Does Temperature Tolerance Increase in Long-Term Domesticated Frankliniella occidentalis Under Constant Temperature?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Insect Populations
2.2. Cold Exposure
2.3. Heat Exposure
2.4. Statistical Analysis
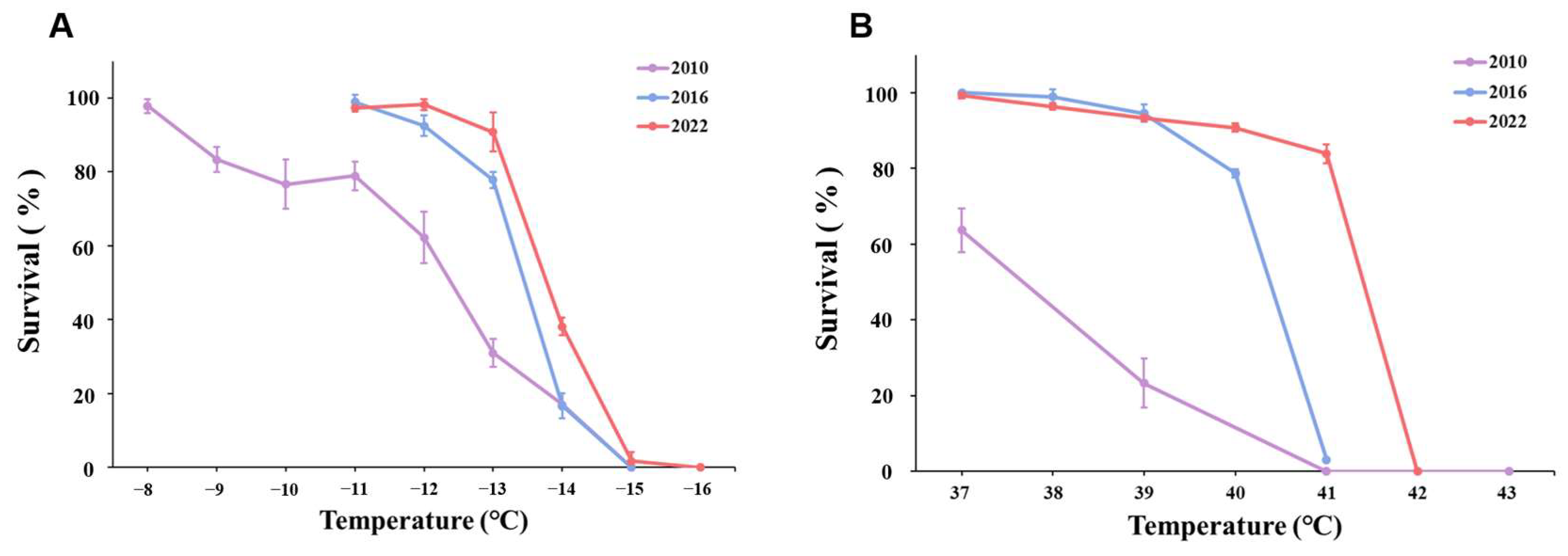
3. Results
3.1. Cold Exposure
3.2. Heat Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.X. Insect Ecology and Forecast; China Agricultural Press: Beijing, China, 2002; pp. 16–27. [Google Scholar]
- Sgrò, C.M.; Terblanche, J.S.; Hoffmann, A.A. What can plasticity contribute to insect responses to climate change? Ann. Rev. Entomol. 2016, 61, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Boardman, L. Cross-talk between low temperature and other environmental factors. Curr. Opin. Insect Sci. 2024, 63, 101193. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Hoffmann, A.A. Comparing phenotypic effects and molecular correlates of developmental, gradual and rapid cold acclimation responses in Drosophila melanogaster. Funct. Ecol. 2012, 26, 84–93. [Google Scholar] [CrossRef]
- Li, H.B.; Shi, L.; Lu, M.X.; Wang, J.J.; Du, Y.Z. Thermal tolerance of Frankliniella occidentalis: Effects of temperature, exposure time, and gender. J. Therm. Biol. 2011, 36, 437–442. [Google Scholar] [CrossRef]
- Li, H.B.; Shi, L.; Wang, J.J.; Du, Y.Z. Rapid cold hardening of Western flower thrips, Frankliniella occidentalis, and its ecological cost. Acta Ecol. Sin. 2011, 31, 7196–7202. [Google Scholar]
- Li, M.Z.; Liu, X.D. Transgenerational effect of heat adaptation induced by heat acclimation inlarvae of the rice leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Crambidae). Acta Entomol. Sin. 2022, 65, 1314–1323. [Google Scholar]
- USDA. Thrips. In United States Department of Agriculture Cooperative Economic Insect Report; USDA: Washington, DC, USA, 1966; Volume 16, p. 262. [Google Scholar]
- Zhang, Y.J.; Wu, Q.J.; Xu, B.Y.; Zhu, G.R. The occurrence and damage of Frankliniella occidentalis (Thysanoptera: Thripidae): A dangerous alien invasive pest in Beijing. Plant Prot. 2003, 4, 58–59. [Google Scholar]
- Chen, X.; Yuan, L.; Du, Y.; Zhang, Y.; Wang, J. Cross-resistance and biochemical mechanisms of abamectin resistance in the western flower thrips, Frankliniella occidentalis. Pestic. Biochem. Physiol. 2011, 101, 34–38. [Google Scholar] [CrossRef]
- Werren, J.H. The evolution of inbreeding in haplodiploid organisms. In The Natural History of Inbreeding and Outbreeding: Theoretical and Empirical Perspectives; University of Chicago Press: Chicago, IL, USA, 1993. [Google Scholar]
- Gaum, W.G.; Giliomee, J.H.; Pringle, K.L. Life history and life tables of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), on English cucumbers. Bull. Entomol. Res. 1994, 84, 219–224. [Google Scholar] [CrossRef]
- Gai, H.T.; Zhi, J.R.; Sun, M. Effects of temperature on the survival and reproduction of Frankliniella occidentalis and F. intonsa. J. Plant Prot. 2011, 38, 521–526. [Google Scholar]
- Liu, L.H.; Zhang, F.; Wu, Z.Q. Effects of Temperature on the Growth, Development and Survival Rate of Frankliniella occidentalis. Acta Ecol. Sin. 2008, 28, 4891–4895. [Google Scholar]
- Yuan, S.Y.; Kong, Q.; Zhang, H.R.; Wang, X.L.; Sun, S.Q.; Li, Z.Y.; Xiao, C. Effects of temperature on the development and survival rate of Frankliniella occidentalis. Jiangsu Agric. Sci. 2011, 39, 138–139. [Google Scholar]
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using SPSS for Windows; Version 12; Open University Press: Maidenhead, UK, 2005. [Google Scholar]
- Scharf, I.; Daniel, A.; MacMillan, H.A.; Katz, N. The effect of fasting and body reserves on cold tolerance in 2 pit-building insect predators. Curr. Zool. 2016, 63, 287–294. [Google Scholar] [CrossRef][Green Version]
- Bonnot, G.; Peypelut, L.; Gérard, F.; Lavenseau, L.; Fleurat-Lessard, F.; Fields, P.G. The effect of cold acclimation and deacclimation on cold tolerance, trehalose and free amino acid levels in Sitophilus granarius and Cryptolestes ferrugineus (Coleoptera). J. Insect Physiol. 1998, 44, 955–965. [Google Scholar] [PubMed]
- MacMillan, H.A.; Andersen, J.L.; Davies, S.A.; Overgaard, J. The capacity to maintain ion and water homeostasis underlies interspecific variation in Drosophila cold tolerance. Sci. Rep. 2015, 5, 18607. [Google Scholar] [CrossRef]
- MacMillan, H.A.; Schou, M.F.; Kristensen, T.N.; Overgaard, J. Preservation of potassium balance is strongly associated with insect cold tolerance in the field: A seasonal study of Drosophila subobscura. Biol. Lett. 2016, 12, 20160123. [Google Scholar] [CrossRef]
- Dai, T.M. The Characteristics of Temperature-Induced DNA Methylation and the Functional Identification of Dnmt1 Gene in Bemisia tabaci Under Temperature Stress Ondition. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2015. [Google Scholar]
- Lu, M.X.; Li, H.B.; Zheng, Y.T.; Shi, L.; Du, Y.Z. Identification, genomic organization and expression profiles of four heat shock protein genes in the western flower thrips, Frankliniella occidentalis. J. Therm. Biol. 2016, 57, 110–118. [Google Scholar] [CrossRef]
- ElSaadi, M.I.; MacMillan, H.A.; Ferguson, L.V. Cold-induced immune activation in chill-susceptible insects. Curr. Opin. Insect Sci. 2023, 58, 101054. [Google Scholar] [CrossRef]
- Teets, M.N.; MacMillan, A.H. Editorial Overview: Insect cold tolerance research reaches a Swift new Era. Curr. Opin. Insect Sci. 2024, 66, 101284. [Google Scholar] [CrossRef]
- Ju, R.T.; Gao, L.; Zhou, X.H.; Li, B. Tolerance to high temperature extremes in an invasive lace bug, Corythucha ciliata (Hemiptera: Tingidae), in subtropical China. PLoS ONE 2013, 8, e54372. [Google Scholar] [CrossRef]
- Salis, L.; Lof, M.; Asch, M.; Visser, M.E. Modeling winter moth Operophtera brumata egg phenology: Nonlinear effects of temperature and developmental stage on developmental rate. Oikos 2016, 125, 1772–1781. [Google Scholar] [CrossRef]
- Huang, L.H.; Wang, C.Z.; Kang, L. Cloning and expression of five heat shock protein genes in relation to cold hardening and development in the leafminer, Liriomyza sativa. J. Insect Physiol. 2009, 55, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Rreitz, S.; Wang, L.X.; Wang, S.Y.; Xue, L.I.; Lei, Z.R. The mRNA expression profiles of five heat shock protein genes from Frankliniella occidentalis at different stages and their responses to temperatures and insecticides. J. Integr. Agr. 2014, 13, 2196–2210. [Google Scholar] [CrossRef]
- Huang, C.; Li, Z.Y.; Wang, F.L.; Zhang, G.F.; Li, C.R.; Li, Y.H. The supercooling point and freezing point of mature larva and pupa of Bactrocera minax (Enderlein). J. Environ. Insects 2014, 36, 17–21. [Google Scholar]
- Yoder, J.A.; Chambers, M.J.; Tank, J.L.; Keeney, G.D. High temperature effects on water loss and survival examining the hardiness of female adults of the spider beetles, Mezium affine and Gibbium aequinoctiale. J. Insect Sci. 2009, 9, 68. [Google Scholar] [CrossRef]
- Chen, H.; Xu, X.L.; Li, Y.P.; Wu, J.X. Characterization of heat shock protein 90, 70 and their transcriptional expression patterns on high temperature in adult of Grapholita molesta (Busck). Insect Sci. 2014, 21, 439–448. [Google Scholar] [CrossRef]
- Wang, H.; Fang, Y.; Wang, L.; Zhu, W.; Ji, H.; Wang, H.; Xu, S.; Sima, Y. Transcriptome analysis of the Bombyx mori fat body after constant high temperature treatment shows differences between the sexes. J. Integr. Agr. 2014, 41, 6039–6049. [Google Scholar] [CrossRef]
- Esperk, T.; Kjærsgaard, A.; Walters, R.J.; Berger, D.; Blanckenhorn, W.U. Plastic and evolutionary responses to heat stress in a temperate dung fly: Negative correlation between basal and induced heat tolerance? J. Evolution. Biol. 2016, 29, 900–915. [Google Scholar] [CrossRef]
- Li, H.B. The Temperature Tolerance and Its Association with HSPs in the Western Flower Thrips. Ph.D. Thesis, Yangzhou University, Yangzhou, China, 2013. [Google Scholar]
- Chidawanyika, F.; Nyamukondiwa, C.; Strathie, L.; Fischer, K. Effects of thermal regimes, starvation and age on heat tolerance of the parthenium beetle Zygogramma bicolorata (Coleoptera: Chrysomelidae) following dynamic and static protocols. PLoS ONE 2017, 12, e0169371. [Google Scholar] [CrossRef]
- Lalouette, L.; Vernon, P.; Amat, H.; Renault, D. Aging and thermal performance in the sub-Antarctic wingless fly Anatalanta aptera (Diptera: Sphaeroceridae): Older is better. Biol. Lett. 2009, 6, 346. [Google Scholar]
- Larsen, K.J.; Lee, R.E. Cold tolerance including rapid cold-hardening and inoculative freezing of fall migrant monarch butterflies in Ohio. J. Insect Physiol. 1994, 40, 859–864. [Google Scholar] [CrossRef]
- Gerken, A.R.; Eller, O.C.; Hahn, D.A.; Morgan, T.J. Constraints, independence, and evolution of thermal plasticity: Probing genetic architecture of long-and short-term thermal acclimation. Proc. Natl. Acad. Sci. USA 2015, 112, 4399–4404. [Google Scholar] [CrossRef]
- Liao, P.; Miao, S.M.; Xu, R.N.; Liu, C.X.; Chen, G.K.; Wang, M.Q.; Mao, J.J.; Zhang, L.S.; Chen, H.Y. Evaluation of a New Liquid Artificial Diet of Arma chinensis Fallou (Hemiptera: Pentatomidae). Chin. J. Biol. Control. 2019, 35, 9–14. [Google Scholar]
- Li, H.B.; Zhang, C.H.; Jia, F.Z.; Dai, C.G.; Hu, Y. Population rejuvenation of Mythimna separata, a prey of the important natural enemy Arma chinensis. Plant Prot. 2022, 48, 127–131+149. [Google Scholar]
- Mackauer, M. Genetic problems in the production of biological control agents. Annu. Rev. Entomol. 1976, 21, 369–385. [Google Scholar] [CrossRef]
- Jesper, G.S.; Matthew, F.A.; John, S.T. Mass-rearing of insects for pest management: Challenges, synergies and advances from evolutionary physiology. Crop Prot. 2012, 38, 87–94. [Google Scholar]
- Xie, Y.Q.; Zhang, H.Z.; Li, Y.Y.; Kong, L.; Xiang, M.; Yang, H.L.; Zhang, L.M.; Ai, H.M.; Zhang, L.S. Population Degradation Rule of Aphidius gifuensis (Hymenoptera: Aphidoidea). Chin. J. Biol. Control. 2020, 36, 163–168. [Google Scholar]
- Pratissoli, D.; Oliveira, H.N.; GoncAlves, J.R.; Zanuncio, J.C.; Holtz, A.M. Changes in Biological Characteristics of Trichogramma pretiosum (Hym.: Trichogrammatidae) Reared on Eggs of Anagasta kuehniella (Lep.: Pyralidae) for 23 Generations. Biocontrol Sci. Technol. 2004, 14, 313–319. [Google Scholar] [CrossRef]
- Simon, J.C.; Delmotte, F.; Rispe, C.; Crease, T. Phylogenetic relationships between parthenogens and their sexual relatives: The possible routes to parthenogenesis in animals. Biol. J. Linn. Soc. 2003, 79, 151–163. [Google Scholar] [CrossRef]
- Price, G.R. Selection and covariance. Nature 1970, 227, 520–521. [Google Scholar] [CrossRef]
- Lushai, G.; Loxdale, H.D. The potential role of chromosome telomere resetting consequent upon sex in the population dynamics of aphids: A hypothesis. Biol. J. Linn. Soc. 2007, 90, 719–728. [Google Scholar] [CrossRef]

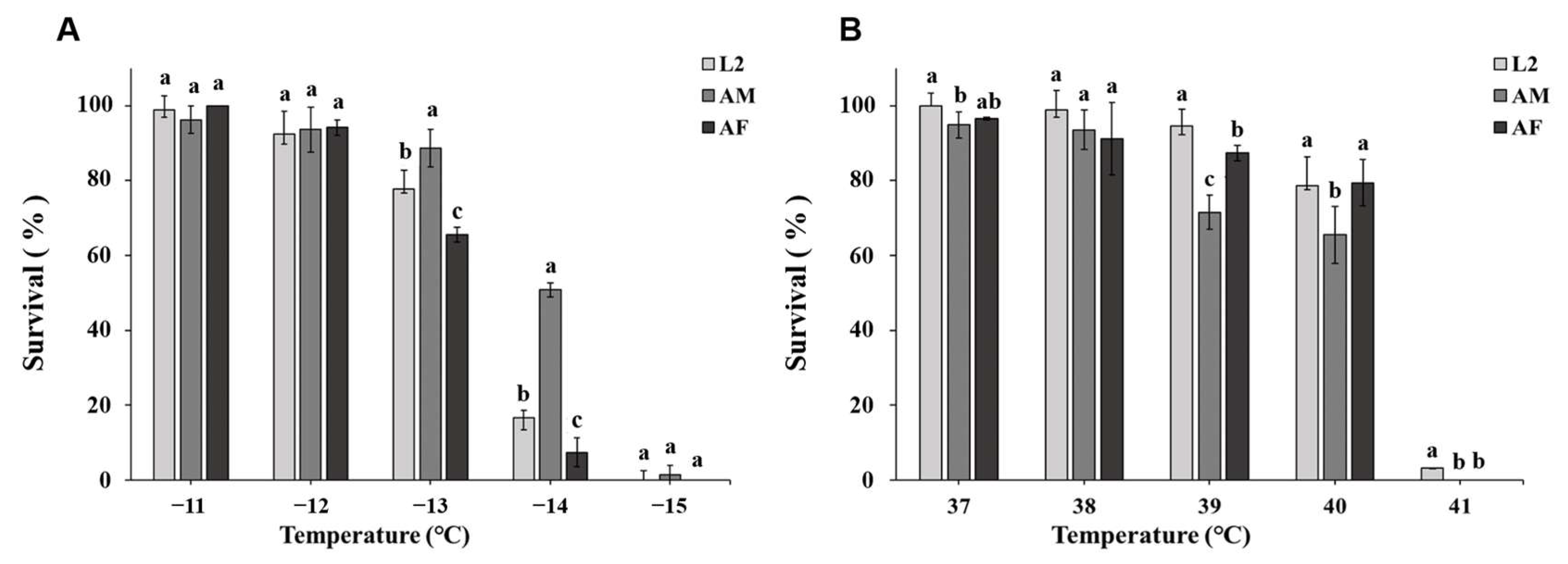
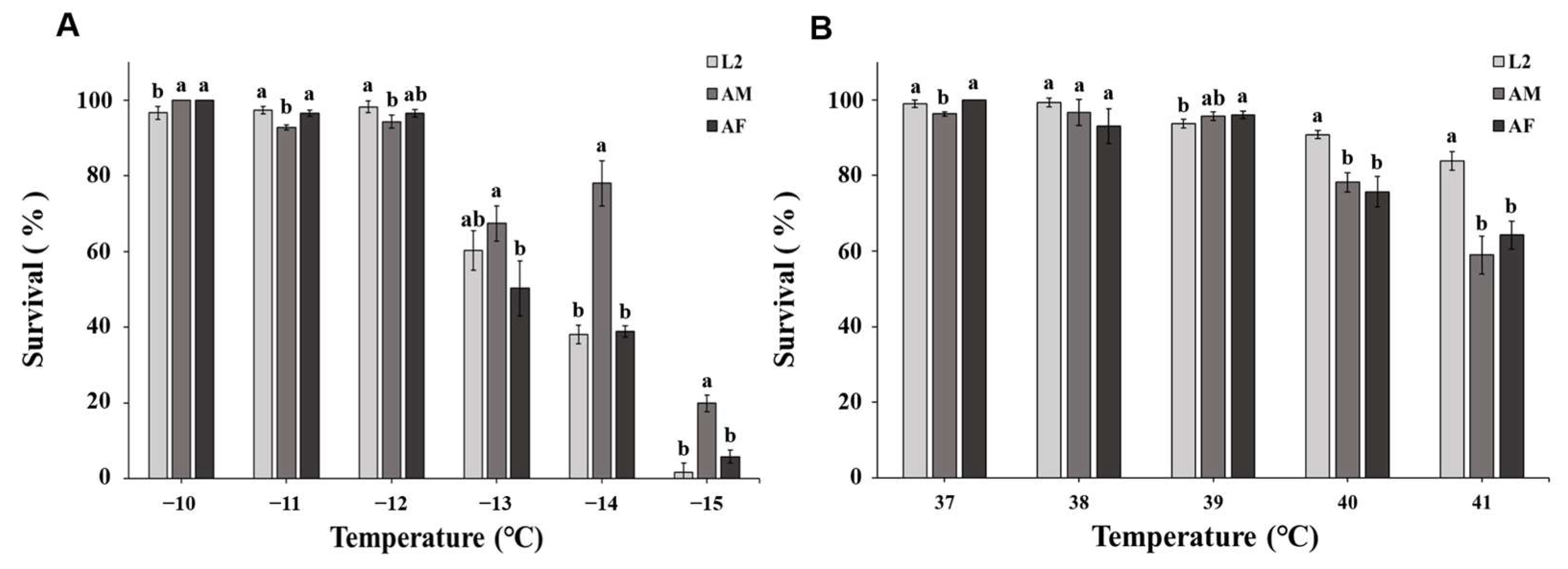
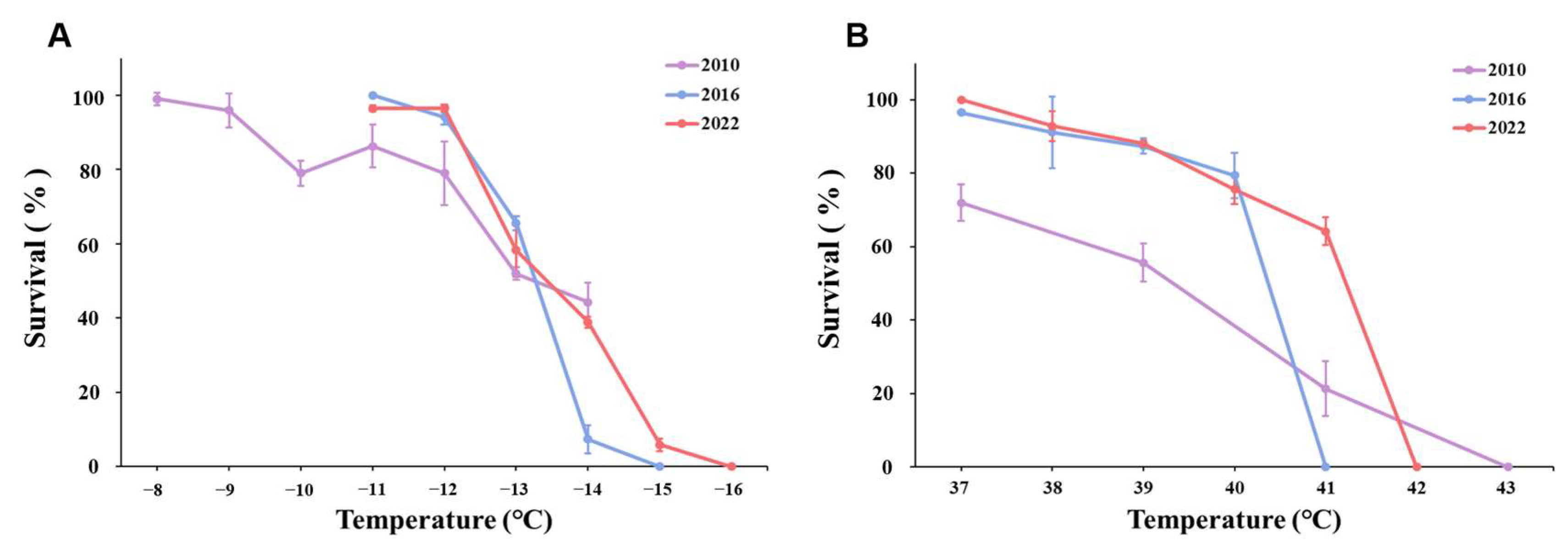
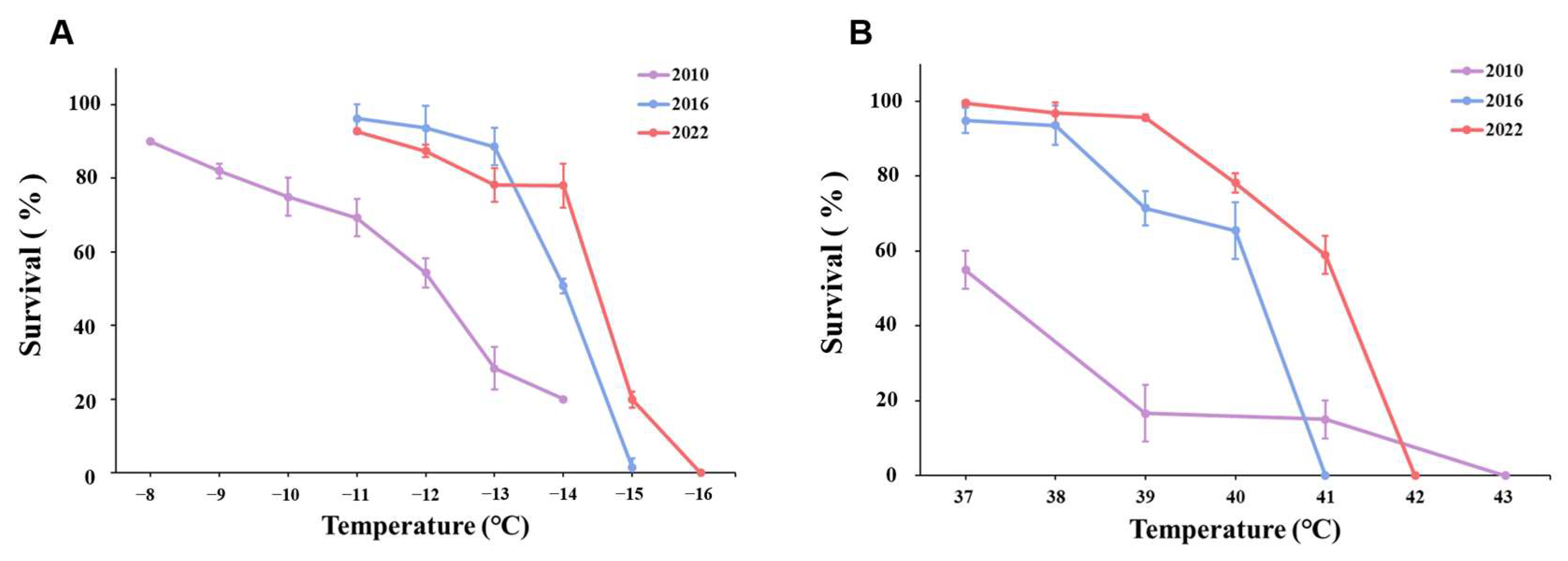
| Developmental Stage | Year | LT50 (°C) | 95% Confidence Limits |
|---|---|---|---|
| AF | 2010 | −12.5 | −13.3~−12.0 a |
| 2016 | −13.2 | −13.4~−12.9 ab | |
| 2022 | −13.4 | −13.2~−13.6 b | |
| AM | 2010 | −11.5 | −12.2~−10.8 a |
| 2016 | −13.8 | −14.2~−13.4 b | |
| 2022 | −13.0 | −12.6~−13.3 b | |
| L2 | 2010 | −12.8 | −11.5~−13.7 a |
| 2016 | −13.3 | −13.8~−12.8 b | |
| 2022 | −13.4 | −12.9~−13.9 b |
| Developmental Stage | Year | LT50 (°C) | 95% Confidence Limits |
|---|---|---|---|
| AF | 2010 | 38.9 | 38.3~39.6 a |
| 2016 | 40.0 | 39.5~40.6 a | |
| 2022 | 39.6 | 38.7~40.8 a | |
| AM | 2010 | 36.9 | 35.9~37.7 b |
| 2016 | 39.7 | 39.3~40.1 a | |
| 2022 | 40.5 | 39.7~41.6 a | |
| L2 | 2010 | 36.9 | 36.1~38.6 b |
| 2016 | 40.2 | 39.8~40.7 a | |
| 2022 | 41.0 | 40.2~41.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, L.; Li, H.; Chang, Y.; Du, Y. Does Temperature Tolerance Increase in Long-Term Domesticated Frankliniella occidentalis Under Constant Temperature? Insects 2025, 16, 557. https://doi.org/10.3390/insects16060557
Shu L, Li H, Chang Y, Du Y. Does Temperature Tolerance Increase in Long-Term Domesticated Frankliniella occidentalis Under Constant Temperature? Insects. 2025; 16(6):557. https://doi.org/10.3390/insects16060557
Chicago/Turabian StyleShu, Lin, Hongbo Li, Yawen Chang, and Yuzhou Du. 2025. "Does Temperature Tolerance Increase in Long-Term Domesticated Frankliniella occidentalis Under Constant Temperature?" Insects 16, no. 6: 557. https://doi.org/10.3390/insects16060557
APA StyleShu, L., Li, H., Chang, Y., & Du, Y. (2025). Does Temperature Tolerance Increase in Long-Term Domesticated Frankliniella occidentalis Under Constant Temperature? Insects, 16(6), 557. https://doi.org/10.3390/insects16060557






