Sexual Development of Silba adipata (Diptera: Lonchaeidae): Effects of Diet, Ultraviolet Light and Fig Latex
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Handling of Samples
2.2. Reproductive System of S. adipata
2.3. Effect of Sugar, Protein and Fig Latex Ingestion on Sexual Development
2.4. Effects of Exposure to Ripe Fruit on Female Sexual Development
2.5. Effect of Ultraviolet Exposure on Female Sexual Development
2.6. Male and Female Responses to Fig Latex
2.7. Adult Longevity
2.8. Statistical Analyses
3. Results
3.1. Reproductive System of Silba adipata
3.1.1. Females
3.1.2. Males
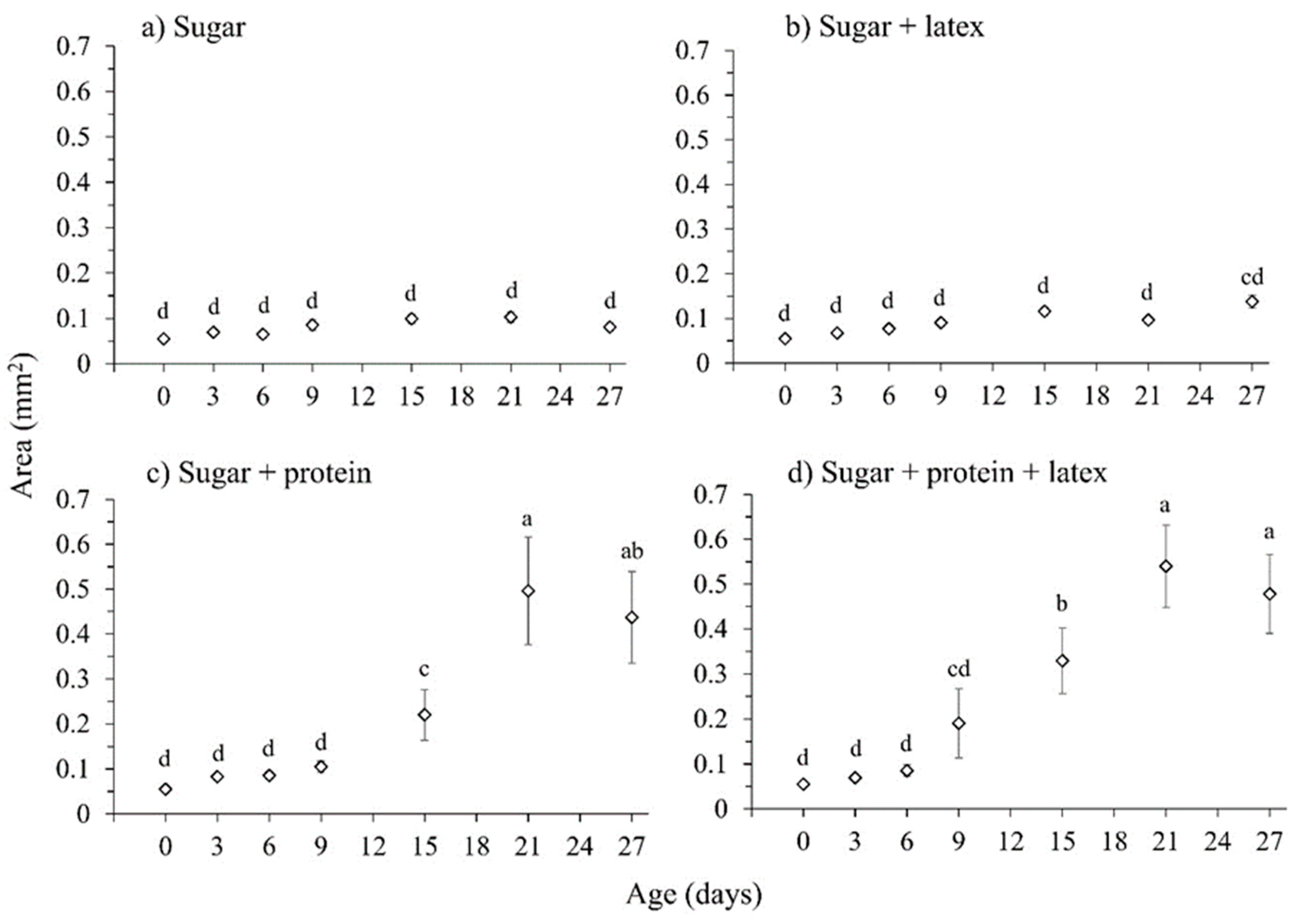
3.2. Effect of Sugar, Protein and Fig Latex Ingestion on Sexual Development
3.2.1. Females
3.2.2. Males
3.3. Effects of Exposure to Ripe Fruit on Female Sexual Development
3.4. Effect of Exposure to Ultraviolet Radiation on Ovary Development
3.5. Male and Female Responses to Fig Latex
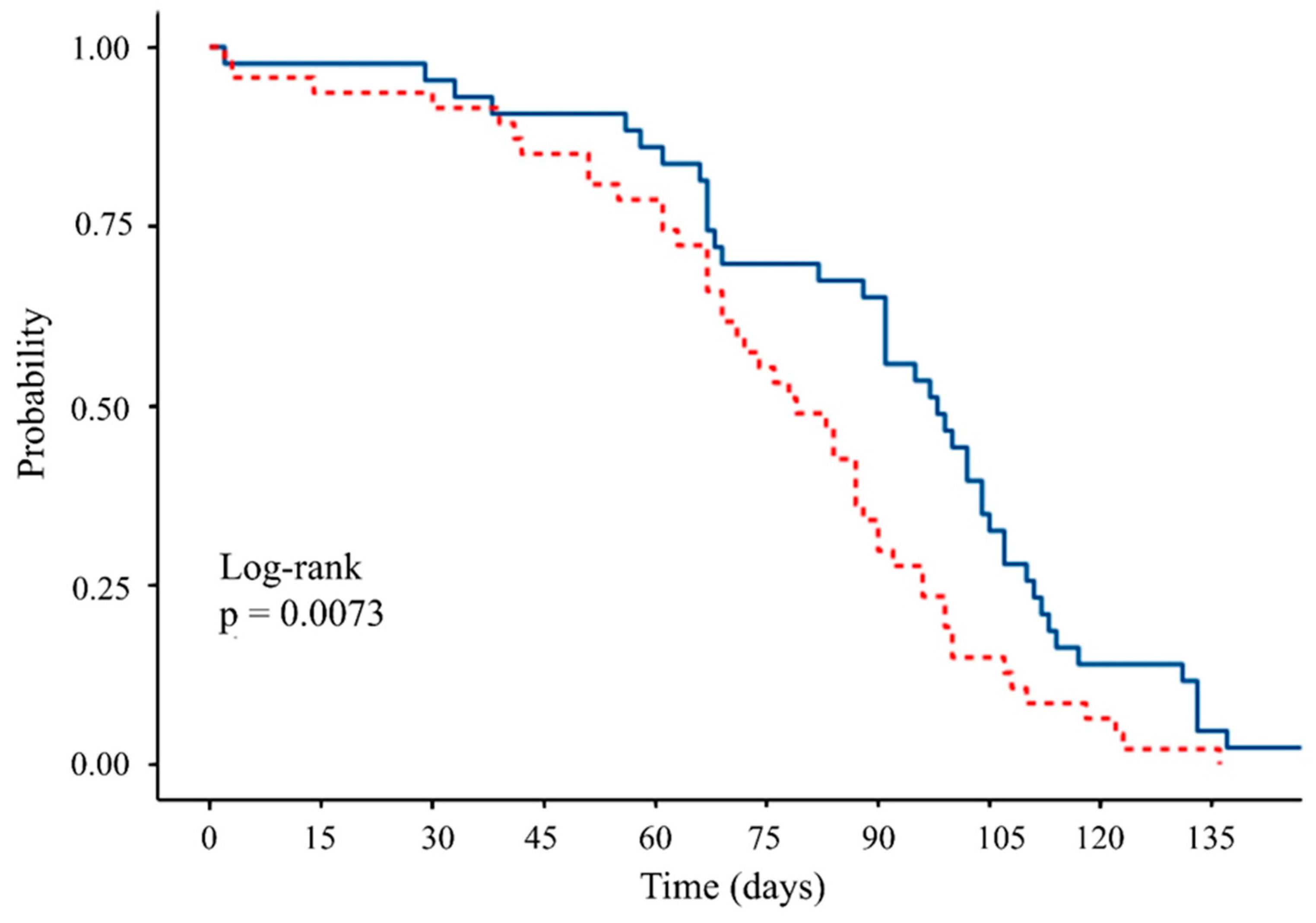
3.6. Longevity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giliomee, J.H.; Venter, E.; Wohlfarter, M. Mediterranean black fig fly, Silba adipata McAlpine (Diptera: Lonchaeidae), recorded from South Africa. Afr. Entomol. 2007, 15, 383–384. [Google Scholar] [CrossRef]
- Britt, K.E.; Gordon, P.E.; Faber, B.A.; Rios, S.I.; Wilson, H. First report of black fig fly, Silba adipata (Diptera: Lonchaeidae), in the United States. J. Integr. Pest Manag. 2022, 13, 12. [Google Scholar] [CrossRef]
- Bautista-Martínez, N.; Meraz-Álvarez, R.; Valdez-Carrasco, J.M.; López-Bautista, E. Black fig fly, Silba adipata McAlpine, in backyards of the State of Mexico. Southwest. Entomol. 2021, 46, 793–796. [Google Scholar] [CrossRef]
- NAPPO, North American Plant Protection Organization. Detection of Black Fig Fly (Silba adipata McAlpine) in the Municipality of Ayala, State of Morelos, 17 March 2020. Available online: https://www.pestalerts.org/nappo/official-pest-reports/926/ (accessed on 1 March 2025).
- SENASICA. Estrategia Operativa Para el Manejo Fitosanitario de la Mosca del Higo Negro, Silba adipata (Diptera: Lonchaeidae). Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. 2021. Available online: https://www.gob.mx/cms/uploads/attachment/file/690628/Estrategia_operativa_mosca_del_higo_2021_compressed.pdf (accessed on 17 October 2024).
- MacGowan, I.; Navarro-de-la-Fuente, L.; Williams, T.; Lasa, R. A new species of Neosilba (Diptera: Lonchaeidae) associated with fig orchards in Mexico. Zootaxa 2025, 5583, 195–200. [Google Scholar] [CrossRef]
- Katsoyannos, B.I. Field observations on the biology and behavior of the black fig fly Silba adipata McAlpine (Diptera: Lonchaeidae), and trapping experiments. Z. Angew. Entomol. 1983, 95, 471–476. [Google Scholar] [CrossRef]
- Silvestri, F. Sulla Lonchaea aristella Beck. (Diptera: Lonchaeidae) dannosa alle infiorescenze e fruttescenze del capririco e del fico. Boll. Lab. Zool. Gen. Agrar. R. Scuola Super. Agric. Portici 1917, 12, 123–146. [Google Scholar]
- Paniagua-Jasso, E.; Tejeda-Reyes, M.A.; Martínez-Castillo, A.M.; Figueroa-de la Rosa, J.I.; García-Banderas, D.V.; Palma-Castillo, L.J.; Illescas-Riquelme, C.P.; Pineda-Guillermo, S. Bioecological parameters of the black fig fly, Silba adipata (Diptera: Lonchaeidae), collected from fig crops in Mexico. Insects 2024, 15, 883. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, F. The Physiology of Insect Reproduction; International Series of Monographs on Pure and Applied Biology: Division, Zoology, 44; University of Minnesota: Minneapolis, MN, USA; Pergamon Press: Oxford, UK, 1970; 307p. [Google Scholar]
- Papaj, D.R. Ovarian dynamics and host use. Annu. Rev. Entomol. 2000, 45, 423–448. [Google Scholar] [CrossRef]
- Aluja, M.; Díaz-Fleischer, F.; Papaj, D.R.; Lagunes, G.; Sivinski, J. Effects of age, diet, female density and host resource on egg load in Anastrepha ludens and Anastrepha obliqua (Diptera: Tephritidae). J. Insect Physiol. 2001, 47, 975–988. [Google Scholar] [CrossRef]
- Mangan, R.L. Adult diet and male-female contact effects on female reproductive potential in Mexican fruit fly (Anastrepha ludens Loew) (Diptera: Tephritidae). J. Econ. Entomol. 2003, 96, 341–347. [Google Scholar] [CrossRef]
- Aluja, M.; Jácome, I.; Macías-Ordoñez, R. Effect of adult nutrition on male sexual performance in four Neotropical fruit fly species of the genus Anastrepha (Diptera: Tephritidae). J. Insect Behav. 2001, 14, 759–775. [Google Scholar] [CrossRef]
- Pérez-Staples, D.; Prabhu, V.; Taylor, P.W. Post-teneral protein feeding enhances sexual performance of Queensland fruit flies. Physiol. Entomol. 2007, 32, 225–232. [Google Scholar] [CrossRef]
- Sivinski, J.; Aluja, M.; Dodson, G.; Freidberg, A.; Headrick, D.; Kaneshiro, K.; Landolt, P. Topics in the evolution of sexual behavior in the Tephritidae. In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behaviour; Aluja, M., Norrbom, A.L., Eds.; CRC: Boca Raton, FL, USA, 2000; pp. 751–792. [Google Scholar]
- Carey, J.R.; Molleman, F. Reproductive aging in tephritid fruit flies. Ann. N. Y. Acad. Sci. 2010, 1204, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Aceves-Aparicio, E.; Pérez-Staples, D.; Arredondo, J.; Corona-Morales, A.; Morales-Mávil, J.; Díaz-Fleischer, F. Combined effects of methoprene and metformin on reproduction, longevity, and stress resistance in Anastrepha ludens (Diptera: Tephritidae): Implications for the sterile insect technique. J. Econ. Entomol. 2021, 114, 142–151. [Google Scholar] [CrossRef]
- Nawade, B.; Shaltiel-Harpaz, L.; Yahyaa, M.; Kabaha, A.; Kedoshim, R.; Bosamia, T.C.; Ibdah, M. Characterization of terpene synthase genes potentially involved in black fig fly (Silba adipata) interactions with Ficus carica. Plant Sci. 2020, 298, 110549. [Google Scholar] [CrossRef]
- Perales-Rosas, D.; Guillén-Sánchez, D.; Valle de la Paz, M.; Hernández-Pérez, R. Biological effectiveness of lures for monitoring Silba adipata in figs in Morelos, Mexico. Southwest. Entomol. 2021, 46, 991–1000. [Google Scholar] [CrossRef]
- Ramos, M.V.; Demarco, D.; da Costa Souza, I.C.; de Freitas, C.D.T. Laticifers, latex, and their role in plant defense. Trends Plant Sci. 2019, 24, 553–567. [Google Scholar] [CrossRef]
- Wink, M. Plant secondary metabolites modulate insect behavior—Steps toward addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Katsoyannos, B.I.; Guerin, P.M. Hexanol: A potent attractant for the black fig fly, Silba adipata. Entomol. Exp. Appl. 1984, 35, 71–74. [Google Scholar] [CrossRef]
- Ben-Yakir, D.; Fereres, A. The effects of UV radiation on arthropods: A review of recent publications (2010–2015). Acta Hortic. 2016, 1134, 335–342. [Google Scholar] [CrossRef]
- Singh, A.; Kishore, K.; Kumar, P.; Khapte, P.S.; Mishra, D.S.; Singh, D.; Kothyari, H.S. Phenological growth and development stages of common fig (Ficus carica L.) under arid climate of India. Folia Hortic. 2023, 35, 395–402. [Google Scholar] [CrossRef]
- McAlpine, D.K. The acalyptrate Diptera with special reference to the Platystomatidae. In Biogeography and Ecology of New Guinea. Monographiae Biologicae; Gressitt, J.L., Ed.; Springer: Dordrecht, The Netherlands, 1982; Volume 42, pp. 659–673. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Pixlr X [Online software]. (2025). Pixlr. Available online: https://pixlr.com (accessed on 2 February 2025).
- Rodriguero, M.S.; Vera, M.T.; Rial, E.; Cayol, J.-P.; Vilardi, J.C. Sexual selection on multivariate phenotype in wild and mass-reared Ceratitis capitata (Diptera: Tephritidae). Heredity 2002, 89, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Jamovi. Jamovi Statistical Software v.2.3.28. 2024. Available online: https://www.jamovi.org (accessed on 15 October 2024).
- Hendrichs, J.; Kenmore, P.; Robinson, A.S.; Vreysen, M.J.B. Area-wide integrated pest management (AW-IPM): Principles, practice and prospects. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 3–33. [Google Scholar]
- Sutter, A.; Price, T.A.R.; Wedell, N. The impact of female mating strategies on the success of insect control technologies. Curr. Opin. Insect Sci. 2021, 45, 75–83. [Google Scholar] [CrossRef]
- Teder, T.; Kaasik, A.; Taits, K.; Tammaru, T. Why do males emerge before females? Sexual size dimorphism drives sexual bimaturism in insects. Biol. Rev. 2021, 96, 2461–2475. [Google Scholar] [CrossRef]
- Landolt, P.J. Reproductive maturation and premating period of the papaya fruit fly, Toxotrypana curvicauda (Diptera: Tephritidae). Fla. Entomol. 1984, 67, 240–244. [Google Scholar] [CrossRef]
- Córdova-García, G.; Navarro-de-la-Fuente, L.; Pérez-Staples, D.; Williams, T.; Lasa, R. Biology and ecology of Delia planipalpis (Stein) (Diptera: Anthomyiidae), an emerging pest of broccoli in Mexico. Insects 2023, 14, 659. [Google Scholar] [CrossRef] [PubMed]
- Raghu, S.; Halcoop, P.; Drew, R.A. Apodeme and ovarian development as predictors of physiological status in Bactrocera cacuminata (Hering) (Diptera: Tephritidae). Aust. J. Entomol. 2003, 42, 281–286. [Google Scholar] [CrossRef]
- McAlpine, J.F.; Munroe, D.D. Swarming of lonchaeid flies and other insects, with description of four new species of Lonchaeidae (Diptera). Can. Entomol. 1968, 100, 1154–1178. [Google Scholar] [CrossRef]
- Yuval, B.; Kaspi, R.; Field, S.A.; Blay, S.; Taylor, P. Effects of post-teneral nutrition on reproductive success of male Mediterranean fruit flies. Fla. Entomol. 2002, 85, 165–170. [Google Scholar] [CrossRef]
- Romanyukha, A.A.; Carey, J.R.; Karkach, A.S.; Yashin, A.I. The impact of diet switching on resource allocation to reproduction and longevity in Mediterranean fruit flies. Proc. B Biol. Sci. 2004, 271, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Meats, A.; Leighton, S.M. Protein consumption by mated, unmated, sterile and fertile adults of the Queensland fruit fly, Bactrocera tryoni, and its relation to egg production. Physiol. Entomol. 2004, 29, 176–182. [Google Scholar] [CrossRef]
- Lahuatte, P.F.; Pérez-Staples, D.; Causton, C.E.; Díaz-Fleischer, F. Influence of larval and adult diets on the maturation of male and female reproductive organs of the avian vampire fly, Philornis downsi (Diptera: Muscidae). Physiol. Entomol. 2024, 49, 328–341. [Google Scholar] [CrossRef]
- Epsky, N.D.; Kendra, P.E.; Schnell, E.Q. History and development of food-based attractants. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T., Epsky, N.D., Jang, E.B., Eds.; Springer: Heidelberg, Germany, 2014; pp. 75–118. [Google Scholar]
- Mazor, M.; Gothilf, S.; Galun, R. The role of ammonia in the attraction of females of the Mediterranean fruit fly to protein hydrolysate baits. Entomol. Exp. Appl. 1987, 43, 25–29. [Google Scholar] [CrossRef]
- Lasa, R.; Williams, T. Addition of ammonium acetate to protein-borax baited traps does not improve attraction of Anastrepha obliqua or Anastrepha serpentina (Diptera: Tephritidae). J. Insect Sci. 2021, 21, 13. [Google Scholar] [CrossRef]
- Hegazy, M.M.; Mekky, R.H.; Afifi, W.M.; Mostafa, A.E.; Abbass, H.S. Composition and biological activities of Ficus carica latex. In Fig (Ficus carica): Production, Processing, and Properties; Ramadan, M.F., Ed.; Springer: Cham, Switzerland, 2023; pp. 597–641. [Google Scholar] [CrossRef]
- Russart, K.L.G.; Nelson, R.J. Artificial light at night alters behavior in laboratory and wild animals. J. Exp. Zool. A Ecol. Integr. Physiol. 2018, 329, 401–408. [Google Scholar] [CrossRef]
- Briscoe, A.D.; Chittka, L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; He, J.; Tomberlin, J.K.; Li, J.; Lei, C.; Sun, M.; Liu, Z.; Yu, Z. An artificial light source influences mating and oviposition of black soldier flies, Hermetia illucens. J. Insect Sci. 2010, 10, 202. [Google Scholar] [CrossRef]
- Weaver, R.E. Effects of simulated moonlight on activity in the desert night snake (Hypsiglena chlorophaea). Northwest Sci. 2011, 85, 497–500. [Google Scholar] [CrossRef]
- Carey, J.R.; Liedo, P.; Müller, H.G.; Wang, J.L.; Vaupel, J.W. Dual modes of aging in Mediterranean fruit fly females. Science 1998, 281, 996–997. [Google Scholar] [CrossRef]
- Salah, M.; Badr, G.; Hetta, H.F.; Khalifa, W.A.; Shoreit, A.A. Fig latex inhibits the growth of pathogenic bacteria invading human diabetic wounds and accelerates wound closure in diabetic mice. Sci. Rep. 2022, 12, 21852. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.; Silva, L.R.; Andrade, P.B.; Valentão, P.; Silva, B.M.; Gonçalves, R.F.; Pereira, J.A.; Guedes de Pinho, P. Further insight into the latex metabolite profile of Ficus carica. J. Agric. Food Chem. 2010, 58, 10855–10863. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Nishida, R.; Jang, E.B.; Shelly, T.E. Pheromones, male lures, and trapping of tephritid fruit flies. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T., Epsky, N., Jang, E., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 15–74. [Google Scholar] [CrossRef]
- Ioannou, C.S.; Papadopoulos, N.T.; Kouloussis, N.A.; Tananaki, C.I.; Katsoyannos, B.I. Essential oils of citrus fruit stimulate oviposition in the Mediterranean fruit fly Ceratitis capitata (Diptera: Tephritidae). Physiol. Entomol. 2012, 37, 330–339. [Google Scholar] [CrossRef]






| Fly Age (Days) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Experiment | 0 d | 3 d | 6 d | 9 d | 15 d | 21 d | 27 d | |
| Diet | Sugar alone | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| (n = 10) | (n = 10) | (n = 9) | (n = 10) | (n = 8) | (n = 9) | (n = 8) | ||
| Sugar + fig latex | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| (n = 10) | (n = 8) | (n = 10) | (n = 11) | (n = 10) | (n = 8) | (n = 11) | ||
| Sugar + hydrolyzed protein | 0% | 0% | 0% | 0% | 8% | 44% | 33% | |
| (n = 10) | (n = 8) | (n = 8) | (n = 11) | (n = 13) | (n = 9) | (n = 12) | ||
| Sugar + hydrolyzed protein + fig latex | 0% | 0% | 0% | 0% | 20% | 46% | 56% | |
| (n = 10) | (n = 8) | (n = 10) | (n = 10) | (n = 15) | (n = 13) | (n = 9) | ||
| Host | Fig host (sugar + hydrolyzed protein) | 0% | 0% | 50% | 20% | |||
| (n = 10) | (n = 8) | (n = 10) | (n = 10) | |||||
| Without fig host (sugar + hydrolyzed protein) | 0% | 0% | 40% | 0% | ||||
| (n = 10) | (n = 10) | (n = 10) | (n = 10) | |||||
| UV | UV light (sugar + hydrolyzed protein) | 0% | 20% | 20% | ||||
| (n = 10) | (n = 10) | (n = 10) | ||||||
| Without UV light (sugar + hydrolyzed protein) | 0% | 12.5% | 50% | |||||
| (n = 10) | (n = 8) | (n = 10) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-del-Castillo, R.; Córdova-García, G.; Pérez-Staples, D.; Birke, A.; Williams, T.; Lasa, R. Sexual Development of Silba adipata (Diptera: Lonchaeidae): Effects of Diet, Ultraviolet Light and Fig Latex. Insects 2025, 16, 495. https://doi.org/10.3390/insects16050495
Díaz-del-Castillo R, Córdova-García G, Pérez-Staples D, Birke A, Williams T, Lasa R. Sexual Development of Silba adipata (Diptera: Lonchaeidae): Effects of Diet, Ultraviolet Light and Fig Latex. Insects. 2025; 16(5):495. https://doi.org/10.3390/insects16050495
Chicago/Turabian StyleDíaz-del-Castillo, Ricardo, Guadalupe Córdova-García, Diana Pérez-Staples, Andrea Birke, Trevor Williams, and Rodrigo Lasa. 2025. "Sexual Development of Silba adipata (Diptera: Lonchaeidae): Effects of Diet, Ultraviolet Light and Fig Latex" Insects 16, no. 5: 495. https://doi.org/10.3390/insects16050495
APA StyleDíaz-del-Castillo, R., Córdova-García, G., Pérez-Staples, D., Birke, A., Williams, T., & Lasa, R. (2025). Sexual Development of Silba adipata (Diptera: Lonchaeidae): Effects of Diet, Ultraviolet Light and Fig Latex. Insects, 16(5), 495. https://doi.org/10.3390/insects16050495






