Imidacloprid Resistance Challenges in Brazilian Strains of Drosophila suzukii (Diptera: Drosophilidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
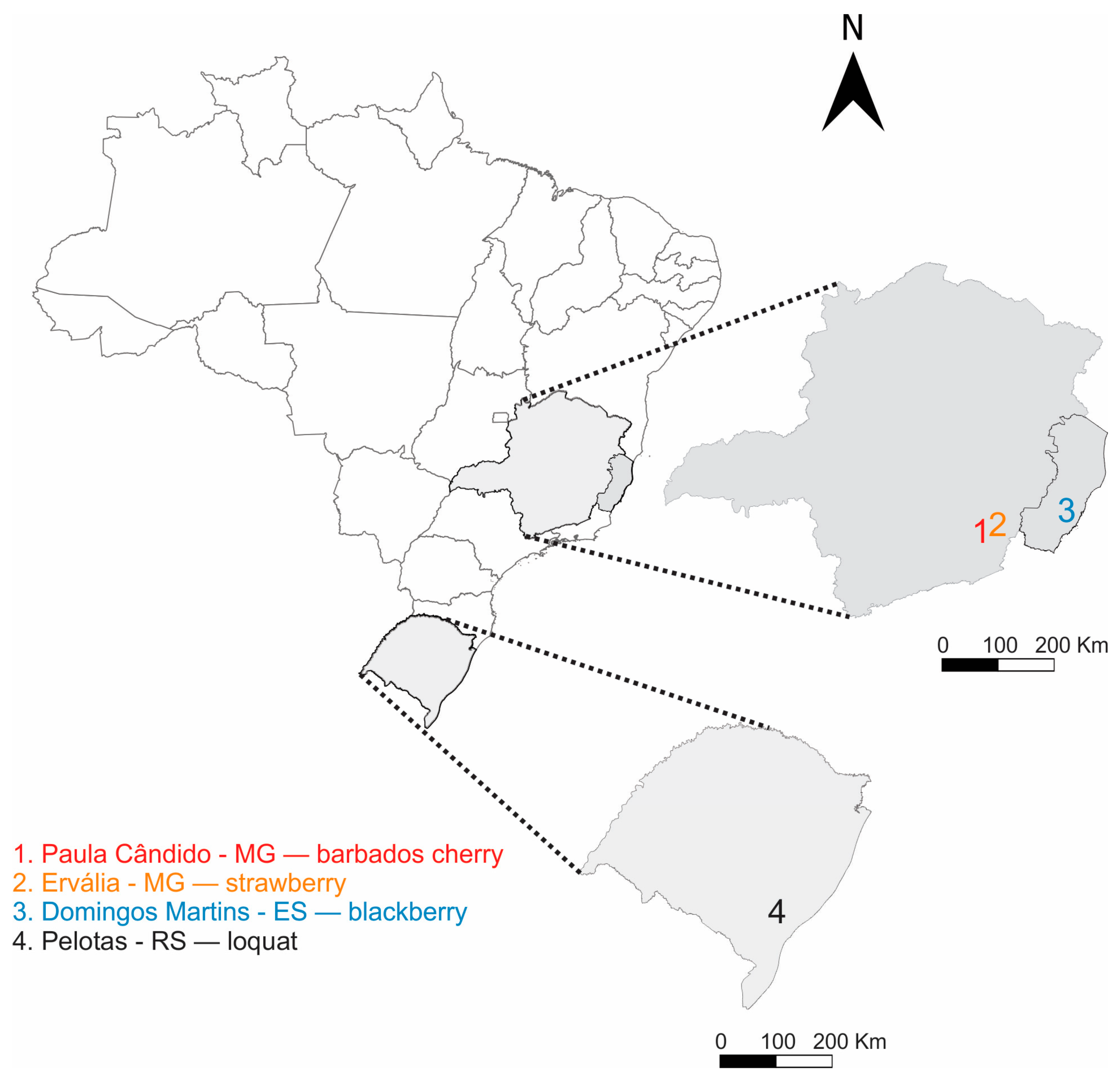
2.1. Insect Rearing and Chemicals
2.2. Toxicity Exposure Protocol
2.3. Insecticide Susceptibility and Resistance Bioassays
2.4. Bioassays with Synergized Imidacloprid in Paula Candido Population
2.5. Data Analysis
3. Results
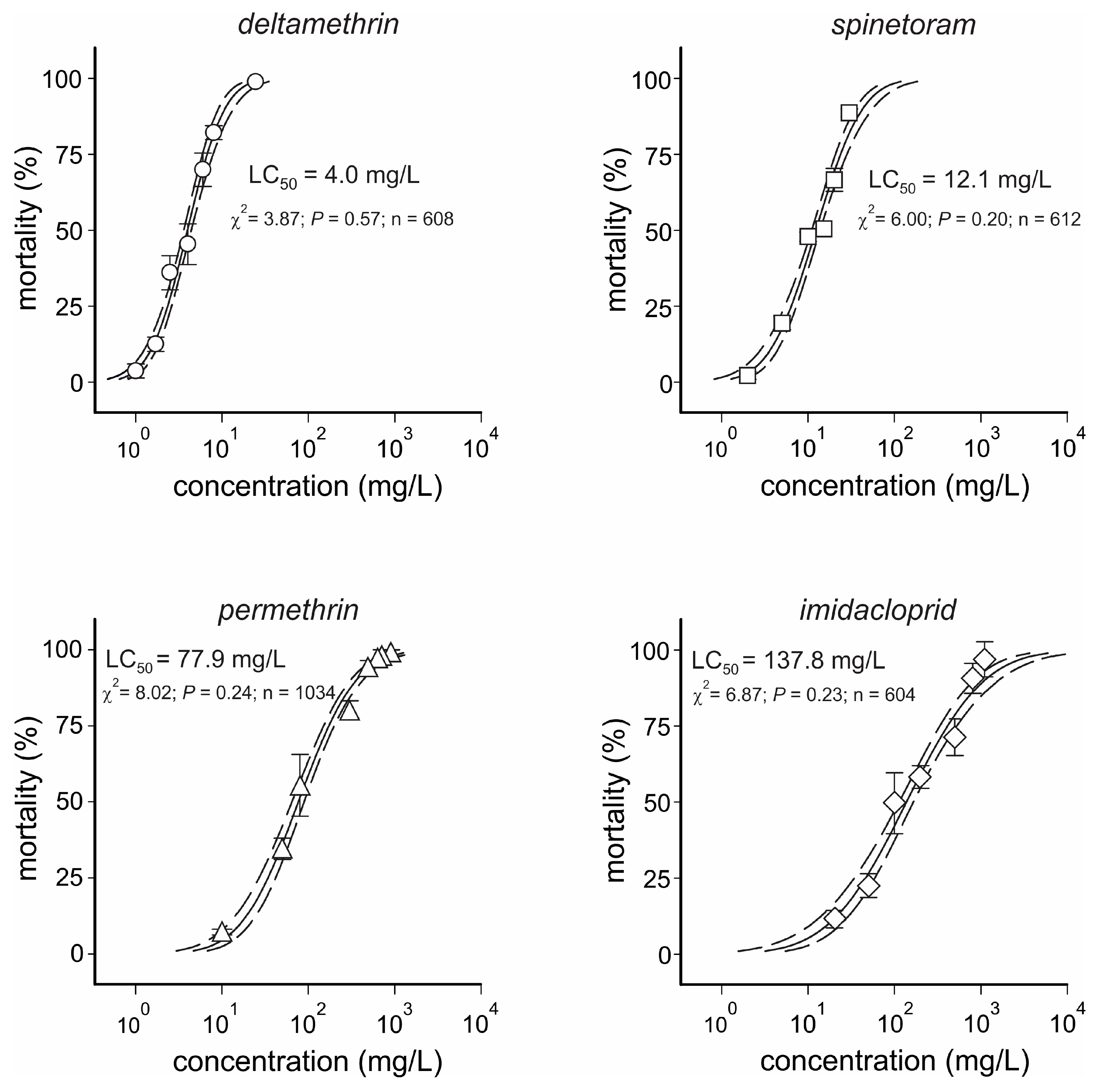
3.1. Concentration–Response Bioassay Data
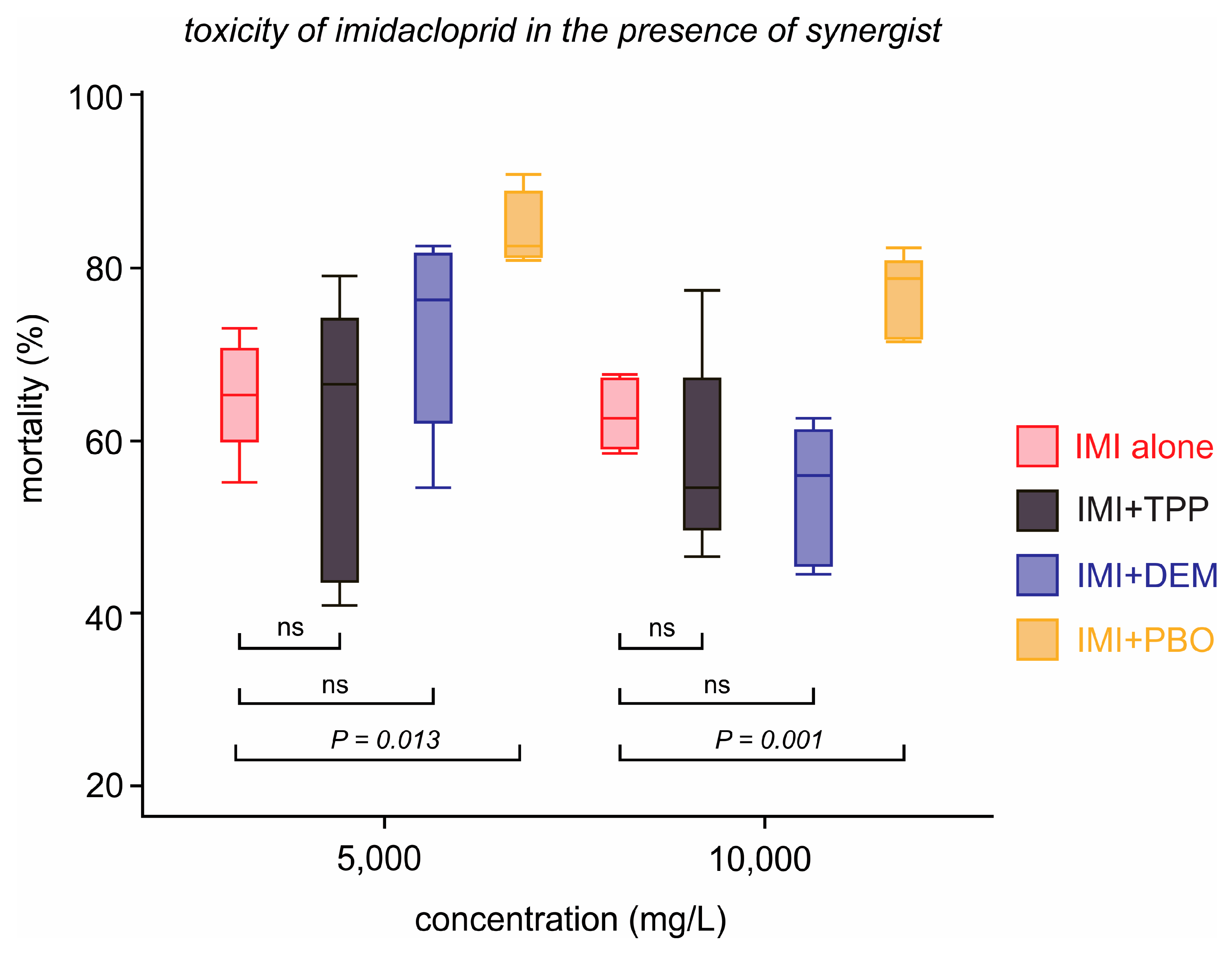
3.2. Synergism Bioassay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emiljanowicz, L.M.; Ryan, G.D.; Langille, A.; Newman, J. Development, Reproductive Output and Population Growth of the Fruit Fly Pest Drosophila suzukii (Diptera: Drosophilidae) on Artificial Diet. J. Econ. Entomol. 2014, 107, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Tochen, S.; Dalton, D.T.; Wiman, N.; Hamm, C.; Shearer, P.W.; Walton, V.M. Temperature-Related Development and Population Parameters for Drosophila suzukii (Diptera: Drosophilidae) on Cherry and Blueberry. Environ. Entomol. 2014, 43, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Little, C.M.; Chapman, T.W.; Hillier, N.K. Plasticity Is Key to Success of Drosophila suzukii (Diptera: Drosophilidae) Invasion. J. Insect Sci. 2020, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Buck, N.; Fountain, M.T.; Potts, S.G.; Bishop, J.; Garratt, M.P.D. The Effects of Non-crop Habitat on Spotted Wing Drosophila (Drosophila suzukii) Abundance in Fruit Systems: A Meta-analysis. Agric. For. Entomol. 2023, 25, 66–76. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Lasa, R.; Funes, C.F.; Buzzetti, K. Drosophila suzukii Management in Latin America: Current Status and Perspectives. J. Econ. Entomol. 2022, 115, 1008–1023. [Google Scholar] [CrossRef]
- Morales, K.B. The Spotted Wing Drosophila in the South of the World: Chilean Case and Its First Productive Impacts. In Invasive Species; El-Shafie, H., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Benito, N.P.; Lopes-da-Silva, M.; Santos, R.S.S.D. Potential spread and economic impact of invasive Drosophila suzukii in Brazil. Pesqui. Agropecuária Bras. 2016, 51, 571–578. [Google Scholar] [CrossRef]
- Cichon, L.; Araque, L.; Garrido, S.; Lago, J.; Cuello, N. Insecticidas Con Posibilidad de Registro En Cereza En Argentina y Sus Implicancias Para La Exportación de Frutas Frescas. Rev. Investig. Agropecu. 2019, 45, 285–291. [Google Scholar]
- Andreazza, F.; Vacacela Ajila, H.E.; Haddi, K.; Colares, F.; Pallini, A.; Oliveira, E.E. Toxicity to and Egg-laying Avoidance of Drosophila suzukii (Diptera: Drosophilidae) Caused by an Old Alternative Inorganic Insecticide Preparation. Pest Manag. Sci. 2018, 74, 861–867. [Google Scholar] [CrossRef]
- Kalfas, I.; De Ketelaere, B.; Beliën, T.; Saeys, W. Optical Identification of Fruitfly Species Based on Their Wingbeats Using Convolutional Neural Networks. Front. Plant Sci. 2022, 13, 812506. [Google Scholar] [CrossRef]
- Rehermann, G.; Spitaler, U.; Sahle, K.; Cossu, C.S.; Donne, L.D.; Bianchi, F.; Eisenstecken, D.; Angeli, S.; Schmidt, S.; Becher, P.G. Behavioral Manipulation of Drosophila suzukii for Pest Control: High Attraction to Yeast Enhances Insecticide Efficacy When Applied on Leaves. Pest Manag. Sci. 2022, 78, 896–904. [Google Scholar] [CrossRef]
- Haye, T.; Girod, P.; Cuthbertson, A.G.S.; Wang, X.G.; Daane, K.M.; Hoelmer, K.A.; Baroffio, C.; Zhang, J.P.; Desneux, N. Current SWD IPM Tactics and Their Practical Implementation in Fruit Crops across Different Regions around the World. J. Pest Sci. 2016, 89, 643–651. [Google Scholar] [CrossRef]
- Shawer, R.; Tonina, L.; Tirello, P.; Duso, C.; Mori, N. Laboratory and Field Trials to Identify Effective Chemical Control Strategies for Integrated Management of Drosophila suzukii in European Cherry Orchards. Crop Prot. 2018, 103, 73–80. [Google Scholar] [CrossRef]
- Disi, J.O.; Sial, A.A. Laboratory Selection and Assessment of Resistance Risk in Drosophila suzukii (Diptera: Drosophilidae) to Spinosad and Malathion. Insects 2021, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Deans, C.; Hutchison, W.D. Propensity for Resistance Development in the Invasive Berry Pest, spotted-wing Drosophila (Drosophila suzukii), under Laboratory Selection. Pest Manag. Sci. 2022, 78, 5203–5212. [Google Scholar] [CrossRef]
- Ganjisaffar, F.; Demkovich, M.R.; Chiu, J.C.; Zalom, F.G. Characterization of Field-Derived Drosophila suzukii (Diptera: Drosophilidae) Resistance to Pyrethroids in California Berry Production. J. Econ. Entomol. 2022, 115, 1676–1684. [Google Scholar] [CrossRef]
- Gress, B.E.; Zalom, F.G. Identification and Risk Assessment of Spinosad Resistance in a California Population of Drosophila suzukii. Pest Manag. Sci. 2019, 75, 1270–1276. [Google Scholar] [CrossRef]
- Krüger, A.P.; Scheunemann, T.; Padilha, A.C.; Pazini, J.B.; Bernardi, D.; Grützmacher, A.D.; Nava, D.E.; Garcia, F.R.M. Insecticide-Mediated Effects on Mating Success and Reproductive Output of Drosophila suzukii. Ecotoxicology 2021, 30, 828–835. [Google Scholar] [CrossRef]
- Schlesener, D.C.H.; Wollmann, J.; Pazini, J.d.B.; Padilha, A.C.; Grützmacher, A.D.; Garcia, F.R.M. Insecticide Toxicity to Drosophila suzukii (Diptera: Drosophilidae) Parasitoids: Trichopria anastrephae (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae). J. Econ. Entomol. 2019, 112, 1197–1206. [Google Scholar] [CrossRef]
- Lisi, F.; Mansour, R.; Cavallaro, C.; Alınç, T.; Porcu, E.; Ricupero, M.; Zappalà, L.; Desneux, N.; Biondi, A. Sublethal Effects of Nine Insecticides on Drosophila suzukii and Its Major Pupal Parasitoid Trichopria drosophilae. Pest Manag. Sci. 2023, 79, 5003–5014. [Google Scholar] [CrossRef]
- Van Timmeren, S.; Mota-Sanchez, D.; Wise, J.C.; Isaacs, R. Baseline Susceptibility of Spotted Wing Drosophila (Drosophila suzukii) to Four Key Insecticide Classes. Pest Manag. Sci. 2018, 74, 78–87. [Google Scholar] [CrossRef]
- Mishra, R.; Chiu, J.C.; Hua, G.; Tawari, N.R.; Adang, M.J.; Sial, A.A. High Throughput Sequencing Reveals Drosophila suzukii Responses to Insecticides. Insect Sci. 2018, 25, 928–945. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, N.R. Investigaton of the State of Insecticide Resistance in Georgia Populations of Drosophila suzukii. Master’s Thesis, University of Georgia, Boca Raton, FL, USA, 2020. [Google Scholar]
- Morais, M.C.; Rakes, M.; Padilha, A.C.; Grützmacher, A.D.; Nava, D.E.; Bernardi, O.; Bernardi, D. Susceptibility of Brazilian Populations of Anastrepha fraterculus, Ceratitis capitata (Diptera: Tephritidae), and Drosophila suzukii (Diptera: Drosophilidae) to Selected Insecticides. J. Econ. Entomol. 2021, 114, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Zanuncio-Junior, J.S.; Fornazier, M.J.; Andreazza, F.; Culik, M.P.; Mendonça, L.d.P.; Oliveira, E.E.; dos S. Martins, D.; Fornazier, M.L.; Costa, H.; Ventura, J.A. Spread of Two Invasive Flies (Diptera: Drosophilidae) Infesting Commercial Fruits in Southeastern Brazil. Fla. Entomol. 2018, 101, 522–525. [Google Scholar] [CrossRef]
- Mendonça, L.P.; Haddi, K.; Godoy, W.A.C. Effects of Co-Occurrence and Intra- and Interspecific Interactions between Drosophila suzukii and Zaprionus indianus. PLoS ONE 2023, 18, e0281806. [Google Scholar] [CrossRef]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; De Toni, D.C.; Valente, V.L.S. The First Records of the Invasive Pest Drosophila suzukii in the South American Continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- Ferronato, P.; Woch, A.L.; Soares, P.L.; Bernardi, D.; Botton, M.; Andreazza, F.; Oliveira, E.E.; Corrêa, A.S. A Phylogeographic Approach to the Drosophila suzukii (Diptera: Drosophilidae) Invasion in Brazil. J. Econ. Entomol. 2019, 112, 425–433. [Google Scholar] [CrossRef]
- Mendonca, L.P.; Oliveira, E.E.; Andreazza, F.; Rezende, S.M.; Faroni, L.R.D.; Guedes, R.N.C.; Haddi, K. Host Potential and Adaptive Responses of Drosophila suzukii (Diptera: Drosophilidae) to Barbados Cherries. J. Econ. Entomol. 2019, 112, 3002–3006. [Google Scholar] [CrossRef]
- AGROFIT. Sistema de Agrotóxicos Fitossanitários—MAPA/CGAF/DFIA/DAS; Ministério da Agricultura Pecuária e Abastecimento: Brasilia, Brazil, 2025. Available online: http://extranet.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 20 January 2025).
- Leite, S.A.; Guedes, R.N.C.; da Costa, D.R.; Colmenarez, Y.C.; Matsumoto, S.N.; dos Santos, M.P.; Coelho, B.S.; Moreira, A.A.; Castellani, M.A. The Effects of Thiamethoxam on Coffee Seedling Morphophysiology and Neotropical Leaf Miner ( Leucoptera coffeella ) Infestations. Pest Manag. Sci. 2022, 78, 2581–2587. [Google Scholar] [CrossRef]
- Zambolim, L. Current Status and Management of Coffee Leaf Rust in Brazil. Trop. Plant Pathol. 2016, 41, 1–8. [Google Scholar] [CrossRef]
- Raga, A.; De Oliveira, D.A.; Souza Filho, M.F.; Sato, M.E.; Siloto, R.C.; Zucchi, R.A.; Cássio, R. Occurrence of Fruit Flies in Coffee Varieties in the State of São Paulo, Brazil. Bol. Sanid. Veg. Y Plagas 2002, 28, 519–524. [Google Scholar]
- Oliveira, I.; Uchoa, M.A.; Fernandes, M.G.; Vieira, C.R.Y.I.; Faccenda, O.; Oliveira, I.S.T. Antixenosis of the Triozid, Triozoida limbata (Hemiptera: Triozidae), to Some Cultivars of Psidium guajava (Myrtaceae) in the Field. Fla. Entomol. 2020, 102, 695. [Google Scholar] [CrossRef]
- Colombi, C.A.; Galli, J.C. Dinâmica Populacional e Evolução de Dano de Triozoida limbata (Hemiptera: Psillydae) Em Goiabeira, Em Jaboticabal, SP. Ciênc. E Agrotecnol. 2009, 33, 412–416. [Google Scholar] [CrossRef]
- Andreazza, F.; Haddi, K.; Oliveira, E.E.; Ferreira, J.A.M. Drosophila suzukii (Diptera: Drosophilidae) Arrives at Minas Gerais State, a Main Strawberry Production Region in Brazil. Fla. Entomol. 2016, 99, 796–798. [Google Scholar] [CrossRef]
- Insecticide Resistance Action Committee. Insecticide Resistance Action Committee. In Proceedings of the 46th Meeting of IRAC International, Brussels, Belgium, 30 March 2011. [Google Scholar]
- SAS Institute Statistical Analysis System: Getting Started with the SAS Learning; SAS 9.4 Statistical Software Platform; SAS Institute: Cary, NC, USA, 2013.
- Robertson, J.L.; Savin, N.E.; Savin, N.E.; Preisler, H.K. Bioassays with Arthropods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780429119682. [Google Scholar]
- Roush, R.T.; Miller, G.L. Considerations for Design of Insecticide Resistance Monitoring Programs. J. Econ. Entomol. 1986, 79, 293–298. [Google Scholar] [CrossRef]
- Oliveira, E.E.; Pippow, A.; Salgado, V.L.; Büschges, A.; Schmidt, J.; Kloppenburg, P. Cholinergic Currents in Leg Motoneurons of Carausius morosus. J. Neurophysiol. 2010, 103, 2770–2782. [Google Scholar] [CrossRef]
- Oliveira, E.E.; Schleicher, S.; Büschges, A.; Schmidt, J.; Kloppenburg, P.; Salgado, V.L. Desensitization of Nicotinic Acetylcholine Receptors in Central Nervous System Neurons of the Stick Insect (Carausius morosus) by Imidacloprid and Sulfoximine Insecticides. Insect Biochem. Mol. Biol. 2011, 41, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Salgado, V.L. Selective Actions of Insecticides on Desensitizing and Non-Desensitizing Nicotinic Acetylcholine Receptors in Cockroach (Periplaneta Americana) Neurons. Pest Manag. Sci. 2021, 77, 3663–3672. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D.; et al. Insecticides, Biologics and Nematicides: Updates to IRAC’s Mode of Action Classification—A Tool for Resistance Management. Pestic. Biochem. Physiol. 2020, 167, 104587. [Google Scholar] [CrossRef]
- Tang, B.; Cheng, Y.; Li, Y.; Li, W.; Ma, Y.; Zhou, Q.; Lu, K. Adipokinetic Hormone Regulates Cytochrome P450-Mediated Imidacloprid Resistance in the Brown Planthopper, Nilaparvata lugens. Chemosphere 2020, 259, 127490. [Google Scholar] [CrossRef]
- Barman, M.; Samanta, S.; Upadhyaya, G.; Thakur, H.; Chakraborty, S.; Samanta, A.; Tarafdar, J. Unraveling the Basis of Neonicotinoid Resistance in Whitefly Species Complex: Role of Endosymbiotic Bacteria and Insecticide Resistance Genes. Front. Microbiol. 2022, 13, 901793. [Google Scholar] [CrossRef]
- Liang, J.; Yang, J.; Hu, J.; Fu, B.; Gong, P.; Du, T.; Xue, H.; Wei, X.; Liu, S.; Huang, M.; et al. Cytpchrome P450 CYP4G68 Is Associated with Imidacloprid and Thiamethoxam Resistance in Field Whitefly, Bemisia tabaci (Hemiptera: Gennadius). Agriculture 2022, 12, 473. [Google Scholar] [CrossRef]
- Smirle, M.J.; Zurowski, C.L.; Ayyanath, M.-M.; Scott, I.M.; MacKenzie, K.E. Laboratory Studies of Insecticide Efficacy and Resistance in Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) Populations from British Columbia, Canada. Pest Manag. Sci. 2017, 73, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Roditakis, E.; Vasakis, E.; Grispou, M.; Stavrakaki, M.; Nauen, R.; Gravouil, M.; Bassi, A. First Report of Tuta absoluta Resistance to Diamide Insecticides. J. Pest Sci. 2015, 88, 9–16. [Google Scholar] [CrossRef]
- Kaplanoglu, E.; Chapman, P.; Scott, I.M.; Donly, C. Overexpression of a Cytochrome P450 and a UDP-Glycosyltransferase Is Associated with Imidacloprid Resistance in the Colorado Potato Beetle, Leptinotarsa decemlineata. Sci. Rep. 2017, 7, 1762. [Google Scholar] [CrossRef]
- Pittendrigh, B.; Reenan, R.; ffrench-Constant, R.H.; Ganetzky, B. Point Mutations in the Drosophila Sodium Channel Gene Para Associated with Resistance to DDT and Pyrethroid Insecticides. Mol. Gen. Genet. 1997, 256, 602–610. [Google Scholar] [CrossRef]
- Perry, T.; Heckel, D.G.; McKenzie, J.A.; Batterham, P. Mutations in Dα1 or Dβ2 Nicotinic Acetylcholine Receptor Subunits Can Confer Resistance to Neonicotinoids in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2008, 38, 520–528. [Google Scholar] [CrossRef]
- Watson, G.B.; Chouinard, S.W.; Cook, K.R.; Geng, C.; Gifford, J.M.; Gustafson, G.D.; Hasler, J.M.; Larrinua, I.M.; Letherer, T.J.; Mitchell, J.C.; et al. A Spinosyn-Sensitive Drosophila melanogaster Nicotinic Acetylcholine Receptor Identified through Chemically Induced Target Site Resistance, Resistance Gene Identification, and Heterologous Expression. Insect Biochem. Mol. Biol. 2010, 40, 376–384. [Google Scholar] [CrossRef]
- Daborn, P.; Boundy, S.; Yen, J.; Pittendrigh, B.; ffrench-Constant, R. DDT Resistance in Drosophila Correlates with Cyp6g1 over-Expression and Confers Cross-Resistance to the Neonicotinoid Imidacloprid. Mol. Genet. Genom. 2001, 266, 556–563. [Google Scholar] [CrossRef]
- Bogwitz, M.R.; Chung, H.; Magoc, L.; Rigby, S.; Wong, W.; O’Keefe, M.; McKenzie, J.A.; Batterham, P.; Daborn, P.J. Cyp12a4 Confers Lufenuron Resistance in a Natural Population of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2005, 102, 12807–12812. [Google Scholar] [CrossRef]
- Van Timmeren, S.; Sial, A.A.; Lanka, S.K.; Spaulding, N.R.; Isaacs, R. Development of a Rapid Assessment Method for Detecting Insecticide Resistance in Spotted Wing Drosophila (Drosophila suzukii Matsumura). Pest Manag. Sci. 2019, 75, 1782–1793. [Google Scholar] [CrossRef]
- Mantilla Afanador, J.G.; Araujo, S.H.C.; Teixeira, M.G.; Lopes, D.T.; Cerceau, C.I.; Andreazza, F.; Oliveira, D.C.; Bernardi, D.; Moura, W.S.; Aguiar, R.W.S.; et al. Novel Lactone-Based Insecticides and Drosophila suzukii Management: Synthesis, Potential Action Mechanisms and Selectivity for Non-Target Parasitoids. Insects 2023, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Bruck, D.J.; Bolda, M.; Tanigoshi, L.; Klick, J.; Kleiber, J.; DeFrancesco, J.; Gerdeman, B.; Spitler, H. Laboratory and Field Comparisons of Insecticides to Reduce Infestation of Drosophila suzukii in Berry Crops. Pest. Manag. Sci 2011, 67, 1375–1385. [Google Scholar] [CrossRef]
- Van Timmeren, S.; Isaacs, R. Control of Spotted Wing Drosophila, Drosophila suzukii, by Specific Insecticides and by Conventional and Organic Crop Protection Programs. Crop Protection 2013, 54, 126–133. [Google Scholar] [CrossRef]
- Main, A.R.; Webb, E.B.; Goyne, K.W.; Mengel, D. Neonicotinoid Insecticides Negatively Affect Performance Measures of Non-target Terrestrial Arthropods: A Meta-analysis. Ecol. Appl. 2018, 28, 1232–1244. [Google Scholar] [CrossRef]
- Andreazza, F.; Bernardi, D.; Baronio, C.A.; Pasinato, J.; Nava, D.E.; Botton, M. Toxicities and Effects of Insecticidal Toxic Baits to Control Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae). Pest Manag. Sci. 2017, 73, 146–152. [Google Scholar] [CrossRef]
- Andreazza, F.; Bernardi, D.; dos Santos, R.S.S.; Garcia, F.R.M.; Oliveira, E.E.; Botton, M.; Nava, D.E. Drosophila suzukii in Southern Neotropical Region: Current Status and Future Perspectives. Neotrop. Entomol. 2017, 46, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion Biology of Spotted Wing Drosophila (Drosophila suzukii): A Global Perspective and Future Priorities. J. Pest. Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of Action Classification and Insecticide Resistance Management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Santos, V.F.; Abeijon, L.M.; Cruz Araújo, S.H.; Garcia, F.R.M.; Oliveira, E.E. The Potential of Plant-Based Biorational Products for the Drosophila suzukii Control: Current Status, Opportunities, and Limitations. Neotrop. Entomol. 2024, 53, 236–243. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Oliveira, E.E. Potential Ecological Interactions and Challenges for the Management of Spotted-Wing Drosophila in Recently Invaded Regions. Neotrop. Entomol. 2024, 53, 186–188. [Google Scholar] [CrossRef]
- Isaacs, R.; Van Timmeren, S.; Gress, B.E.; Zalom, F.G.; Ganjisaffar, F.; Hamby, K.A.; Lewis, M.T.; Liburd, O.E.; Sarkar, N.; Rodriguez-Saona, C.; et al. Monitoring of Spotted-Wing Drosophila (Diptera: Drosophilidae) Resistance Status Using a RAPID Method for Assessing Insecticide Sensitivity Across the United States. J. Econ. Entomol. 2022, 115, 1046–1053. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreazza, F.; Garcia, F.R.M.; Silva, P.B.d.; Barbosa, L.B.; de Oliveira, J.M.; Araújo, G.N.; Oliveira, E.E. Imidacloprid Resistance Challenges in Brazilian Strains of Drosophila suzukii (Diptera: Drosophilidae). Insects 2025, 16, 494. https://doi.org/10.3390/insects16050494
Andreazza F, Garcia FRM, Silva PBd, Barbosa LB, de Oliveira JM, Araújo GN, Oliveira EE. Imidacloprid Resistance Challenges in Brazilian Strains of Drosophila suzukii (Diptera: Drosophilidae). Insects. 2025; 16(5):494. https://doi.org/10.3390/insects16050494
Chicago/Turabian StyleAndreazza, Felipe, Flávio Roberto Mello Garcia, Pedro Bento da Silva, Lucas Bretas Barbosa, Joel Marques de Oliveira, Gabriel Netto Araújo, and Eugenio E. Oliveira. 2025. "Imidacloprid Resistance Challenges in Brazilian Strains of Drosophila suzukii (Diptera: Drosophilidae)" Insects 16, no. 5: 494. https://doi.org/10.3390/insects16050494
APA StyleAndreazza, F., Garcia, F. R. M., Silva, P. B. d., Barbosa, L. B., de Oliveira, J. M., Araújo, G. N., & Oliveira, E. E. (2025). Imidacloprid Resistance Challenges in Brazilian Strains of Drosophila suzukii (Diptera: Drosophilidae). Insects, 16(5), 494. https://doi.org/10.3390/insects16050494








