Optimizing Artificial Diet Composition for Enhanced Development and Fertility of Amblyseius swirskii
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing of Amblyseius swirskii and Its Prey Organisms
2.2. Rearing Conditions of Amblyseius swirskii
2.3. Preparation of Artificial Diets
2.4. Experimental Design
2.5. Vitality Indices Calculation
2.5.1. Determination of Fertility
2.5.2. Determination of Egg Hatching Rate
2.5.3. Determination of Adult Survival (Lifespan)
2.5.4. Determination of Feeding Intensity in First-Generation Amblyseius swirskii
2.6. Genetic Identification Based on ITS Region of rDNA
2.6.1. DNA Extraction
2.6.2. PCR Amplification
2.6.3. ITS Gene Sequencing
2.7. Statistical Analysis
3. Results
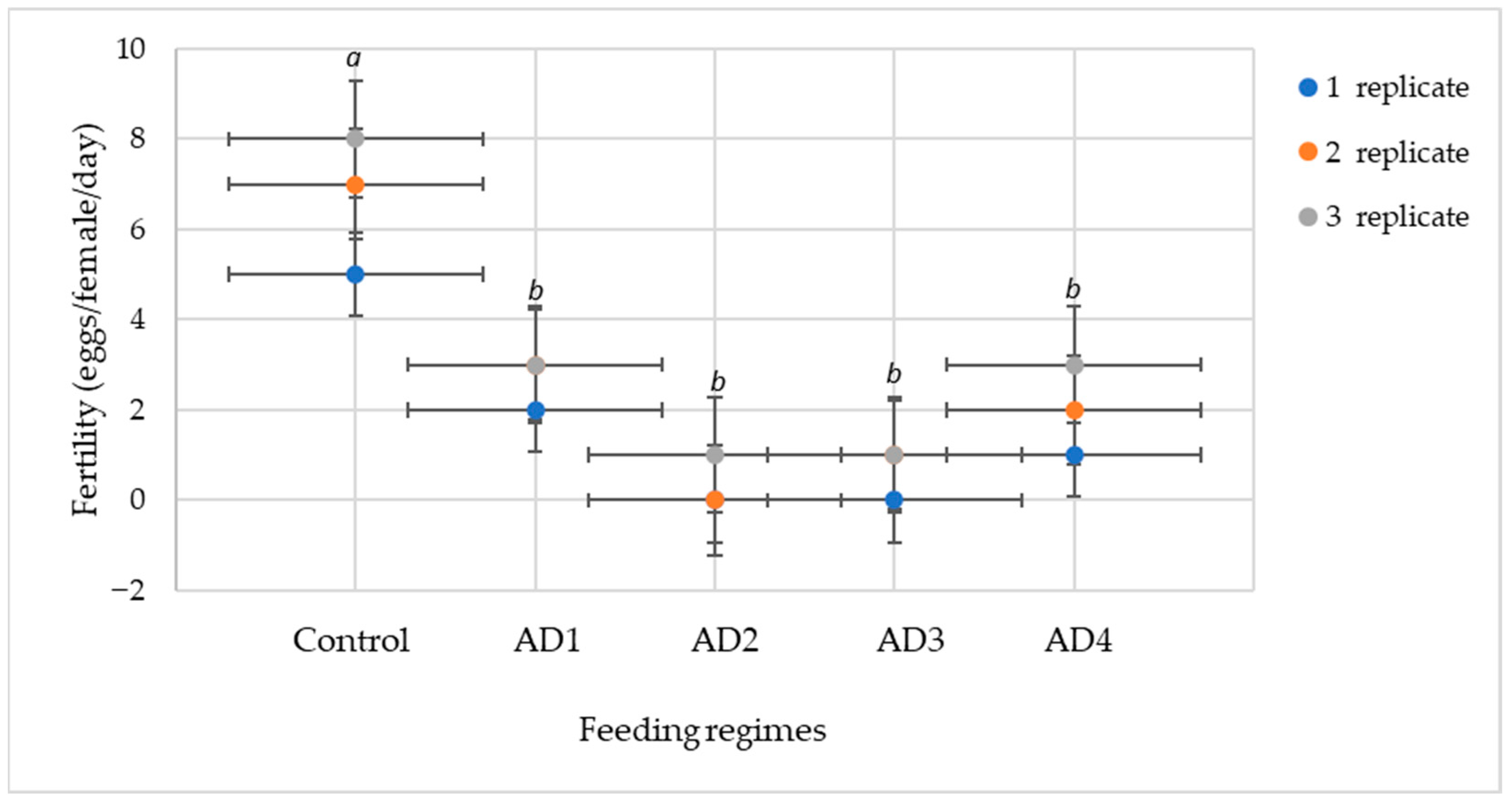
3.1. Development and Fertility of A. swirskii Under Different Feeding Regimes
3.2. Analysis of Nymph Emergence
3.2.1. Nymph Hatching
3.2.2. Nymph Survival
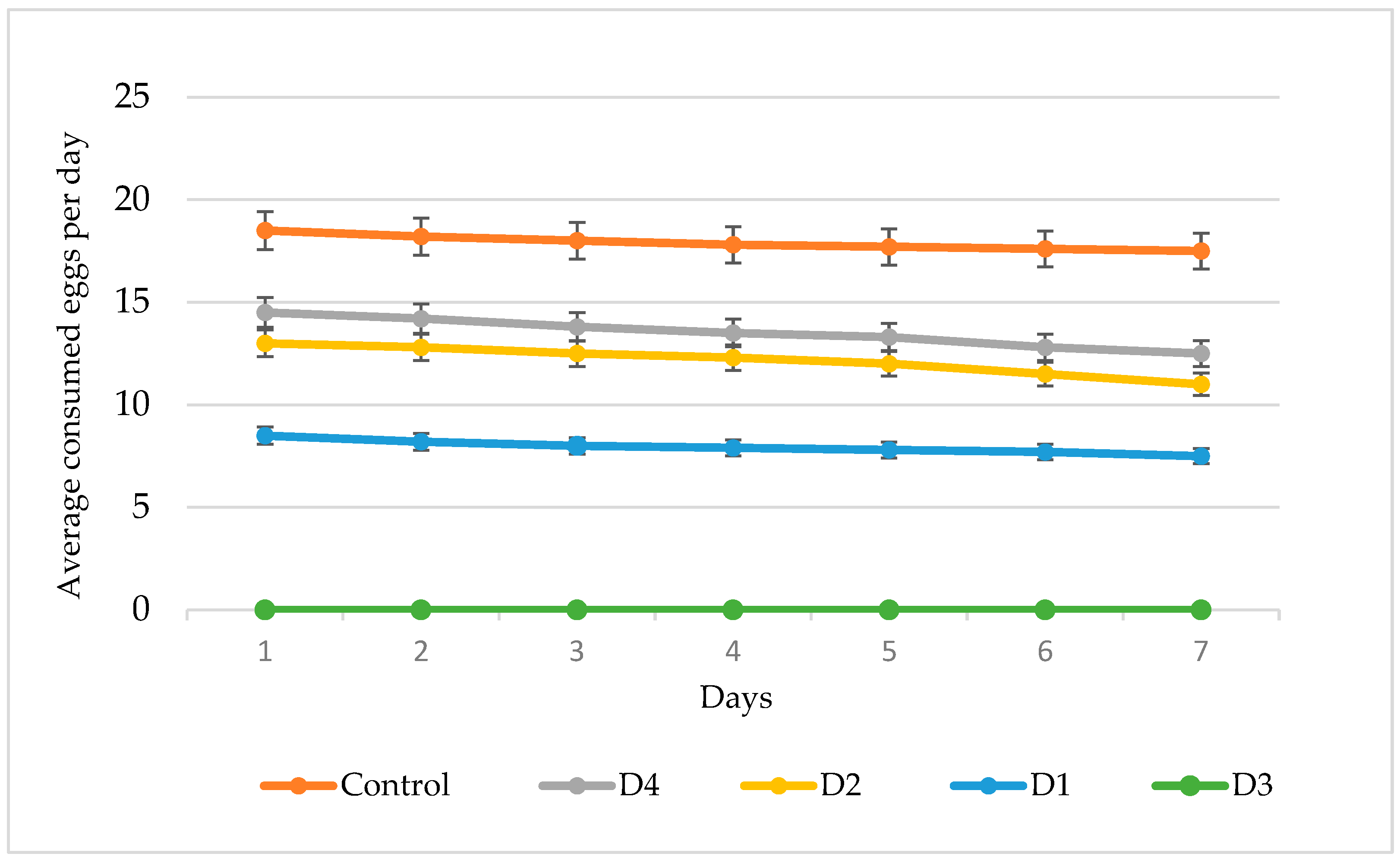
3.3. Feeding Dynamics and Lifespan of Amblyseius swirskii on Different Diets
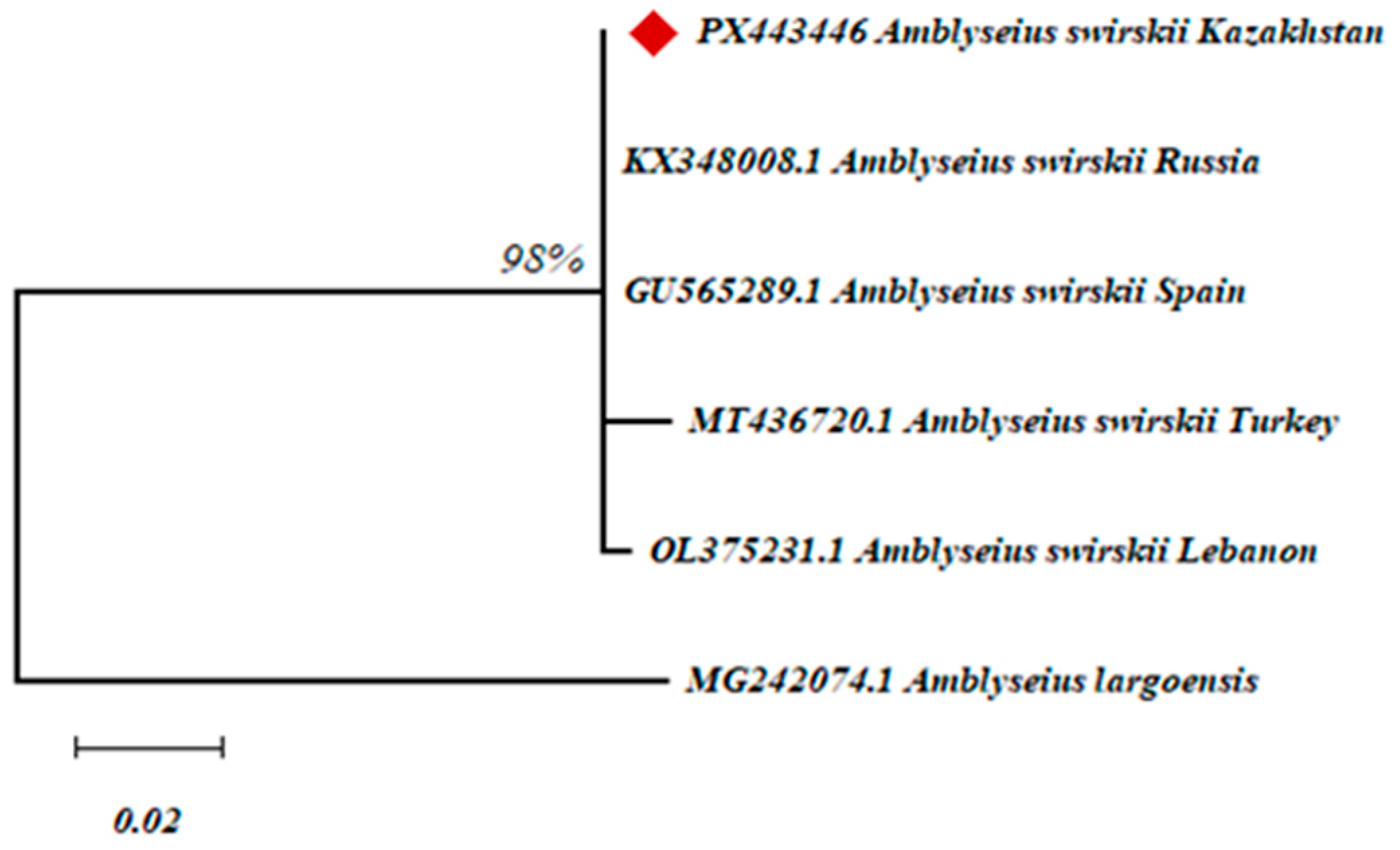
3.4. Molecular Genetic Identification of A. swirskii
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Arcot, Y.; Medina, R.F.; Bernal, J.; Cisneros-Zevallos, L.; Akbulut, M.E.S. Integrated Pest Management: An Update on the Sustainability Approach to Crop Protection. ACS Omega 2024, 9, 41130–41147. [Google Scholar] [CrossRef]
- Shamshiri, R.R.; Kalantari, F.; Ting, K.C.; Thorp, K.R.; Hameed, I.A.; Weltzien, C.; Ahmad, D.; Shad, Z.M. Advances in Greenhouse Automation and Controlled Environment Agriculture: A Transition to Plant Factories and Urban Agriculture. Int. J. Agric. Biol. Eng. 2018, 11, 1–22. [Google Scholar] [CrossRef]
- Tsapko, V. Phytophages of Greenhouse Cucumber and Their Control. In Proceedings of the Student Science—A Glimpse into the Future; Krasnoyarsk State Agrarian University, Federal State Budgetary Educational Institution of Higher Education: Krasnoyarsk, Russia, 2020; Volume 1, pp. 23–26. [Google Scholar]
- Kozlova, Y. Biologization of The Agricultural Crops Protection Systems Against Diseases. Vestn. Agrar. Nauk. 2022, 1, 17–22. [Google Scholar]
- Ismailov, V.; Agasyeva, I.; Nastasy, A.; Nefedova, M.; Besedina, E.; Komantsev, A. The Application of Entomophagous and Acariphagous Species in Biological Protection Systems of an Apple Orchard (Malus Domestica Borkh). Horticulturae 2023, 9, 379. [Google Scholar] [CrossRef]
- Adly, D.; Sanad, A.S. Comparative Evaluation of Biological Control Programs and Chemical Pesticides for Managing Insect and Mite Pests in Cucumber Greenhouses: A Sustainable Approach for Enhanced Pest Control and Yield. Egypt. J. Biol. Pest Control 2024, 34, 42. [Google Scholar] [CrossRef]
- Kumar, M. Recent Trends in Plant Protection, 1st ed.; New India Publishing Agency: Delhi, India, 2024; ISBN 978-93-5887-978-0. [Google Scholar]
- Migeon, A. The Jean Gutierrez Spider Mite Collection. Zookeys 2015, 489, 15–24. [Google Scholar] [CrossRef]
- Available online: https://www.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.4-SC-4-17.English.pdf (accessed on 19 October 2025).
- Available online: https://pan-international.org/wp-content/uploads/Consolidated-List-of-Bans-Explanatory-2017April.pdf (accessed on 19 October 2025).
- Available online: https://www.gov.kz/memleket/entities/agroindust/documents/details/41094 (accessed on 9 June 2020).
- Zamotajlova, D.A.; Popova, E.V.; Khudyakova, E.V.; Stepantsevich, M.N. The Entomophages Use for Crop Protection: Ecological and Economic-Mathematical Modeling and Forecasting. IOP Conf. Ser. Earth Environ. Sci. 2022, 1069, 012047. [Google Scholar] [CrossRef]
- Argento, S.; Garcia, G.; Treccarichi, S. Sustainable and Low-Input Techniques in Mediterranean Greenhouse Vegetable Production. Horticulturae 2024, 10, 997. [Google Scholar] [CrossRef]
- Bout, A.; Ris, N.; Multeau, C.; Mailleret, L. Augmentative Biological Control Using Entomophagous Arthropods. In Extended Biocontrol; Fauvergue, X., Rusch, A., Barret, M., Bardin, M., Jacquin-Joly, E., Malausa, T., Lannou, C., Eds.; Springer: Dordrecht, The Netherlands, 2022; pp. 43–53. ISBN 978-94-024-2149-1. [Google Scholar]
- Sulaymanov, O.; Jumaev, R. Entomophagous Species of Litter (Aleyrodidae). E3S Web Conf. 2023, 389, 03097. [Google Scholar] [CrossRef]
- Mukhametov, A.; Benin, D.; Mutalibova, G.; Snegirev, D.; Pshidatok, S. The Impact of Entomophages on the Population Dynamics of Major Greenhouse Cucumber Pests. CropForage Turfgrass Manag. 2023, 9, e20196. [Google Scholar] [CrossRef]
- Kamaev, I.O.; Mironova, M.K. Phytosanitary Risk of Herbivorous Mites (Arachnida: Acariformes). Plant Quar. Sci. Pract. 2018, 3, 13–20. [Google Scholar]
- Fouly, A.H.; Ata, T.E.; Awadalla, S.S.; Marouf, E.A. Influence of Two Phytoseiid Predacious Mites on Population Density of the Two-Spotted Spider Mite, Tetranycus urticae Koch on Eggplant (Solanum melanogena L.) in Protected Cultivation (Acar: Tetranychidae: Phytoseiidae). J. Plant Prot. Pathol. 2025, 16, 219–224. [Google Scholar] [CrossRef]
- Suchalkin, F.A. Development of Biological Control Method for Tobacco Thrips on Cucumbers in Protected Ground. Abstract of Dissertation for the Degree of Candidate of Biological Sciences. Bachelor’s Thesis, All-Union Research Institute of Phytopathology, Golitsino, Russia, 1987. [Google Scholar]
- Sweelam, M.; Nasreldin, M. Biological Aspects and Life-Tables of the Predatory Mites, Amblyseius swirskii Athias-Henriot and Neoseiulus californicus (McGregor), Reared on Four Types of Food. Acarines J. Egypt. Soc. Acarol. 2023, 17, 45–55. [Google Scholar] [CrossRef]
- Sierra-Monroy, J.A.; Deere, J.A.; Cárdenas, K.A.M.; Montserrat, M.; Janssen, A. A Predatory Mite as Potential Biological Control Agent of the Invasive Thrips parvispinus. In BioControl; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Novljan, M.; Bohinc, T.; Kreiter, S.; Döker, I.; Trdan, S. The Indigenous Species of Predatory Mites (Acari: Phytoseiidae) as Biological Control Agents of Plant Pests in Slovenia. Acarologia 2023, 63, 1048–1061. [Google Scholar] [CrossRef]
- Dalir, S.; Fathipour, Y.; Khanamani, M.; Hajiqanbar, H. Assessing Performance of Amblyseius swirskii as a Predatory Mite of Tetranychus urticae and Frankliniella occidentalis: Life Table and Foraging Behaviour Studies. Int. J. Acarol. 2024, 50, 587–594. [Google Scholar] [CrossRef]
- Bolckmans, K.; van Houten, Y.; Hoogerbrugge, H. Biological Control of Whiteflies and Western Flower Thrips in Greenhouse Sweet Peppers with the Phytoseiid Predatory Mite Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae). Available online: https://www.researchgate.net/publication/242278027 (accessed on 19 October 2025).
- Yaşar, İ.; Kök, Ş.; Kasap, İ. Investigation of the Synergistic Effect of Two Predatory Mites, Phytoseiulus persimilis and Amblyseius swirskii (Acari: Phytoseiidae), in the Biological Control of Tetranychus urticae (Acari: Tetranychidae). Int. J. Acarol. 2024, 50, 315–319. [Google Scholar] [CrossRef]
- Al-Azzazy, M.M.; Alhewairini, S.S. Life History of the Two Predacious Mites Species, Amblyseius swirskii, and Neoseiuluscucumeris (Acari: Phytoseiidae), as Biological Control Agents of the Date Palm Mite, Oligonychusafrasiaticus (Acari: Tetranychidae). Braz. J. Biol. 2024, 84, e283484. [Google Scholar] [CrossRef]
- Lopez, L. Meet Amblyseius swirskii(Acari: Phytoseiidae): A Commonly Used Predatory Mite in Vegetable Crops. J. Integr. Pest Manag. 2023, 14, 20. [Google Scholar] [CrossRef]
- Pavlyushin, V. Biological Plant Protection in Greenhouse, Intensive and Organic Farming. BIO Web Conf. 2020, 18, 00024. [Google Scholar] [CrossRef]
- Krasavina, L.P.; Trapeznikova, O.V. Assessment of Different Species of Fodder Mites For Mass Rearing of the Predatory Mites Amblyseius swirskii And Neoseiuluscucumeris (Mesostigmata, Phytoseiidae) Under Laboratory Conditions. Plant Prot. News 2020, 103, 177–181. [Google Scholar] [CrossRef]
- Khulbe, A.; Batra, P. Insect-Pests and Diseases in Greenhouse Cultivation and Their Biological Control. In Protected Cultivation; Apple Academic Press: New York, NY, USA, 2024; p. 38. ISBN 978-1-003-40259-6. [Google Scholar]
- Alpysbayeva, K.; Sharipova, D.; Nurmanov, B.; Seitzhan, A.; Naimanova, B. Enhancing Biological Control Efficiency: Predatory Potential of Phytoseiulus persimilis Against Tetranychus urticae in Greenhouse Conditions. Org. Farm. 2024, 10, 133–141. [Google Scholar] [CrossRef]
- Joshi, M.D.; Srivastava, A.K.; Ashaq, M.; Jaggi, S.; Gupta, P.; Hasan, W.; Gupta, S. Biocontrol Agents and Plant Protection. Uttar Pradesh J. Zool. 2024, 45, 109–131. [Google Scholar] [CrossRef]
- Kozlova, E.G.; Anisimov, A.I.T.; Moor, V.V.; All-Russian Institute of Plant Protection; Saint-Petersburg State Agrarian University; “Agrolider” Co., Ltd. Comparison of Efficiency of Different Populations of Predatory Mite Phytoseiulus persimilis in Conditions of Production Greenhouses. Plant Prot. News 2018, 3, 23–28. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Vangansbeke, D.; Lü, X.; De Clercq, P. Development and Reproduction of the Predatory Mite Amblyseius Swirskii on Artificial Diets. BioControl 2013, 58, 369–377. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Vangansbeke, D.; De Clercq, P. Artificial and Factitious Foods Support the Development and Reproduction of the Predatory Mite Amblyseius swirskii. Exp. Appl. Acarol. 2014, 62, 181–194. [Google Scholar] [CrossRef]
- Riahi, E.; Fathipour, Y.; Talebi, A.A.; Mehrabadi, M. Attempt to Develop Cost-Effective Rearing of Amblyseius swirskii (Acari: Phytoseiidae): Assessment of Different Artificial Diets. J. Econ. Entomol. 2017, 110, 1525–1532. [Google Scholar] [CrossRef]
- Ogawa, Y.; Osakabe, M. Development, Long-Term Survival, and the Maintenance of Fertility in Neoseiuluscalifornicus (Acari: Phytoseiidae) Reared on an Artificial Diet. Exp. Appl. Acarol. 2008, 45, 123–136. [Google Scholar] [CrossRef]
- Okassa, M.; Tixier, M.-S.; Kreiter, S. Morphological and Molecular Diagnostics of Phytoseiulus persimilis and Phytoseiulus macropilis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2010, 52, 291–303. [Google Scholar] [CrossRef]
- San, P.P.; Tuda, M.; Nakahira, K.; Takagi, M. Optimal Rearing Medium for the Population Growth of the Predatory Mite, Amblyseius swirskii (Athias-Henriot) (Acari: Phytoseiidae). Egypt. J. Biol. Pest Control 2020, 30, 130. [Google Scholar] [CrossRef]
- Calvo, F.J.; Knapp, M.; Van Houten, Y.M.; Hoogerbrugge, H.; Belda, J.E. Amblyseius swirskii: What Made This Predatory Mite Such a Successful Biocontrol Agent? Exp. Appl.Acarol. 2015, 65, 419–433. [Google Scholar] [CrossRef]
- Andreeva, I.V.; Zenkova, A.A.; Tsvetkova, V.P.; Gerne, D.Y. Gerne The Use of Inorganic Substrates in the Technology of Reproduction Predatory Mites of Phytoseiulus. Innov. Food Saf. 2018, 1, 7–15. Available online: https://innfoodsecr.elpub.ru/jour/article/view/393/247 (accessed on 19 October 2025).
- Navajas, M.; Lagnel, J.; Fauvel, G.; De Moraes, G. Sequence Variation of Ribosomal Internal Transcribed Spacers (ITS) in Commercially Important Phytoseiidae Mites. Exp. Appl. Acarol. 1999, 23, 851–859. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Aphalo, P.J. OpenIntro statistics, by david m. diez, christopher d. barr and mine cetinkaya-rundel. UV4Plants Bull. 2017, 2016, 51–53. [Google Scholar] [CrossRef]
- Bolckmans, K.J.F.; van Houten, Y.M. Mite Composition, Use Thereof, Method for Rearing the Phytoseiid Predatory Mite Amblyseius swirskii, Rearing System for Rearing Said Phytoseiid Mite and Methods for Biological Pest Control on a Crop. 2006. Available online: https://patents.google.com/patent/WO2006057552A1/en (accessed on 19 October 2025).
- Abou-Awad, B.A.; Reda, A.S.; Elsawi, S.A. Effects of Artificial and Natural Diets on the Development and Reproduction of Two Phytoseiid Mites Amblyseius gossipi and Amblyseius swirskii (Acari: Phytoseiidae). Int. J. Trop. Insect Sci. 1992, 13, 441–445. [Google Scholar] [CrossRef]

| Development Stage | C | D1 | D2 | D3 | D4 |
|---|---|---|---|---|---|
| Larval | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 |
| Nymphal | 2.1 ± 0.1 | 2.5 ± 0.2 | 2.0 ± 0.1 | 2.3 ± 0.1 | 1.9 ± 0.1 |
| Pre-imaginal | 3.1 ± 0.1 ab | 3.6 ± 0.2 a | 2.9 ± 0.1 b | 3.3 ± 0.1 a | 2.8 ± 0.1 b |
| Imaginal | 9.9 ± 0.2 ab | 10.5 ± 0.3 a | 9.6 ± 0.2 b | 10.3 ± 0.3 a | 10.4 ± 0.3 a |
| HSD | − | − | 0.3 | − | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alpysbayeva, K.; Adilkhankyzy, A.; Seitzhan, A.; Anuarbekov, K.; Naimanova, B.; Turbekova, S. Optimizing Artificial Diet Composition for Enhanced Development and Fertility of Amblyseius swirskii. Insects 2025, 16, 1105. https://doi.org/10.3390/insects16111105
Alpysbayeva K, Adilkhankyzy A, Seitzhan A, Anuarbekov K, Naimanova B, Turbekova S. Optimizing Artificial Diet Composition for Enhanced Development and Fertility of Amblyseius swirskii. Insects. 2025; 16(11):1105. https://doi.org/10.3390/insects16111105
Chicago/Turabian StyleAlpysbayeva, Karlygash, Ainura Adilkhankyzy, Assel Seitzhan, Kanat Anuarbekov, Balzhan Naimanova, and Shyryn Turbekova. 2025. "Optimizing Artificial Diet Composition for Enhanced Development and Fertility of Amblyseius swirskii" Insects 16, no. 11: 1105. https://doi.org/10.3390/insects16111105
APA StyleAlpysbayeva, K., Adilkhankyzy, A., Seitzhan, A., Anuarbekov, K., Naimanova, B., & Turbekova, S. (2025). Optimizing Artificial Diet Composition for Enhanced Development and Fertility of Amblyseius swirskii. Insects, 16(11), 1105. https://doi.org/10.3390/insects16111105






