in Insect Antimicrobial Innate Immunity: Functional Molecules and Cellular Pathways. Cell. Signal. 2021, 83, 110003. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Vogel, P.; Alber, G.; Schaub, G. The Innate Immune System of Mammals and Insects. Contrib. Microbiol. 2008, 15, 21–44. [Google Scholar] [PubMed]
- Nishide, Y.; Kageyama, D.; Yokoi, K.; Jouraku, A.; Tanaka, H.; Futahashi, R.; Fukatsu, T. Functional Crosstalk across IMD and Toll Pathways: Insight into the Evolution of Incomplete Immune Cascades. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182207. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.B.; Kim, C.H.; Lee, H.; Kwon, H.M.; Park, J.W.; Ryu, J.H.; Kurokawa, K.; Ha, N.C.; Lee, W.J.; Lemaitre, B.; et al. Proteolytic Cascade for the Activation of the Insect Toll Pathway Induced by the Fungal Cell Wall Component. J. Biol. Chem. 2009, 284, 19474–19481. [Google Scholar] [CrossRef] [PubMed]
- Bang, I.S. JAK/STAT Signaling in Insect Innate Immunity. Entomol. Res. 2019, 49, 339–353. [Google Scholar] [CrossRef]
- Ding, B.; Zhang, C.; He, L.; Zeng, Q.; Zhang, S.; Yang, H.; Yang, H. JAK/STAT Signaling Pathway Is Involved in Antibacterial Immunity in the Green Peach Aphid Myzus persicae (Sulzer). Pestic. Biochem. Physiol. 2024, 205, 106168. [Google Scholar] [CrossRef] [PubMed]
- Farahani, S.; Bandani, A.R.; Alizadeh, H.; Goldansaz, S.H.; Whyard, S. Differential Expression of Heat Shock Proteins and Antioxidant Enzymes in Response to Temperature, Starvation, and Parasitism in the Carob Moth Larvae, Ectomyelois ceratoniae (Lepidoptera: Pyralidae). PLoS ONE 2020, 15, e0228104. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Lavilla, N.; García-Reina, A.; De la Rúa, P. Mild Thermal Stress Does Not Negatively Affect Immune Gene Expression in the Bumblebee Bombus terrestris. Apidologie 2021, 52, 163–173. [Google Scholar] [CrossRef]
- Shen, Y.; Gu, J.; Huang, L.H.; Zheng, S.C.; Liu, L.; Xu, W.H.; Feng, Q.L.; Kang, L. Cloning and Expression Analysis of Six Small Heat Shock Protein Genes in the Common Cutworm, Spodoptera litura. J. Insect. Physiol. 2011, 57, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Paim, R.M.M.; Araujo, R.N.; Leis, M.; Sant’anna, M.R.V.; Gontijo, N.F.; Lazzari, C.R.; Pereira, M.H. Functional Evaluation of Heat Shock Proteins 70 (HSP70/HSC70) on Rhodnius prolixus (Hemiptera, Reduviidae) Physiological Responses Associated with Feeding and Starvation. Insect Biochem. Mol. Biol. 2016, 77, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Chen, X.; Liu, W.; Zhang, Z.; Wang, Y.; You, K.; Li, Y.; Zhang, R.; Zhou, Q. Characterization of Heat Shock Protein 70 Transcript from Nilaparvata lugens (Stål): Its Response to Temperature and Insecticide Stresses. Pestic. Biochem. Physiol. 2017, 142, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Llorente, L.; Aquilino, M.; Herrero, Ó.; de la Peña, E.; Planelló, R. Characterization and Expression of Heat Shock and Immune Genes in Natural Populations of Prodiamesa olivacea (Diptera) Exposed to Thermal Stress. Ecotoxicol. Environ. Saf. 2023, 263, 115359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, H.; Li, R.; Li, X.; Xu, Y.; Dai, X.; Zhou, Y.; Wang, H. Comparative Transcriptome Analysis of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) Reveals Novel Insights into Heat Stress Tolerance in Insects. BMC Genom. 2017, 18, 974. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.H.; Zheng, L.X.; Chu, J.; Liang, X.H.; Wang, J.; Gao, X.W.; Wu, F.A.; Sheng, S. Characterization, and Functional Analysis of Hsp70 and Hsp90 Gene Families in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Front. Physiol. 2021, 12, 753914. [Google Scholar] [CrossRef] [PubMed]
- Dahlgaard, J.; Loeschcke, V.; Michalak, P.; Justesen, J. Induced Thermotolerance and Associated Expression of the Heat-Shock Protein Hsp70 in Adult Drosophila melanogaster. Funct. Ecol. 1998, 12, 786–793. [Google Scholar] [CrossRef]
- Krebs, R.A.; Feder, M.E. Tissue-Specific Variation in Hsp70 Expression and Thermal Damage in Drosophila melanogaster Larvae. J. Exp. Biol. 1997, 200, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Bahrndorff, S.; Mariën, J.; Loeschcke, V.; Ellers, J. Dynamics of Heat-Induced Thermal Stress Resistance and Hsp70 Expression in the Springtail, Orchesella cincta. Funct. Ecol. 2009, 23, 233–239. [Google Scholar] [CrossRef]
- Richards, E.H.; Dani, M.P.; Lu, Y.; Butt, T.; Weaver, R.J. Effect of Stress on Heat Shock Protein Levels, Immune Response and Survival to Fungal Infection of Mamestra brassicae Larvae. J. Insect Physiol. 2017, 96, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Becnel, J.J.; Clark, G.G.; Linthicum, K.J. Expression of AeaHsp26 and AeaHsp83 in Aedes aegypti (Diptera: Culicidae) Larvae and Pupae in Response to Heat Shock Stress. J. Med. Entomol. 2010, 47, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, X.; Zhang, W.; Zhang, B.; Ma, C. Sen Dynamics of Heat Shock Protein Responses to Thermal Stress Changes after Metamorphosis in a Lepidopteran Insect. Arch. Insect Biochem. Physiol. 2021, 107, e21791. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I.; Kowalski, P.; Jakubowicz, T. Humoral Immune Response of Galleria mellonella Larvae after Infection by Beauveria bassiana under Optimal and Heat-Shock Conditions. J. Insect Physiol. 2009, 55, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I.; Taszłow, P. Heat Shock Affects Host-Pathogen Interaction in Galleria mellonella Infected with Bacillus thuringiensis. J. Insect Physiol. 2013, 59, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, Y.; Zhou, Z.; Zhang, H.; Guo, J.; Wan, F. Heat Shock Factor Is Involved in Regulating the Transcriptional Expression of Two Potential Hsps (AhHsp70 and AhsHsp21) and Its Role in Heat Shock Response of Agasicles hygrophila. Front. Physiol. 2020, 11, 562204. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zou, Q.; Zheng, H.; Zhang, F.; Tang, B.; Wang, S. Three Heat Shock Proteins from Spodoptera exigua: Gene Cloning, Characterization and Comparative Stress Response during Heat and Cold Shocks. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 159, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Si, F.L.; Qiao, L.; He, Q.Y.; Zhou, Y.; Yan, Z.T.; Chen, B. HSP Superfamily of Genes in the Malaria Vector Anopheles sinensis: Diversity, Phylogenetics and Association with Pyrethroid Resistance. Malar. J. 2019, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Gerenday, A.; Park, Y.-J.; Lan, Q.; Fallon, A.M. Expression Of A Heat-Inducible Gene In Transfected Mosquito Cells. Insect Biochem. 1989, 19, 679–686. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, F.X.; Cai, M.J.; Zhao, W.L.; Li, X.R.; Wang, J.X.; Zhao, X.F. The Hormone-Dependent Function of Hsp90 in the Crosstalk between 20-Hydroxyecdysone and Juvenile Hormone Signaling Pathways in Insects Is Determined by Differential Phosphorylation and Protein Interactions. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 5184–5192. [Google Scholar] [CrossRef] [PubMed]
- El-Saadi, M.I.; MacMillan, H.A.; Ferguson, L.V. Cold-Induced Immune Activation in Chill-Susceptible Insects. Curr. Opin. Insect Sci. 2023, 58, 101054. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Tang, T.; Song, Q.; Wang, Z.; He, K.; Liu, X.; Song, J.; Wang, L.; Yang, Y.; Feng, C. Transcription Analysis of the Stress and Immune Response Genes to Temperature Stress in Ostrinia furnacalis. Front. Physiol. 2019, 10, 1289. [Google Scholar] [CrossRef] [PubMed]
- Koštál, V.; Tollarová-Borovanská, M. The 70 KDa Heat Shock Protein Assists during the Repair of Chilling Injury in the Insect, Pyrrhocoris apterus. PLoS ONE 2009, 4, e4546. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martinez, G.; Benoit, J.B.; Rinehart, J.P.; Elnitsky, M.A.; Lee, R.E.; Denlinger, D.L. Dehydration, Rehydration, and Overhydration Alter Patterns of Gene Expression in the Antarctic Midge, Belgica antarctica. J. Comp. Physiol. B 2009, 179, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Gusev, O.; Cornette, R.; Kikawada, T.; Okuda, T. Expression of Heat Shock Protein-Coding Genes Associated with Anhydrobiosis in an African Chironomid Polypedilum vanderplanki. Cell Stress Chaperones 2011, 16, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Lopez-Martinez, G.; Phillips, Z.P.; Patrick, K.R.; Denlinger, D.L. Heat Shock Proteins Contribute to Mosquito Dehydration Tolerance. J. Insect Physiol. 2010, 56, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Lopez-Martinez, G.; Teets, N.M.; Phillips, S.A.; Denlinger, D.L. Responses of the Bed Bug, Cimex lectularius, to Temperature Extremes and Dehydration: Levels of Tolerance, Rapid Cold Hardening and Expression of Heat Shock Proteins. Med. Vet. Entomol. 2009, 23, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Pockley, A.G. Heat Shock Proteins as Regulators of the Immune Response. Lancet 2003, 362, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-N.; Liu, Y.; Xin, Z.-Z.; Zhu, X.-Y.; Ge, B.-M.; Li, C.-F.; Wang, D.; Bian, X.-G.; Yang, L.; Chen, L.; et al. A Small Heat Shock Protein 21 (SHSP21) Mediates Immune Responses in Chinese Oak Silkworm Antheraea pernyi. Int. J. Biol. Macromol. 2018, 111, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dai, L.; Wang, L.; Qian, C.; Wei, G.; Li, J.; Zhu, B.; Liu, C. Inhibitors of Eicosanoid Biosynthesis Influencing the Transcripts Level of SHSP21.4 Gene Induced by Pathogen Infections, in Antheraea pernyi. PLoS ONE 2015, 10, e0121296. [Google Scholar] [CrossRef] [PubMed]
- Altincicek, B.; Knorr, E.; Vilcinskas, A. Beetle Immunity: Identification of Immune-Inducible Genes from the Model Insect Tribolium castaneum. Dev. Comp. Immunol. 2008, 32, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Lyupina, Y.V.; Dmitrieva, S.B.; Timokhova, A.V.; Beljelarskaya, S.N.; Zatsepina, O.G.; Evgen’ev, M.B.; Mikhailov, V.S. An Important Role of the Heat Shock Response in Infected Cells for Replication of Baculoviruses. Virology 2010, 406, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.B.; Rochon, D. Cucumber Necrosis Virus Recruits Cellular Heat Shock Protein 70 Homologs at Several Stages of Infection. J. Virol. 2016, 90, 3302–3317. [Google Scholar] [CrossRef] [PubMed]
- Merkling, S.H.; Overheul, G.J.; Van Mierlo, J.T.; Arends, D.; Gilissen, C.; Van Rij, R.P. The Heat Shock Response Restricts Virus Infection in Drosophila. Sci. Rep. 2015, 5, 12758. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhao, Y.; Wang, D.; Chen, Z. Mode of Action of Heat Shock Protein (HSP) Inhibitors against Viruses through Host HSP and Virus Interactions. Genes 2023, 14, 792. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.F.; Pullin, J.S.K.; Reavy, B. Inhibition of the Induction of Heat Shock Proteins in Drosophila melanogaster Cells Infected with Insect Picornaviruses. FEBS Lett. 1981, 128, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, W.; Xiao, M.; Ou, S.; Hu, Q. Destruxin A Induces and Binds HSPs in Bombyx mori Bm12 Cells. J. Agric. Food Chem. 2017, 65, 9849–9853. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I.; Kowalski, P. Galleria mellonella Infected with Bacillus thuringiensis Involves Hsp90. Open Life Sci. 2013, 8, 561–569. [Google Scholar] [CrossRef]
- Banfi, D.; Mastore, M.; Bianchi, T.; Brivio, M.F. The Expression Levels of Heat Shock Protein 90 (HSP90) in Galleria mellonella Following Infection with the Entomopathogenic Nematode Steinernema carpocapsae and Its Symbiotic Bacteria Xenorhabdus nematophila. Insects 2025, 16, 201. [Google Scholar] [CrossRef] [PubMed]
- Wrońska, A.K.; Boguś, M.I. Heat Shock Proteins (HSP 90, 70, 60, and 27) in Galleria mellonella (Lepidoptera) Hemolymph Are Affected by Infection with Conidiobolus coronatus (Entomophthorales). PLoS ONE 2020, 15, e0228556. [Google Scholar] [CrossRef] [PubMed]

| HSP | Stressors | Functions |
|---|---|---|
| HSP 90 |
|
|
| HSP 60/70 |
|
|
| HSPs small |
|
|
| Detailed information on the HSPs summarized in this table can be found in supplementary material | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banfi, D.; Bianchi, T.; Mastore, M.; Brivio, M.F. The Role of Heat Shock Proteins in Insect Stress Response, Immunity, and Climate Adaptation. Insects 2025, 16, 741. https://doi.org/10.3390/insects16070741
Banfi D, Bianchi T, Mastore M, Brivio MF. The Role of Heat Shock Proteins in Insect Stress Response, Immunity, and Climate Adaptation. Insects. 2025; 16(7):741. https://doi.org/10.3390/insects16070741
Chicago/Turabian StyleBanfi, Davide, Tommaso Bianchi, Maristella Mastore, and Maurizio Francesco Brivio. 2025. "The Role of Heat Shock Proteins in Insect Stress Response, Immunity, and Climate Adaptation" Insects 16, no. 7: 741. https://doi.org/10.3390/insects16070741
APA StyleBanfi, D., Bianchi, T., Mastore, M., & Brivio, M. F. (2025). The Role of Heat Shock Proteins in Insect Stress Response, Immunity, and Climate Adaptation. Insects, 16(7), 741. https://doi.org/10.3390/insects16070741









