Host-Affected Body Coloration Dynamics in Perina nuda Larvae: A Quantitative Analysis of Color Variations and Endogenous Plant Influences
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Plants
2.2. Origin of Insects
2.3. Chlorophyll and Carotenoid Content of Leaves
2.4. Nutrient and Phenolic Compound Content of Leaves
2.5. Insect and Leaf Color
2.6. Statistical Analysis
3. Results
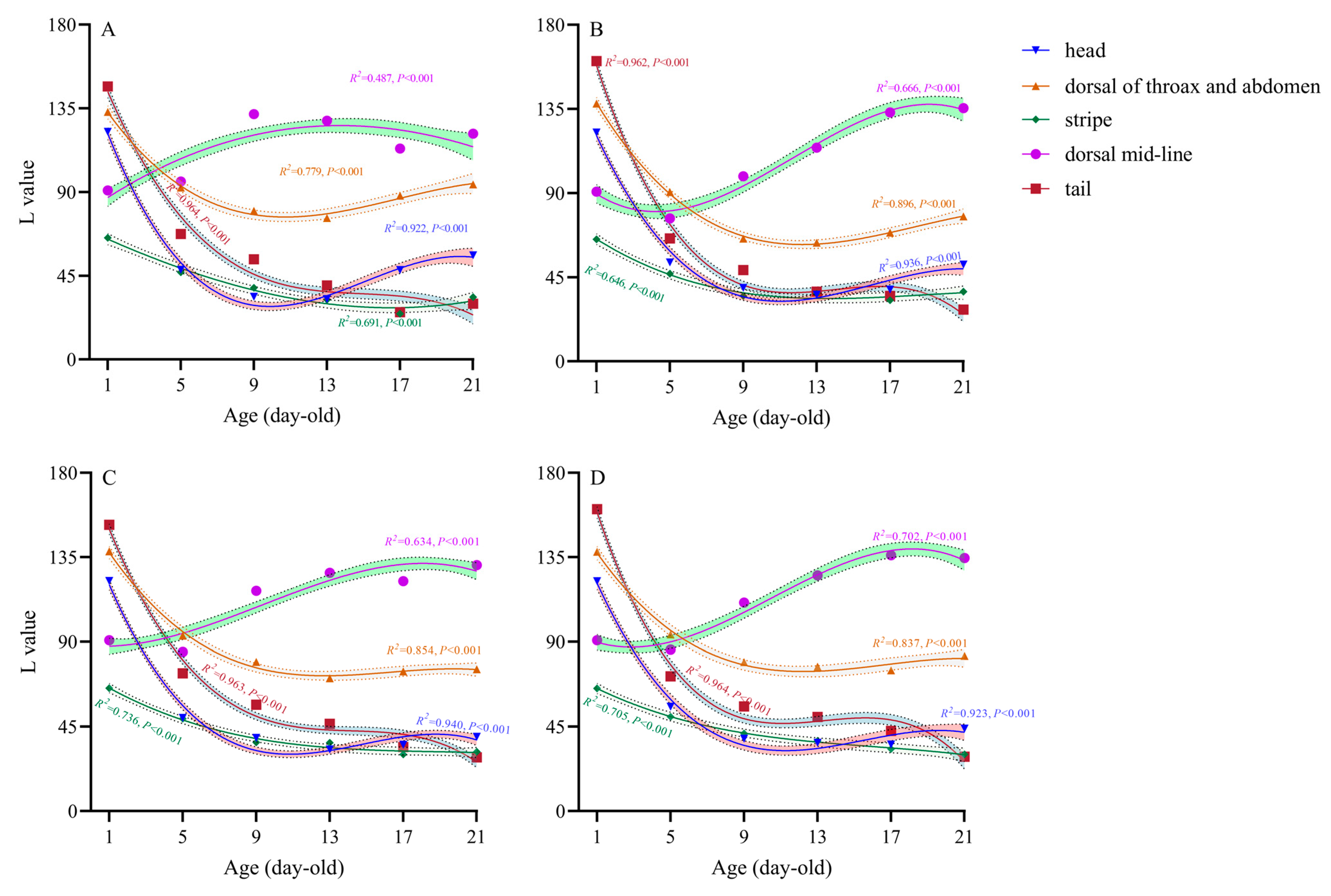
3.1. Changing Trend of the Body Coloration of P. nuda Larvae in Different Ages
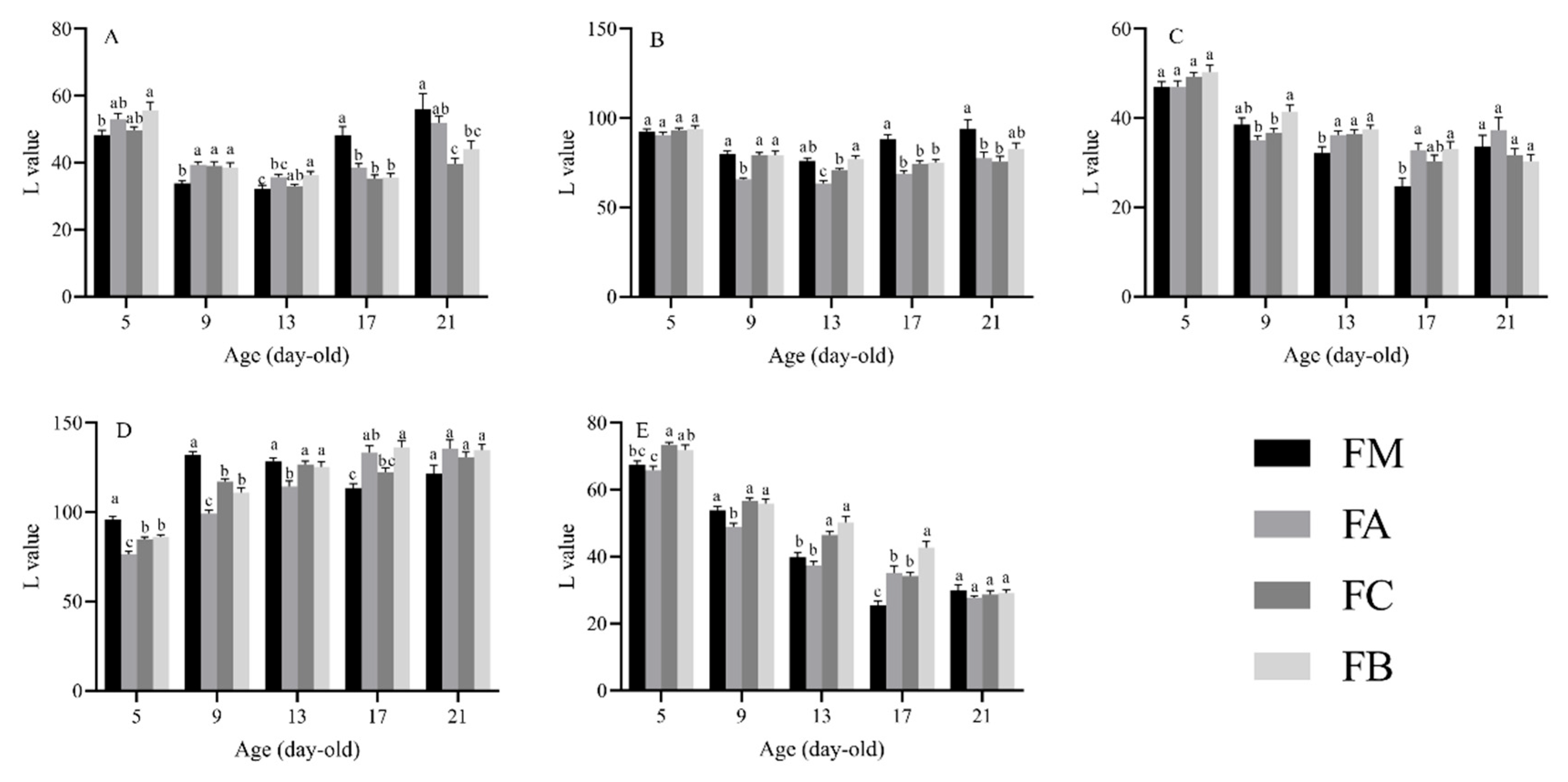
3.2. Effects of Different Hosts on Body Coloration of Different Parts of P. nuda Larvae
3.2.1. Head
3.2.2. Dorsal Thorax and Abdomen
3.2.3. Stripe
3.2.4. Dorsal Mid-Line
3.2.5. Tail
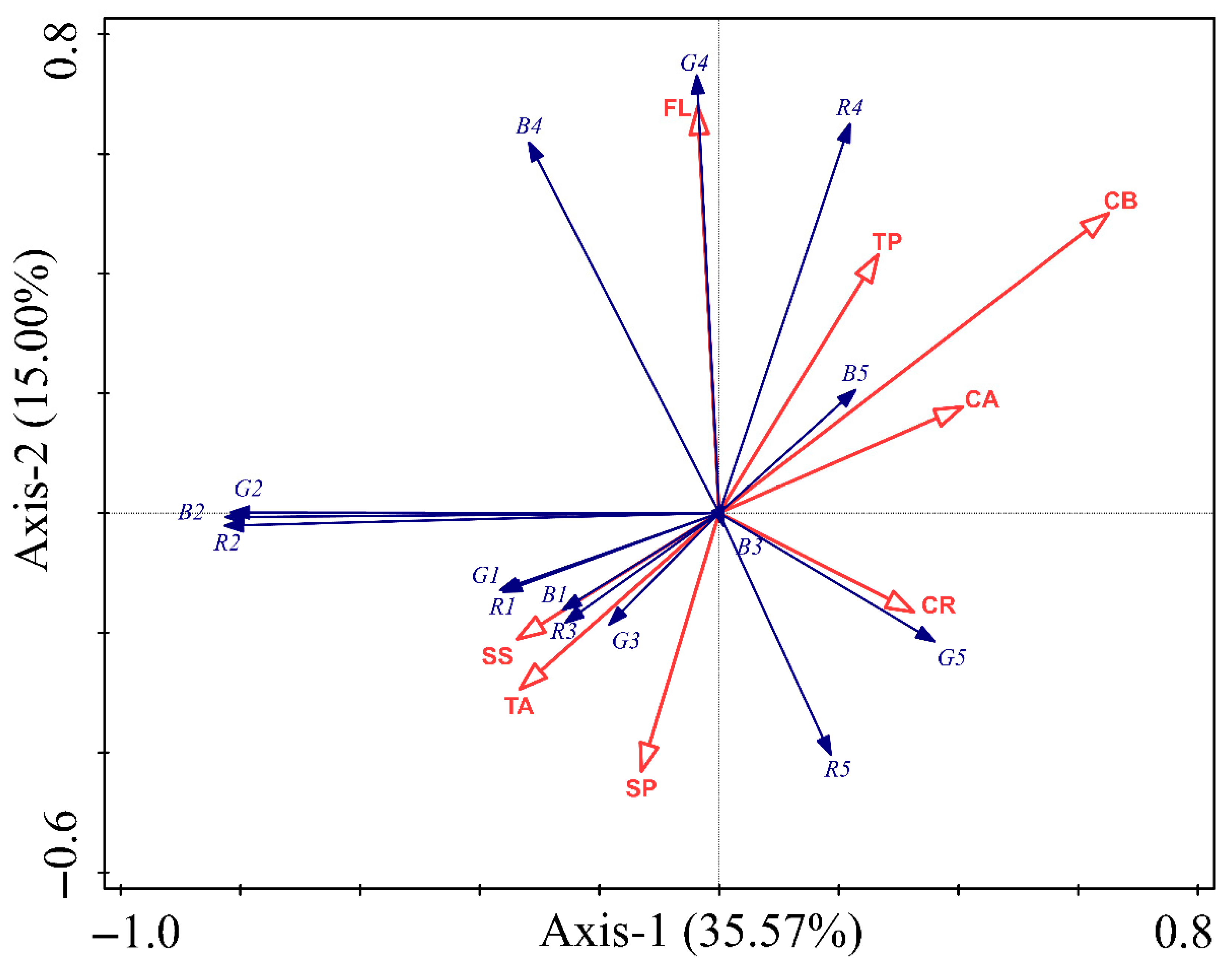
3.3. Correlation Between Body Coloration of 20-Day-Old Perina Nuda Larvae and Endogenous Substances in Host Plants
3.4. Linear Relationship Between Leaf Color and Body Coloration of 20-Day-Old P. nuda Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraser, B.; Murphy, C.; Bunting, F. Real World Color Management: Industrial-Strength Production Techniques; Peachpit Press: Berkeley, CA, USA, 2003. [Google Scholar]
- Vidal, M.; Garcia-Arrona, R.; Bordagaray, A.; Ostra, M.; Albizu, G. Simultaneous determination of color additives tartrazine and allura red in food products by digital image analysis. Talanta 2018, 184, 58–64. [Google Scholar] [CrossRef]
- Jin, X.; Yi, K.; Xu, J. MoADNet: Mobile asymmetric dual-stream networks for real-time and lightweight RGB-D salient object detection. IEEE Trans. Circuits Syst. Video Technol. 2022, 32, 7632–7645. [Google Scholar] [CrossRef]
- Carney, M.N.; Johnston, W.M. A novel regression model from RGB image data to spectroradiometric correlates optimized for tooth colored shades. J. Dent. 2016, 51, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Ellers, J.; Boggs, C.L. Functional ecological implications of intraspecific differences in wing melanization in Colias butterflies. Biol. J. Linn. Soc. Lond. 2004, 82, 79–87. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Beldade, P. Development and evolution of insect pigmentation: Genetic mechanisms and the potential consequences of pleiotropy. Semin. Cell Dev. Biol. 2009, 20, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Bonebrake, T.C.; Ashton, L.A.; Kitching, R.L.; Cao, M.; Sun, Z.; Ho, J.C.; Nakamura, A. Colors of night: Climate-morphology relationships of geometrid moths along spatial gradients in southwestern China. Oecologia 2018, 188, 537–546. [Google Scholar] [CrossRef]
- Bond, A.B.; Kamil, A.C. Visual predators select for crypticity and polymorphism in virtual prey. Nature 2002, 415, 609–613. [Google Scholar] [CrossRef]
- Hoekstra, H.E. Genetics, Development and evolution of adaptive pigmentation in vertebrates. Heredity 2006, 97, 222–234. [Google Scholar] [CrossRef]
- True, J.R. Insect melanism: The molecules matter. Trends Ecol. Evol. 2003, 18, 640–647. [Google Scholar] [CrossRef]
- Umbers, K.D.L.; Herberstein, M.E.; Madin, J.S. Colour in insect thermoregulation: Empirical and theoretical tests in the colour-changing grasshopper, Kosciuscola tristis. J. Insect Physiol. 2013, 59, 81–90. [Google Scholar] [CrossRef]
- Karl, I.; Geister, T.L.; Fischer, K. Intraspecific variation in wing and pupal melanization in copper butterflies (Lepidoptera: Lycaenidae). Biol. J. Linn. Soc. Lond. 2009, 98, 301–312. [Google Scholar] [CrossRef]
- Mavárez, J.; Salazar, C.A.; Bermingham, E.; Salcedo, C.; Jiggins, C.D.; Linares, M. Speciation by hybridization in Heliconius butterflies. Nature 2006, 441, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.R.P.; Chanderkala, L.C.L.; Divya, S.D.S. Ecological significance of wing spot dimorphism in Drosophila biarmipes. Acta Entomol. Sinica 2013, 56, 1267–1274. [Google Scholar]
- Tsuchida, T. Molecular basis and ecological relevance of aphid body colorations. Curr. Opin. Insect Sci. 2016, 17, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bel, Y.; Porcar, M.; Socha, R.; Němec, V.; Ferré, J. Analysis of pteridines in Pyrrhocoris apterus (L.) (Heteroptera, Pyrrhocoridae) during development and in body-color mutants. Arch. Insect Biochem. Physiol. 1997, 34, 83–98. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Williams, B.L.; Selegue, J.E.; Carroll, S.B. Drosophila pigmentation evolution: Divergent genotypes underlying convergent phenotypes. Proc. Natl. Acad. Sci. USA 2003, 100, 1808–1813. [Google Scholar] [CrossRef]
- Théry, M.; Casas, J. The multiple disguises of spiders: Web colour and decorations, body colour and movement. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 471–480. [Google Scholar] [CrossRef]
- Nino, M.C.; Reddivari, L.; Osorio, C.; Kaplan, I.; Liceaga, A.M. Insects as a source of phenolic compounds and potential health benefits. J. Insects Food Feed 2021, 7, 1077–1087. [Google Scholar] [CrossRef]
- Futahashi, R.; Fujiwara, H. Regulation of 20-hydroxyecdysone on the larval pigmentation and the expression of melanin synthesis enzymes and yellow gene of the swallowtail butterfly, Papilio xuthus. Insect Biochem. Mol. Biol. 2007, 37, 855–864. [Google Scholar] [CrossRef]
- Pener, M.P. Locust Phase Polymorphism and Its Endocrine Relations. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 1991; pp. 1–79. [Google Scholar]
- Umbers, K.D.L.; Fabricant, S.A.; Gawryszewski, F.M.; Seago, A.E.; Herberstein, M.E. Reversible colour change in Arthropoda: Arthropod colour change. Biol. Rev. Camb. Philos. Soc. 2014, 89, 820–848. [Google Scholar] [CrossRef]
- Wang, X.; Kang, L. Molecular mechanisms of phase change in locusts. Annu. Rev. Entomol. 2014, 59, 225–244. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Zhang, Y.; Li, Y.; Yu, Y.; Jiang, X.; Pan, W. Insect cytokine growth-blocking peptide may regulate density-dependent phase trait of cuticular melanization in the larval armyworm, Mythimna separata. J. Asia Pac. Entomol. 2020, 23, 498–503. [Google Scholar] [CrossRef]
- Ramniwas, S.; Kajla, B.; Dev, K.; Parkash, R. Direct and correlated responses to laboratory selection for body melanisation in Drosophila melanogaster: Support for the melanisation–desiccation resistance hypothesis. J. Exp. Biol. 2013, 216, 1244–1254. [Google Scholar] [PubMed]
- Yang, M.; Wang, Y.; Liu, Q.; Liu, Z.; Jiang, F.; Wang, H.; Guo, X.; Zhang, J.; Kang, L. A β-carotene-binding protein carrying a red pigment regulates body-color transition between green and black in locusts. eLife 2019, 8, e41362. [Google Scholar] [CrossRef] [PubMed]
- Rassart, M.; Colomer, J.-F.; Tabarrant, T.; Vigneron, J.P. Diffractive hygrochromic effect in the cuticle of the hercules beetle Dynastes hercules. New J. Phys. 2008, 10, 033014. [Google Scholar] [CrossRef]
- Eacock, A.; Rowland, H.M.; Van’t Hof, A.E.; Yung, C.J.; Edmonds, N.; Saccheri, I.J. Adaptive colour change and background choice behaviour in peppered moth caterpillars is mediated by extraocular photoreception. Commun. Biol. 2019, 2, 286. [Google Scholar] [CrossRef]
- Yamasaki, A.; Shimizu, K.; Eujisaki, K. Effect of host plant part on larval body-color polymorphism in Helicoverpa armigera (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 2009, 102, 76–84. [Google Scholar] [CrossRef]
- Li, J.J.; Gong, B.Y.; Wen, Y.C.; Peng, Y.P.; Yang, H.Z.; Wen, L.Z. Influence of light intensity on the body colour of Prodenia litura (Fabricius) larvae based on computer visualization. Chin. J. Appl. 2014, 51, 200–211. [Google Scholar]
- Peng, Y.P.; Wen, L.Z.; Yi, Q.; Hu, L. Quantitative analysis of body coloration change of Prodenia litura (Lepidoptera: Noctuidae) larvae in relation to food color using computer vision technology. Acta Entomol. Sinica 2015, 58, 559–568. [Google Scholar]
- Liao, S.; Lin, H.; Wang, J.; Wang, Q.; Wei, H.; Chen, H. Effects of different Ficus feeding experiences on host preference of Perina nuda larvae (Lepidoptera: Lymantriidae). J. Econ. Entomol. 2024, 117, 209–217. [Google Scholar] [CrossRef]
- Wang, C. Life history of the Perina nuda (Fabricius) and virus production in the infected pupae. Chin. J. Entomol. 1995, 15, 59–68. [Google Scholar]
- Zeng, L.Q.; He, X.Y.; Pan, A.F.; Cai, S.P. Biological properties of Perina nuda in Fuzhou. J. Zhejiang For. Sci. Technol. 2019, 39, 49–54. [Google Scholar]
- Ong, P.Y.; Tan, S.T. Comparison of total phenolic content and antioxidant activities in selected coloured plants. Br. Food J. 2020, 122, 3193–3201. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Shi, L.; Shen, P.; Li, Y. Leaf color formation mechanisms in Alternanthera bettzickiana elucidated by metabolite and transcriptome analyses. Planta 2022, 255, 59. [Google Scholar] [CrossRef]
- Chutipaijit, S.; Cha-Um, S.; Sompornpailin, K. High contents of proline and anthocyanin increase protective response to salinity in Oryza Sativa L. spp. indica. Aust. J. Crop Sci. 2011, 5, 1191–1198. [Google Scholar]
- Farzad, M.; Griesbach, R.; Hammond, J.; Weiss, M.R.; Elmendorf, H.G. Differential expression of three key anthocyanin biosynthetic genes in a color-changing flower, Viola cornuta cv. Yesterday, Today and Tomorrow. Plant Sci. 2003, 165, 1333–1342. [Google Scholar] [CrossRef]
- Zhu, Y.R.; Li, J.X. Liao Involvement of anthocyanins in the resistance to chilling-induced oxidative stress in Saccharum officinarum L. Leaves. Plant Physiol. Biochem. 2013, 73, 427–433. [Google Scholar] [CrossRef]
- Yin, G.; Wang, Y.; Xiao, Y.; Yang, J.; Wang, R.; Jiang, Y.; Huang, R.; Liu, X.; Jiang, Y. Relationships between leaf color changes, pigment levels, enzyme activity, photosynthetic fluorescence characteristics and chloroplast ultrastructure of Liquidambar formosana Hance. J. For. Res. 2022, 33, 1559–1572. [Google Scholar] [CrossRef]
- Favre, N.; Bárcena, A.; Bahima, J.V.; Martínez, G.; Costa, L. Pulses of low intensity light as promising technology to delay postharvest senescence of broccoli. Postharvest Biol. Technol. 2018, 142, 107–114. [Google Scholar] [CrossRef]
- Hapsari, B.W.; Manikharda; Setyaningsih, W. Methodologies in the analysis of phenolic compounds in Roselle (Hibiscus sabdariffa L.): Composition, biological activity, and beneficial effects on human health. Horticulturae 2021, 7, 35. [Google Scholar] [CrossRef]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef] [PubMed]
- Auguy, F.; Abdel-Lateif, K.; Doumas, P.; Badin, P.; Guerin, V.; Bogusz, D.; Hocher, V. Activation of the isoflavonoid pathway in actinorhizal symbioses. Funct. Plant Biol. 2011, 38, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shi, Q.; Niu, L.; Zhang, Y. Transcriptomic analysis of leaf in tree peony reveals differentially expressed pigments genes. Molecules 2017, 22, 324. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zou, Z.; Zhang, X.; Zhou, L.; Wang, Y.; Fang, W.; Zhu, X. Metabolic analyses reveal different mechanisms of leaf color change in two purple-leaf tea plant (Camellia sinensis L.) cultivars. Hortic. Res. 2018, 5, 7. [Google Scholar] [CrossRef]
- Fescemyer, H.W.; Erlandson, C.M. Influence of diet on the density-dependent phase polymorphism of velvetbean caterpillars (Lepidoptera: Noctuidae). Environ. Entomol. 1993, 22, 933–941. [Google Scholar] [CrossRef]
- Grayson, J.; Edmunds, M. The causes of colour and colour change in caterpillars of the poplar and eyed hawkmoths (Laothoe populi and Smerinthus ocellata). Biol. J. Linn. Soc. Lond. 1989, 37, 263–279. [Google Scholar] [CrossRef]
- Gunn, A. The The determination of larval phase coloration in the African armyworm, Spodoptera exempta and its consequences for thermoregulation and protection from UV light. Entomol. Exp. Appl. 1998, 86, 125–133. [Google Scholar] [CrossRef]
- Tojo, S. Variation in phase polymorphism in the common cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 1991, 26, 571–578. [Google Scholar] [CrossRef][Green Version]
- Yoshida, A.; Yabu, S.; Otaki, J.M. The plastic larval body coloration of the pale grass blue butterfly Zizeeria maha (Lepidoptera: Lycaenidae) in response to the host plant color: The maternal effect on Crypsis. Insects 2023, 14, 202. [Google Scholar] [CrossRef]
- Zeuss, D.; Brandl, R.; Brändle, M.; Rahbek, C.; Brunzel, S. Global warming favours light-coloured insects in Europe. Nat. Commun. 2014, 5, 3874. [Google Scholar] [CrossRef]
- Freeman, M. Michael Freeman’s Perfect Exposure: The Professional’s Guide to Capturing Perfect Digital Photographs; Focal Press: Oxford, UK, 2009. [Google Scholar]
- Oetama, V.S.P.; Pentzold, S.; Boland, W. The fate of chlorophyll in phytophagous insects goes beyond nutrition. Z. Naturforsch. C. 2021, 76, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Badgaa, A.; Büchler, R.; Wielsch, N.; Walde, M.; Heintzmann, R.; Pauchet, Y.; Svatos, A.; Ploss, K.; Boland, W. The Green Gut: Chlorophyll degradation in the gut of Spodoptera littoralis. J. Chem. Ecol. 2015, 41, 965–974. [Google Scholar] [CrossRef]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Du, M.-H.; Yan, Z.-W.; Hao, Y.-J.; Yan, Z.-T.; Si, F.-L.; Chen, B.; Qiao, L. Suppression of Laccase 2 severely impairs cuticle tanning and pathogen resistance during the pupal metamorphosis of Anopheles sinensis (Diptera: Culicidae). Parasites Vect. 2017, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jeon, E.J.; Lee, J.; Hwang, H.; Cho, S.-W.; Lee, H. A phenol-amine superglue inspired by insect sclerotization process. Adv. Mater. 2020, 32, e2002118. [Google Scholar] [CrossRef]
- Marais, J.; Deavours, B.; Dixon, R.A.; Ferreira, D. The Science of Flavonoids; Springer: New York, NY, USA, 2006; pp. 1–46. [Google Scholar]
- Oroño, L.; Aluja, M.; Ovruski, S.; Rull, J.; Interdonato, R.; Prado, F.E.; Hilal, M. Dynamics of soluble sugars and secondary metabolites in fruit of Juglans australis attacked by Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae). Arthropod-Plant Interact. 2019, 13, 411–421. [Google Scholar] [CrossRef]
- Britton, S.; Davidowitz, G. The effect of diet on melanin pigmentation in animals. Funct. Ecol. 2023, 37, 206–217. [Google Scholar] [CrossRef]
- Brakefield, P.M. Industrial melanism: Do we have the answers? Trends Ecol. Evol. 1987, 2, 117–122. [Google Scholar] [CrossRef]
- Ankersmit, G.W.; Bell, C.; Dijkman, H.; Mace, N.; Rietstra, S.; Schröder, J.; de Visser, C. Incidence of parasitism by Aphidius rhopalosiphi in colour forms of the aphid Sitobion avenae. Entomol. Exp. Appl. 1986, 40, 223–229. [Google Scholar] [CrossRef]
- Losey, J.E.; Harmon, J.; Ballantyne, F.; Brown, C. A polymorphism maintained by opposite patterns of parasitism and predation. Nature 1997, 388, 269–272. [Google Scholar] [CrossRef]
- Braendle, C.; Weisser, W.W.J. Variation in escape behavior of red and green clones of the pea aphid. J. Insect Behav. 2001, 14, 497–509. [Google Scholar] [CrossRef]
- Saastamoinen, M.; Ikonen, S.; Wong, S.C.; Lehtonen, R.; Hanski, I. Plastic larval development in a butterfly has complex environmental and genetic causes and consequences for population dynamics. J. Anim. Ecol. 2013, 82, 529–539. [Google Scholar] [CrossRef] [PubMed]
- van Oystaeyen, A.; van Zweden, J.S.; Huyghe, H.; Drijfhout, F.; Bonckaert, W.; Wenseleers, T. Chemical strategies of the beetle Metoecus paradoxus, social parasite of the wasp Vespula vulgaris. J. Chem. Ecol. 2015, 41, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.P. Research on Influence of Temperature, Humidity and Food Color on Body Color of Lepidoptera Noctuidae Larvae. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2015. Available online: https://kns.cnki.net/kcms2/article/abstract?v=R2bxtEM5djBYdYyBRgvnvEad1_HmVEY4VAPCjPKC7sfUUf6H8pqH6dYQLvLuDkK14wbTnFp3Ts0QCJVR3B1zgYDv11s-9nhBGpibwEJkE2uewWxal6h3KCYHpCHmXYZpbr3s9-scp-RkDoTh1VgNO2dEhw-4M-_vxqqYCVAtRzd9fFiVv-psFpU15xSJwpHVGdhmHbYbPHo=&uniplatform=NZKPT&language=CHS (accessed on 13 July 2025).

| Source | df | F | p |
|---|---|---|---|
| A | 5 | 3134.899 | <0.001 |
| H | 3 | 20.564 | <0.001 |
| B | 4 | 5990.234 | <0.001 |
| A × H | 15 | 12.027 | <0.001 |
| A × B | 20 | 813.310 | <0.001 |
| H × B | 12 | 13.674 | <0.001 |
| A × H × B | 60 | 8.533 | <0.001 |
| Error | 3390 | — | — |
| Total | 3510 | — | — |
| Corrected total | 3509 | — | — |
| Endogenous Substance | Explain the Amount of Variation | Contribution/% | Pseudo F Statistic | p |
|---|---|---|---|---|
| SS | 1.9 | 3.4 | 0.5 | 0.702 |
| SP | 5.1 | 8.9 | 1.3 | 0.270 |
| TA | 5 | 8.7 | 1.4 | 0.242 |
| FL | 9.1 | 15.9 | 2.1 | 0.086 |
| TP | 2.8 | 5 | 0.8 | 0.574 |
| CA | 8.2 | 14.3 | 2.1 | 0.074 |
| CB | 19 | 33.2 | 4.2 | 0.008 |
| CR | 6 | 10.6 | 1.5 | 0.218 |
| Body Part | Regression Model | VIFmax | F | p | R2 |
|---|---|---|---|---|---|
| Head | YRb = −9.640 + 0.159 Rh + 0.469 Gh − 0.076 Bh | 9.314 | 4.215 | 0.008 | 0.138 |
| YGb = 6.932 + 0.216 Rh + 0.192 Gh − 0.074 Bh | 9.314 | 3.481 | 0.020 | 0.117 | |
| YBb = 12.953 + 0.095 Rh + 0.094 Gh − 0.001 Bh | 9.314 | 2.159 | 0.099 | 0.076 | |
| Dorsal thorax and abdomen | YRb = 84.31 + 0.329 Rh + 0.081 Gh − 0.271 Bh | 9.314 | 0.818 | 0.488 | 0.030 |
| YGb = 88.762 + 0.373 Rh − 0.135 Gh − 0.336 Bh | 9.314 | 0.979 | 0.407 | 0.036 | |
| YBb = 86.007 + 0.414 Rh − 0.365 Gh − 0.218 Bh | 9.314 | 0.905 | 0.442 | 0.033 | |
| Stripe | YRb = −13.727 − 0.426 Rh + 0.649 Gh + 0.051 Bh | 9.314 | 2.091 | 0.108 | 0.074 |
| YGb = −6.752 − 0.396 Rh + 0.558 Gh + 0.028 Bh | 9.314 | 1.539 | 0.211 | 0.055 | |
| YBb = −10.974 − 0.402 Rh + 0.527 Gh + 0.074 Bh | 9.314 | 1.755 | 0.163 | 0.062 | |
| Dorsal mid-line | YRb = 148.010 − 0.060 Rh + 0.052 Gh + 0.100 Bh | 9.314 | 0.155 | 0.951 | 0.004 |
| YGb = 171.122 − 0.009 Rh − 0.358 Gh + 0.050 Bh | 9.314 | 0.875 | 0.458 | 0.032 | |
| YBb = 125.068 − 0.091 Rh − 0.561 Gh + 0.288 Bh | 9.314 | 1.657 | 0.183 | 0.059 | |
| Tail | YRb = 104.703 + 0.575 Rh − 0.627 Gh − 0.181 Bh | 9.314 | 2.092 | 0.108 | 0.074 |
| YGb = 15.556 + 0.150 Rh − 0.094 Gh − 0.004 Bh | 9.314 | 1.076 | 0.364 | 0.039 | |
| YBb = −5.829 − 0.179 Rh + 0.205 Gh + 0.091 Bh | 9.314 | 4.789 | 0.004 | 0.154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.; Mao, X.; Liu, Y.; Luo, G.; Wang, J.; Lin, H.; Tang, M.; Chen, H. Host-Affected Body Coloration Dynamics in Perina nuda Larvae: A Quantitative Analysis of Color Variations and Endogenous Plant Influences. Insects 2025, 16, 728. https://doi.org/10.3390/insects16070728
Liao S, Mao X, Liu Y, Luo G, Wang J, Lin H, Tang M, Chen H. Host-Affected Body Coloration Dynamics in Perina nuda Larvae: A Quantitative Analysis of Color Variations and Endogenous Plant Influences. Insects. 2025; 16(7):728. https://doi.org/10.3390/insects16070728
Chicago/Turabian StyleLiao, Songkai, Xinjie Mao, Yuan Liu, Guihua Luo, Jiajin Wang, Haoyu Lin, Ming Tang, and Hui Chen. 2025. "Host-Affected Body Coloration Dynamics in Perina nuda Larvae: A Quantitative Analysis of Color Variations and Endogenous Plant Influences" Insects 16, no. 7: 728. https://doi.org/10.3390/insects16070728
APA StyleLiao, S., Mao, X., Liu, Y., Luo, G., Wang, J., Lin, H., Tang, M., & Chen, H. (2025). Host-Affected Body Coloration Dynamics in Perina nuda Larvae: A Quantitative Analysis of Color Variations and Endogenous Plant Influences. Insects, 16(7), 728. https://doi.org/10.3390/insects16070728






