Overwintering Capacity of the Mediterranean Fruit Fly in the Dalmatia Region of Croatia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects Collection and Handling
Fruits Handling and Larvae Collection for Adult and Pupae Overwintering Tests
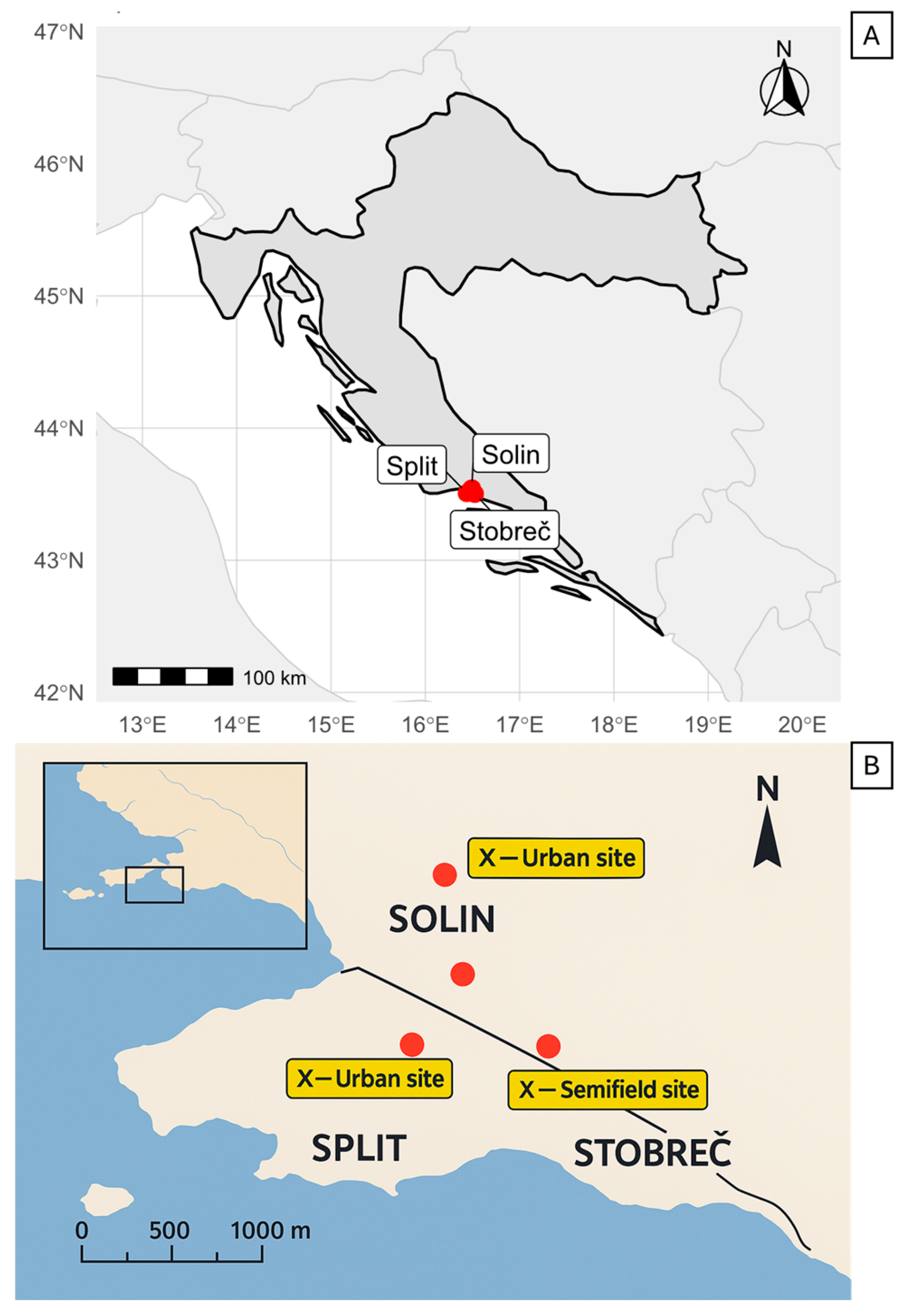
2.2. Overwintering Sites
2.2.1. Outdoor Conditions (Open-Field Conditions)
2.2.2. Storehouse in Field (Semi-Field Conditions)
2.2.3. Basement of a Building (Urban Conditions)
2.3. Experimental Design
2.3.1. Adults Overwintering Trials
2.3.2. Pupae Overwintering Trials
2.4. Statistics
3. Results
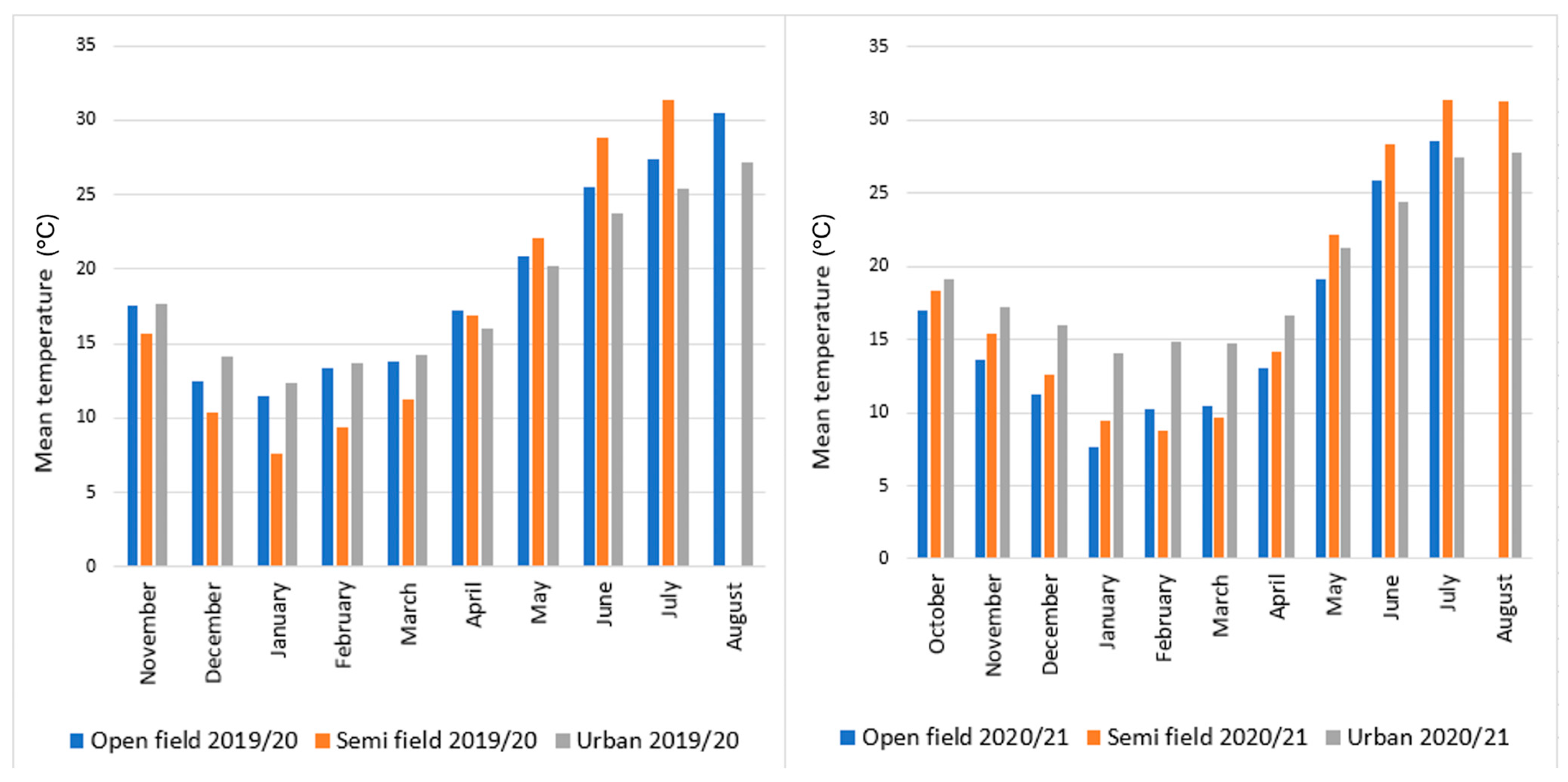
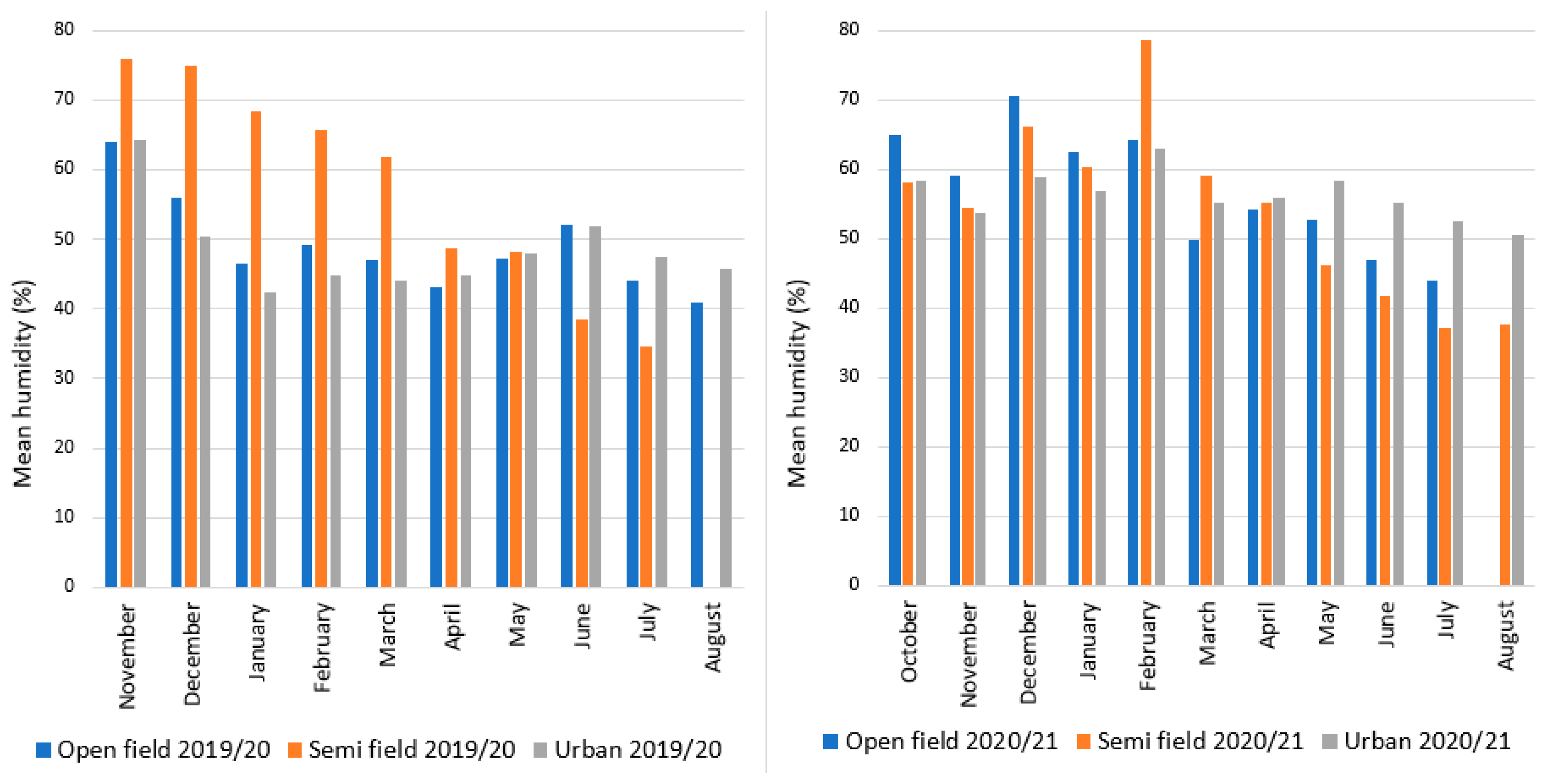
3.1. Climatic Profile of the Overwintering Sites
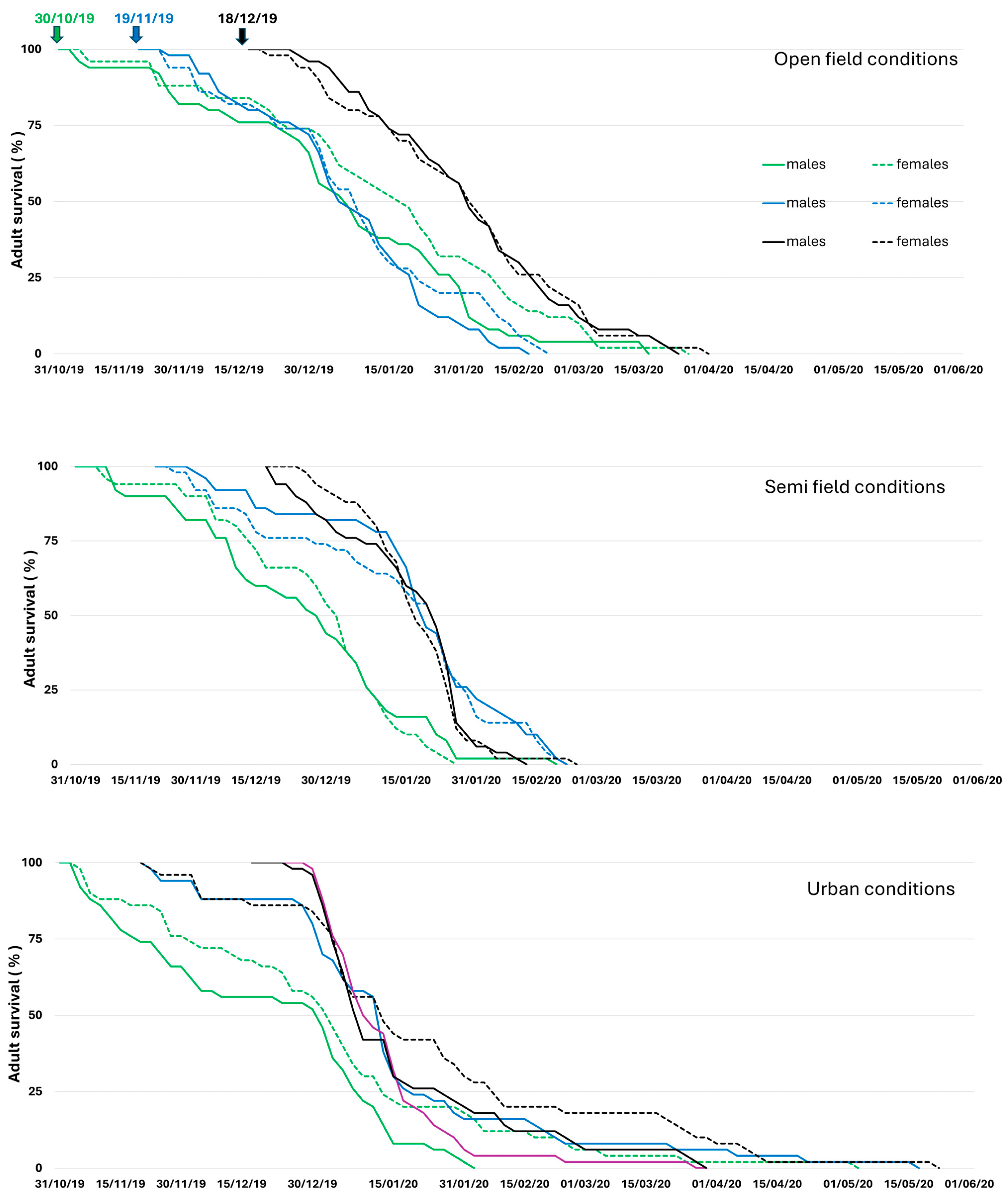
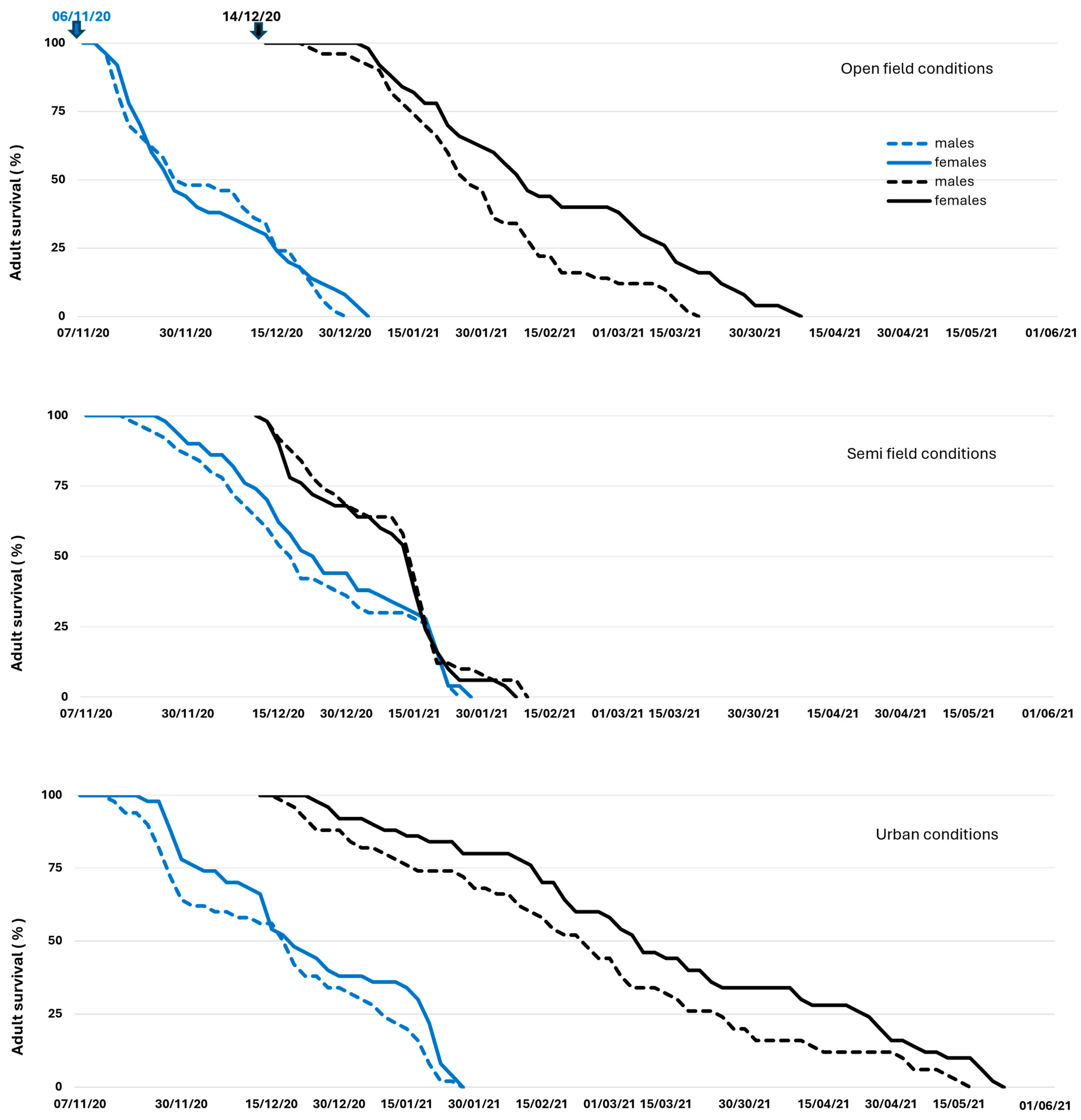
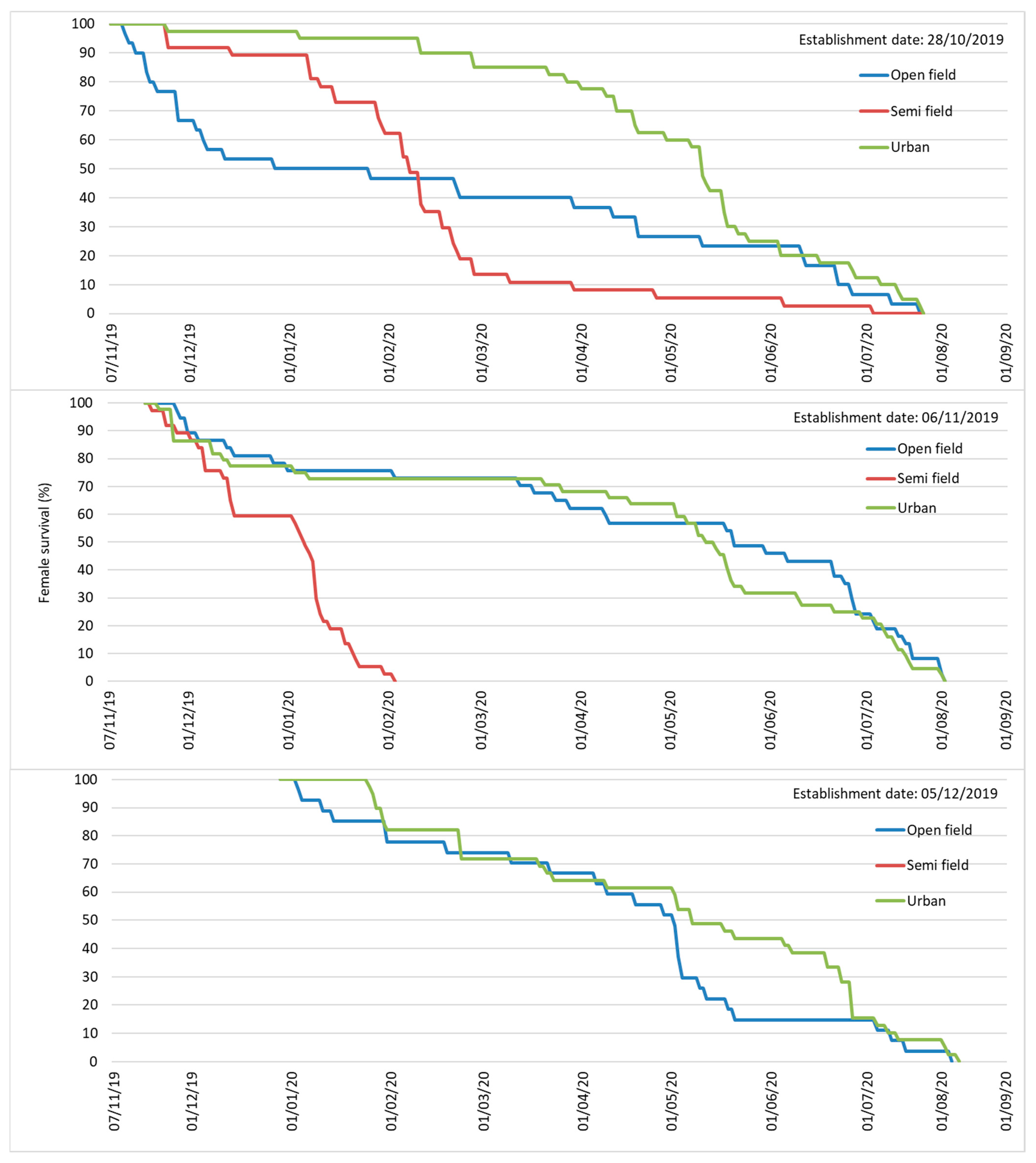
3.2. Adults Overwintering
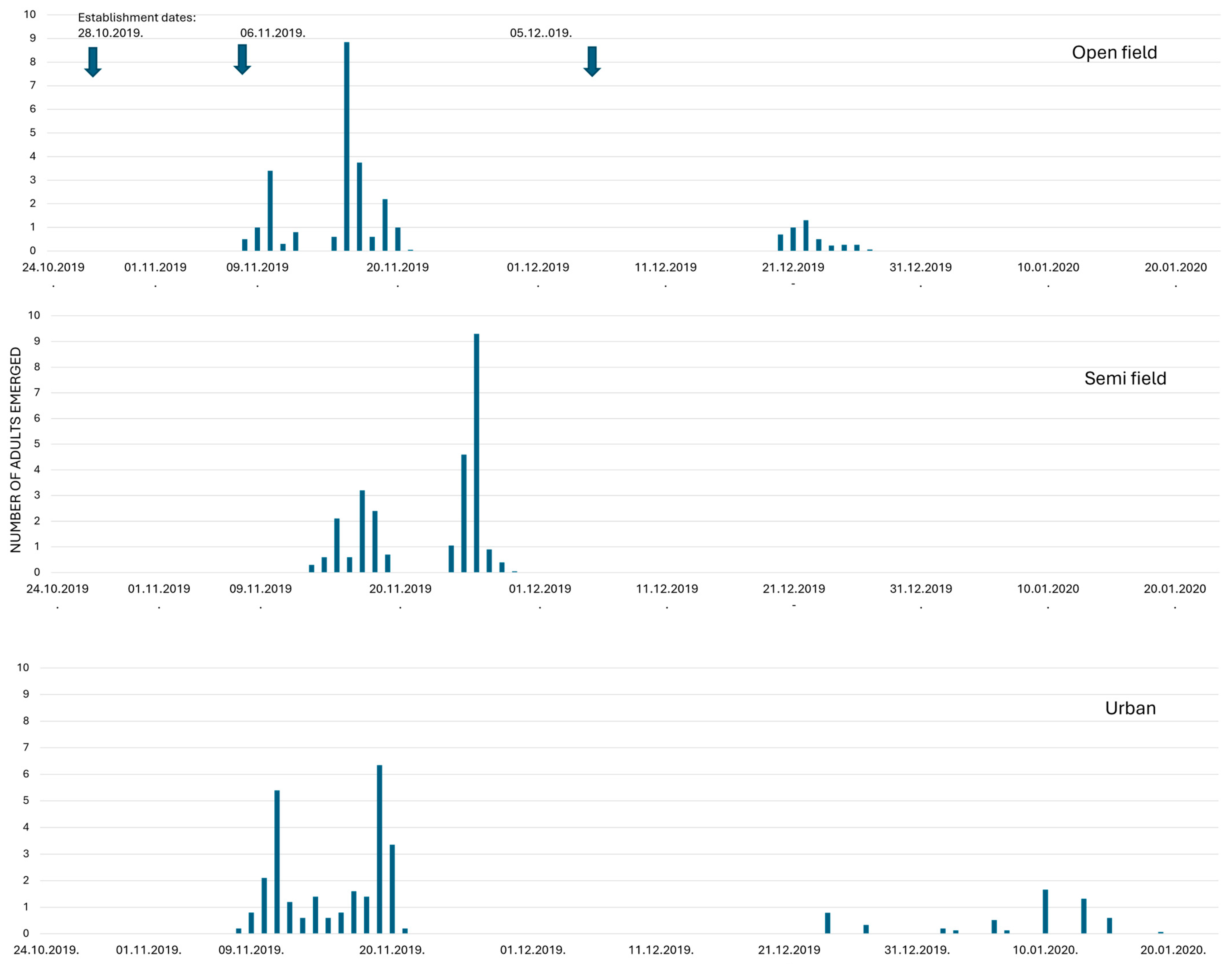
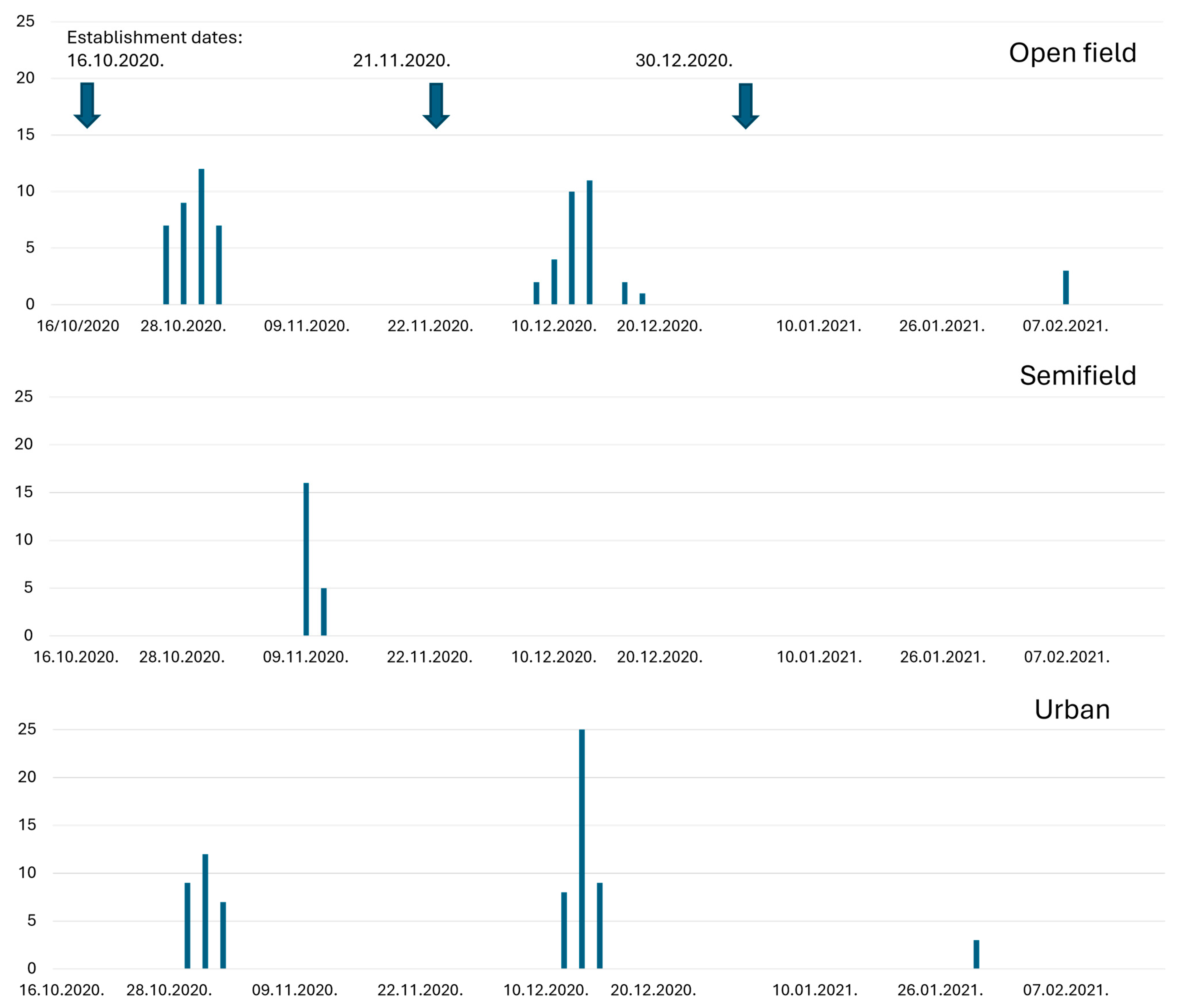
3.3. Pupae Overwintering
Adult Demographic Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liquido, N.J.; Cunningham, R.T.; Nakagawa, S. Host plants of Mediterranean fruit fly (Diptera: Tephritidae) on the island of Hawaii (1949–1985 Survey). J. Econ. Entomol. 1990, 83, 1863–1878. [Google Scholar] [CrossRef]
- Papadopoulos, N.T. Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 2318–2322. [Google Scholar]
- De Meyer, M. Systematic revision of the subgenus Ceratitis MacLeay ss. (Diptera, Tephritidae). Zool. J. Lin. Soc. 2000, 128, 439–467. [Google Scholar] [CrossRef]
- Fimiani, P. The Mediterranean region. In Fruit Flies: Their Biology, Natural Enemies and Control; Robinson, A.S., Hooper, G., Eds.; Elsevier: New York, NY, USA, 1989; pp. 37–50. [Google Scholar]
- Malacrida, A.R.; Gomulski, L.M.; Bonizzoni, M.; Bertin, S.; Gasperi, G.; Guglielmino, C.R. Globalization and fruitfly invasion and expansion: The Medfly paradigm. Genetica 2007, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gomulski, L.M.; Dimopoulos, G.; Xi, Z.; Soares, M.B.; Bonaldo, M.F.; Malacrida, A.R.; Gasperi, G. Gene discovery in an invasive tephritid model pest species, the Mediterranean fruit fly, Ceratitis capitata. BMC Genom. 2008, 9, 243. [Google Scholar] [CrossRef]
- Deschepper, P.; Vanbergen, S.; Virgilio, M.; Sciarretta, A.; Colacci, M.; Rodovitis, V.G.; Jaques, J.A.; Bjeliš, M.; Bourtzis, K.; Papadopoulos, N.T.; et al. Global invasion history with climate-related allele frequency shifts in the invasive Mediterranean fruit fly (Diptera, Tephritidae: Ceratitis capitata). Sci. Rep. 2024, 14, 25549. [Google Scholar] [CrossRef]
- Israely, N.; Ritte, U.; Oman, S.D. Inability of Ceratitis capitata (Diptera: Tephritidae) to overwinter in the Judean hills. J. Econ. Entomol. 2004, 97, 33–42. [Google Scholar] [CrossRef]
- Mwatawala, M.; Virgilio, M.; Joseph, J.; de Meyer, M. Niche partitioning among two Ceratitis rosa morphotypes and other Ceratitis pest species (Diptera, Tephritidae) along an altitudinal transect in Central Tanzania. Zookeys 2015, 540, 429–442. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Carey, J.R.; Katsoyannos, B.I.; Kouloussis, N.A. Overwintering of the Mediterranean fruit fly (Diptera: Tephritidae) in northern Greece. Ann. Entomol. Soc. Am. 1996, 89, 526–534. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Katsoyannos, B.I.; Carey, J.R.; Kouloussis, N.A. Seasonal and annual occurrence of the Mediterranean fruit fly (Diptera: Tephritidae) in Northern Greece. Ann. Entomol. Soc. Am. 2001, 94, 41–50. [Google Scholar] [CrossRef]
- Rigamonti, I.; Agosti, M.; Malacrida, A.R. Distribuzione e danni della Mosca Mediterranea della Frutta Ceratitis capitata Wied. (Diptera Tephritidae) in Lombardia (Italia settentrionale). In Proceedings of the 19. Congresso Nazionale Italiano di Entomologia Catania, Catania, Italy, 10–15 June 2002; pp. 581–587. [Google Scholar]
- Rigamonti, I.E. Contributions to the knowledge of Ceratitis capitata Wied. (Diptera, Tephritidae) in Northern Italy. I Observations on biology. Boll. Zool. Agr. Bachic. 2004, 2, 89–100. [Google Scholar]
- Rigamonti, I.E. Contributions to the knowledge of Ceratitis capitata Wied. (Diptera, Tephritidae) in Northern Italy. II Overwintering in Lombardy. Boll. Zool. Agr. Bachic. 2004, 2, 101–116. [Google Scholar]
- Rigamonti, I. La Ceratitis capitata in Lombardia; IRIS: Milan, Italy, 2005; pp. 42–47. [Google Scholar]
- Zanoni, S. Study of the Bio-Ethology of Ceratitis capitata Wied. in Trentino and Development of Sustainable Strategies for Population Control. Ph.D. Thesis, Universita’ Deglistudi del Molise, Molise, Italy, 2018. [Google Scholar]
- Zanoni, S.; Baldessari, M.; De Cristofaro, A.; Angeli, G.; Ioratti, C. Susceptibility of selected apple cultivars to the Mediterranean fruit fly. J. Appl. Entomol. 2019, 143, 74453. [Google Scholar] [CrossRef]
- Cayol, J.P.; Causse, R. Mediterranean fruit fly Ceratitis capitata Wiedemann (Dipt., Trypetidae) back in Southern France. J. Appl. Entomol. 1993, 116, 94–100. [Google Scholar] [CrossRef]
- König, S.; Steinmöller, S.; Baufeld, P. Origin and potential for overwintering of Ceratitis capitata (Wiedemann) captured in an official survey in Germany. J. Plant Dis. Prot. 2022, 129, 1201–1215. [Google Scholar] [CrossRef]
- Egartner, A.; Lethmayer, C. Invasive Fruit Flies of economic importance in Austria—Monitoring activities 2017. In Proceedings of the 9th International Conference on Integrated Fruit Production, Thessaloniki, Greece, 4–8 September 2016; Ioriatti, C., Damos, P., Escudero-Colomar, L.A., Linder, C., Stensvand, A., Eds.; IOBC-WPRS: Zürich, Switzerland, 2016; pp. 45–49. [Google Scholar]
- Egartner, A.; Lethmayer, C.; Gottsberger, R.A.; Blümel, S. Recent records of Ceratitis sp. and Bactrocera spp. (Tephritidae, Diptera) in Austria. In Proceedings of the 4th International TEAM Meeting, La Grande-Motte, France, 5–9 October 2020; p. 90. [Google Scholar]
- Gutierrez, A.P.; Ponti, L. Assessing the invasive potential of the Mediterranean fruit fly in California and Italy. Biol. Invasions 2011, 13, 2661–2676. [Google Scholar] [CrossRef]
- Sultana, S.; Baumgartner, J.B.; Dominiak, B.C.; Royer, R.E.; Beaumont, L.J. Impacts of climate change on high priority fruit fly species in Australia. PLoS ONE 2020, 15, e0213820. [Google Scholar] [CrossRef]
- Lemić, D.; Bjeliš, M.; Ninčević, P.; Pajač Živković, I.; Popović, L.; Virić Gašparić, H.; Benitez, A.H. Medfly Phenotypic Plasticity as A Prerequisite for Invasiveness and Adaptation. Sustainability 2021, 13, 12510. [Google Scholar] [CrossRef]
- Gilioli, G.; Sperandio, G.; Colturato, M.; Pasquali, S.; Gervasio, P.; Wilstermann, A.; Raja Dominic, A.; Schrader, G. Non-linear physiological responses to climate change: The case of Ceratitis capitata distribution and abundance in Europe. Biol. Invasions 2021, 24, 261–279. [Google Scholar] [CrossRef]
- Ninčević, P. Phenotypic Plasticity of the Mediterranean Medfly (Ceratitis capitata) Is a Prerequisite for Invasiveness and Adaptation to Different Agroecological Conditions. Master’s Thesis, University of Zagreb, Zagreb, Hrvatska, 2021; pp. 1–56. [Google Scholar]
- Schlichting, C.D. The role of phenotypic plasticity in diversification. In Phenotypic Plasticity: Functional and Conceptual Approaches; deWitt, T.J., Scheiner, S.M., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 191–200. [Google Scholar]
- Moraiti, C.A.; Verykouki, E.; Papadopoulos, N.T. Chill coma recovery of Ceratitis capitata adults across the Northern Hemisphere. Sci. Rep. 2022, 12, 17555. [Google Scholar] [CrossRef]
- Ninčević, P.; Lemić, D. Agro-ecological factors of mandarine Cultivation—Cause of morphological variability of Mediterranean fruit fly. Glas. Biljn. Zaštite 2020, 20, 75–76. [Google Scholar]
- Lemić, D.; Benitez, H.A.; Bjeliš, M.; Ordenes-Claveria, R.; Ninčević, P.; Mikac, K.M.; Pajač Živković, I. Agroecological effect and sexual shape dimorphism in Medfly Ceratitis capitata (Diptera: Tephritidae) an example in Croatian populations. Zool. Anz. 2020, 288, 118–124. [Google Scholar] [CrossRef]
- Lux, A.S. Individual-Based Modeling Approach to Assessment of the Impacts of Landscape Complexity and Climate on Dispersion, Detectability and Fate of Incipient Medfly Populations. Front. Physiol. 2018, 8, 1121. [Google Scholar] [CrossRef] [PubMed]
- Szyniszewska, A.M.; Tatem, A.J. Global Assessment of Seasonal Potential Distribution of Mediterranean Fruit Fly, Ceratitis capitata (Diptera: Tephritidae). PLoS ONE 2014, 9, e111582. [Google Scholar] [CrossRef] [PubMed]
- Duyck, P.F.; Quilici, S. Survival and development of different life stages of three Ceratitis spp. (Diptera: Tephritidae) reared at five constant temperatures. Bull. Entomol. Res. 2002, 92, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Cayol, J.P. Box thorn, key early season host of the Mediterranean fruit fly. Int. J. Pest Manag. 1996, 42, 325–329. [Google Scholar] [CrossRef]
- Diamantidis, A.D.; Carey, J.R.; Nakas, C.T.; Papadopoulos, N.T. Population specific demography and invasion potential in medfly. Ecol. Evol. 2011, 1, 479–488. [Google Scholar] [CrossRef]
- Papadogiorgou, G.D.; Moraiti, C.A.; Papadopoulos, N.T. Life—History of two mediterannean fruit fly populations on key overwintering hosts and different temperatures. In Proceedings of the 4th International TEAM Meeting, La Grande-Motte, France, 5–9 October 2020; p. 64. [Google Scholar]
- Nyamukondiwa, C.; Kleynhans, E.; Terblanche, J.S. Phenotypic plasticity of thermal tolerance contributes to the invasion potential of Mediterranean fruit flies (Ceratitis capitata). Ecol. Entomol. 2010, 35, 565–575. [Google Scholar] [CrossRef]
- Nyamukondiwa, C.; Terblanche, J.S. Within-generation variation of critical thermal limits in adult Mediterranean and Natal fruit flies Ceratitis capitata and Ceratitis rosa: Thermal history affects short-term responses to temperature. Physiol. Entomol. 2010, 35, 255–264. [Google Scholar] [CrossRef]
- Szyniszewska, A.M.; Bieszczak, H.; Kozyra, K.; Papadopoulos, N.T.; De Meyer, M.; Nowosad, J.; Ota, N.; Kritikos, D.J. Evidence that recent climatic changes have expanded the potential geographical range of the Mediterranean fruit fly. Sci. Rep. 2024, 14, 2515. [Google Scholar] [CrossRef]
- Weldon, C.W.; Nyamukondiwa, C.; Karsten, M.; Chown, S.L.; Terblanche, J.S. Geographic variation and plasticity in climate stress resistance among southern African populations of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Sci. Rep. 2018, 8, 9849. [Google Scholar] [CrossRef]
- Tominić, A. Muha voćnih plodova (Ceratitis capitata Wied.) na primorju. Biljn. Proizv. 1951, 3, 132–136. [Google Scholar]
- Kovačević, Ž. Voćna mušica kao ekološi problem. Agron. J. 1960, 4, 161–170. [Google Scholar]
- Popović, L.; Bjeliš, M.; Ivić, D.; Deak, S.; Mustapić, P. Potreba provođenja higijene u voćnjacima mandarina i zbrinjavanje otpada u otkupnim centrima. Glas. Biljn. Zaštite 2015, 15, 57. [Google Scholar]
- Bjeliš, M.; Popović, L.; Kiridžija, M.; Ortiz, G.; Pereira, R. Suppression of Mediterranean Fruit Fly Using Sterile Insect Technique in Neretva River Valley of Croatia. In Proceedings of the 9th International Symposium on Fruit Flies of Ecconomic Importance, Bangkok, Thailand, 12–16 May 2014; Beatriz, S.M., Teresa, V., Rui, P., Orankanok, W., Eds.; Siam Print Co. Ltd.: Bangkok, Thailand, 2016; pp. 24–29. [Google Scholar]
- Bjeliš, M.; Popović, L.; Tavra, I.; Strikić, F.; Sever, Z.; Moraiti, C.A.; Papadopoulos, N.; Ortiz Moreno, G.; Pereira, R. Overwintering of Mediterranean Fruit fly Adults in Dalmatia and Implications to Current Strategy of SIT Suppression Program in Neretva Valley. In Proceedings of the 11th International Symposium on Fruit Flies of Economic Importance Abstract Book, International Symposium on Fruit Flies of Economic Importance, Sydney, Australia, 13–18 November 2022; Rempoulakis, P., Rempoulakis, C., Eds.; NSW Department of Primary Industries: Sydney, Australia, 2022; p. 181. [Google Scholar]
- Bjeliš, M. Report on project activities FAO/IAEA RER 5012. In Supporting the Managment of Fruit Flies in the Balkans and Eastern Mediterranean for 2016; Croatian Centre for Agriculture, Food and Rural Affairs: Opuzen, Croatia, 2017; pp. 1–55. [Google Scholar]
- Peñarrubia-María, E.; Avilla, J.; Escudero-Colomar, L.A. Survival of wild adults of Ceratitis capitata (Wiedemann) under natural winter conditions in North East Spain. Psyche 2012, 1, 497087. [Google Scholar] [CrossRef]
- Wernicke, M.; Egartner, A.; Blümel, S.; Moraiti, C.A.; Papadopoulos, N.T. Overwintering potential of the Mediterranean fruit fly (Diptera: Tephritidae) in Austria. J. Econ. Entomol. 2024, 117, 1983–1994. [Google Scholar] [CrossRef]
- Katsoyannos, B.I.; Kouloussis, N.A.; Carey, J.R. Seasonal and annual occurrence of Mediterranean fruit flies (Diptera: Tephritidae) on Chios Island, Greece: Differences between two neighboring citrus orchards. Ann. Entomol. Soc. Am. 1998, 91, 43–51. [Google Scholar] [CrossRef]
- Mavrikakis, P.G.; Economopoulos, A.P.; Carey, J.R. Continuous winter reproduction and growth of the Mediterranean fruit fly (Diptera: Tephritidae) in Heraklion, Crete, Southern Greece. Environ. Entomol. 2000, 29, 1180–1187. [Google Scholar] [CrossRef]
- De Lima, C.P.F.; Broughton, S. Investigation of Fruit Damage in Citrus in South West Western Australia; Horticultural Research and Development Corporation: Sydney, Australia, 2001; p. 23. [Google Scholar]
- Papadogiorgou, G.D.; Papadopoulos, A.G.; Moraiti, C.A.; Verykouki, E.; Papadopoulos, N.T. Latitudinal variation in survival and immature development of Ceratitis capitata populations reared in two key overwintering hosts. Sci. Rep. 2024, 14, 467. [Google Scholar] [CrossRef]
- Nyamukondiwa, C.; Weldon, C.W.; Chown, S.L.; le Roux, P.C.; Terblanche, J.S. Thermal biology, population fluctuations and implications of temperature extremes for the management of two globally significant insect pests. J. Insect Physiol. 2013, 59, 1199–1211. [Google Scholar] [CrossRef]
- Manrakhan, A.; Daneel, J.H.; Stephen, P.R.; Hattingh, V. Cold tolerance of immature stages of Ceratitis capitata and Bactrocera dorsalis (Diptera: Tephritidae). J. Econ. Entomol. 2022, 115, 482–492. [Google Scholar] [CrossRef]
- Papadogiorgou, G.D.; Moraiti, C.A.; Nestel, D.; Terblanche, J.S.; Verykouki, E.; Papadopoulos, N.T. Acute cold stress and supercooling capacity of Mediterranean fruit fly populations across the Northern Hemisphere (Middle East and Europe). J. Insect Physiol. 2023, 147, 104519. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.E.; Sinclair, B.J. The impacts of repeated cold exposure on insects. J. Exp. Biol. 2012, 215, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Sgrò, C.M.; Terblanche, J.S.; Hoffmann, A.A. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 2016, 61, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Bowler, K.; Terblanche, J.S. Insect thermal tolerance: What is the role of ontogeny, ageing and senescence? Biol. Rev. 2008, 83, 339–355. [Google Scholar] [CrossRef]
- Papadogiorgou, G.D.; Papadopoulos, N.T. Temperature and Host Fruit During Immature Development Shape Adult Life History Traits of Different Ceratitis capitata Populations. Insects 2025, 16, 65. [Google Scholar] [CrossRef]
- Mutamiswa, R.; Tarusikirwa, V.L.; Nyamukondiwa, C.; Cuthbert, R.N.; Chidawanyika, F. Thermal stress exposure of pupal oriental fruit fly has strong and trait-specific consequences in adult flies. Physiol. Entomol. 2023, 48, 35–44. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, L.; Zhao, J.; Li, N.; Qin, D.; Xiao, C.; Lu, Y.; Guo, Z. Rapid cold hardening and cold acclimation promote cold tolerance of oriental fruit fly, Bactrocera dorsalis (Hendel) by physiological substances transformation and cryoprotectants accumulation. Bull. Entomol. Res. 2023, 113, 574–586. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; De Meyer, M.; Terblanche, J.S.; Kriticos, D.J. Fruit flies: Challenges and opportunities to stem the tide of global invasions. Annu. Rev. Entomol. 2024, 69, 355–373. [Google Scholar] [CrossRef]
- Bjeliš, M.; Tavra, I.; Strikić, F.; Stojić; M.; Nestel, D. Invasion of Ceratitis capitata W. (Diptera, Tephritidae) from coastal to inland areas of Dalmatia region of Croatia: E-traps as an improved detection tool//IOBC-WPRS Working Group. In Integrated Control in Citrus Fruit Crops; Rodovitis, V.G., Campos-Rivela, J.M., Martínez-Ferrer, M.T., Eds.; International Organization for Biological and Integrated Control of Noxious Animals and Plants, West Palearctic Regional Section (IOBC-WPRS): Darmstadt, Germany, 2022; pp. 66–72. [Google Scholar]
- Bali, E.-M.D.; Moraiti, C.A.; Ioannou, C.S.; Mavraganis, V.; Papadopoulos, N.T. Evaluation of Mass Trapping Devices for Early Seasonal Management of Ceratitis Capitata (Diptera: Tephritidae) Populations. Agronomy 2021, 11, 1101. [Google Scholar] [CrossRef]
- Argov, Y.; Gazit, Y. Biological control of the Mediterranean fruit fly in Israel: Introduction and establishment of natural enemies. Biol. Control 2008, 46, 502–507. [Google Scholar] [CrossRef]
- Beris, E.I.; Papachristos, D.P.; Fytrou, A.; Antonatos, S.A.; Kontodimas, D.C. Pathogenicity of three entomopathogenic fungi on pupae and adults of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). J. Pest Sci. 2013, 86, 275–284. [Google Scholar] [CrossRef]
- Konstantopoulou, M.A.; Mazomenos, B.E. Evaluation of Beauveria bassiana and B. brongniartii strains and four wild-type fungal species against adults of Bactrocera oleae and Ceratitis capitata. Biocontrol 2005, 50, 293–305. [Google Scholar] [CrossRef]
- Kapranas, A.; Chronopoulou, A.; Lytra, I.C.; Peters, A.; Milonas, P.G.; Papachristos, D.P. Efficacy and residual activity of commercially available entomopathogenic nematode strains for Mediterranean fruit fly control and their ability to infect infested fruits. Pest Manag. Sci. 2021, 77, 3964–3969. [Google Scholar] [CrossRef] [PubMed]
- Kapranas, A.; Chronopoulou, A.; Peters, A.; Antonatos, S.; Lytra, I.; Milonas, P.; Papachristos, D. Early and off-season biological control of medfly with entomopathogenic nematodes: From laboratory experiments to successful field trials. Biol. Contr. 2023, 179, 105173. [Google Scholar] [CrossRef]
- Miranda, M.; Alonso, R.; Alemany, A. Field evaluation of medfly (Dipt., Tephritidae) female attractants in a Mediterranean agrosystem (Balearic Islands, Spain). J. Appl. Entomol. 2001, 125, 333–339. [Google Scholar] [CrossRef]
- El-Sayed, A.; Suckling, D.; Wearing, C.; Byers, J. Potential of mass trapping for long-term pest management and eradication of invasive species. J. Econ. Entomol. 2006, 99, 1550–1564. [Google Scholar] [CrossRef]
- Bjeliš, M.; Rodovitis, V.G.; Lemic, D.; Kaniouras, P.; Gančević, P.; Papadopoulos, N.T. Invasion History and Dispersion Dynamics of the Mediterranean Fruit Fly in the Balkan Peninsula. Insects 2024, 15, 975. [Google Scholar] [CrossRef]


| Adult Overwintering | Number of Replications | |
|---|---|---|
| Site/Season | 2019/2020 | 2020/2021 |
| Open-field | 10 × 3 establishment dates: 30 October 2019, 18 November 2019, 18 December 2019 | 10 × 2 establishment dates 6 November 2020, 14 December 2020 |
| Semi-field | 10 × 3 establishment dates: 30 October 2019, 18 November 2019, 18 December 2019 | 10 × 2 establishment dates 6 November 2020, 14 December 2020 |
| Urban | 10 × 3 establishment dates: 30 October 2019, 18 November 2019, 18 December 2019 | 10 × 2 establishment dates 6 November 2020, 14 December 2020 |
| Establishment Dates | Replications (Pupae/Replication) | |||
|---|---|---|---|---|
| Site | Season 2019/20 | Season 2020/21 | Season 2019/2020 | Season 2020/2021 |
| Open-field | 28 October 2019 6 November 2019 5 December 2019 | 16 October 2020 21 November 2020 30 December 2020 | 10 (20) 10 (40) 10 (30) | 10 (20) 10 (20) 9 (20) |
| Semi-field | 28 October 2019 6 November 2019 5 December 2019 | 16 October 2020 21 November 2020 30 December 2020 | 10 (20) 10 (40) 10 (30) | 10 (20) 10 (20) 10 (20) |
| Urban | 28 October 2019 6 November 2019 5 December 2019 | 16 October 2020 21 November 2020 30 December 2020 | 10 (20) 10 (40) 10 (30) | 10 (20) 10 (20) 8 (20) |
| Establishment Date | Average Life Span (Days ± SE) | |||||
|---|---|---|---|---|---|---|
| Open-Field Conditions | Semi-Field Conditions | Urban Conditions | ||||
| Males | Females | Males | Females | Males | Females | |
| 30 October 2019 | 68.7 ± 4.3 | 77.8 ± 4.5 | 55.4 ± 3.3 | 58.1 ± 2.7 | 48.1 ± 3.8 | 63.1 ± 5.2 |
| 19 November 2019 | 51.2 ± 2.7 | 53.1 ± 3.3 | 63.3 ± 3.0 | 56.5 ± 3.6 | 60.6 ± 4.8 | 69.1 ± 5.6 |
| 18 December 2019 | 46.2 ± 3.0 | 47.0 ± 3.4 | 30.2 ± 2.1 | 31.2 ± 1.6 | 27.1 ± 2.1 | 31.6 ± 3.3 |
| 6 November 2020 | 27.1 ± 2.3 | 26.9 ± 2.4 | 49.2 ± 2.6 | 47.9 ± 3.0 | 46.0 ± 3.1 | 43.5 ± 3.4 |
| 14 December 2020 | 64.4 ± 3.9 | 50.3 ± 3.2 | 26.4 ± 2.1 | 27.3 ± 2.0 | 89.8 ± 5.9 | 71.0 ± 5.7 |
| Source of Variance | B ± SE | Exp (B) | p |
|---|---|---|---|
| 2019–2020 | |||
| Overwintering site | <0.001 | ||
| Open-field | −0.269 ± 0.085 | 0.764 | 0.002 |
| Semi-field | −0.180 ± 0.085 | 1.197 | 0.034 |
| Establishment date | <0.001 | ||
| 30 October 2019 | −1.097 ± 0.086 | 0.334 | <0.001 |
| 19 November 2019 | −1.041 ± 0.088 | 0.353 | <0.001 |
| Sex | −0.180 ± 0.067 | 0.835 | 0.008 |
| 2020–2021 | |||
| Overwintering site | <0.001 | ||
| Open-field | 0.917 ± 0.187 | 2.502 | 0.000 |
| Semi-field | 2.629 ± 0.214 | 13.862 | 0.000 |
| Establishment date | <0.001 | ||
| 6 November 2020 | 0.008 ± 0.214 | 1.008 | 0.971 |
| 14 December 2020 | −2.555 ± 0.230 | 0.078 | 0.000 |
| Sex | −0.180 ± 0.067 | 0.835 | 0.008 |
| Source of Variance | B ± SE | Exp (B) | p |
|---|---|---|---|
| 2019–2020 | |||
| Overwintering site | <0.001 | ||
| Open-field | 0.019 ± 0.113 | 1.019 | 0.865 |
| Semi-field | 0.367 ± 0.113 | 1.444 | 0.001 |
| Establishment date | <0.001 | ||
| 30 October 2019 | −1.552 ± 0.122 | 0.212 | <0.001 |
| 19 November 2019 | −2.958 ± 0.115 | 0.052 | <0.001 |
| 2020–2021 | |||
| Overwintering site | <0.001 | ||
| Open-field | 0.669 ± 0.167 | 1.952 | <0.001 |
| Semi-field | 1.980 ± 0.251 | 7.244 | <0.001 |
| Establishment date | <0.001 | ||
| 16 October 2020 | −0.800 ± 0.205 | 0.449 | <0.001 |
| 22 November 2020 | −0.473 ± 0.213 | 0.623 | 0.026 |
| Source of Variance | B ± SE | Exp (B) | p |
|---|---|---|---|
| 2019–2020 | |||
| Overwintering site | <0.001 | ||
| Open-field | 4.031 ± 0.361 | 56.292 | <0.001 |
| Semi-field | −2.991 ± 0.141 | 0.050 | <0.001 |
| Establishment date | <0.001 | ||
| 30 October 2019 | 5.545 ± 0.361 | 255.855 | <0.001 |
| 19 November 2019 | 6.080 ± 0.360 | 437.211 | <0.001 |
| Over. Site * Establishment date | <0.001 | ||
| Open-field * 30 October 2019 | −3.687 ± 0.386 | 0.025 | <0.001 |
| Open-field * 19 November 2019 | −3.148 ± 0.365 | 0.043 | <0.001 |
| Semi-field * 30 October 2019 | 0.572 ± 0.166 | 1.772 | 0.001 |
| 2020–2021 | |||
| Overwintering site | <0.001 | ||
| Open-field | 8.784 ± 40.919 | 0.000 | 0.830 |
| Semi-field | −4.967 ± 0764 | 0.007 | <0.001 |
| Establishment date | <0.001 | ||
| 16 October 2020 | 6.391 ± 0.766 | 255.855 | <0.001 |
| 22 November 2020 | 6.080 ± 0.360 | 437.211 | <0.001 |
| Over. Site * Establishment date | <0.001 | ||
| Open-field * 16 October 2020 | 10.402 ± 40.920 | 32,931.043 | 0.799 |
| Open-field * 22 November 2020 | 9.099 ± 40.920 | 8943.478 | 0.824 |
| Overwintering Site | Establishment Date | N | Average ± SE | |
|---|---|---|---|---|
| Lifespan (Days) | Fecundity (Eggs/Female) | |||
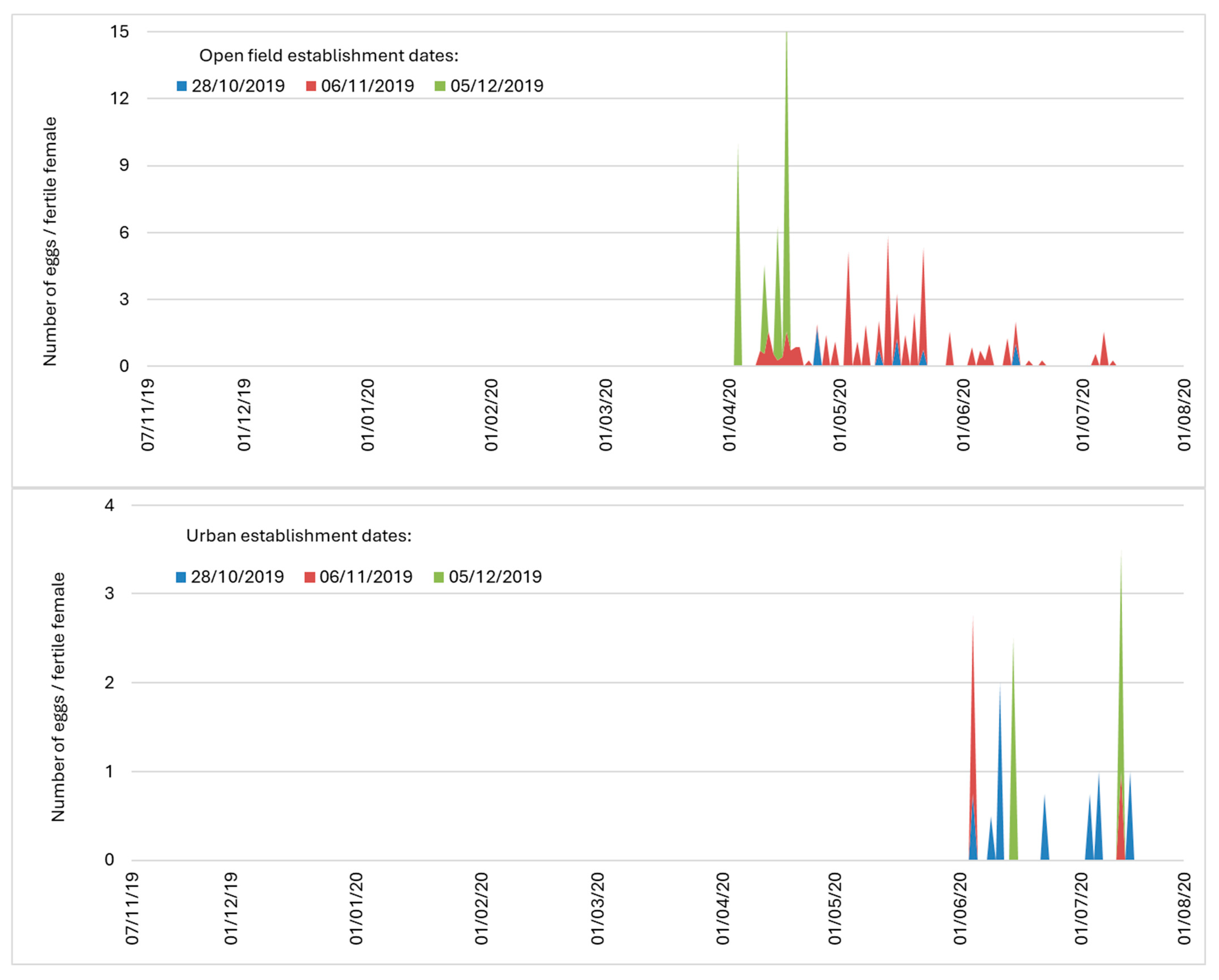
| Open-field | 28 October 2019 | 30 | 104.1 ± 16.1 | 0.73 ± 0.4 |
| 6 November 2019 | 37 | 143.6 ± 15.0 | 8.62 ± 3.6 | |
| 5 December 2019 | 22 | 104.3 ± 13.9 | 3.64 ± 0.5 | |
| Semi-field | 28 October 2019 | 32 | 87.8 ± 8.8 | 0 |
| 6 November 2019 | 37 | 41.5 ± 3.8 | 0 | |
| 5 December 2019 | 0 | |||
| Urban area | 28 October 2019 | 35 | 168.2 ± 9.1 | 0.77 ± 0.4 |
| 6 November 2019 | 39 | 137.9 ± 14.4 | 0.15 ± 0.1 | |
| 5 December 2019 | 30 | 110.8 ± 11.4 | 0.17 ± 0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjeliš, M.; Tavra, I.; Strikić, F.; Popović, L.; Moraiti, C.A.; Rodovitis, V.G.; Papadopoulos, N.T. Overwintering Capacity of the Mediterranean Fruit Fly in the Dalmatia Region of Croatia. Insects 2025, 16, 1104. https://doi.org/10.3390/insects16111104
Bjeliš M, Tavra I, Strikić F, Popović L, Moraiti CA, Rodovitis VG, Papadopoulos NT. Overwintering Capacity of the Mediterranean Fruit Fly in the Dalmatia Region of Croatia. Insects. 2025; 16(11):1104. https://doi.org/10.3390/insects16111104
Chicago/Turabian StyleBjeliš, Mario, Ivan Tavra, Frane Strikić, Luka Popović, Cleopatra A. Moraiti, Vasilis G. Rodovitis, and Nikos T. Papadopoulos. 2025. "Overwintering Capacity of the Mediterranean Fruit Fly in the Dalmatia Region of Croatia" Insects 16, no. 11: 1104. https://doi.org/10.3390/insects16111104
APA StyleBjeliš, M., Tavra, I., Strikić, F., Popović, L., Moraiti, C. A., Rodovitis, V. G., & Papadopoulos, N. T. (2025). Overwintering Capacity of the Mediterranean Fruit Fly in the Dalmatia Region of Croatia. Insects, 16(11), 1104. https://doi.org/10.3390/insects16111104








