The Trade-Off Between the Increased Colony Nurturing Ability and the Decreased Lifespan of Worker Bees (Apis mellifera)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Bees
2.2. Transcriptome Analysis
2.3. LC/MS Non-Targeted Lipidome Analysis
2.4. RNA Extraction and cDNA Synthesis
2.5. Fluorescent Real-Time Quantitative PCR (qPCR)
2.6. Statistical Analysis
3. Results
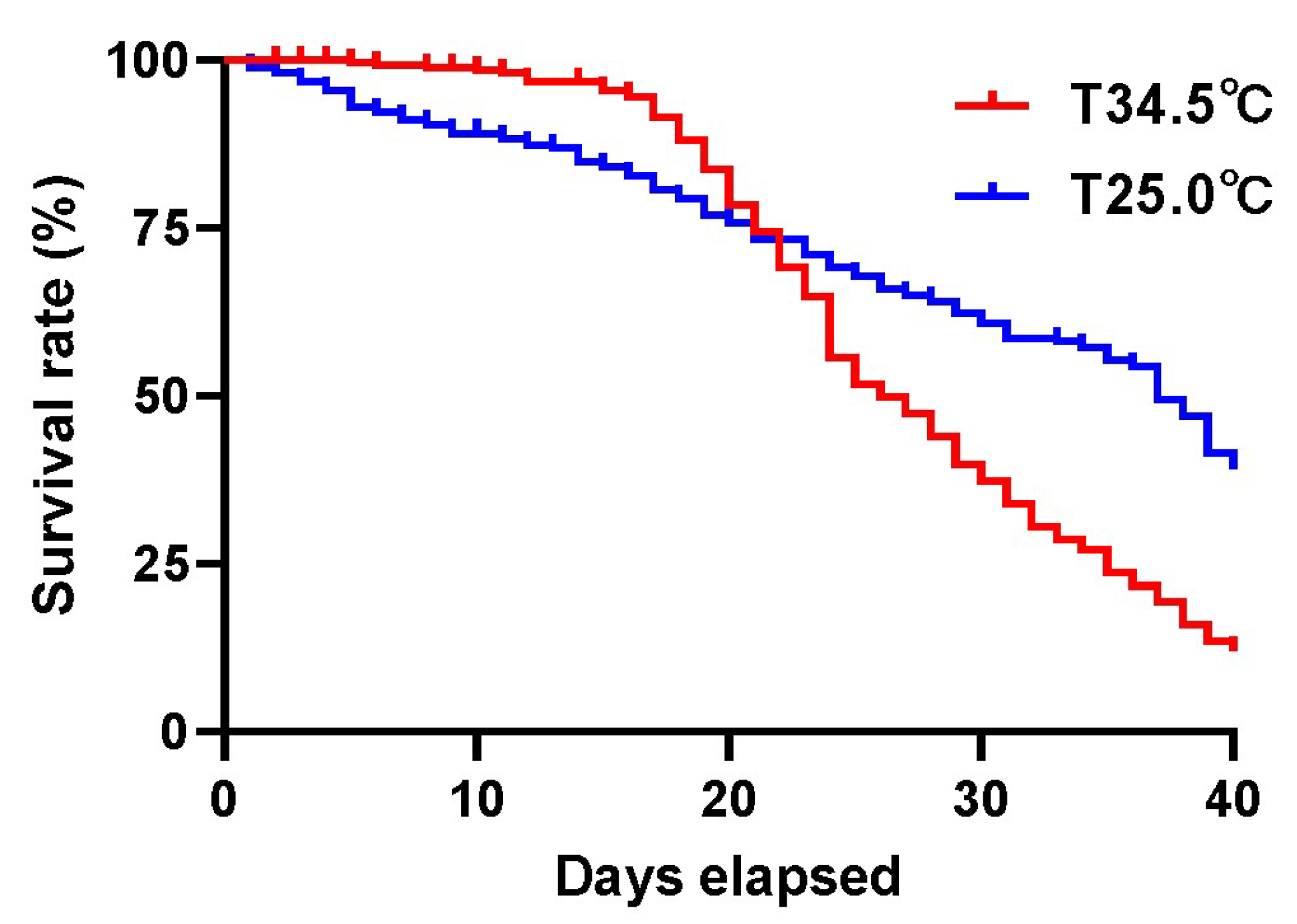
3.1. High Colony Temperature Decreased the Survivorship of Adult Bees
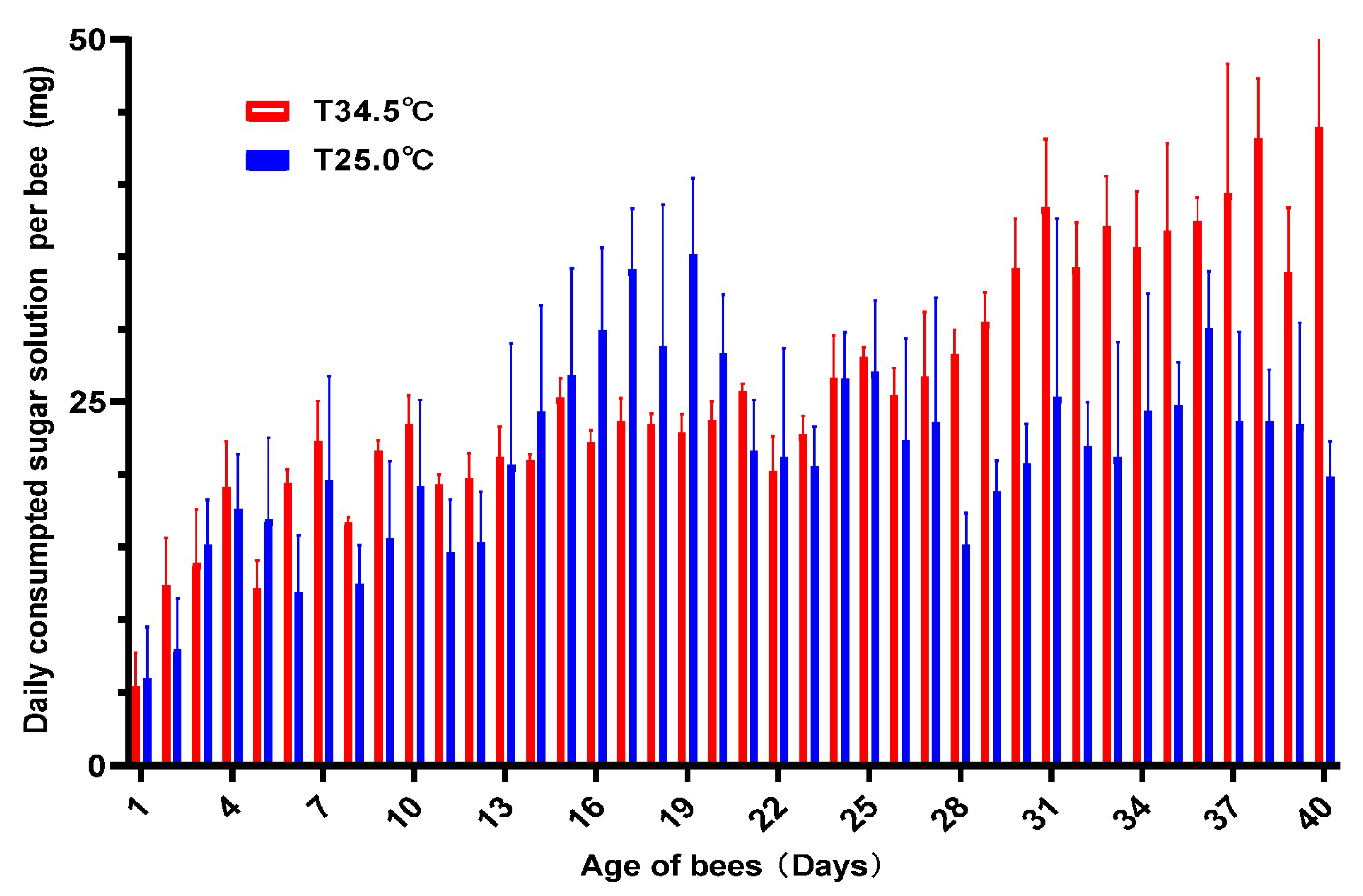
3.2. High Temperature Increased the Sugar Consumption
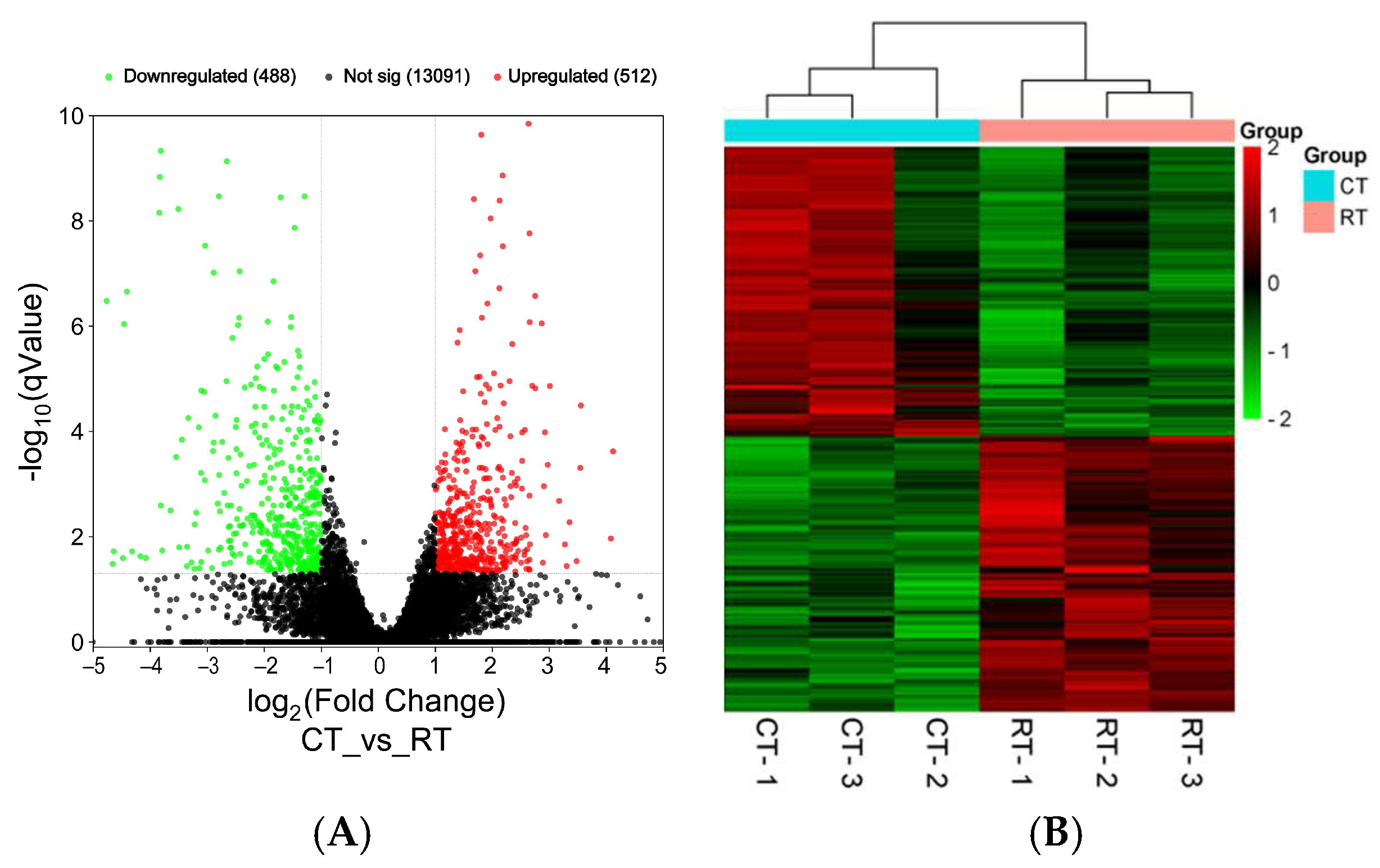
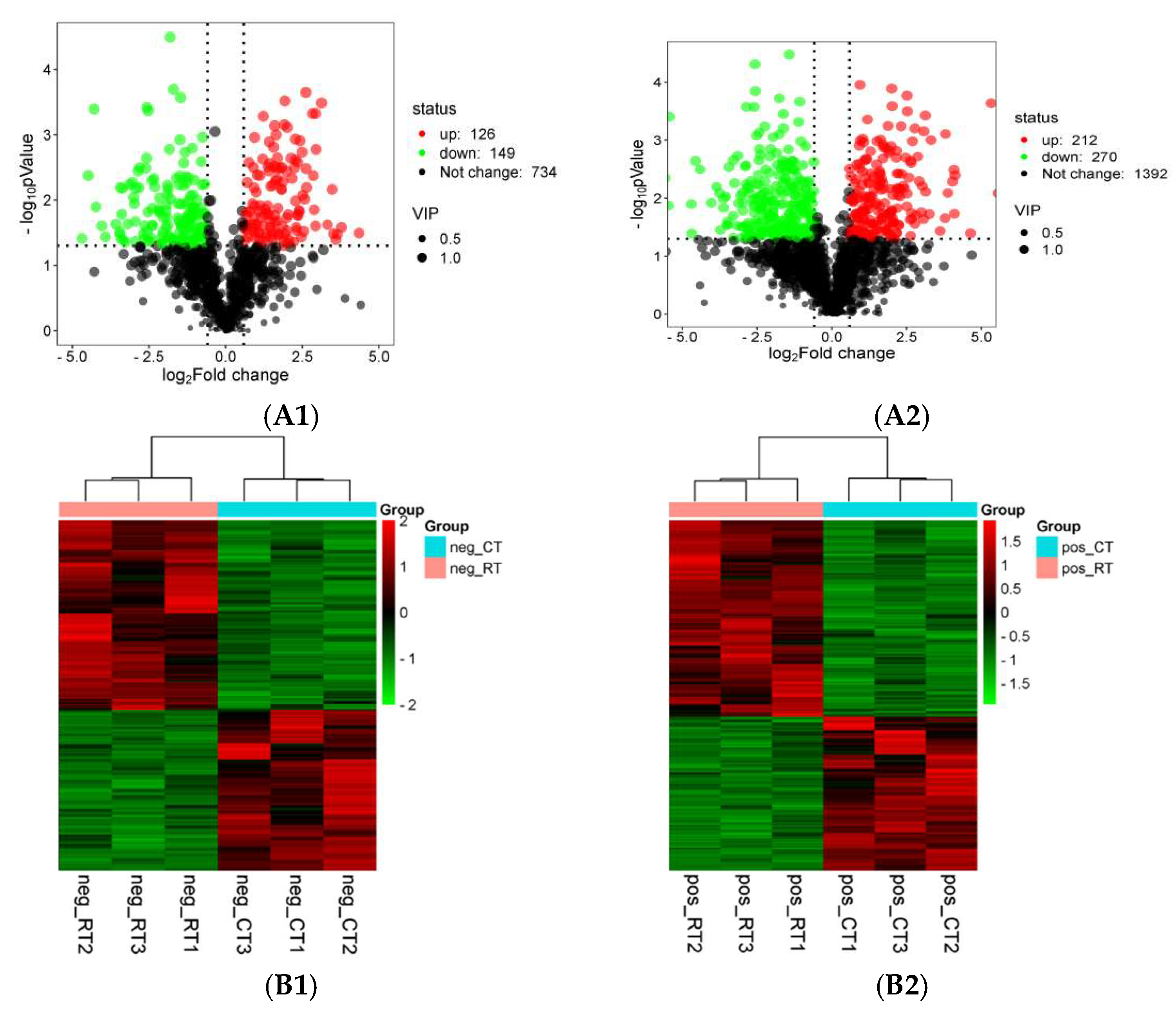
3.3. Transcriptome Sequencing
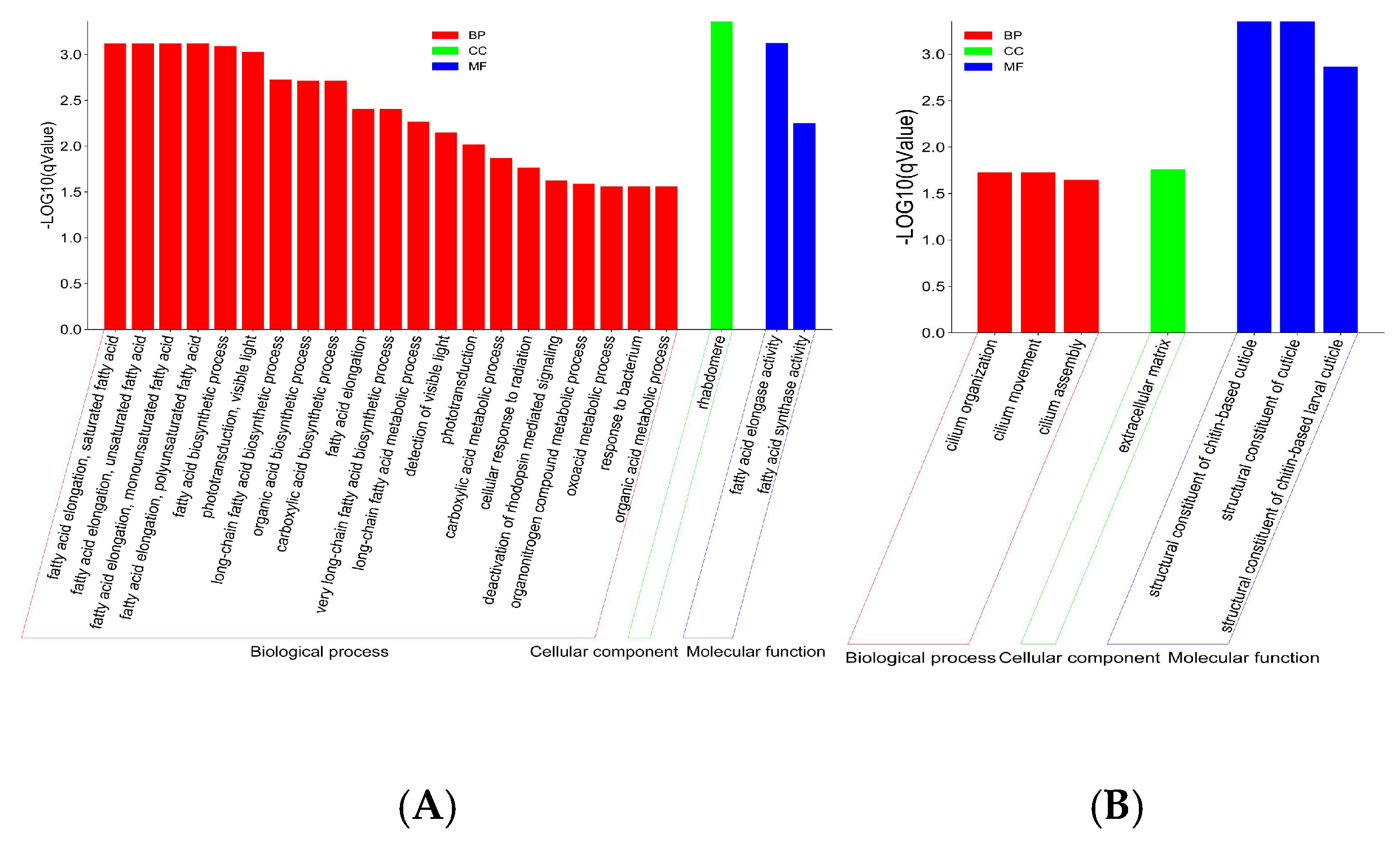
3.4. GO Enrichment
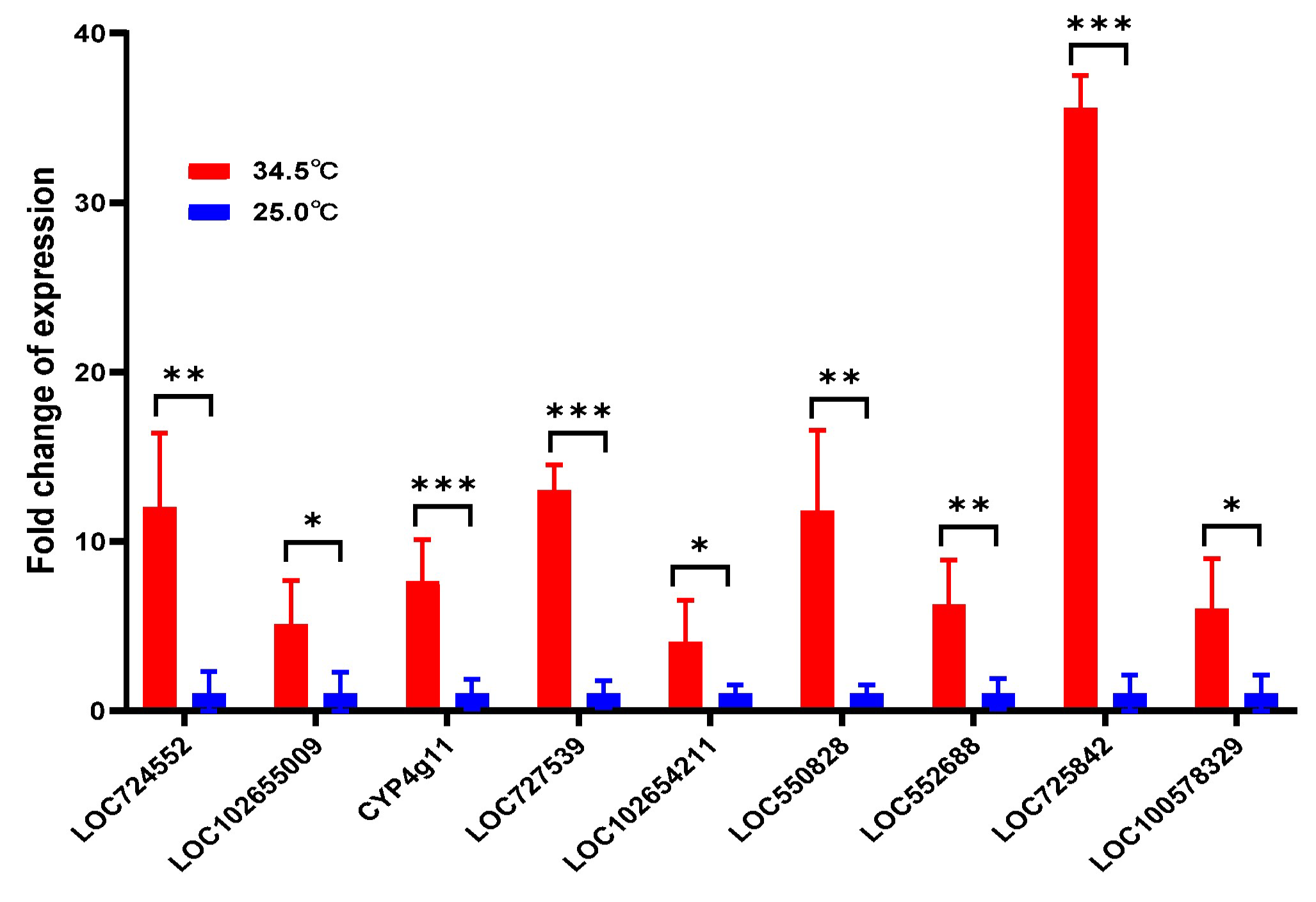
3.4.1. Upregulated Genes Were Enriched in Fatty Acid Biosynthesis and Metabolism
3.4.2. Downregulated Genes Were Enriched in the Structural Constituent of the Cuticle
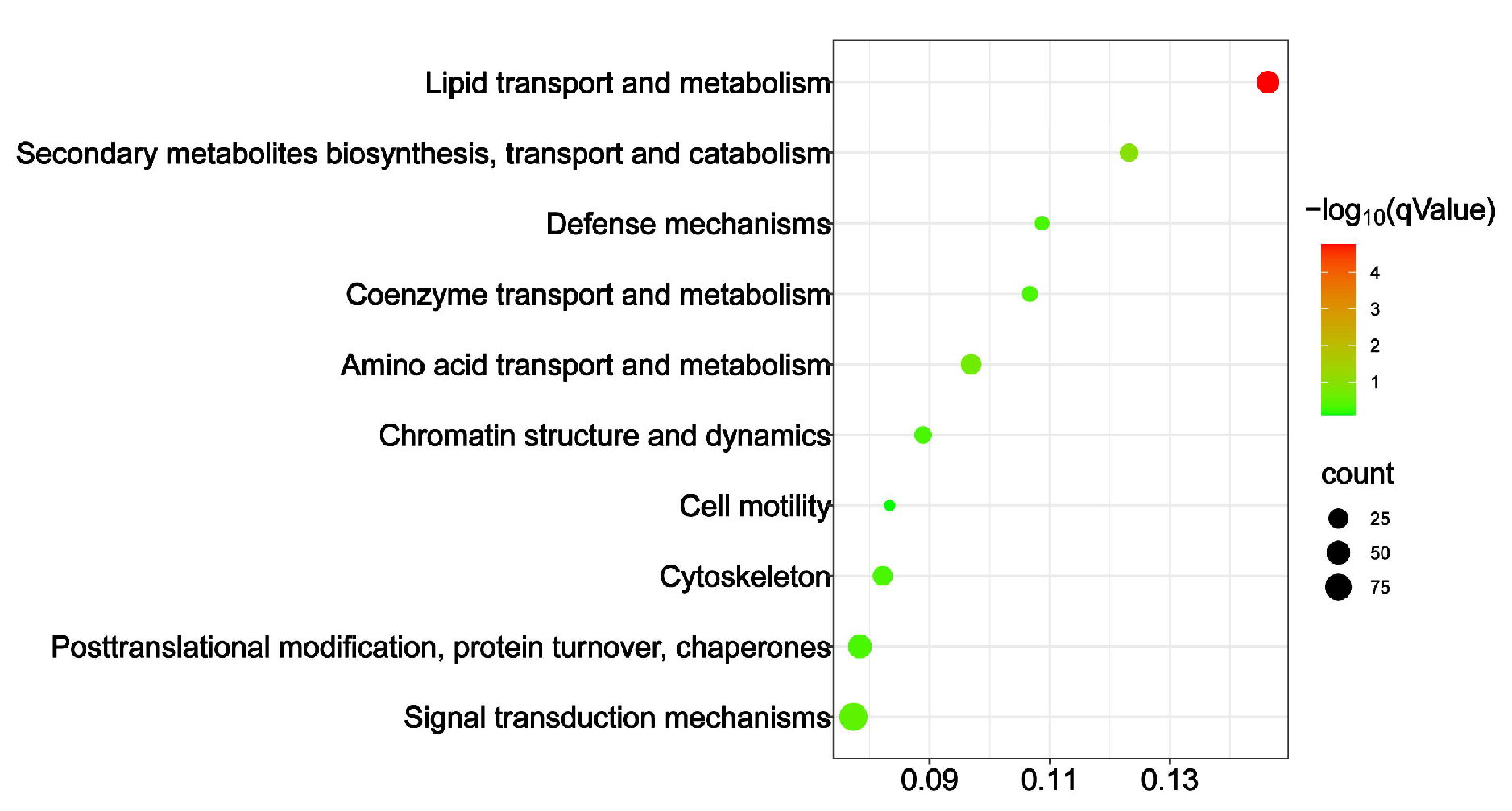
3.5. KOG Enrichment
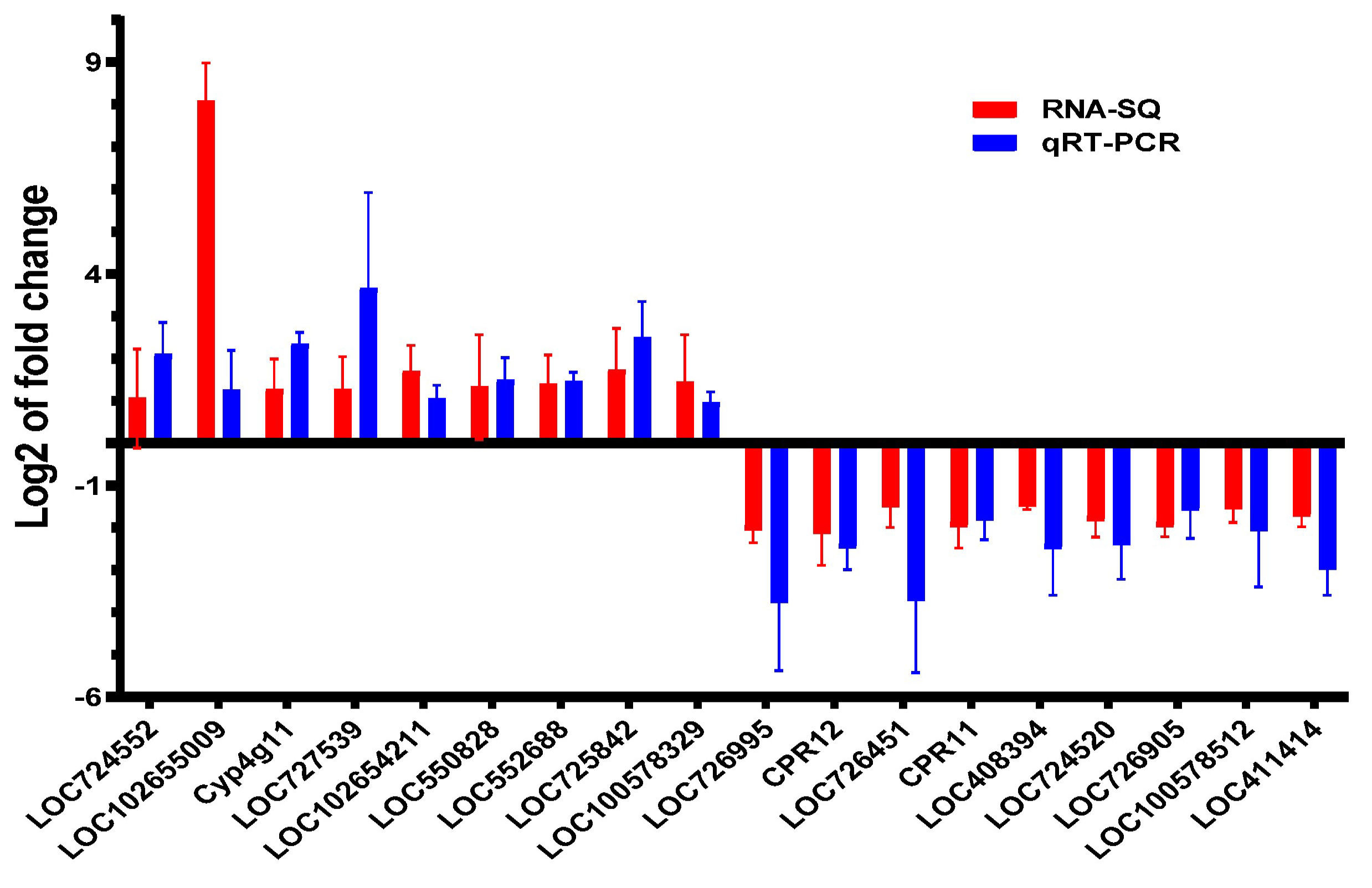
3.6. qRT-PCR Verification
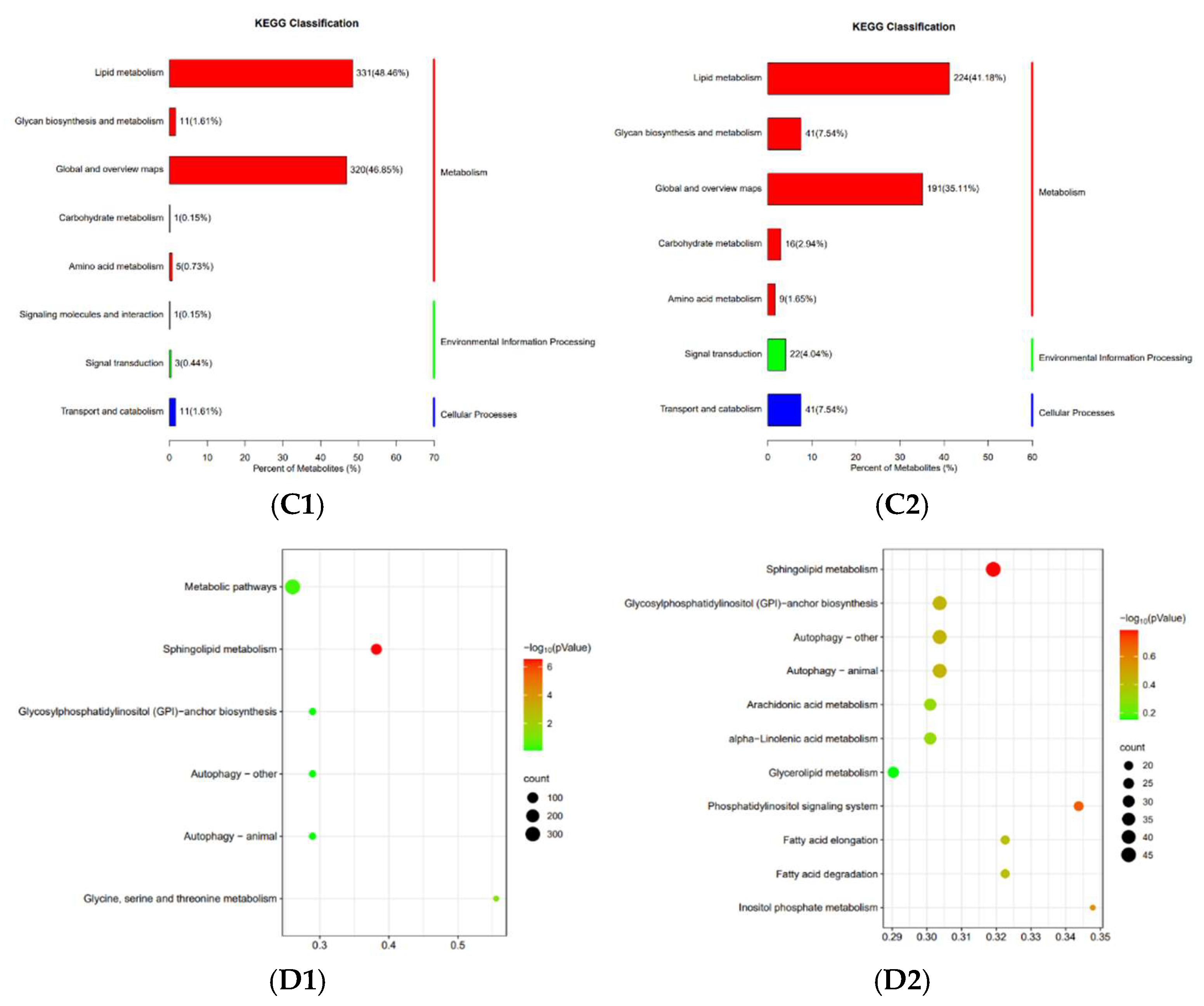
3.7. Lipidomic Analysis
3.8. Upregulated Fatty Acid Genes in Young Adult Bees Reared at 34.5 °C
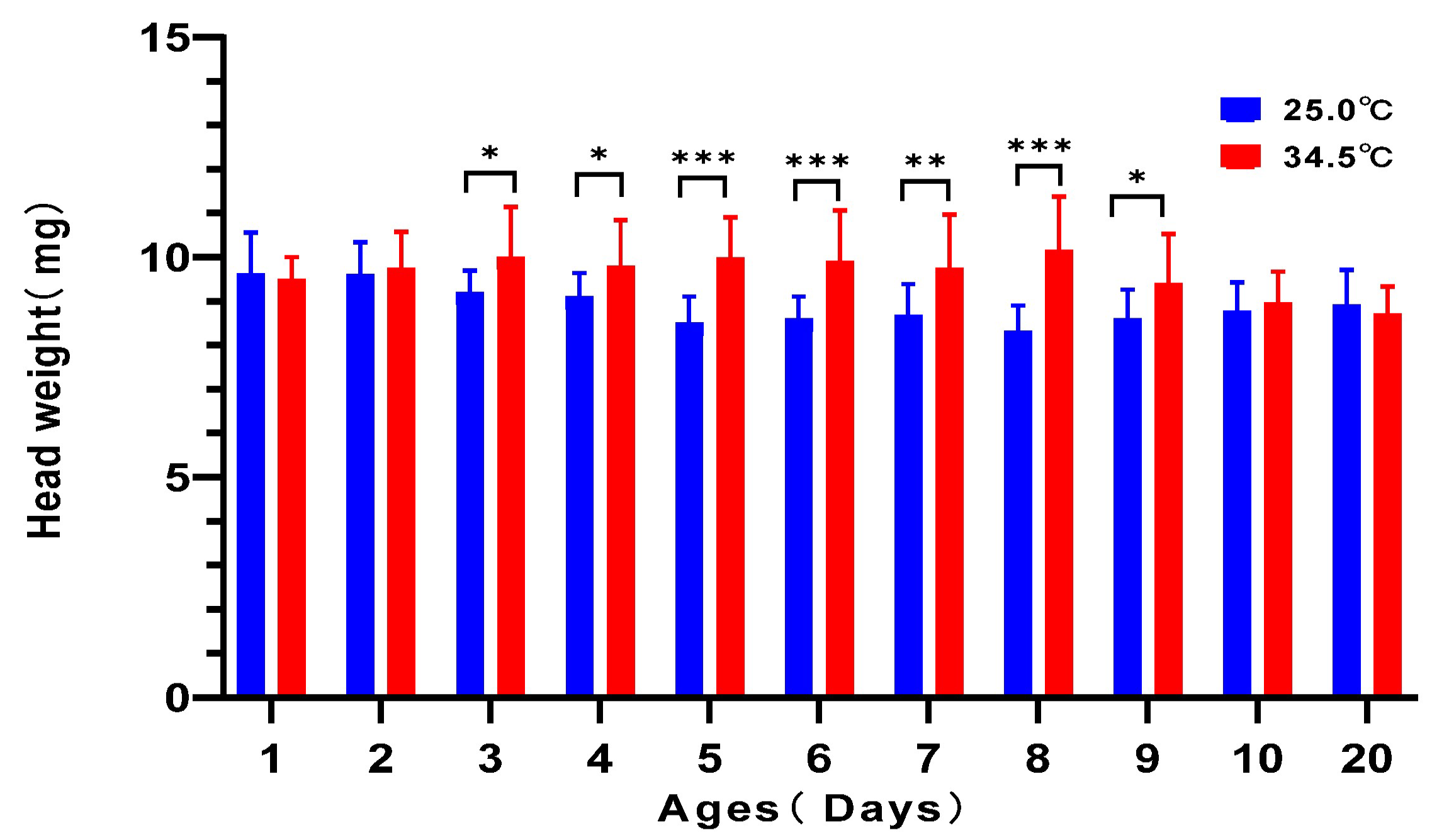
3.9. Heavier Head Weight in Young Adult Bees Reared at 34.5 °C
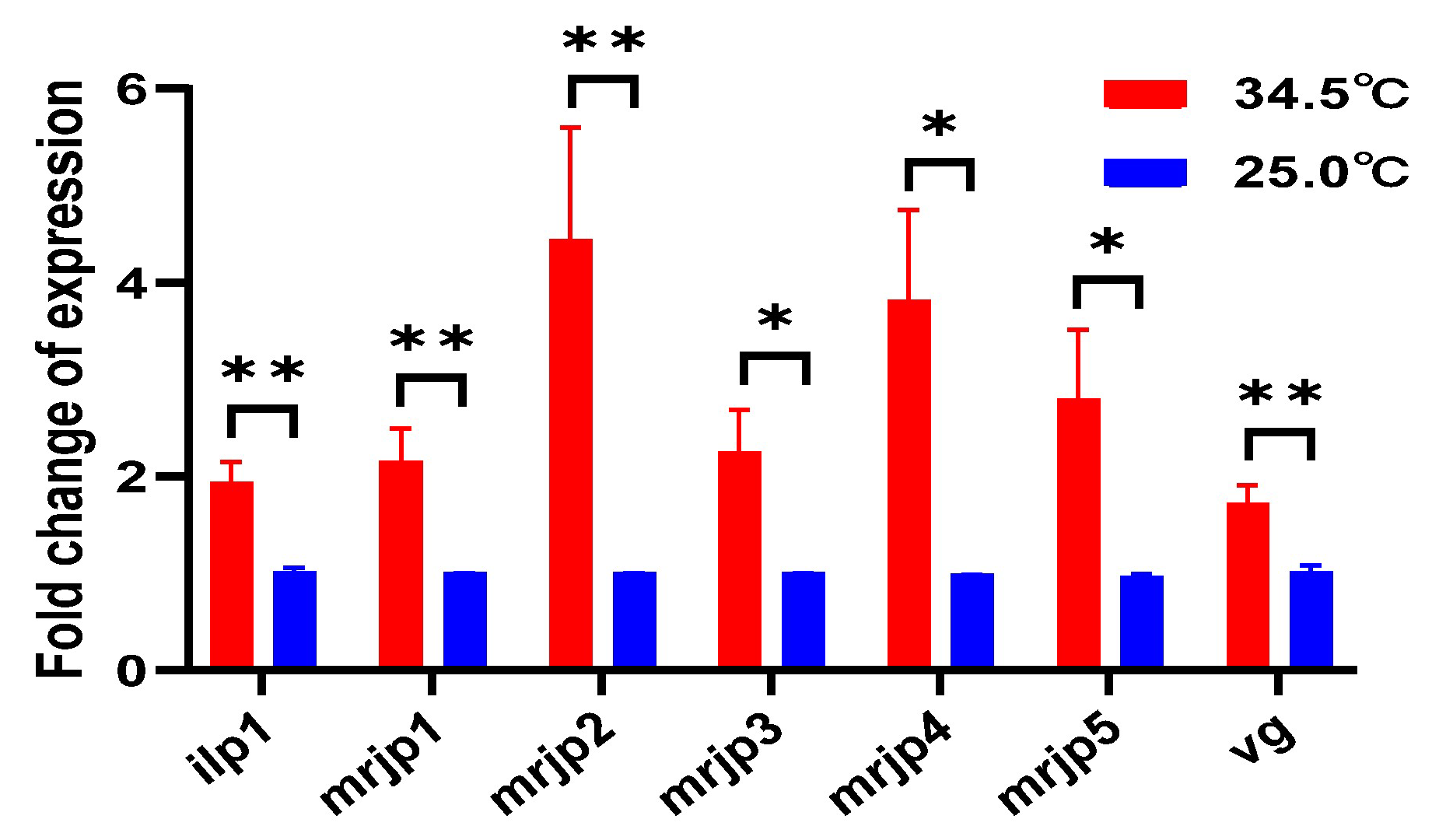
3.10. Upregulated Royal Jelly Secretion-Related Genes in Young Adult Bees Reared at 34.5 °C
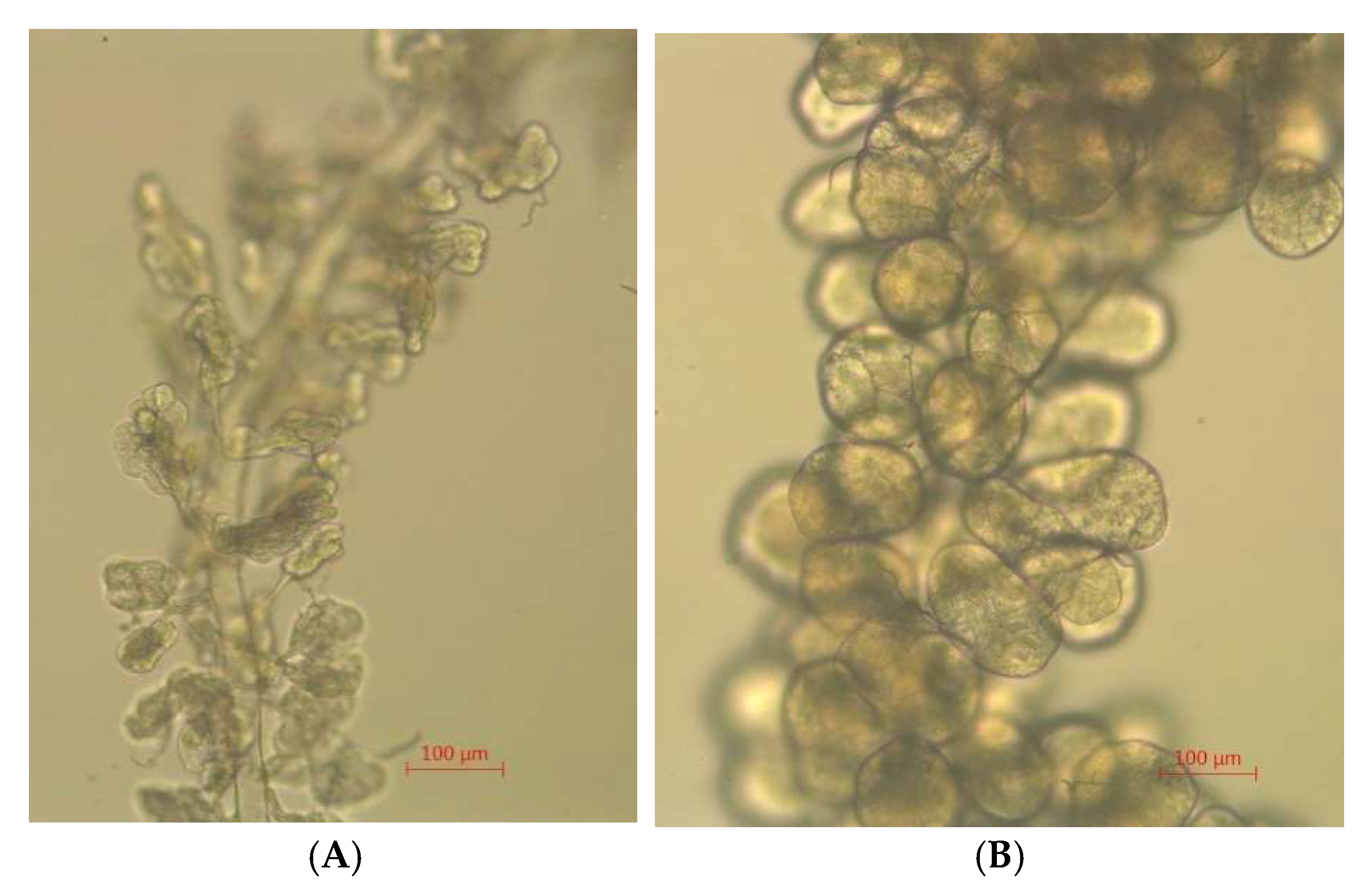
3.11. Developed Hypopharyngeal Gland in Young Adult Bees Reared at 34.5 °C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abarca, M.; Spahn, R. Direct and indirect effects of altered temperature regimes and phenological mismatches on insect populations. Curr. Opin. Insect Sci. 2021, 47, 67–74. [Google Scholar] [CrossRef]
- Ngando, F.J.; Zhang, X.; Qu, H.; Zhang, C.; Yang, F.; Feng, Y.; Shang, Y.; Chen, S.; Ren, L.; Guo, Y. Analysis of the Influence of Changing and Fixed Temperatures on the Growth and Pteridine Content in the Head of Adults Sarcophaga crassipalpis (Diptera: Sarcophagidae). Animals 2023, 13, 2402. [Google Scholar] [CrossRef]
- Asseng, S.; Spänkuch, D.; Hernandez-Ochoa, I.M.; Laporta, J. The upper temperature thresholds of life. Lancet. Planet. Health 2021, 5, e378–e385. [Google Scholar] [CrossRef]
- Precht, H.; Christophersen, J.; Hensel, H.; Larcher, W. Homeothermy and Poikilothermy. In Temperature and Life; Springer: Berlin/Heidelberg, Germany, 1973; pp. 505–508. [Google Scholar]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef]
- Wu, G.; Baumeister, R.; Heimbucher, T. Molecular Mechanisms of Lipid-Based Metabolic Adaptation Strategies in Response to Cold. Cells 2023, 12, 1353. [Google Scholar] [CrossRef] [PubMed]
- Keil, G.; Cummings, E.; de Magalhães, J.P. Being cool: How body temperature influences ageing and longevity. Biogerontology 2015, 16, 383–397. [Google Scholar] [CrossRef]
- Flouris, A.D.; Piantoni, C. Links between thermoregulation and aging in endotherms and ectotherms. Temperature 2015, 2, 73–85. [Google Scholar] [CrossRef]
- Huang, W.; Campbell, T.; Carbone, M.A.; Jones, W.E.; Unselt, D.; Anholt, R.R.H.; Mackay, T.F.C. Context-dependent genetic architecture of Drosophila life span. PLoS Biol. 2020, 18, e3000645. [Google Scholar] [CrossRef]
- Tabarean, I.; Morrison, B.; Marcondes, M.C.; Bartfai, T.; Conti, B. Hypothalamic and dietary control of temperature-mediated longevity. Ageing Res. Rev. 2010, 9, 41–50. [Google Scholar] [CrossRef]
- Lee, H.J.; Alirzayeva, H.; Koyuncu, S.; Rueber, A.; Noormohammadi, A.; Vilchez, D. Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes. Nat. Aging 2023, 3, 546–566. [Google Scholar] [CrossRef]
- Conti, B. Considerations on temperature, longevity and aging. Cell. Mol. Life Sci. 2008, 65, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, J.; Kim, Y.; Lee, S.V. Regulatory systems that mediate the effects of temperature on the lifespan of Caenorhabditis elegans. J. Neurogenet. 2020, 34, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kenyon, C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr. Biol. 2009, 19, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xiao, R.; Ronan, E.A.; He, Y.; Hsu, A.L.; Liu, J.; Xu, X.Z. Environmental Temperature Differentially Modulates C. elegans Longevity through a Thermosensitive TRP Channel. Cell Rep. 2015, 11, 1414–1424. [Google Scholar] [CrossRef]
- Lee, M.C.; Yoon, D.S.; Lee, Y.; Choi, H.; Shin, K.H.; Park, H.G.; Lee, J.S. Effects of low temperature on longevity and lipid metabolism in the marine rotifer Brachionus koreanus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 250, 110803. [Google Scholar] [CrossRef]
- Johnston, R.K.; Snell, T.W. Moderately lower temperatures greatly extend the lifespan of Brachionus manjavacas (Rotifera): Thermodynamics or gene regulation? Exp. Gerontol. 2016, 78, 12–22. [Google Scholar] [CrossRef]
- Buffenstein, R. The Naked Mole-Rat: A New Long-Living Model for Human Aging Research. J. Gerontol. Ser. A 2005, 60, 1369–1377. [Google Scholar] [CrossRef]
- Carrillo, A.E.; Flouris, A.D. Caloric restriction and longevity: Effects of reduced body temperature. Ageing Res. Rev. 2011, 10, 153–162. [Google Scholar] [CrossRef]
- Sestini, E.A.; Carlson, J.C.; Allsopp, R. The effects of ambient temperature on life span, lipid peroxidation, superoxide dismutase, and phospholipase A2 activity in Drosophila melanogaster. Exp. Gerontol. 1991, 26, 385–395. [Google Scholar] [CrossRef]
- Pamplona, R.; Portero-Otín, M.; Riba, D.; Ledo, F.; Gredilla, R.; Herrero, A.; Barja, G. Heart fatty acid unsaturation and lipid peroxidation, and aging rate, are lower in the canary and the parakeet than in the mouse. Aging 1999, 11, 44–49. [Google Scholar] [CrossRef]
- Xiao, R.; Liu, J.; Xu, X.Z. Thermosensation and longevity. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2015, 201, 857–867. [Google Scholar] [CrossRef]
- Palani, S.N.; Sellegounder, D.; Wibisono, P.; Liu, Y. The longevity response to warm temperature is neurally controlled via the regulation of collagen genes. Aging Cell 2023, 22, e13815. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, B.; Dong, Y.; Gong, J.; Xu, T.; Liu, J.; Xu, X.Z. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 2013, 152, 806–817. [Google Scholar] [CrossRef]
- Vakkayil, K.L.; Hoppe, T. Temperature-Dependent Regulation of Proteostasis and Longevity. Front. Aging 2022, 3, 853588. [Google Scholar] [CrossRef]
- Dixit, A.; Bhattacharya, B. Sensory perception of environmental cues as a modulator of aging and neurodegeneration: Insights from Caenorhabditis elegans. J. Neurosci. Res. 2021, 99, 2416–2426. [Google Scholar] [CrossRef]
- Florides, G.A.; Christodoulides, P. Global warming and carbon dioxide through sciences. Environ. Int. 2009, 35, 390–401. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, A.; Thakur, I.S. Carbon dioxide sequestration by chemolithotrophic oleaginous bacteria for production and optimization of polyhydroxyalkanoate. Bioresour. Technol. 2016, 213, 249–256. [Google Scholar] [CrossRef]
- Bebber, D.P. Range-expanding pests and pathogens in a warming world. Annu. Rev. Phytopathol. 2015, 53, 335–356. [Google Scholar] [CrossRef]
- Tonnang, H.E.; Sokame, B.M.; Abdel-Rahman, E.M.; Dubois, T. Measuring and modelling crop yield losses due to invasive insect pests under climate change. Curr. Opin. Insect Sci. 2022, 50, 100873. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- St. Leger, R.J. Insects and their pathogens in a changing climate. J. Invertebr. Pathol. 2021, 184, 107644. [Google Scholar] [CrossRef]
- White, S.A.; Dillon, M.E. Climate warming and bumble bee declines: The need to consider sub-lethal heat, carry-over effects, and colony compensation. Front. Physiol. 2023, 14, 1251235. [Google Scholar] [CrossRef]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a Warming Climate: Insect Responses to Extreme High Temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef]
- Abou-Shaara, H. The foraging behaviour of honey bees, Apis mellifera: A review. Vet. Med. 2014, 59, 1–10. [Google Scholar] [CrossRef]
- Stabentheiner, A.; Kovac, H. Energetic optimisation of foraging honeybees: Flexible change of strategies in response to environmental challenges. PLoS ONE 2014, 9, e105432. [Google Scholar] [CrossRef]
- Stabentheiner, A.; Kovac, H.; Mandl, M.; Käfer, H. Coping with the cold and fighting the heat: Thermal homeostasis of a superorganism, the honeybee colony. J. Comp. Physiol. A 2021, 207, 337–351. [Google Scholar] [CrossRef]
- Stabentheiner, A.; Kovac, H.; Brodschneider, R. Honeybee Colony Thermoregulation–Regulatory Mechanisms and Contribution of Individuals in Dependence on Age, Location and Thermal Stress. PLoS ONE 2010, 5, e8967. [Google Scholar] [CrossRef]
- Jones, J.C.; Helliwell, P.; Beekman, M.; Maleszka, R.; Oldroyd, B.P. The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005, 191, 1121–1129. [Google Scholar] [CrossRef]
- Simone, M.; Evans, J.D.; Spivak, M. Resin Collection and Social Immunity in Honey Bees. Evolution 2009, 63, 3016–3022. [Google Scholar] [CrossRef]
- Dell, A.I.; Pawar, S.; Savage, V.M. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl. Acad. Sci. USA 2011, 108, 10591–10596. [Google Scholar] [CrossRef] [PubMed]
- Koeniger, N. The biology of the honey bee. Insectes Sociaux 1988, 35, 316–318. [Google Scholar] [CrossRef]
- Neuparth, T.; Costa, F.O.; Costa, M.H. Effects of temperature and salinity on life history of the marine amphipod Gammarus locusta. Implications for ecotoxicological testing. Ecotoxicology 2002, 11, 61–73. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Pamplona, R.; Buffenstein, R.; Buttemer, W.A. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 2007, 87, 1175–1213. [Google Scholar] [CrossRef]
- Zhou, L.; Tong, H.; Tang, H.; Pang, S. Fatty acid desaturation is essential for C. elegans longevity at high temperature. Mech. Ageing Dev. 2021, 200, 111586. [Google Scholar] [CrossRef]
- Ford, J.H. Saturated fatty acid metabolism is key link between cell division, cancer, and senescence in cellular and whole organism aging. Age 2010, 32, 231–237. [Google Scholar] [CrossRef]
- Johnson, A.A. Lipid Hydrolase Enzymes: Pragmatic Prolongevity Targets for Improved Human Healthspan? Rejuvenation Res. 2020, 23, 107–121. [Google Scholar] [CrossRef]
- Hernández-García, E.; Rosenbaum, T. Lipid modulation of thermal transient receptor potential channels. Curr. Top. Membr. 2014, 74, 135–180. [Google Scholar] [CrossRef]
- Taberner, F.J.; Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. TRP channels interaction with lipids and its implications in disease. Biochim. Biophys. Acta 2015, 1848, 1818–1827. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, S.; Oh, U.; Hwang, S.W. Endogenous lipid-derived ligands for sensory TRP ion channels and their pain modulation. Arch. Pharmacal Res. 2010, 33, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, J.P. A ménage à trois made in heaven: G-protein-coupled receptors, lipids and TRP channels. Cell Calcium 2011, 50, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Sisignano, M.; Bennett, D.L.; Geisslinger, G.; Scholich, K. TRP-channels as key integrators of lipid pathways in nociceptive neurons. Prog. Lipid Res. 2014, 53, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Raghu, P. Regulation of Drosophila TRPC channels by protein and lipid interactions. Semin. Cell Dev. Biol. 2006, 17, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Calla, B.; MacLean, M.; Liao, L.-H.; Dhanjal, I.; Tittiger, C.; Blomquist, G.J.; Berenbaum, M.R. Functional characterization of CYP4G11—A highly conserved enzyme in the western honey bee Apis mellifera. Insect Mol. Biol. 2018, 27, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Huang, X.; Pang, S. Regulation of the lysosome by sphingolipids: Potential role in aging. J. Biol. Chem. 2022, 298, 102118. [Google Scholar] [CrossRef]
- Mignard, V.; Dubois, N.; Lanoé, D.; Joalland, M.P.; Oliver, L.; Pecqueur, C.; Heymann, D.; Paris, F.; Vallette, F.M.; Lalier, L. Sphingolipid distribution at mitochondria-associated membranes (MAMs) upon induction of apoptosis. J. Lipid Res. 2020, 61, 1025–1037. [Google Scholar] [CrossRef]
- Fugio, L.B.; Coeli-Lacchini, F.B.; Leopoldino, A.M. Sphingolipids and Mitochondrial Dynamic. Cells 2020, 9, 581. [Google Scholar] [CrossRef]
- Worgall, T.S. Sphingolipid synthetic pathways are major regulators of lipid homeostasis. Adv. Exp. Med. Biol. 2011, 721, 139–148. [Google Scholar] [CrossRef]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Hepowit, N.L.; Moon, B.; Ebert, A.C.; Dickson, R.C.; MacGurn, J.A. Art2 mediates selective endocytosis of methionine transporters during adaptation to sphingolipid depletion. J. Cell Sci. 2023, 136, jcs260675. [Google Scholar] [CrossRef]
- Cutler, R.G.; Thompson, K.W.; Camandola, S.; Mack, K.T.; Mattson, M.P. Sphingolipid metabolism regulates development and lifespan in Caenorhabditis elegans. Mech. Ageing Dev. 2014, 143–144, 9–18. [Google Scholar] [CrossRef]
- Cutler, R.G.; Mattson, M.P. Sphingomyelin and ceramide as regulators of development and lifespan. Mech. Ageing Dev. 2001, 122, 895–908. [Google Scholar] [CrossRef]
- Huang, X.; Liu, J.; Dickson, R.C. Down-regulating sphingolipid synthesis increases yeast lifespan. PLoS Genet. 2012, 8, e1002493. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Withers, B.R.; Dickson, R.C. Sphingolipids and lifespan regulation. Biochim. Biophys. Acta 2014, 1841, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, X.; Withers, B.R.; Blalock, E.; Liu, K.; Dickson, R.C. Reducing sphingolipid synthesis orchestrates global changes to extend yeast lifespan. Aging Cell 2013, 12, 833–841. [Google Scholar] [CrossRef]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Xue, X.; Wu, L.; Wang, K. Chemical Composition of Royal Jelly. In Bee Products-Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar]
- Fluri, P.; Lüscher, M.; Wille, H.; Gerig, L. Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees. J. Insect Physiol. 1982, 28, 61–68. [Google Scholar] [CrossRef]
- Robinson, G.E. Regulation of Division of Labor in Insect Societies. Annu. Rev. Entomol. 1992, 37, 637–665. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Huang, H.; Yang, M.; Ma, G.; Huang, X.; Huang, S.; Duan, X.; Li, J. The Trade-Off Between the Increased Colony Nurturing Ability and the Decreased Lifespan of Worker Bees (Apis mellifera). Insects 2025, 16, 558. https://doi.org/10.3390/insects16060558
Sun C, Huang H, Yang M, Ma G, Huang X, Huang S, Duan X, Li J. The Trade-Off Between the Increased Colony Nurturing Ability and the Decreased Lifespan of Worker Bees (Apis mellifera). Insects. 2025; 16(6):558. https://doi.org/10.3390/insects16060558
Chicago/Turabian StyleSun, Chaoxia, Hongji Huang, Mei Yang, Guoshuai Ma, Xinyao Huang, Shaokang Huang, Xinle Duan, and Jianghong Li. 2025. "The Trade-Off Between the Increased Colony Nurturing Ability and the Decreased Lifespan of Worker Bees (Apis mellifera)" Insects 16, no. 6: 558. https://doi.org/10.3390/insects16060558
APA StyleSun, C., Huang, H., Yang, M., Ma, G., Huang, X., Huang, S., Duan, X., & Li, J. (2025). The Trade-Off Between the Increased Colony Nurturing Ability and the Decreased Lifespan of Worker Bees (Apis mellifera). Insects, 16(6), 558. https://doi.org/10.3390/insects16060558






